Abstract
The Kanzius non-invasive radio-frequency hyperthermia system (KNiRFH) has been investigated as a treatment option for hepatic hyperthermia cancer therapy. The treatment involves exposing the patient to an external high-power RF (13.56 MHz) electric field, whereby the propagating waves penetrate deep into the tumor causing targeted heating based on differential tissue dielectric properties. However, a comprehensive examination of the Kanzius system alongside any associated toxicities and its ability to induce hepatic hyperthermia in larger animal models, such as swine, are the subjects of the work herein. Ten Yucatan female mini-swine were treated with the KNiRFH system. Two of the pigs were treated a total of 17 times over a five-week period to evaluate short- and long-term KNiRFH-associated toxicities. The remaining eight pigs were subjected to single exposure sessions to evaluate heating efficacy in liver tissue. Our goal was to achieve a liver target temperature of 43°C and to evaluate toxicities and burns post-treatment. Potential toxicities were evaluated by contrast-enhanced MRI of the upper abdomen and blood work, including complete metabolic panel, complete blood count, and liver enzymes. The permittivities of subcutaneous fat and liver were also measured, which were used to calculate tissue specific absorption rates (SAR). Our results indicate negligible KNiRFH-associated toxicities; however, due to fat overheating, liver tissue temperature did not exceed 38.5°C. This experimental limitation was corroborated by tissue permittivity and SAR calculations of subcutaneous fat and liver. Significant steps must be taken to either reduce subcutaneous fat heating or increase localized heating, potentially through the use of KNiRFH-active nanomaterials, such as gold nanoparticles or single-walled carbon nanotubes, which have previously shown promising results in murine cancer models.
Keywords: Hyperthermia, swine, hepatic, Kanzius
The Kanzius non-invasive radiofrequency hyperthermia system (KNiRFH) is comprehensively examined, including potential short- and long-term side effects and efficacy in inducing hepatic temperature changes. Although the system does not cause any toxic side-effects, even after periodic and regular exposure (up to 6 weeks), the system is incapable of achieving any clinically relevant temperature changes in hepatic tissue, which is primarily due to the large specific absorption rate of adipose tissue compared to hepatic tissue.
I. Introduction
Malignancies of the liver may arise primarily as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma, or secondarily as metastases from other primary sites including breast, lung, pancreas, or colorectal cancers. It is not a rare disease: HCC is the sixth most common cancer worldwide, and synchronous liver metastases are found in approximately 14.5% of colorectal cancer cases, which itself is the third most common cancer worldwide [1], [2]. Although surgery is the treatment of choice in suitable candidates, a significant proportion of liver malignancies are unresectable due to tumor extent, location, or poor hepatic reserve. Existing loco-regional therapies for unresectable disease include invasive radiofrequency or microwave ablation, as well as transcatheter arterial chemoembolization. Though effective at controlling tumor growth, if not reducing tumor burden, these therapies are not typically curative and disease recurrence is common. Over the last few decades, non-invasive radiofrequency hyperthermia (NiRFH) has been proposed to augment loco-regional therapies to improve response rate and increase survival, although certain technical challenges have limited its adoption into routine clinical practice [3].
NiRFH as an oncologic treatment involves non-invasively increasing tumor temperature to the range of 39-45°C to enhance susceptibility to cytotoxic agents and/or ionizing radiation therapy, in contrast to invasive radiofrequency (RF) ablation in which temperatures of 60°C or greater are achieved to cause coagulative tissue necrosis. Hyperthermia is thought to potentiate chemotherapeutics at the cellular level by altering the structure of cytosolic and nuclear proteins, as well as by inhibiting DNA repair mechanisms [3], [4]. Treatment with 40-42°C mild hyperthermia has also been shown to increase tumor oxygenation and blood flow, which may lead to greater accumulation of chemotherapeutics within tumors and heightened tumor susceptibility to radiation due to increased production of reactive oxygen species [5], [6]. Additionally, whereas certain modalities of delivering hyperthermia such as microwave, ultrasound, and infrared have limited penetration depths, RF energy is uniquely capable of penetrating into deep tissues within the human body.
Clinical trials involving the use of NiRFH, usually as adjunctive therapy to ionizing radiation and/or cytotoxic chemotherapy, have been performed and reported in a variety of malignancies including cancers of the central nervous system, head and neck, lung, breast, esophagus, rectum, cervix, bladder, soft tissue sarcomas, and skin [7], [8]. However, NiRFH has not been systematically evaluated to treat malignancies of the liver. Difficulties in heating efficacy and issues with skin burns have been reported, demonstrating that challenges in applying NiRFH successfully and reproducibly to the liver are a valid concern [9]–[16].
The purpose of this study was to investigate the safety and heating efficacy of repeated RF field treatments to the liver in a physiologically relevant swine model using the Kanzius non-invasive radiofrequency hyperthermia system (KNiRFH), which operates at 13.56 MHz, a harmonic of the industrial-scientific-medical (ISM) designated bands. Experimental findings regarding heating efficacy were then correlated with a model of tissue heating in electromagnetic fields based on specific absorption rate (SAR) of different organs and tissues. The KNiRFH system has been used over the last decade to investigate localized and targeted heating of hepatocellular, pancreatic and breast cancer models using nanomaterials such as gold nanoparticles, [17]–[20] quantum dots, [21] and single-walled carbon nanotubes [22], [23] However, a thorough and comprehensive evaluation of the safety and efficacy of this system as a stand-alone unit in a clinically relevant animal species has yet to be done and sets the premise of the work contained herein.
II. Methods and Materials
The experiment was performed in two series. All experiments were performed in swine with non-tumor-bearing normal livers. The first series was centered on evaluating safety/toxicity of repeated RF exposure in two pigs over six weeks. The second series was focused on determining heating efficacy of RF treatment in the livers of eight pigs under single exposure conditions.
A. First Series
1). Animal Model
Two female Yucatan miniature swine (Sinclair Bioresources; Auxvasse, MO) were housed and raised according to Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC) guidelines. All experimental procedures were done in accordance with the Baylor College of Medicine Institutional Animal Care and Use Committee’s (IACUC) approval of protocol #AN6460. No special diet was given. At the time of experimental treatment, the pigs were 12 months of age and weighed 62–70 kg.
2). Experimental Setup First Series
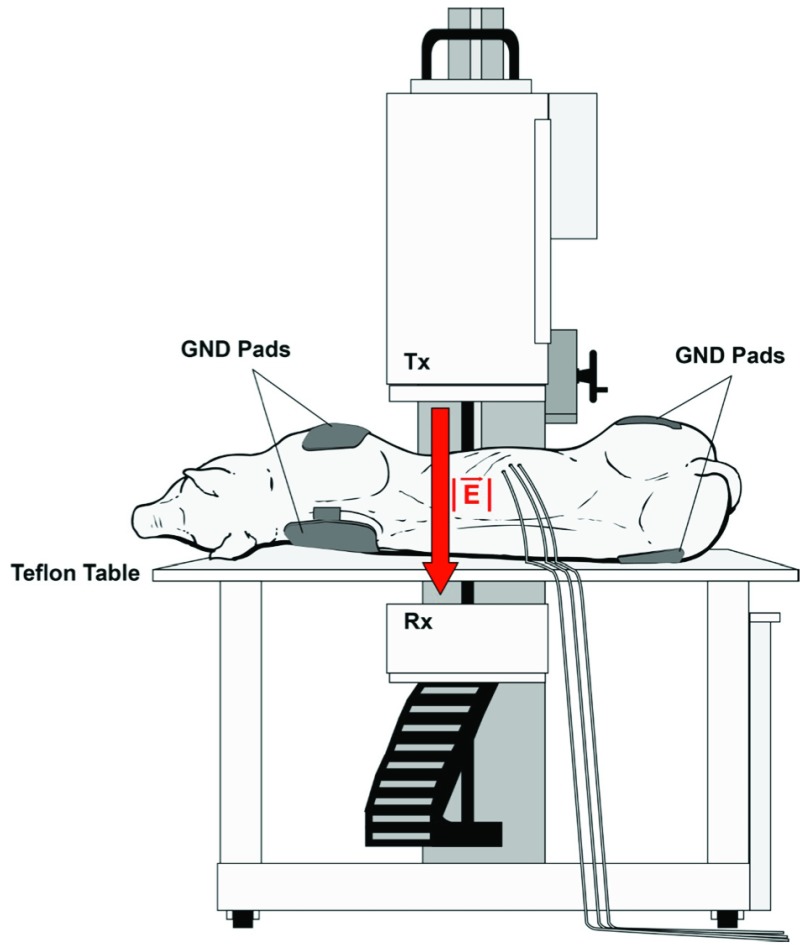
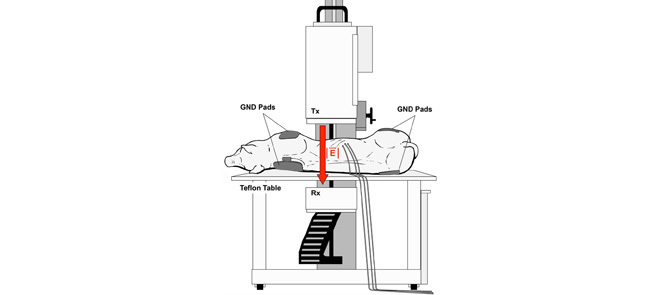
For each of the two pigs, KNiRFH treatments were delivered three times a week for five weeks and twice more on the sixth week for a total of seventeen treatments. Repeated exposures of this duration and frequency aims to mimic treatment schedules that would be anticipated for human use of a clinically adopted KNiRFH device, based off of previous reports of similar NiRFH devices [9]–[16]. For each treatment, general anesthesia was induced with intramuscular injection of telazol (4.4 mg/kg) and xylazine (2.2 mg/kg). Anesthesia was maintained with inhaled 1–3% isoflurane with supplemental O2. The pigs were placed in the supine to left lateral decubitus position on a non-conductive polytetrafluoroethylene table. The transcutaneous ultrasound-confirmed anatomic location of the liver was centered between the transmitting and receiving heads of the KNiRFH device. A total of four return electrode gel pads were placed bilaterally onto each shoulder and rump to ground the animals in the high intensity electrical field to prevent potentially injurious electrical arcing (Figure 1).
Figure 1.
Experimental RF treatment setup depicting pig positioning upon a Teflon table between the transmitting (Tx) and receiving (Rx) heads of the KNiRFH device and placement of grounding pads.
Temperature was measured with fiber optic probes (TF4; Photon Control, Burnaby, Canada) that were placed into the dermis and subcutaneous tissue overlying the liver for every treatment. These probes have been verified to be thermally and electrically inert under RF exposure at relevant output power. For selected treatments, two fiber optic probes were placed into the liver parenchyma via 14–16 gauge angiocatheters under ultrasound guidance. Liver probe placement was performed on average once a week to prevent excessive needle/catheter liver injury from repeated probe placements. Skin temperature was further monitored during KNiRFH treatment for potential cutaneous “hot spots” with a tripod mounted infrared camera (FLIR-T62101; FLIR Systems, Inc., Boston, MA).
3). KNiRFH Treatment
Treatments were delivered via the KNiRFH system (Therm Med, LLC, Erie, PA) that operates at a frequency of 13.56 MHz (Figure 1). A full system schematic can be found in the literature [24]. The transmitting head of the device was brought to a variable height of 3–6 cm (adjusted to minimize reflected power) from the nearest skin surface of the pig. With concurrent temperature monitoring, treatment began at 100–200 W of power followed by incremental ramp ups in device power to obtain a target liver tissue temperature of 43°C. Device power did not exceed 1200 W. Once target temperature was reached, external air convection cooling was utilized to reduce and control dermal and subcutaneous tissue temperature. Once the target temperature of 43°C was reached, or if any other tissue (i.e. dermis or subcutaneous tissue) reached 45°C or greater, RF device power was decreased to maintain steady-state for a treatment time of twenty minutes. At the conclusion of each treatment, the pigs were closely monitored until full recovery.
4). Imaging and Blood Analysis
Magnetic resonance imaging (Magnetom Trio 3T; Siemens, Erlangen, Germany) of the upper abdomen was performed before and after the six week series of treatment to evaluate the effect of repeated KNiRFH treatment on normal liver parenchyma. Non-contrast images were acquired using T1 VIBE and T2 HASTE sequences. Arterial, portal venous, and delayed (5 minute) phase contrast images were obtained under T1 VIBE sequence with gadoteridol (ProHance 0.1 mmol/kg; Bracco Diagnostic Inc., Milan, Italy) before and after KNiRFH.
Whole blood was sampled from ear veins once weekly prior to, during, and at the conclusion of the six weeks of KNiRFH treatment. Further samples were drawn immediately after treatments to assess for possible acute changes. Samples were analyzed for complete blood count (CBC) with differentiation and a complete metabolic panel including serum electrolytes, creatinine, blood urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), direct and indirect bilirubin, creatine kinase (CK), and lactate dehydrogenase (LDH).
5). Statistical Methods
Statistical analysis was performed on Prism 6 (GraphPad Software, Inc., La Jolla, CA). Comparison of blood/serum values before and after RF treatment was performed with paired two-tailed t-test. Linear regression was used to analyze blood/serum trends over time. A p-value of less than 0.05 was selected to determine statistical significance.
B. Second Series
1). Experimental Setup Second Series
Eight Yucatan female miniature swine were 6–12 months of age, weighed 37–50 kg, and were housed and maintained in a similar manner as the first series. In contrast to the first series, fiber optic temperature probes were placed under direct visualization into the liver via open abdominal surgery. For this surgical procedure, a midline laparotomy under general anesthesia was performed to gain access to the abdominal cavity, and subsequently the abdominal incision was closed with sutures after the probes were positioned and secured. KNiRFH treatment parameters were identical as in the first series with the exception that these were single treatment exposures.
2). Tissue Permittivity Analysis
After KNiRFH treatment but prior to euthanasia, pigs in the second series underwent in-vivo tissue permittivity measurements (Agilent 85070E [coaxial probe] and Agilent E4991A [impedance analyzer], Agilent Technologies, Santa Clara, CA) via the abdominal incision. The probe, which measures both real and imaginary permittivity in the 10 MHz to 3 GHz frequency range, was calibrated according to manufacturer specifications and brought into direct contact with tissue for measurement. Tissue measurements of subcutaneous fat, liver, stomach, spleen, small intestine, pancreas, and kidney were performed in this manner.
III. Results
A. KNiRFH Treatment and Heating Efficacy
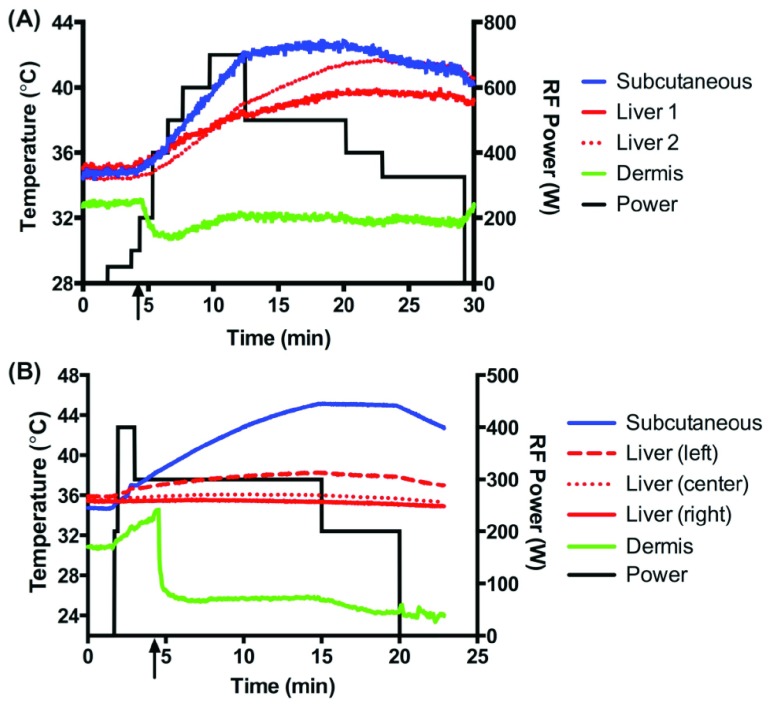
The KNiRFH treatments were well tolerated by the pigs in series 1 and no external signs of injury or behavioral changes were observed during the six weeks of treatment. A sample heating plot of the first series is shown in Figure 2A. Regarding hyperthermia of the liver, the rates of achieving ≥40°C, ≥41°C, or ≥42°C in one or more liver probes for a given treatment session were 61%, 50%, and 39%, respectively. External cooling was effective at controlling dermal and, to a lesser extent, subcutaneous tissue temperatures. Despite external cooling, subcutaneous overheating limited further attempts to achieve liver heating in 50% of the cases in which liver temperature did not exceed 40°C, with the remaining cases demonstrating no substantial heating in any of the tissues measured (dermis, subcutaneous fat, and liver).
Figure 2.
Representative temperature-time plot of KNiRFH of measured tissues in the first (A) and second (B) series. Device power is plotted on the secondary axis. Black arrow designates initiation of air convection cooling.
There was some discordance in temperature measurements between the two liver probes. In analyzing the subgroup of treatments in which one or more liver probes achieved ≥40°C, ≥41°C, or ≥42°C, the percentage of treatments in which the two probes showed a temperature difference of greater than 2°C was 55%, 56%, and 71%, respectively. In the second series in which the temperature probes were placed surgically, liver temperature did not exceed 38.5°C in all eight of the pigs treated. A sample plot is shown in Figure 2B.
B. Blood/Serum
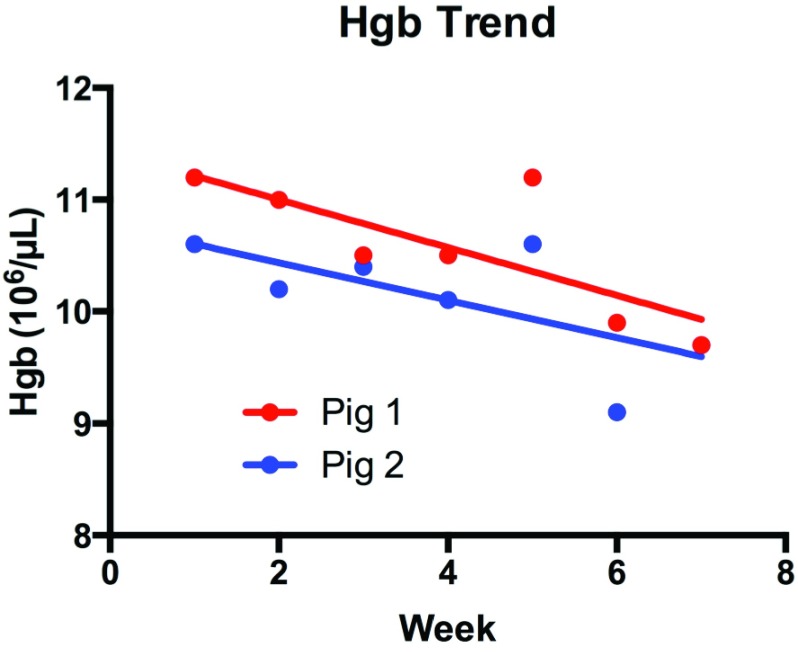
KNiRFH treatment did not result in a significant change in white blood cell (WBC) acutely (p=0.26, Supplementary Information Fig 1.) or longitudinally over six weeks (p=0.41 and 0.10, Supplementary Information Fig 2.). Platelet count did not significantly change from baseline and remained in the range of 550-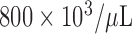 . Hemoglobin decreased both acutely (p=0.02 Supplementary Information Fig 3.) and over time (p=0.045 and 0.10, Figure 3) by an average of 11.5% and 11.0%, respectively.
. Hemoglobin decreased both acutely (p=0.02 Supplementary Information Fig 3.) and over time (p=0.045 and 0.10, Figure 3) by an average of 11.5% and 11.0%, respectively.
Figure 3.
Change in hemoglobin over six weeks of KNiRFH (p=0.045 and 0.10 for pigs 1 and 2 respectively).
Liver enzymes aspartate and alanine transaminases (AST and ALT, respectively) remained within normal physiologic levels (<50 U/L) with no significant elevation (p>0.11, Supplementary Information Fig 4.) at any time during or after the 6 weeks of KNiRFH field treatment. KNiRFH treatment did not impact serum electrolytes (Na, K, Cl, CO2, Ca, Mg, Phosp), BUN, creatinine, total bilirubin, or GGT. Mild, transient elevations in CK and LDH were observed but were not clinically noteworthy.
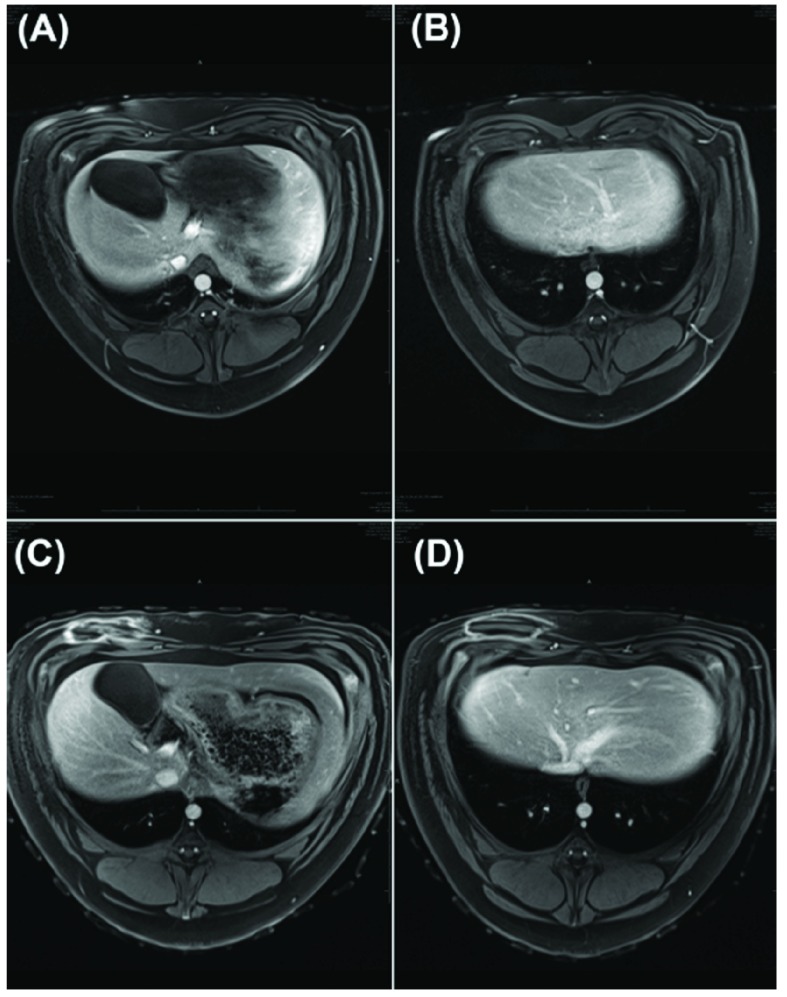
Figure 4.
MR images (T2 HASTE with contrast, arterial phase) of liver prior (A-B) and after (C-D) RF treatment.
C. Magnetic Resonance Imaging
Non-contrast T1 VIBE and T2 HASTE and triple-phase T1 VIBE contrast images were interpreted by a licensed radiologist. Comparison between images prior to and after the total of 17 RF treatments revealed no evidence of liver or surrounding organ injury (Figure 4). No significant differences in hepatic parenchyma density or characteristics and associated vasculature/ducts were identified.
D. Tissue Permittivity
Averaged real (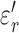 ) and imaginary (
) and imaginary (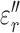 ) relative permittivities of measured tissues are shown in Supplementary Information Fig 5 and 6. At 13.56 MHz,
) relative permittivities of measured tissues are shown in Supplementary Information Fig 5 and 6. At 13.56 MHz, 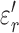 and
and 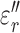 for subcutaneous fat were (mean ± standard deviation) 17.2 ± 3.6 and 132 ± 45, respectively;
for subcutaneous fat were (mean ± standard deviation) 17.2 ± 3.6 and 132 ± 45, respectively; 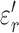 and
and 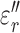 for liver were 195 ± 11 and 698 ± 69, respectively. These permittivity measurements were used to estimate the heating rate of tissues in the RF field by calculating the specific absorption rate (SAR), a parameter that quantifies the amount of electromagnetic energy converted into heat in a particular material [25]:
for liver were 195 ± 11 and 698 ± 69, respectively. These permittivity measurements were used to estimate the heating rate of tissues in the RF field by calculating the specific absorption rate (SAR), a parameter that quantifies the amount of electromagnetic energy converted into heat in a particular material [25]:
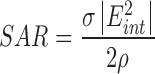 |
where 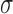 represents conductivity,
represents conductivity, 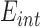 is the internal electric field, and
is the internal electric field, and 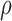 is the mass density. The internal electric field is in part dependent upon the three-dimensional permittivity milieu. Permittivity, which describes a material’s response to electric fields, becomes a complex parameter in time-varying electromagnetic fields and is defined as:
is the mass density. The internal electric field is in part dependent upon the three-dimensional permittivity milieu. Permittivity, which describes a material’s response to electric fields, becomes a complex parameter in time-varying electromagnetic fields and is defined as:
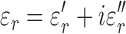 |
where 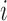 equals
equals 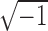 and
and 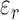 ,
, 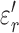 , and
, and 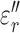 represent the complex, real, and imaginary relative permittivities, respectively. Furthermore, conductivity is related to permittivity and frequency (
represent the complex, real, and imaginary relative permittivities, respectively. Furthermore, conductivity is related to permittivity and frequency (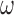 ) by:
) by:
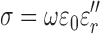 |
where 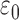 is the permittivity of free space.
is the permittivity of free space.
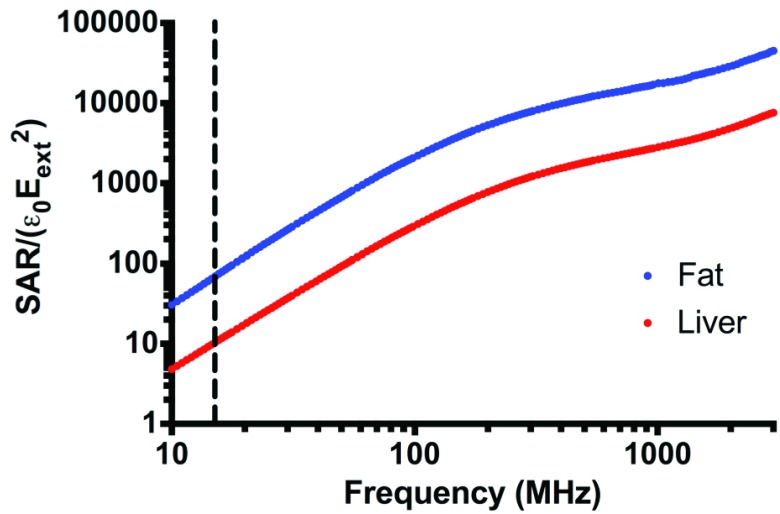
Figure 5.
Relative SAR for subcutaneous fat and liver from 10 MHz to 3 GHz. Dotted line indicates 13.56 MHz.
Because the internal electric field and hence SAR are geometry-dependent, a geometry must be defined before tissue SAR can be compared. In the simplified approximation of infinite tissue planes of subcutaneous fat and liver exposed to a capacitive RF device (Supplementary Information Fig 7.) boundary conditions at the material interfaces leads to the equalities [26]:
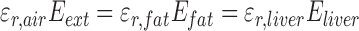 |
Combining equations 1–4 and substituting 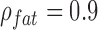 g/mL and
g/mL and 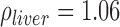 g/mL [27], relative SAR for subcutaneous fat and liver was obtained (Figure 5). Throughout the frequency spectrum measured, the SAR for subcutaneous fat was 5.8 to 7.3-fold greater than that of liver.
g/mL [27], relative SAR for subcutaneous fat and liver was obtained (Figure 5). Throughout the frequency spectrum measured, the SAR for subcutaneous fat was 5.8 to 7.3-fold greater than that of liver.
IV. Discussion and Conclusions
NiRFH as an oncologic treatment modality has garnered research interest and enthusiasm. Clinical use of NiRFH has been primarily focused on select cancers, i.e. breast, cervix, rectum, bladder, esophagus, and soft tissue sarcomas. Notably, tumors in these locations are either superficially located or arise within natural orifices and, therefore, RF applicators can be placed in close proximity to these tumors for effective heating. NiRFH in the liver and other intra-abdominal or retroperitoneal organs is ostensibly less well studied due to the difficulty associated with predictably and reliably heating these regions. It is known that deep heating can be limited due to overheating of subcutaneous fat, particularly with capacitively-coupled RF devices, which is somewhat ameliorated by aggressive surface cooling with water boluses. Moreover, invasive thermometry of intra-abdominal organs poses a greater risk of injury to surrounding structures compared to superficial or intra-cavitary (i.e. within the rectum or cervix) temperature probes. Our current experiments sought to determine if these challenges were surmountable to achieve reliable therapeutic hyperthermia of the liver with our KNiRFH field device operating at 13.56 MHz. This ISM-approved frequency is commonly used for medical hyperthermia devices because 13.56 MHz and other selected harmonic frequencies do not infringe on communication hardware such as cell phones, satellites, and wireless transmission systems.
Results showed that repeated treatment with our KNiRFH system produced no detectable toxicity or tissue injury. In our pigs, there was no evidence of abnormalities based on behavioral, external visible, metabolic/physiologic (based on serum electrolytes, liver function tests, and renal function), or radiologic (based on MRI) analysis. White blood cell and platelet counts remained within normal range throughout the treatment duration. The only statistically significant change was a decrease in hemoglobin from an average of 10.9 to 8.7 mg/dL (Figure 3). This decrease did not result in clinically apparent problems for the animals, and no treatment was needed. Importantly, the etiology of hemoglobin decrease may not be due specifically to RF treatment; repeated blood draws, bleeding from the liver surface after thermal probe placement, and exposure to anesthetics are other possible reasons for the anemia [28], [29].
That no skin burns or fat necrosis occurred during this study is noteworthy as it is a commonly reported complication in prior clinical trials (Table 1). We avoided skin burns or subcutaneous fat necrosis by fastidiously and uniformly applying skin cooling techniques: air convection prevents surface moisture that can be a locus for hot spots from developing or accumulating, as compared to water boluses in RF devices applied directly to the skin that promote moisture accumulation. Pigs are also less disposed to sweating than humans. Moreover, judicious monitoring of the subcutaneous fat overlying the liver and adjusting RF device power such that the subcutaneous fat did not exceed 45°C also limits the occurrence of fat necrosis from overheating. These results suggest that KNiRFH treatment when performed with proper temperature monitoring and treatment parameters can be delivered safely. However, extraordinary measures are required to overcome the biophysical problem of adipose tissue overheating caused by the high specific absorption rate (SAR) of fatty tissue when shortwave RF energy is applied.
TABLE 1. Overview of Previous NiRFH Systems and Associated Parameters.
| Author | Year | #Pat | #TM | LTT | RF Device | Freq (MHz) | Power (W) | S-cool | TT (mins) | MTher | Heating Success |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moffat et al.9,27 | 1983, 1985 | 178 | 38 | MET | IIMS-100 Capacitive | 13.56 | 65–360 | NR | 60–230 | TC/FO | 100% < 40°C |
| Petrovich et al.13 | 1989 | 49 | 49 | MET | BSD-1000 Phased array | 55 | 780 | NR | 45–60 | TC | 43% < 40°C |
| Nagata et al.10,11 | 1990, 1997 | 173 | 77 | HM | Thermotron RF-8 Capacitive | 8 | 800–1300 | WB | 40–50 | TC | 40% < 40°C |
| Hamazoe et al.26 | 1991 | 17 | 17? | HM | Thermotron RF-8 Capacitive | 8 | 700–1300 | NR | 40–50 | TC | 47% < 42°C |
| Seong et al.14 | 1994 | 84 | 16 | uHCC | Thermotron RF-8 Capacitive | 8 | 600–800 | SB | 30 | TC | 6.3% < 40°C |
| Urata et al.15 | 1995 | 7 | 7 | HM | BSD-1000 Phased array | 60–90 | NR | WB | 15–30 | TR | 0% < 40°C |
| Yamamoto & Tanaka16 | 1997 | 45 | 26 | HM | Thermotron RF-8 Capacitive | 8 | 500–1100 | SB | 50 | TC | 65% < 42°C |
| Noh et al.12 | 2014 | 3 pigs | 3 | NL | Celsius TCS Capacitive | 13.56 | 40–200 | WB | 60 | FO | 100% < 40°C |
NR designates not recorded. Patient numbers (#Pat) Number of temperatures measured (#TM) Liver Tissue Type (LTT); MET (Metastatic); Hepatic Malignancies (HM); unresectable HCC (uHCC); Normal Liver (NM); Surface cooling technique (S-cool); Treatment Time (TT, mins), method of thermometry (MTher); Water bolus (WB); Saline bolus (SB); Thermal Couple (TC); Fiber Optic (FO); Thermistor (TR).
In regards to heating efficacy, this study demonstrates that liver hyperthermia is not reliably achieved with the KNiRFH device in swine. In the first series of swine we treated, the rate of RF-induced liver hyperthermia of 41°C was only 50%. In the second series of animals where accuracy of intrahepatic probe placement was assured with an open laparotomy approach, the highest liver temperature reached was no greater than 38.5°C. We attribute this difference to the uncertainty of fiber optic temperature probe placement in the first series in which the probes were placed via a transcutaneous angiocatheter under ultrasound guidance. Subsequent retraction or displacement of the temperature probe during positioning of the animals for KNiRFH treatment, however, likely resulted in unintended movement of the probes out of the tissue of interest. Furthermore, because the probes are not echogenic under ultrasound, their precise position in the liver could not be confirmed in the first series of animals. This set of problems could account for the heterogeneity in liver temperature measurements from the first series. It is clear that proper positioning and confirmation of thermal probes is essential.
We found that liver heating was limited by undesirable heating of subcutaneous fat tissue (Figure 2), which was approximately 3–4 cm thick in the animals used in these experiments. This finding is consistent with prior reports in the literature, in which it has been asserted that deep organ hyperthermia with capacitive RF devices is feasible only in lean patients. Our inability to generate adequate liver hyperthermia is not unusual, as numerous other studies have documented difficulty in heating the liver with RF energy. A compilation of studies investigating the use of NiRFH on liver and liver tumors is shown in Table 1 [9]–[16], [29]–[31]. Most studies reported an inability to generate hyperthermia of greater than 40°C in 40% to 100% of patients in which thermometry was performed. In a study by Seong et al. hyperthermia was successful in 93.7% of cases in which thermometry was performed, however, thermometry was only performed on 19% of patients undergoing NiRFH [14]. Clearly, selection bias cannot be ruled out and this data is inadequate to support certainty of liver heating and for regulatory organization-required approval of a medical device that operates with a high degree of precision with reproducible results in all patients. Furthermore, invasive thermocouples and thermistors may be sources of significant measurement error as they contain metallic leads and wires, which can not only perturb electromagnetic fields around the probe, but also induce currents within the wires leading to inaccurate registered temperature values [25].
The calculated SAR between fat and other abdominal organs corroborates the limitation in obtaining liver hyperthermia via KNiRFH applications because of fat overheating. Based on our measurements of tissue permittivity, the SAR of subcutaneous fat is 6.6-fold greater than that of the liver at 13.56 MHz. The increased vascularity and high blood flow rate of liver tissue compared to subcutaneous fat also serves as a heat sink, making liver tissue more difficult to heat. Thus, a capacitive 13.56 MHz RF device does not appear capable of generating therapeutically relevant and reproducible liver or other deep tissue hyperthermia. Increasing the operating frequency permits the focusing of electric fields due to its shorter wavelength at the cost of decreased tissue penetration. From the standpoint of tissue SAR, our measurements show that increasing the electric field frequency would have no benefit, since the SAR of subcutaneous fat consistently remains approximately six-fold greater than that of liver from 10 MHz to 3 GHz.
Attaining reliable external RF field-induced hyperthermia of the liver continues to be a challenge. Furthermore, we have not even addressed in our animal study the heterogeneity in human populations related both to obesity rates and differences in bioelectrical properties of the liver [32], [33]. Individual humans vary greatly in their body mass index and in the amount and thickness of subcutaneous adipose tissue. The inability to overcome the high SAR of fatty tissue using shortwave RF field devices will be problematic in a significant proportion of humans with even mild to moderate, much less morbid obesity. Achieving dependable deep tissue hyperthermia to treat malignant disease in the liver, pancreas, gastrointestinal organs, retroperitoneal organs, or pelvic organs will be thwarted by deposition of the RF field energy in adipose tissue. For the liver specifically, the electrical properties of malignant liver tumors and of the liver itself can vary greatly depending on the type of tumor and the quality of the liver, i.e. normal liver versus steatotic versus cirrhotic liver [33]. Our animals were all healthy, relatively lean pigs with normal livers and we were not able to produce RF-induced liver hyperthermia adequately or repeatedly.
Clinically, NiRFH has been prescribed by calculating or estimating the “thermal dose,” defined by the cumulative equivalence minutes at 43°C (CEM43), which originates from the Arrhenius relationship based on a threshold temperature of 43°C [34]–[36]. It is clear that accurate, real-time tissue thermometry is an essential therapeutic parameter if reliable electromagnetic hyperthermia devices are to be used routinely as part of cancer treatment. However, in many clinical studies patients have been treated with NiRFH without tissue thermometry (Table 1), thus it is impossible to determine what thermal dose was delivered to tumors in these patients treated with no or inadequate tumor and normal tissue temperature monitoring. Invasive thermometry can be particularly challenging with regards to hepatocellular carcinoma as this disease commonly arises in the setting of existing viral or alcoholic cirrhosis, which leads to problematic comorbidities such as bleeding diatheses and ascites [14]. Serious complications such as pneumothorax, hemorrhage, or bowel injury are possible using invasive thermal probes, and while unlikely to happen in a single instance of probe placement, would cumulatively become a significant risk when multiple probes are invasively placed over many treatment cycles in numerous patients.
Improved thermometric techniques are needed to characterize thermal dose delivered to target tissues accurately and to establish reproducible clinical protocols. The ideal method would be able to provide a real-time three-dimensional thermal map of all relevant body tissues during hyperthermia treatment. The technique of magnetic resonance (MR) thermometry utilizing the proton resonance frequency shift method may eventually meet the above criteria and integration with NiRFH devices in a hybrid system has been investigated to some extent [37]–[40]. Currently, problems with spatial resolution due to respiration and body movement limit this technology from adequately resolving the concerns regarding use of RF electromagnetic field therapy to produce tumor tissue hyperthermia sufficient to provide therapeutic benefit while avoiding injury or toxicity related to heating of normal tissues or structures. Successful application of non-invasive, accurate real-time thermography techniques has the potential to minimize injuries by detecting internal body hot spots and improve understanding of energy/heat distribution leading to device and protocol optimization.
Ultimately, increasing the relevancy of RF electromagnetic field-induced tumor hyperthermia as a therapeutic modality for cancer will require improved modeling and individual patient treatment planning to overcome the very real problem of the high SAR, and resultant rapid heating, of adipose tissue when applying many otherwise clinically useful RF range frequencies.
With proper thermal monitoring of tissues, RF energy can be safely applied repeatedly to pigs without injuries observable in behavioral, serum/blood, and MRI analyses. Generating adequate liver hyperthermia, however, continues to be a challenge as our KNiRFH device was unable to heat the liver above 38.5°C in swine. Problematic heating of subcutaneous tissue remains the limiting factor. This experimental finding was corroborated by tissue permittivity measurements and analysis of SAR comparing subcutaneous fat and liver tissues across a range of radiofrequencies.
For KNiRFH to undergo rigorous clinical trial affirmation of improved treatment response when used with cytotoxic, biologic, or immunotherapeutic agents; with ionizing radiation; or with novel cancer-specific targeting approaches to enhance intracellular hyperthermia in cancer cells, the critical concerns that must be addressed include abrogation of heating in subcutaneous and other adipose tissue, development of accurate real-time non-invasive thermography techniques, improved biophysical understanding of the interactions between electromagnetic fields and malignant and normal tissues, and development of timely planning modules to customize treatment schema based on specific patient cancer type and body habitus.
Acknowledgment
The authors thank the BCM Core for Advanced Magnetic Resonance Imaging (CAMRI) for their MRI support (especially Lacey Berry, Krista Runge, and Michael Beauchamp) and the Center for Comparative Medicine for veterinary assistance (especially Dalis Collins, Paul Johnson, and Jenni Adams). The authors also give much thanks to Deborah Taylor and Eboni Lewis for surgical assistance.
Biographies
Jason C. Ho received the B.S. degree in physics from the University of Utah, with a focus on nanooptic research, in 2006, and the M.D. degree from The Ohio State University in 2012, where he is currently pursuing the General Surgery Residency with the Baylor College of Medicine. He was involved in investigating the effects of radiofrequency energy on in vitro and animal models of tumors. His research interests include bioelectromagnetics.
Lam Nguyen received the bachelor’s degree in biomedical engineering from the University of Houston and the master’s degree in management information system from the University of Houston at Clear Lake. He was with the Baylor College of Medicine as a Research Assistant, where he specialized in analysis and design. He is currently involved in patent filings in the field of hyperthermia to treat arterial encompassment in pancreatic cancer and related problems.
Justin J. Law received the B.S. degree in chemistry from Texas A&M University in 2009 and the Ph.D. degree in chemistry from Rice University in 2015. His Ph.D. thesis was entitled “Ultrashort Single-Walled Carbon Nanotubes: A Platform for Medical Imaging and Therapy.” In 2015, he joined the Baylor College of Medicine as a Post-Doctoral Associate, with a focus on noninvasive radiofrequency hyperthermia and treatments for liver and pancreatic cancer.
Matthew J. Ware received the master’s degree in nanomedicine from the Center for Nanohealth, in 2011, and the Ph.D. degree in nanomedicine from the Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, USA, in collaboration with the Center for Nanohealth, Swansea University, U.K., in 2014. In 2015, he was recruited for a postdoctoral appointment in the fields of nanomedicine and surgical research with the team of Dr. S. Curley, Baylor College of Medicine, Houston, specializing in developing noninvasive radiofrequency therapy for pancreatic and hepatic cancers. His research focused on innovative nanomedicine approaches for the treatment of pancreatic cancer.
V. Keshishian, photograph and biography not available at the time of publication.
N. C. Lara received the B.S. degree in chemistry from the California Institute of Technology, and the M.A. and Ph.D. degrees in chemistry from Rice University. As part of her graduate research, she was involved in the heat-producing interactions between conductive media and the Kanzius noninvasive radiofrequency field device. She has also identified safe, bio-compatible materials with potential for optimizing radiofrequency heating in biological tissues. Her Ph.D. was a funded through the 2013 NSF Graduate Research Fellow Program.
Trac Nguyen received the master’s degree in biomedical engineering from the University of Houston, Texas. His research focused on the recovery of voluntary movements in patients who undergo neuro-rehabilitation by investigating the cortical habituation of acoustic startle reflexes through EEG.
Steven A. Curley received the M.D. and F.A.C.S. degrees. He is currently a Professor of Surgery and the Chief of Surgical Oncology with the Baylor College of Medicine. He has been involved in electromagnetic (EM) device and EM field research for biomedical purposes for over 25 years. He has been involved with the Kanzius noninvasive radiofrequency field device since 2005. His goal is to produce more effective and less toxic multimodality cancer therapeutics. His research interests are developing devices to produce thermal damage to advanced or unresectable malignant tumors.
Stuart J. Corr received the B.Eng. degree (Hons.) in electronics with music from the University of Glasgow, U.K., in 2005, the M.Eng. degree in electronic systems with a focus on nanoelectronics and photonics, and the Ph.D. degree in engineering from Dublin City University, U.K., in 2008 and 2011, respectively. He is currently an Assistant Professor and the Director of Technology Development with the Department of Surgery, Baylor College of Medicine. He has been involved in the field of nanomediated radiofrequency cancer hyperthermia since 2009. He has authored several peer-reviewed papers and book chapters in the above-mentioned field. His current research interests lie in the field of medical device fabrication, 3-D printing technologies, and space nanomedicine.
Funding Statement
This work was supported by the Kanzius Research Foundation.
References
- [1].Ananthakrishnan A., Gogineni V., and Saeian K., “Epidemiology of primary and secondary liver cancers,” Seminars Interventional Radiol., vol. 23, pp. 47–63, Mar. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., and Bouvier A.-M., “Epidemiology and management of liver metastases from colorectal cancer,” Ann. Surgery, vol. 244, pp. 254–259, Aug. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wust P., et al. , “Hyperthermia in combined treatment of cancer,” Lancet Oncol., vol. 3, pp. 487–497, Aug. 2002. [DOI] [PubMed] [Google Scholar]
- [4].Takemoto M., et al. , “The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours,” Int. J. Hyperthermia, vol. 19, pp. 193–203, Mar-Apr 2003. [DOI] [PubMed] [Google Scholar]
- [5].Shakil A., Osborn J. L., and Song C. W., “Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia,” Int. J. Radiat. Oncol., Biol., Phys., vol. 43, pp. 859–865, Mar. 1999. [DOI] [PubMed] [Google Scholar]
- [6].Song C. W., Park H. J., Lee C. K., and Griffin R., “Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment,” Int. J. Hyperthermia, vol. 21, pp. 761–767, Dec. 2005. [DOI] [PubMed] [Google Scholar]
- [7].Datta N. R., et al. , “Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future,” Cancer Treatment Rev., vol. 41, pp. 742–753, Nov. 2015. [DOI] [PubMed] [Google Scholar]
- [8].Hurwitz M. and Stauffer P., “Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care,” Seminars Oncol., vol. 41, pp. 714–729, Dec. 2014. [DOI] [PubMed] [Google Scholar]
- [9].Moffat F. L., et al. , “Further experience with regional radiofrequency hyperthermia and cytotoxic chemotherapy for unresectable hepatic neoplasia,” Cancer, vol. 55, no. 6, pp. 1291–1295, Mar. 1985. [DOI] [PubMed] [Google Scholar]
- [10].Nagata Y., et al. , “Radiofrequency thermotherapy for malignant liver tumors,” Cancer, vol. 65, pp. 1730–1736, Apr. 1990. [DOI] [PubMed] [Google Scholar]
- [11].Nagata Y., et al. , “Clinical results of radiofrequency hyperthermia for malignant liver tumors,” Int. J. Radiat. Oncol., Biol., Phys., vol. 38, pp. 359–365, May 1997. [DOI] [PubMed] [Google Scholar]
- [12].Noh J. M., et al. , “In vivo verification of regional hyperthermia in the liver,” Radiat. Oncol. J., vol. 32, pp. 256–261, Dec. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petrovich Z., et al. , “Deep regional hyperthermia of the liver: A clinical study of 49 patients,” Amer. J. Clin. Oncol., vol. 12, pp. 378–383, Oct. 1989. [DOI] [PubMed] [Google Scholar]
- [14].Seong J., Lee H. S., Han K. H., Chon C. Y., Suh C. O., and Kim G. E., “Combined treatment of radiotherapy and hyperthermia for unresectable hepatocellular carcinoma,” Yonsei Med. J., vol. 35, pp. 252–259, Sep. 1994. [DOI] [PubMed] [Google Scholar]
- [15].Urata K., Uehara S., Hayashi H., Matsumata T., Takenaka K., and Sugimachi K., “Radiofrequency hyperthermia for malignant liver tumors: The clinical results of seven patients,” Hepato-Gastroenterol., vol. 42, pp. 492–496, Sep-Oct 1995. [PubMed] [Google Scholar]
- [16].Yamamoto K. and Tanaka Y., “Radiofrequency capacitive hyperthermia for unresectable hepatic cancers,” J. Gastroenterol., vol. 32, pp. 361–366, Jun. 1997. [DOI] [PubMed] [Google Scholar]
- [17].Glazer E. S. and Curley S. A., “Non-invasive radiofrequency ablation of malignancies mediated by quantum dots, gold nanoparticles and carbon nanotubes,” Therapeutic Del., vol. 2, pp. 1325–1330, Oct. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Glazer E. S., Massey K. L., Zhu C., and Curley S. A., “Pancreatic carcinoma cells are susceptible to noninvasive radio frequency fields after treatment with targeted gold nanoparticles,” Surgery, vol. 148, pp. 319–324, Aug. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raoof M., et al. , “Stability of antibody-conjugated gold nanoparticles in the endolysosomal nanoenvironment: Implications for noninvasive radiofrequency-based cancer therapy,” Nanomed., Nanotechnol., Biol., Med., vol. 8, pp. 1096–1105, Oct. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raoof M., et al. , “Gold nanoparticles and radiofrequency in experimental models for hepatocellular carcinoma,” Nanomed., Nanotechnol., Biol., Med., vol. 10, pp. 1121–1130, Aug. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Glazer E. S. and Curley S. A., “Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles,” Cancer, vol. 116, pp. 3285–3293, Jul. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gannon C. J., et al. , “Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field,” Cancer, vol. 110, pp. 2654–2665, Dec. 2007. [DOI] [PubMed] [Google Scholar]
- [23].Raoof M., et al. , “Remotely triggered cisplatin release from carbon nanocapsules by radiofrequency fields,” Biomaterials, vol. 34, pp. 1862–1869, Feb. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kanzius J. and Roy R., “RF systems and methods for processing salt water,” WO Patent 2008 064002 A2, Sep. 24, 2009.
- [25].Gandhi O. P., Biological Effects and Medical Applications of Electromagnetic Fields. Englewood Cliffs, NJ, USA: Prentice-Hall, 1990. [Google Scholar]
- [26].Guy A. W., Lehmann J. F., and Stonebridge J. B., “Therapeutic applications of electromagnetic power,” Proc. IEEE, vol. 62, no. 1, pp. 55–75, Jan. 1974. [Google Scholar]
- [27].Chan S. C., et al. , “Estimating liver weight of adults by body weight and gender,” World J. Gastroenterol., vol. 12, pp. 2217–2222, Apr. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chan F.-T., Chang G.-R., Wang H.-C., and Hsu T.-H., “Anesthesia with isoflurane and sevoflurane in the crested serpent eagle (Spilornis cheela hoya): Minimum anesthetic concentration, physiological effects, hematocrit, plasma chemistry and behavioral effects,” J. Veterinary Med. Sci., vol. 75, pp. 1591–1600, Dec. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gothelf A., Hojman P., and Gehl J., “Change in hemoglobin levels due to anesthesia in mice: An important confounder in studies on hematopoietic drugs,” Biol. Procedures Online, vol. 11, pp. 325–330, Dec. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hamazoe R., Maeta M., Murakami A., Yamashiro H., and Kaibara N., “Heating efficiency of radiofrequency capacitive hyperthermia for treatment of deep-seated tumors in the peritoneal cavity,” J. Surgical Oncol., vol. 48, pp. 176–179, Nov. 1991. [DOI] [PubMed] [Google Scholar]
- [31].Moffat F. L., et al. , “Effect of radiofrequency hyperthermia and chemotherapy on primary and secondary hepatic malignancies when used with metronidazole,” Surgery, vol. 94, pp. 536–542, Oct. 1983. [PubMed] [Google Scholar]
- [32].Apovian C. M., “The obesity epidemic—Understanding the disease and the treatment,” New England J. Med., vol. 374, pp. 177–179, Jan. 2016. [DOI] [PubMed] [Google Scholar]
- [33].Stauffer P. R., Rossetto F., Prakash M., Neuman D. G., and Lee T., “Phantom and animal tissues for modelling the electrical properties of human liver,” Int. J. Hyperthermia, vol. 19, pp. 89–101, Jan-Feb 2003. [DOI] [PubMed] [Google Scholar]
- [34].Dewey W. C., “Arrhenius relationships from the molecule and cell to the clinic,” Int. J. Hyperthermia, vol. 25, pp. 3–20, Feb. 2009. [DOI] [PubMed] [Google Scholar]
- [35].Overgaard J., “Some problems related to the clinical use of thermal isoeffect doses,” Int. J. Hyperthermia, vol. 3, pp. 329–336, Jul-Aug 1987. [DOI] [PubMed] [Google Scholar]
- [36].Sapareto S. A. and Dewey W. C., “Thermal dose determination in cancer therapy,” Int. J. Radiat. Oncol., Biol., Phys., vol. 10, no. 6, pp. 787–800, Jun. 1984. [DOI] [PubMed] [Google Scholar]
- [37].Kuroda K., “Non-invasive MR thermography using the water proton chemical shift,” Int. J. Hyperthermia, vol. 21, pp. 547–560, Sep. 2005. [DOI] [PubMed] [Google Scholar]
- [38].Lüdemann L., Wlodarczyk W., Nadobny J., Weihrauch M., Gellermann J., and Wust P., “Non-invasive magnetic resonance thermography during regional hyperthermia,” Int. J. Hyperthermia, vol. 26, pp. 273–282, Jan. 2010. [DOI] [PubMed] [Google Scholar]
- [39].Winter L., et al. , “Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: Electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla,” PLoS ONE, vol. 8, no. 4, p. e61661, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yuan J., Mei C.-S., Panych L. P., McDannold N. J., and Madore B., “Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry,” Quant. Imag. Med. Surgery, vol. 2, pp. 21–32, Mar. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]