ABSTRACT
The present study investigates the role of quorum sensing (QS) molecules expressed by C. sakazakii in biofilm formation and extracellular polysaccharide expression. The QS signaling was detected using Chromobacterium violaceum 026 and Agrobacterium tumefaciens NTL4(pZLR4) based bioassay. Long chain N-acyl-homoserine lactones (AHLs) with C6- C18 chain length were identified using High Performance Liquid Chromatography and Liquid Chromatography-High Resolution Mass Spectrometry. A higher Specific Biofilm Formation (SBF) index (p < 0.05) with the presence of genes associated with cellulose biosynthesis (bcsA, bcsC and bcsG) was observed in the strains. AHLs and their mechanisms can serve as novel targets for developing technologies to eradicate and prevent biofilm formation by C. sakazakii.
KEYWORDS: biofilm, cronobacter sakazakii, extracellular polymeric substances, mass spectroscopy, N-acyl homoserine lactone, quorum sensing, virulence
Cronobacter sakazakii is an opportunistic pathogen associated with serious diseases in adults as well as infants with more severe invasive infections in neonates such as meningitis and necrotizing enterocolitis.1 Epidemiologically, over 90% of these infections have been related to ingestion of contaminated powdered infant formula (PIF).2 The pathogen has been reported to form biofilms on enteral feeding tubes, silicon, stainless steel, polycarbonate, glass, and polyvinyl chloride (PVC) in order to survive the stressful growth conditions.3 According to the US National Institutes of Health announcement more than 80% of all microbial infections involving the formation of biofilms.4 Various factors such as flagella, outer membrane proteins, extracellular polysaccharide substances (EPS) and environmental conditions contribute to biofilm formation.5 Among these factors, the EPS has been shown to play a substantial part in determining the architecture of a biofilm.6
It has been stated that the bacterial processes such as the formation of biofilm, virulence and bioluminescence, are mediated by quorum sensing (QS).7,8 QS is a cell-to-cell communication system often mediated by the production of signaling molecules, or autoinducers (AI). The expression of QS molecules is regulated by the density and type of community and expression of virulence components of the infecting pathogen. These QS regulatory mechanisms are also being suggested as novel targets for developing advanced strategies to prevent infections.9 Previous studies from Lehner et al. and da Silva Araujo et al. reported the presence of 2 and 3 QS molecules in different Cronobacter spp.10,11 Therefore, this study investigated the biofilm formation, EPS composition, and identification of other QS molecules in C. sakazakii. The study also elucidated the interaction between biofilm formation, EPS production and AHL production.
For the quantification of biofilm formation, a specific biofilm formation (SBF) index was calculated as SBF = (AB-CW)/G in which AB = OD540nm of stained attached bacteria and CW = OD540nm of stained control wells containing bacteria-free medium only and G = OD600nm of cells growth in suspended culture. A strong SBF index was observed for all the tested strains at the interface of polystyrene microtiter well (p < 0 .05) (Table 1). The strong index indicates that the strains significantly formed a biofilm under prolonged periods of incubation (48 h). The presence of cellulose biosynthesis genes (bcsC, bcsA and bcsG) was further analyzed among the C. sakazakii strains (Table 1). All the tested strains possessed gene encoding for peptidoglycan hydrolase (flgJ) whereas the genes encoding for flagellar synthesis had different prevalences among the strains.
Table 1.
Biofilm formation, EPS composition and prevalence of biofilm forming genes among C. sakazakii strains.
| Biofilm Formation and Quantification |
EPS Composition |
Genes involved in biofilm formation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. sakazakii strains | BF = AB-CW | BF = AB/CW | SBF = (AB-CW)/G | Total Carbohydrate (µg/ml) | Total Protein (µg/ml) | bcsC | bcsG | bcsA | flgJ | fliD | flhE |
| ATCC 12868 | 3.2 | 8.06 | 2.95cNote. | 79.21bNote. | 0.58aNote. | + | + | + | + | + | + |
| E604 | 3.7 | 9.32 | 4.73dNote. | 87.06dNote. | 7.11dNote. | + | + | + | + | + | – |
| N13 | 1.87 | 4.71 | 1.90aNote. | 55.31aNote. | 5.06cNote. | + | + | – | + | – | + |
| N15 | 2.98 | 7.51 | 2.27bNote. | 82.76cNote. | 4.70bNote. | + | − | + | + | + | − |
a–d = Different letters in the same column indicate that the values are significantly different (p < 0.05) as measured by 2 sided Tukey's – post-hoc range test between replications
The composition of EPS expressed by C. sakazakii possessed very low protein:carbohydrate content and EPS expression differed among the isolates (p < 0 .05) (Table 1). The FTIR spectroscopy of the EPS revealed the presence of COOH groups (1600 cm−1 to 1725 cm−1) and -OH (2800 cm−1 to 3600 cm−1) groups, indicating that the samples were exopolysaccharide. The presence of -CH- vibrations in lipids, amide I in proteins, amide II in protein and -COC- group vibration in carbohydrates was indicated by the peaks obtained at wave no. range 3,200–2,800, 1,800–1,600 and 1,600–1,500, respectively.
The C. sakazakii strains produced colourless colonies with the CV026 bioassay and produced blue green color with the A. tumefaciens NTL4(pZLR4) bioassay indicating that the strains were expressing long chain AHL QS signaling molecules. Growth kinetic studies revealed that the expression of the AHL signal appeared only after 6 h of incubation. Thereafter, the production of AHL was directly proportional to the growth phase of the tested strains (Fig S1).
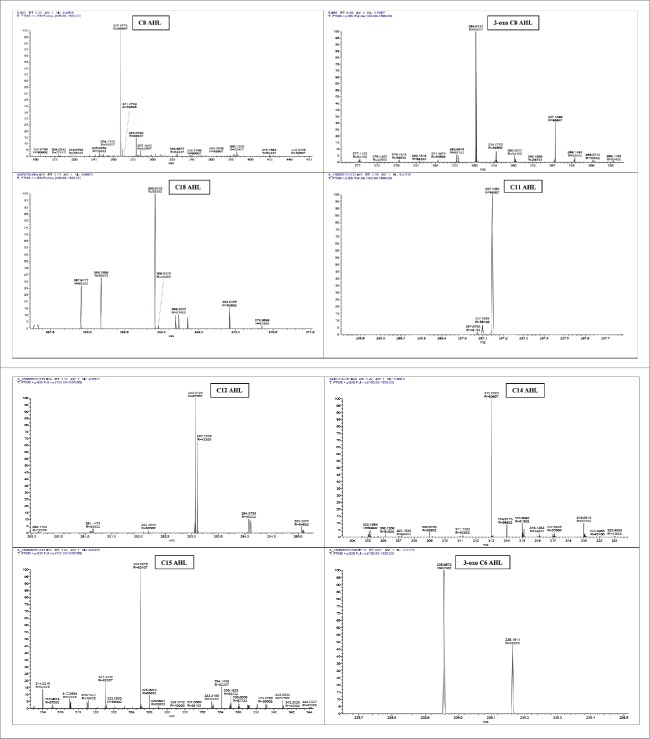
The FTIR analysis of the AHL showed the presence of a lactone ring, N-H bond, C-O bond by peak at 1664.33 cm−1, 1428.99 cm−1and 1179.90 cm−1, respectively. HPLC analysis provided a chromatogram showing that 3-oxo-C8 AHL (standard) possessed 2 peaks which were observed at 8.92 min and 85.11 min, indicating elution of both solvent (DMSO) and 3-oxo-C8, respectively. A single peak was observed in a test sample at 84.74 min showed the presence of AHL. The LC-HRMS analysis of AHL confirmed the presence of N-undecanoyl-L-AHL, N-dodecanoyl-L-AHL, N-tetradecanoyl-L-AHL, N-pentadecanoyl-L-AHL, N-(β-ketocaproyl)-L-AHL, N-octanoyl-L-AHL, N-3-oxo-octanoyl-L-AHL and N-octadecanoyl-L-AHL (Fig. 1).
Figure 1.
LC-HRMS profiles of AHL signals: Detection of AHLs by mass spectrometry analysis of supernatants extracts of C. sakazakii. A.C8 AHL (Retention time: 22.8 sec; m/z: 267.07) B. 3-oxo-C8 AHL (Retention time: 22.8 sec; m/z: 283.07) C. C18 ASL (Retention time: 18.6 sec; m/z: 368.91) D. C11 AHL (Retention time: 22.8 sec; m/z: 287.14) E. C12 AHL (Retention time: 22.2 sec; m/z: 283.07) F. C14 ASL (Retention time: 25.2 sec; m/z: 313.008) G. C15 AHL (Retention time: 25.2; m/z: 324.98) H. 3-oxo-C6 AHL (Retention time: 30.6 sec; m/z: 235.95)
The formation of biofilms by pathogens on biotic and abiotic surfaces increases the risk of infections by increasing their resistance or tolerance to environmental stresses and resistance to disinfectants and other commonly used antimicrobial compounds.3 Owing to the ubiquitous nature of the organism and the high severity of infection, a multilocus sequence typing (MLST) method has been developed for the rapid and reliable identification and discrimination between different Cronobacter spp12 The confirmed C. sakazakii strains and standard C. sakazakii strains were investigated for their ability to form biofilms using a microtitre plate assay in the present study. For the quantification of the biofilm, a SBF index was calculated for each strain which represents the most convenient mathematical formula to quantify the expression of bacterial biofilms.13 Strain E604 demonstrated a strong SBF as compared to other strains tested. Lehner et al.10 did not find any significant difference in biofilm forming abilities between the C. sakazakii isolates from human, environmental and foods whereas, Lee et al.14 reported significant variation in biofilm formation among different food isolates of Cronobacter spp. The variation in the biofilm forming abilities is reported to be strain-specific as reported in other studies in C. sakazakii.15 Our results revealed that the genes for biosynthesis of cellulose are present in tested C. sakazakii strains which also possessed a higher SBF index value. The expression of genes encoding cellulose biosynthesis (encoded by 2 operons bcsABZC and bcsEFG) has been reported in all Cronobacter spp except in C. sakazakii sequence type (ST) 13 and clonal complex (CC) 100.16 The study also reported the presence of enterobacterial common antigen (ECA) gene cluster (ECA1) in all the C. sakazakii.16 The study by Hu et al.17 also reported the prevalence/presence of bcsA (93.3%), bcsB (95%) and bcsC (100%) in 180 C. sakazakii isolates and also in C. sakazakii BAA-894 indicating that bcsABC genes are necessary to produce cellulose, and are involved in biofilm formation. These authors also suggested that the lack of bcsAB genes in some C. sakazakii strains might be due to mutation or loss of the bcsAB genes which seems to happen more frequently in C. sakazakii than that in other Cronobacter species. Our results also indicated the presence of flgJ in all the strains with the presence of fliD and flhE varying among the strains. These findings signified that the bcsABC genes are some of the genes present that may direct or regulate the biofilm formation. However, pleiotropic mechanisms still need to be understood since biofilm formation consists of series of complex processes which might be commonly regulated by the interaction between different genes and various environmental conditions.18,19 The presence of cellulose in the biofilm matrix was observed in all the strains using calcofluor plate assay (Fig S2)
Since the composition of the EPS can influence biofilm architecture, we investigated the total carbohydrates and concentration of proteins of the strains. The protein: carbohydrate ratio was very low, but significantly differed in all the tested strains. No correlation was observed between the EPS production and biofilm formation which may be due to difference in cell surface hydrophobicity due to low protein:carbohydrate20 and the varied levels of production of EPS components among different strains.10
It is well known that the biofilm-forming ability of bacteria is mainly due to production of AHL mediated QS.19 In our study, we did not observe any QS signal molecule using the CV026 biosensor indicating low production levels of short acyl AHLs (C4-AHL to C8-AHL). These short acyl AHLs can only be detected at higher concentrations as earlier reported in P. aeruginosa which either failed to produce short chain AHLs or the level of signals was very low.21 However, the production of a blue-green chromogenic reaction in the A. tumefaciens NTL4(pZLR4) and X-gal indicated the expression of long chain AHLs as signaling molecules in C. sakazakii. Further LC-HRMS analysis confirmed the expression of long chain AHLs among the tested strains. Taken together, the biosensor assay results and mass spectra data suggest that there is unequivocal confirmation of the presence of long chain AHL in the C. sakazakii.
Our study demonstrated production of long-chain AHLs among different C.sakzakii strains. Earlier, the GC-MS study of AHL produced by C. sakazakii confirmed the identification of 3 molcules: N-heptanoyl-AHL, N-dodecanoyl-AHL and N-tetradecanoyl-AHL.11 Previously, Lehner et al.10 tentatively indicated the presence of 2 different types of AHLs (3-oxo- C6-AHL and 3-oxo-C8-AHL) by Thin Layer Chromatography (TLC) which predicted the ability of Cronobacter spp. to produce cell-to-cell signaling molecules. Still, there are no other reports on the expression of QS molecules and their role in biofilm formation in C. sakazakii.
Our results reflect that the strains which have significant levels of long chain AHLs expression also showed higher SBF indices. These results are in accordance with the findings of Taghadosi et al.22 who reported that E. coli isolates with the highest AHL levels also exhibited strong adherence to microplate wells. Khajanchi et al.23 proposed that AHL-mediated QS signaling system modulates the virulence of Aeromonas hydrophila by regulating the expression of T6SS, metalloprotease and biofilm formation. Various other studies have addressed the regulatory consequence of cell-signaling mechanisms arbitrated by increases expression of AHL on the process of biofilm formation in different Gram-negative bacteria, including P. aeruginosa, Burkholderia cepacia and Serratia liquefaciens.24-26
To our best knowledge, this is the first report describing the expression of long chain AHLs in C. sakazakii and confirmed the presence of 3-oxo-C8-AHL. Since QS regulates the expression of many important phenotypes including virulence production, therefore the study of QS in C. sakazakii may lead to better understanding its survival in the different environments and hosts. Natural products isolated from plants that show anti-QS activity may provide a solution to prevent infection caused by QS pathogens.
The strains confirmed as C. sakazakii based on fusA loci (Table S1) of MLST analysis as suggested by Baldwin et al were used for further study.12 The isolates N13 and N15 (Isolate id 1669 and 1670 in http://pubmlst.org/cronobacter/) along with the 2 standard strains of C. sakazakii ATCC 12868 and E604 (kindly gifted by Dr. Ben Davies Tall, FDA, USA) were investigated. All the C. sakazakii strains were maintained in Tryptic Soy Broth medium (TSB). The two biosensors strains of Chromobacterium violaceum CV026 (kindly gifted by Dr. Paul Williams, University of Nottingham) and Agrobacteruim tumefaciens NTL4 (pZLR4) (kindly gifted by Dr. Stephen K Farrand, University of Illinois, US), were used for the detection of AHLs.27 The C. violaceum strain CV026 was cultured in Luria Bertani (LB) medium supplemented with 100 μg/ml ampicillin and 30 μg/ml kanamycin whereas A. tumefaciens strain NTL4(pZLR4) was cultured in nutrient broth (NB) medium containing gentamicin (50 μg/ml) at 28°C for 24 h.
Biofilm formation was quantified according to the method of Boddey et al.28 with slight modifications. Briefly, 230 μl of the TSB was added into each well of 96-well polystyrene plate followed by the addition of 20 μl of overnight grown bacterial culture. After incubation at 37°C for 48 h, the plates were rinsed 3 times with deionized water and the adhered cells were stained with crystal violet (CV) (1.0%, w/v) for 15 min. The CV was rinsed with water and the stained cells were liberated by glacial acetic acid (33%, v/v) following 30 min incubation. The sterile TSB was used as negative control and the absorbance was measured at 540 nm using Go scan microplate reader (Thermo, USA). The extent of biofilm formation was measured as depicted by Naves et al.13: (i) BF = AB-CW, where BF is the biofilm formation, AB is the OD540nm of stained attached bacteria and CW is the OD540nm of stained control wells containing bacteria-free medium only; (ii) BF = AB/CW; and (iii) SBF = (AB- CW)/G in which SBF is the Specific Biofilm Formation index and G is the OD600nm of ells growth in suspended culture. The SBF index in C. sakazakii strains was classified semi-quantitatively in 3 categories on the basis of absorbance for each of the formula used: strong (>2.5), moderate (1.5–2.5) and weak (<1.5).
The DNA was extracted from overnight grown culture using phenol:chloroform:iso-amyl alcohol method. The primers and PCR parameters for detection of genes (bcsC, bcsG, bscA, flgJ, fliD and flhE) involved in biofilm formation were reported in Ye et al.15 The PCR mixture (25 µl) consisted of 1 µl of DNA, 2.5 µl of 10 × PCR buffer, 0.5 µl of 25 mM MgCl2, 0.5 µl of 10 mM dNTP (Promega) mix, 1 µl each of 10 pM primers, 0.25 µl of 5 U Taq DNA polymerase and nuclease free water. The amplified products were analyzed in 1.5% agarose gel by staining with ethidium bromide.
The EPS was extracted by the modified procedure of Onbasli and Aslim.29 Five ml of overnight culture in TSB was centrifuged at 15,000 g for 5 min and boiled for 15 min at 100 °C. A 100 ml of trichloroacetic acid solution was added to the suspension and incubated for 1 h at 37°C. The mixture was kept in ice water for 30 min and centrifuged at 15,000 g for 20 min. The supernatant containing EPS was pooled with equal volume of ethanol. The mixture was kept at −20°C for 1 h and then centrifuged at 15,000 g for 20 min again. The precipitate was washed using 95% ethanol and centrifuged at 15,000 g for 20 min. The final precipitate was dissolved in 1 ml of deionized distilled water and was evaluated for the total carbohydrate content and protein using Phenol-Sulfuric Acid (PSA) method and Bradford method, respectively.30,31 The Fourier transform infrared (FTIR) (Agilent Cary 630 FTIR system) spectroscopy was done using KBr as the reference.32
The AHL production among the isolates was screened using well diffusion and test tube assay described in Mukherji and Prabhune.33 For the well diffusion assay, the test cultures were added in wells of the LB agar plate overlaid with CV026. The testing for AHL production against A. tumefaciensNTL4(pZLR4) was done in a similar way supplementing the agar with50 mg/ml X-gal. The production of violacein and blue halo around colony was taken as an AHL-positive response of CV026 and A. tumefaciens NTL4(pZLR4), respectively.
For test tube assay, a 500 μl of overnight broth culture of A. tumefaciensNTL4(pZLR4) was added to 10 ml of NB medium along with 1 ml inoculum of C. sakazakii strains and kept for overnight at 28 °C. A 2 ml of culture medium was withdrawn and centrifuged at 15,000 g for 10 min. The cell pellet was solubilised in 2 ml of dimethyl sulfoxide (DMSO), and centrifuged and the development of a blue-green color indicating the presence of acyl-AHL was measured at 630 nm.
The extraction of AHLs was done as described by Shaw et al.34 The strains were cultured in 500 ml of TSB for 18 h at 37 °C and 200 rpm. The supernatant of early stationary-phase culture was extracted twice with equal volumes of acidified ethyl acetate (0.1% formic acid). The organic phases were combined, dried over anhydrous magnesium sulfate and evaporated to dryness by rotary evaporation at 37 °C. The residue was resuspended in 500 μl of ethyl acetate and stored at −20°C. Aliquots (100 μL) of the extract were withdrawn from the top layer and placed in sample vials for FTIR, HPLC and mass spectrometry analysis.
The FTIR spectroscopic analysis of extracted AHL was performed using Agilent Cary 630 FTIR spectrometer.22 The reverse phase HPLC was done on C18 column of 250 × 4.6 mm using 3-oxo-C8 AHL as standard on Hitachi Chromeline HPLC system with UV detector.35 The mobile phase included mixture of acetonitrile and deionized water in the ratio 1: 99,30: 70, 50:50,70:30 for 15 min each followed by the ratio 99:1 for 30 min. The flow rate was maintained at 0.5 ml/min at 37°C. The LC-HRMS analysis of extracted AHL in acetonitrile was performed on Thermo Scientific, Hybrid Quadrapole Q-Exactive orbitrap mass spectrometer. The chromatographic separation was carried out using LC (Accela 1250 pump), Thermo Scientific Hypersil ODS C18 column of length 5 cm with particle size of 1.9 μm.36 The mass spectrometer was operated in a positive electrospray ionization mode in 70,000 full widths at half-height maximum resolution with mass range m/z 300 to 800. The operation conditions were as follows: spray voltage at 3.6 kV, capillary temperature at 320 °C, S-lens RF level at 50, automatic gain control (AGC) at 1 × 106, and maximum injection time at 120ms. Nitrogen was used as the sheath gas, auxiliary gas, and sweep gas, set at 45, 10, and 2, respectively (arbitrary units). The isocratic solvent system of acetonitrile and water was used in the ratio 99:1 holding for 5 min. Flow rate was adjusted at 350 μl/min. A volume of 1.5 μl of the sample was injected and full LC-HRMS scan was performed using positive polarity. The data were analyzed with Thermo Scientific Xcalibur software. The type of AHL was detected based on the m/z (mass to charge ratio).
The mean values (n = 3) were calculated for SBF index, carbohydrate and protein content and the comparison between the means was done by ANOVA and Tukey's multiple comparison test (p < 0.05) by SPSS software.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors wish to thank Department of Science and Technology (DST), grant number SB/FT/LS-426/2012, Govt. of India, Department of Biotechnology (DBT), grant number BT/Bio-CARe/07/605/2010-11, Government of India and Jaypee University of Information Technology (JUIT), Solan, India for providing financial support and essential facilities required for the research work.
References
- [1].Farmer JJ., III My 40-year history with Cronobacter/Enterobacter sakazakii–lessons learned, myths debunked, and recommendations. Front Pediatr 2015; 3:84; PMID:26640778; http://dx.doi.org/ 10.3389/fped.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singh N, Goel G, Raghav M. An insight into putative virulence factors determining the pathogenicity of Cronobacter sakazakii. Virulence 2015; 6:433-40; PMID:25950947; http://dx.doi.org/ 10.1080/21505594.2015.1036217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim H, Ryu JH, Beuchat LR. Attachment and biofilm formation by Enterobacter sakazakii on stainless steel and enteral feeding tubes. Appl Environ Microbiol 2006; 73:5846-56; http://dx.doi.org/ 10.1128/AEM.00654-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National Institute of Health: Research on microbial biofilms: 2002 ; PA Number: PA-03–047; http://grants.nih.gov/grants/guide/pa-files/PA-03-047.html [Google Scholar]
- [5].Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 1998; 30:285-93; PMID:9791174; http://dx.doi.org/ 10.1046/j.1365-2958.1998.01061.x [DOI] [PubMed] [Google Scholar]
- [6].Zogaj X, Bokranz W, Nimtz M, Römling U. Production of cellulose and curli fimbrae by members of the family Enterobacteraiceae isolated from the human gastrointestinal tract. Infect Immun 2003; 71:4151-58; PMID:12819107; http://dx.doi.org/ 10.1128/IAI.71.7.4151-4158.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol 2001; 55:165-99; PMID:11544353; http://dx.doi.org/ 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- [8].Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 2005; 21:319-46; PMID:16212498; http://dx.doi.org/ 10.1146/annurev.cellbio.21.012704.131001 [DOI] [PubMed] [Google Scholar]
- [9].Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology 2006; 152:895-04; PMID:16549654; http://dx.doi.org/ 10.1099/mic.0.28601-0 [DOI] [PubMed] [Google Scholar]
- [10].Lehner A, Riedel K, Eberl L, Breeuwer P, Diep B, Stephan R. Biofilm formation, extracellular polysaccharide production, and cell-to-cell signalling in various Enterobacter sakazakii strains: aspects promoting environmental persistence. J Food Protect 2005; 68:2287-94 [DOI] [PubMed] [Google Scholar]
- [11].da Silva Araujo FD, Esper LMR, Kuaye AY, Sircili MP, Marsaioli AJ. N-acyl-homoserine lactones from Enterobacter sakazakii (Cronobacter spp) and their degradation by Bacillus cereus enzymes. J Agr Food Chem 2012; 60:585-92; http://dx.doi.org/ 10.1021/jf203846f [DOI] [PubMed] [Google Scholar]
- [12].Baldwin A, Loughlin M, Caubilla-Barron J, Kucerova E, Manning G, Dowson C, Forsythe S. Multilocus sequence typing of Cronobacter sakazakii and Cronobacter malonaticus reveals stable clonal structures with clinical significance which do not correlate with biotypes. BMC Microbiol 2009; 9:223; PMID:19852808; http://dx.doi.org/ 10.1186/1471-2180-9-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Naves P, Del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, Rodríguez-Cerrato V, Ponte MC, Soriano F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method‐dependent. J Appl Microbiol 2008; 105:585-90; PMID:18363684; http://dx.doi.org/ 10.1111/j.1365-2672.2008.03791.x [DOI] [PubMed] [Google Scholar]
- [14].Lee YD, Park JH, Chang H. Detection, antibiotic susceptibility and biofilm formation of Cronobacter spp. from various foods in Korea. Food Control 2012; 24:225-30; http://dx.doi.org/ 10.1016/j.foodcont.2011.09.023 [DOI] [Google Scholar]
- [15].Ye Y, Ling N, Jiao R, Wu Q, Han Y, Gao J. Effects of Ca2+ and Mg2+ on the biofilm formation of Cronobacter Sakazakii Strains from powdered infant formula. J Food Saf 2015; 35:416-21; http://dx.doi.org/ 10.1111/jfs.12190 [DOI] [Google Scholar]
- [16].Ogrodzki P, Forsythe S. Capsular profiling of the Cronobacter genus and the association of specific Cronobacter sakazakii and C. malonaticus capsule types with neonatal meningitis and necrotizing enterocolitis. BMC genomics 2015; 16:1; PMID:25553907; http://dx.doi.org/ 10.1186/s12864-015-1960-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu L, Grim CJ, Franco AA, Jarvis KG, Sathyamoorthy V, Kothary MH, McCardell BA, Tall BD. Analysis of the cellulose synthase operon genes, bcsA, bcsB, and bcsC in Cronobacter species: Prevalence among species and their roles in biofilm formation and cell–cell aggregation. Food Microbiol 2015; 52:97-105; PMID:26338122; http://dx.doi.org/ 10.1016/j.fm.2015.07.007 [DOI] [PubMed] [Google Scholar]
- [18].Simoes M, Simoes LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT-Food Sci Technol 2010; 43:573-83; http://dx.doi.org/ 10.1016/j.lwt.2009.12.008 [DOI] [Google Scholar]
- [19].Surette MG, Bassler BL. Quorum sensing in Escherichia coli and Salmonella typhimurium. P Natl Acad Sci 1998; 95:7046-50; http://dx.doi.org/ 10.1073/pnas.95.12.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin HS, Kang ST, Nam SY. Effect of carbohydrates to protein in EPS on sludge settling characteristics. Biotechnol Bioprocess Eng 2000; 5:460-4; http://dx.doi.org/ 10.1007/BF02931948 [DOI] [Google Scholar]
- [21].Boşgelmez-Tınaz G, Ulusoy S, Arıdoğan B, Eroğlu F, Kaya S. N-butanoyl-l-homoserine lactone (BHL) deficient Pseudomonas aeruginosa isolates from an intensive care unit. Microbiol Res 2005; 160:399-03; PMID:16255145; http://dx.doi.org/ 10.1016/j.micres.2005.03.005 [DOI] [PubMed] [Google Scholar]
- [22].Taghadosi R, Shakibaie MR, Masoumi S. Biochemical detection of N-Acyl homoserine lactone (AHL) from biofilm-forming uropathogenic Escherichia coli (UPEC) isolated from urinary tract infection (UTI) samples. Rep Biochem Mol Biol 2015; 3:1-6 [PMC free article] [PubMed] [Google Scholar]
- [23].Khajanchi BK, Sha J, Kozlova EV, Erova TE, Suarez G, Sierra JC, Popov VL, Horneman AJ, Chopra AK. N-Acyl homoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology 2009; 155:3518-31; PMID:19729404; http://dx.doi.org/ 10.1099/mic.0.031575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heydorn A, Ersbøll B, Kato J, Hentzer M, Parsek MR, Tolker-Nielsen T, Givskov M, Molin S. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl Environ Microb 2002; 68:2008-17; http://dx.doi.org/ 10.1128/AEM.68.4.2008-2017.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov M, Molin S, Eberl L. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 2001; 147:2517-28; PMID:11535791; http://dx.doi.org/ 10.1099/00221287-147-9-2517 [DOI] [PubMed] [Google Scholar]
- [26].Labbate M, Queck SY, Koh KS, Rice SA, Givskov M, Kjelleberg S. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J Bacteriol 2004; 186:692-98; PMID:14729694; http://dx.doi.org/ 10.1128/JB.186.3.692-698.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McLean RJC, Whiteley M, Stickler DJ, Fuqua WC. Evidence of autoinducer activity in naturally-occurring biofilms. FEMS Microbiol Lett 1997; 154:259-63; PMID:9311122; http://dx.doi.org/ 10.1111/j.1574-6968.1997.tb12653.x [DOI] [PubMed] [Google Scholar]
- [28].Boddey JA, Flegg CP, Pay CJ, Beacham IR, Peak IR. Temperature-regulated microcolony formation by Burkholderia pseudomallei requires pilA and enhance associated with cultured human cells. Infect Immun 2006; 74:5374-81; PMID:16926432; http://dx.doi.org/ 10.1128/IAI.00569-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onbasli D, Aslim B. Effects of some organic pollutants on the exopolysaccharides (EPSs) produced by some Pseudomonas spp. strains. J Hazard Mater 2009; 168:64-7; PMID:19304385; http://dx.doi.org/ 10.1016/j.jhazmat.2009.01.131 [DOI] [PubMed] [Google Scholar]
- [30].Dubois M, Gillesk A, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for determination of sugars and related substances. Anal Chem 1956; 28:350-56; http://dx.doi.org/ 10.1021/ac60111a017 [DOI] [Google Scholar]
- [31].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/ 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- [32].Mancuso Nichols CA, Garon S, Bowman JP, Raguénès G, Guézennec J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J Appl Microbiol 2004; 96:1057-66; PMID:15078522; http://dx.doi.org/ 10.1111/j.1365-2672.2004.02216.x [DOI] [PubMed] [Google Scholar]
- [33].Mukherji R, Prabhune A. A new class of bacterial quorum sensing antagonists: glycomonoterpenols synthesized using linalool and α terpineol. World J Microb Biot 2015; 31:841-49; http://dx.doi.org/ 10.1007/s11274-015-1822-5 [DOI] [PubMed] [Google Scholar]
- [34].Shaw PD, Ping G, Daly SL, Cha C, Cronan JE, Rinehart KL, Farrand SK. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA 1997; 94:6036-41; PMID:9177164; http://dx.doi.org/ 10.1073/pnas.94.12.6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Teplitski M, Eberhard A, Gronquist M, Gao M, Robinson JB, Bauer WD. Chemical identification of N-acyl homoserine lactone quorum sensing signals produced in Sinorhizobium meliloti strains in defined medium. Arch Microbiol 2003; 180:494-97; PMID:14593447; http://dx.doi.org/ 10.1007/s00203-003-0612-x [DOI] [PubMed] [Google Scholar]
- [36].Fekete A, Frommberger M, Rothballer M, Li X, Englmann M, Fekete J, Hartmann A, Eberl L, Schmitt-Kopplin P. Identification of bacterial N-acyl homoserine lactones (AHLs) with a combination of ultra-performance liquid chromatography (UPLC), ultra-high-resolution mass spectrometry, and in-situ biosensors. Anal Bioanal Chem 2007; 387:455-67; PMID:17165024; http://dx.doi.org/ 10.1007/s00216-006-0970-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.