Abstract
Lilium longiflorum cv. Nellie White, commonly known as Easter lily, is an important floral crop with an annual wholesale value of over $26 million in the United States. The root-lesion nematode, Pratylenchus penetrans, is a major pest of lily due to the significant root damage it causes. In this study, we investigated the cytological aspects of this plant–nematode interaction using bright-field and transmission electron microscopy. We took advantage of an in vitro culture method to multiply lilies and follow the nematode infection over time. Phenotypic reactions of roots inoculated with P. penetrans were evaluated from 0 to 60 d after nematode infection. Symptom development progressed from initial randomly distributed discrete necrotic areas to advanced necrosis along entire roots of each inoculated plant. A major feature characterizing this susceptible host response to nematode infection was the formation of necrosis, browning, and tissue death involving both root epidermis and cortical cells. Degradation of consecutive cell walls resulted in loss of cell pressure, lack of cytoplasmic integrity, followed by cell death along the intracellular path of the nematode’s migration. Pratylenchus penetrans was never seen in the vascular cylinder as the layer of collapsed endodermal cells presumably blocked the progression of nematodes into this area of the roots. This study presents the first detailed cytological characterization of P. penetrans infection of Easter lily plants.
Keywords: cell biology, Easter lily, electron microscopy, Lilium longiflorum, Pratylenchus penetrans, root-lesion nematode
Root-lesion nematodes (RLN) are considered the third most important group of plant-parasitic nematodes in terms of the economic losses caused in agriculture worldwide (Jones et al., 2013). Among the approximately 80 species described so far for the genus Pratylenchus (Fosu-Nyarko and Jones, 2016), P. penetrans (Cobb, 1917) Filipjev and Schuurmans Stekhoven, 1941, is considered one of the most important species of this genus due to its large distribution, wide host range of at least 400 plants, and its impact on economically important crops (Castillo and Vovlas, 2007). Infestations of this species present a major problem in the production of bulb flower crops, such as lily plants. Lily is one of the most economically important monocot flower bulb worldwide, used as cut flower, pot plant, grown in gardens, and in western Asia it can be used for edible or medical purposes (Bakhsaie et al., 2016). Economic production losses due to P. penetrans of several lily species include Lilium speciosum Thunb. and Lilium regale E.H. Wilson ‘Fire King’ in the Netherlands (Maas et al., 1978), Lilium longiflorum Thunb. in the United States (Westerdahl et al., 1993) and South Korea (Kang et al., 2013). In the United States, P. penetrans is considered one of the most damaging plant pathogens to Easter lily production (L. longiflorum cv. Nellie White), a floral crop with a pot plant wholesale value of approximately $26 million (Bakhsaie et al., 2016), whereas in South Korea lily production represents an annual value of $34 million (Kang et al., 2013). Disease symptoms on Easter lilies in the field are generally characterized by stunted shoot and root growth and chlorotic foliage. In highly infested fields, lily plants may not even be able to emerge from bulbs, whereas in fields having a moderate level of P. penetrans infestation, symptoms are not manifest until late in the growing season (Westerdahl et al., 2003).
The pattern of infection of different Pratylenchus species (e.g. Procambarus alleni Ferris, 1961, Pratylenchus coffeae Goodey, 1951, P. penetrans, Pratylenchus scribneri Steiner, 1943, Pratylenchus thornei Sher and Allen, 1953, Pratylenchus vulnus Allen and Jensen, 1951 and Pratylenchus zeae Graham, 1951) has been followed using a diversity of macro- or microscopic analyses in a variety of economically important crops or trees, such as alfalfa (Medicago sativa L.) (Townshend and Stobbs, 1981; Townshend et al., 1989), apple, (Malus pumila Miller) (Pitcher et al., 1960), carrot (Daucus carota subsp. sativus (Hoffm.) Schübl. and G. Martens) (Rohde, 1963), celery (Apium graveolens (Mill.) Pers.) (Townshend, 1963a), plantain (Musa × paradisiaca L.) (Pinochet, 1978), pea (Pisum sativum L.) (Oyekan et al., 1972), strawberry (Fragaris × ananassa Duchesne) (Townshend, 1963b; Kurppa and Vrain, 1985), snap beans (Phaseolus vulgaris L) and lima beans (Phaseolus lunatus L.) (Thomason et al., 1976), soybean (Glycines max (L.) Merr.) (Acosta and Malek, 1981), red clover (Trifolium pratense L.) and birdsfoot trefoil (Lotus corniculatus L.) (Townshend and Stobbs, 1981), rape (Brassica napus L.), oil radish (Raphanus sativus L.), tobacco (Nicotiana tabacum L.), potato (Solanum tuberosum L.) (Zunke, 1990a; Zunke, 1990b), corn (Zea mays subsp. mays L.) (Ogiga and Estey, 1975; Han et al., 1995), chickpea (Cicer arientinum, L.) (Castillo et al., 1998), sugarcane (Saccharum officinarum L.) (Kathiresan and Mehta, 2002), coffee (Coffea arabica L.) (Devi et al., 2009), and peach (Prunus persica (L.) Batsch) (Mountain and Patrick, 1959). However, microscopic analyses have not been performed for lily plants or other economically important bulb flower crops.
A major feature characterizing the host response to Pratylenchus spp. is the formation of lesions, necrotic areas, browning, and cell death of the root cells (Castillo and Vovlas, 2007; Fosu-Nyarko and Jones, 2016). As Pratylenchus spp. are able to migrate and move into and out of the roots, this provides open avenues for other secondary pathogens, including soil fungi and bacteria (Castillo and Vovlas, 2007). Infection of Pratylenchus spp. can occur along the entire length of the root, causing extensive damage to the epidermis and root cortex, and in particular hosts the root endodermis can be also affected (Jones et al., 2013). The severe damage due to both nematode intracellular migration and feeding activity, leads to the reduction of the plant root system, and consequent weakening of the plant’s capacity to acquire nutrients and water from the soil (Linsell et al., 2014; Fosu-Nyarko and Jones, 2016).
Current progress in Lilium genetic biotechnology and breeding of lily cultivars has been promoted by the establishment of in vitro culture systems that allows rapid production of plants, and constitutes a great resource for validation of new potential plant products for breeders and bulb producers (Bakhsaie et al., 2016). We took advantage of an in vitro culture methodology already established for multiplying lilies (Kamo and Han, 2008), and used this system to follow P. penetrans infection and development of root-lesion disease in L. longiflorum cv. Nellie White, using macro- and microscopic analyses. Herein, characterized in detail the mechanism of infection and general cell phenotype response of lily roots to P. penetrans infection.
Materials and Methods
Plant material
Lilium longiflorum cv. Nellie White plants were grown in vitro in Magenta jars containing Murashige and Skoog (MS) basal medium with vitamins (M519; PhytoTechnology, Shawnee Mission, KS) supplemented with 3% sucrose and solidified with 0.2% Phytagel (Sigma Aldrich, St. Louis, MO). Plants were subcultured every 3 to 4 mon and grown at 25°C under cool-white fluorescent lights (40–60 µmol⋅m−2⋅s−1) with a 12-hr photoperiod until used for nematode infection. Bulbs of stock cultures were individually transferred to petri dishes containing the same medium. Plants were allowed to grow until they had a root with >5 cm, and then they were used for the following experiments.
Nematode challenge assays
Pratylenchus penetrans (mixed stage population) were recovered from in vitro cultures of excised root cultures of maize (Zea mays L. cv. ‘Iochief’) maintained in MS medium (Rebois and Huettel, 1986). Nematodes were extracted by placing infected roots on a wire sieve in a sterilized glass bowl filled with distilled water containing 50 mg/liter carbenicillin and 50 mg/liter kanamycin. After 3 d, the sieve was removed and the solution containing the nematodes was poured into a 50-ml Falcon tube and centrifuged for 4 min at 4,000g and 4°C. The supernatant was removed with a sterile 10-ml pipette, and the nematode pellet was then resuspended with sterilized water containing both antibiotics mentioned above.
Nematode infections were carried out in four independent assays using 25 plants in each assay. Bulbs were placed in a depression created within the MS agar medium in order to allow roots to develop in the bottom of the petri dishes, and grown using the same conditions described above. The main roots (>5 cm) of 20 plants were inoculated with approximately 500 sterile nematodes (all stages), whereas five plants not inoculated with P. penetrans and grown in the same conditions were included as control. The nematode infection process and root-lesion disease development was followed either macroscopically or by light microscopy from 0 to 60 days after infection (DAI).
Acid fuchsin staining
In order to follow P. penetrans penetration, migration and reproduction within infected plants, roots inoculated from 1 to 30 DAI were extracted from the bulbs and stained with acid fuchsin following Byrd et al. (1983). Root tissues were then destained using a clearing solution (equal volumes of lactic acid, glycerol, and distilled water) for 2 to 4 hr at room temperature. After rinsing several times with tap water, roots containing nematodes were stored in acidified glycerol (five drops of 1.0 M HCl in 50 ml of glycerol), and observed using a Nikon Eclipse 50i light microscope.
Transmission electron microscopy
For transmission electron microscopy (TEM) analyses, infected nematode lily roots containing different levels of necrotic tissues (mild to strong necrotic regions) were collected. The infected roots were selected in agreement with the macroscopic symptoms observed at different time points after nematode infection (1–30 DAI). Roots from agar cultures were dissected under fixative into 1-mm pieces, and placed under vacuum for 30 min. Tissue was fixed for 2 hr at room temperature in 2.5% glutaraldehyde, 0.05 M sodium cacodylate, 0.005 M CaCl2 (pH 7.0), then refrigerated at 4°C overnight. Tissue was rinsed six times with 0.05 M sodium cacodylate, 0.005 M CaCl2 buffer, and postfixed in 1% buffered osmium tetroxide for 2 hr at room temperature. The tissue was then rinsed six times in the same buffer, dehydrated in a graded ethanol series followed by two exchanges of propylene oxide, infiltrated in a graded series of LX-112 resin/propylene oxide, and polymerized in LX-112 resin at 45°C for 18 hr then raised to 65°C for 24 hr. Silver–gold sections of 60 to 90 nm were cut on a Reichert/AO Ultracut ultramicrotome with a Diatome diamond knife and mounted onto 100-mesh carbon/formvar-coated copper grids or onto oval slot grids. Grids were stained with 4% uranyl acetate for 10 min and 3% lead citrate for 5 min, then imaged at 80 kV with a Hitachi HT-7700 transmission electron microscope.
Results
Development of symptoms in lily roots infected with P. penetrans
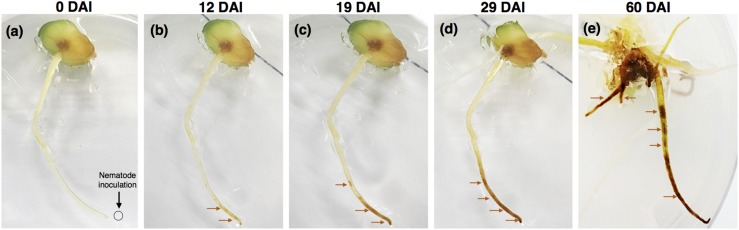
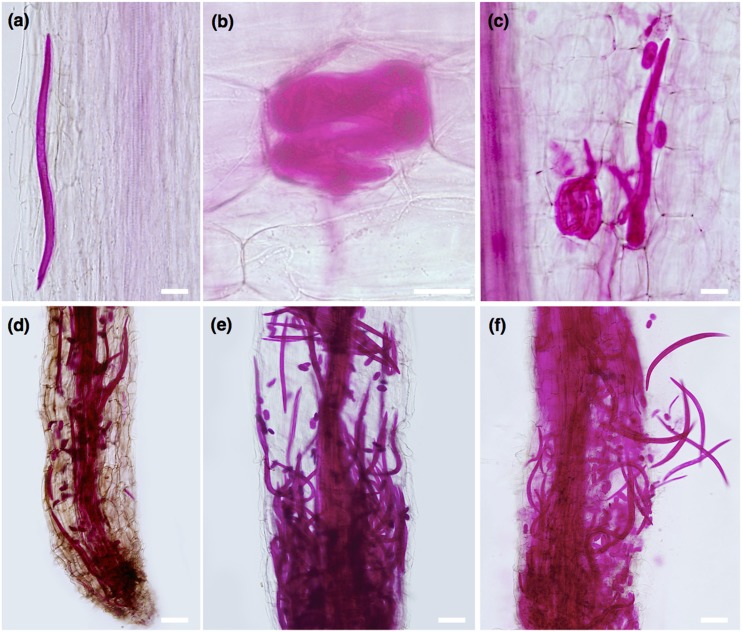
The development of symptoms caused by P. penetrans in lily roots were characterized initially by randomly distributed discrete lesions or browning areas along the root tip and elongation zone, to well advanced necrotic areas along the entire root of the plant at more advanced time points (Fig. 1). Symptoms were not observed in control roots that displayed a white light color (data not shown). The severity of symptoms of the roots increased along the different time points was studied (0–60 DAI), and was followed by acid fuchsin staining (Fig. 2). Although in the first day, a few nematodes were observed migrating within the root (Fig. 2A), or established in the epidermal and cortical root cells (Fig. 2B,C), after 4 DAI an increased number of nematodes was observed within the infected roots (Fig. 2D–F). Accumulation of nematodes was mainly found in the cortical tissue associated with the extensive distribution of lesions. All nematode stages (juveniles, males, and females) were found within the root tissues, including a high number of eggs laid by females. At later stages of infection (up to 60 DAI), roots exhibited severe necrotic brownish-black lesions, some of which coalesced to form longer necrotic tissue areas.
Fig. 1.
Symptom development in lily roots after root-lesion nematode (Pratylenchus penetrans) infection. A gradual development of lesions was observed in infected roots from 1 to 60 days after nematode infection (DAI). As nematodes were inoculated at the root tip, lesions were first distributed in this area, followed by progression of necrotic lesions along the entire root. The first four images are sequential time points after nematode infection using the same plant.
Fig. 2.
Acid fuchsin staining of Pratylenchus penetrans in different time points after lily root infection. A. Nematode migration within root tissues at 1 day after infection (DAI). B. Nematode coiled within epidermis root cell. C. At 4 DAI, females and egg deposition were observed within root tissues. D, E. After 12 DAI, a high increase of nematodes (eggs, juveniles, and adults) was observed along different areas of infected roots. F. Coalescence of necrotic root tissues at later time points. Scale bars: A–C = 50 µm, D–F = 100 µm.
In vivo microcopy analyses of lily infection by P. penetrans
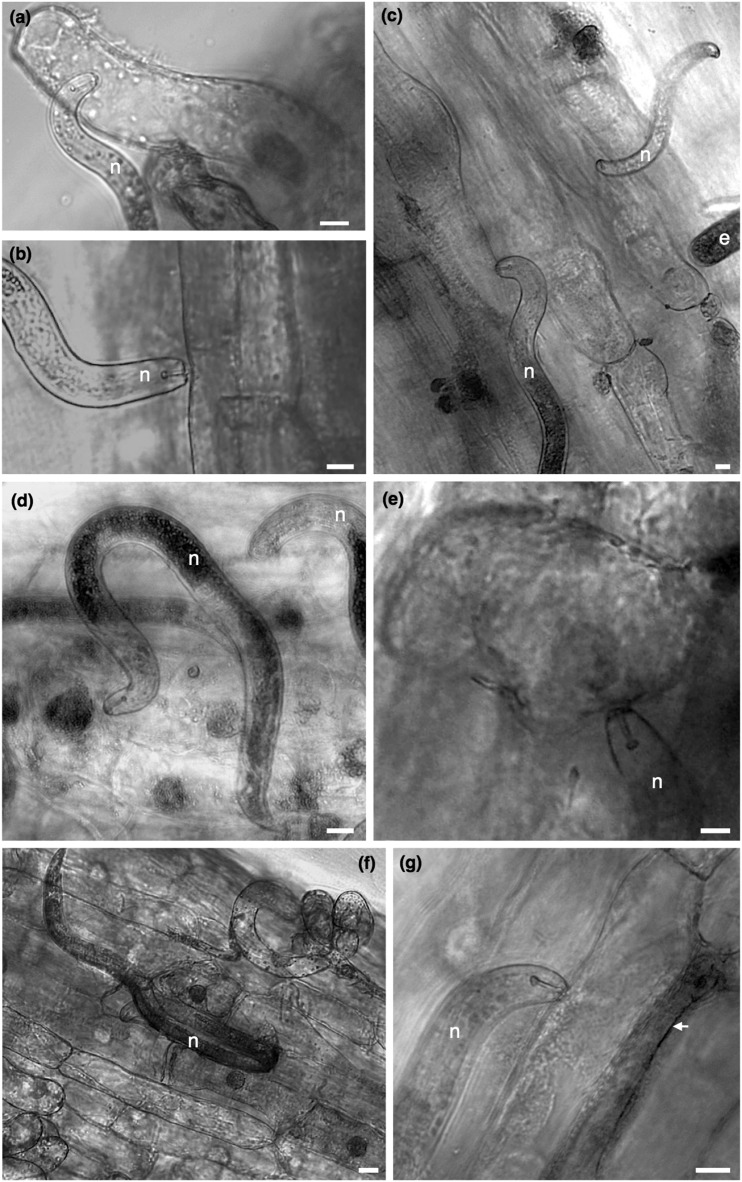
A few hours after nematode infection, all motile stages (juveniles and adult stages) were found near the root elongation zone. Nematodes were seen probing or feeding ectoparasitically on the epidermis of the main root (Fig. 3B), and occasionally juvenile stages were also seen exploring lily root hairs (Fig. 3A). One DAI, most nematodes were still in the medium dispersed along the root apical meristem and elongation zone, whereas a few nematodes could be seen probing/feeding on epidermal root cells (Fig. 3C). At this time point, a few eggs laid by adult females were often observed along the epidermal layer of roots or in the medium close to the main root. Three to five days after inoculation feeding occurred both ecto- or endoparasitically, with nematodes distributed within the first centimeters of the root, at different depths of the root (Fig. 3D–F). Cells punctured or fed upon nematodes displayed in some cases a darkly stained coloration of the cytoplasm surrounding the nuclei, and enlargement of the nucleus was frequently observed (Fig. 3D). As nematodes feed on root cells, these cells were observed to be shrunken (Fig. 3F) or containing a dense granular cytoplasm (Fig. 3G), and nematodes were seen associated for long periods with the same cell, whereas in other cases nematodes fed in different cells along the same area of the root. Interestingly, in some cases cells adjacent to the nematode, or cells that the nematode was feeding on, were also affected showing a dark coloration and collapsed morphology (Fig. 3G). As nematode infection proceeded (beyond 12DAI), necrotic areas were formed and cell collapse was observed in both epidermal and cortical cells in relation to the nematode activity.
Fig. 3.
Light microscopy of lily roots infected with Pratylenchus penetrans. A. Root-lesion juvenile nematode feeding on root hairs. B, C. A mixture of stages feeding/probing on epidermal cells of the main roots. D, E. Early stages of cellular reactions of lily root cells to P. penetrans, showing enlargement of the nuclei and occurrence of a darkened nucleus in some cells. F. Cells with shrunken cytoplasm. G. Nematode feeding cell with granular cytoplasm. In some cases, cells adjacent to nematode feeding cells (G, indicated by an arrow) were also affected revealing a dark coloration and collapsed morphology. Scale bars = 20 µm.
TEM of lily roots on P. penetrans infection
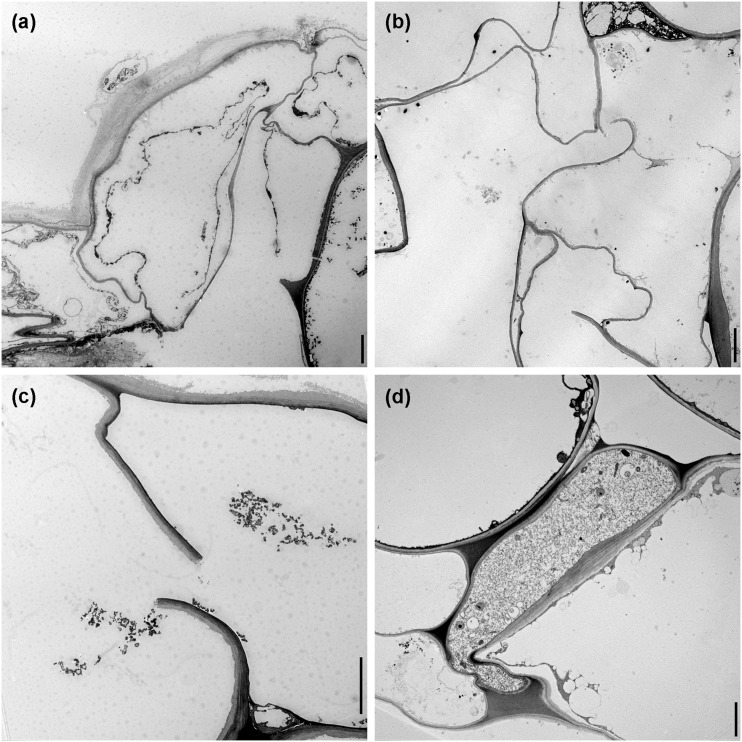
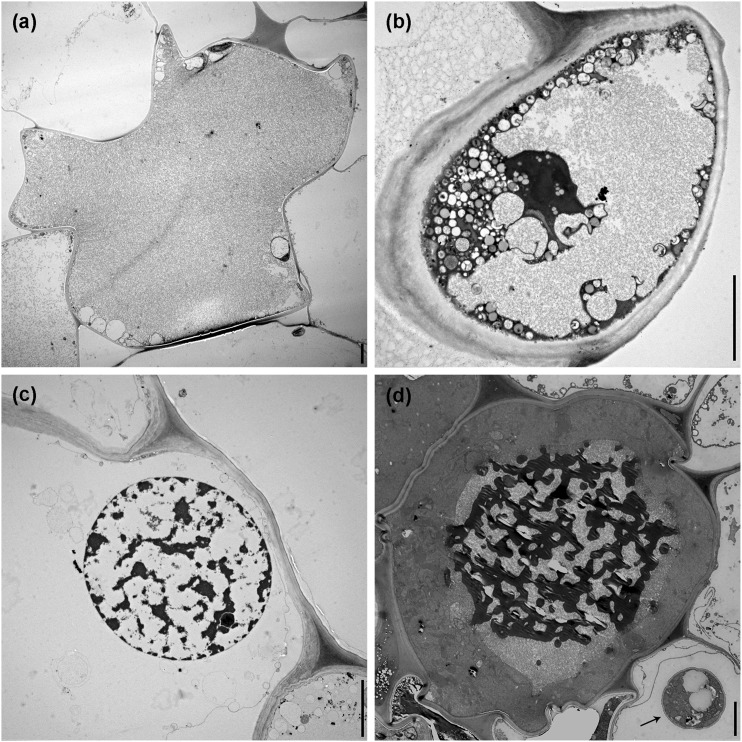
In transverse sections of nematode-infected roots, sectors of epidermal and cortical root cells with damage were observed by TEM. Epidermal cells affected by nematodes showed several levels of destruction of the cytoplasm (Fig. 4A), and there was often degeneration of their nuclei. In the case of root cortical tissue, cells fed upon by nematodes were lysed and generally devoid of cytoplasmic content (Fig. 4B,C), whereas in other cases cytoplasmic damage was prominent, as the contents of the cells became disorganized, discolored, organelles disintegrated, and filled with unrecognizable cellular debris. As nematodes migrated through the cortex layers, cavities among cells were observed, as a result of the breakdown of the contiguous cortical parenchyma cells. In some sections compressed cortex cells could be observed, probably associated with nematode activity and loss of turgor pressure of the cell (Fig. 4D).
Fig. 4.
Transmission electron microscopy images of lily roots infected with Pratylenchus penetrans. A. Deformed and necrotic epidermal cells. B, C. Cortical cells devoid of cytoplasmic contents with ruptured cell walls, creating large cavities within the cortex. D. Cortical cells adjacent to nematodes often showing a compressed phenotype. Scale bars = 5 µm.
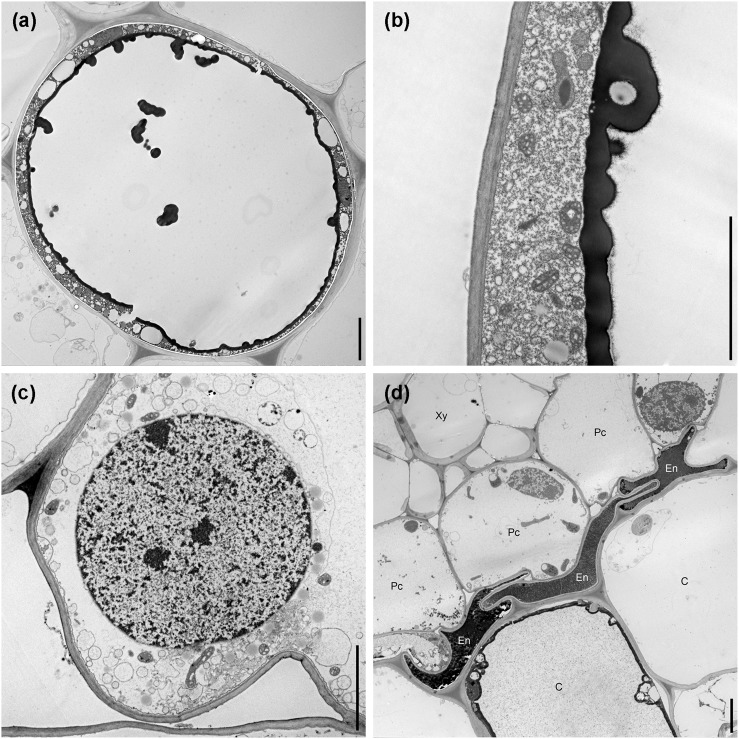
Cortical cells adjacent to the nematode body or adjacent to the cells fed upon by the nematode also seemed to be affected, showing slight to pronounced changes of the cell morphology and contents, accompanied by the accumulation of granular material in the vacuole and cytoplasm, disorganization of the organelles, condensation of chromatin within the nucleus, and the accumulation of electron-dense deposits within the cell (Fig. 5A–D). In some cells these dense tannin-like deposits formed rounded globules, which accumulated in the vacuoles, and were deposited along the inner layer of the tonoplast of the cells (Fig. 6A,B), and in some cases surrounding the cell nucleus. In some cells adjacent to nematode activity, the presence of numerous elongated mitochondria with a variety of shapes was also common, and high number of vesicles (Fig. 6C) was also observed. During the onset of nematode migration into deeper layers, we observed a disrupted and compressed endodermis, showing dense granular cytoplasm, extensive darkening, and electron-dense deposits (Fig. 6D). Nematodes were never found migrating or feeding within the vascular tissue of lily roots.
Fig. 5.
Typical characteristics of cortical cells adjacent to Pratylenchus penetrans activity, illustrated by transmission electron microcopy. A. Accumulation of granular material in the vacuole and cytoplasm. B. Breakdown of organelles and disorganization of the cytoplasm. C. Condensation of chromatin in the nucleus. D. Accumulation of electron-dense deposits within the vacuole, arrow indicates a nematode presenting within the cortex. Scale bars = 5 µm.
Fig. 6.
Additional characteristics of Pratylenchus penetrans–infected lily roots, illustrated by transmission electron microcopy. A. Deposition of tannin-like globules frequently observed in the vacuoles of cortical cells. B. Tannin-like globules are deposited onto the inner layer of the tonoplast forming a thick electron dense layer. C. Increased production of vesicles and abnormally shaped mitochondria. D. Compression of the endodermis due to the loss of structural rigidity of the cortex. Xy = xylem, Pc = pericycle, En = endodermis, C = cortex. Scale bars = 5 µm.
Discussion
The reaction of lily roots on infection by P. penetrans was herein characterized and followed by various macro- and microscopy methodologies. A hallmark of RLN infection is the formation of necrotic areas along the plant’s roots inherent to nematode feeding and migration activities (Fosu-Nyarko and Jones, 2016). To follow up P. penetrans’ pattern of infection, nematodes were initially inoculated adjacent to the lily root tip, allowing the nematodes to migrate into the roots. Depending on the host plant, P. penetrans seems to preferentially aggregate and penetrate around and above the elongation zone of several plants, e.g., rape, oil radish, tobacco, or potato (Zunke, 1990b), whereas in other cases, e.g., strawberry, P. penetrans penetrates roots in the region of root hair development (Kurppa and Vrain, 1985). Although the initial penetration site of P. penetrans in lily roots was mainly at the elongation zone (near the area where nematodes were initially inoculated), these nematodes seemed capable of penetrating lily roots in any part of the root, as nematodes were observed entering the entire root as disease and nematode development progressed.
The development of root-lesion disease was characterized by continuous browning and discoloration of the roots, a typical reaction of the plant to Pratylenchus spp. (Castillo and Vovlas, 2007), as a consequence of the nematode tissue damage, and cell death involving mainly epidermal and cortex cells of the root. When contact is made with the root, the nematode activity can be separated into different stages: cell probing, root penetration, feeding and migration, and reproduction (Zunke, 1990a; Linsell et al., 2014). Similar to other hosts, lily root hairs can provide some source of food for P. penetrans, as nematodes were often seen feeding from these cells. Nevertheless, the great majority of nematodes were found feeding ectoparasitically on root epidermal cells or endoparasitically in the root cortex.
In terms of cellular changes, the host reaction of lilies on P. penetrans infection was analogous to other hosts (Thomason et al., 1976; Acosta and Malek, 1981; Townshend et al., 1989; Zunke, 1990a), which involved destruction of the epidermal and cortex cells, severe disintegration of the cytoplasm from cells parasitized by the nematode, and in some cases the occurrence of hypertrophied nuclei. Different levels of degeneration were observed on cells fed upon by the nematode, with cells completely devoid of cytoplasmic content, containing degenerated organelles, and loss of an intact membrane. The degradation of epidermal and cortical cells is a typical characteristic of root-lesion disease, followed by cell death, as a consequence of the nematode’s migration within the roots. Sections of infected lily roots displayed different levels of degeneration within the cells adjacent to the cortical cells pierced by the nematode, or in close contact with the nematode’s path. Degenerate cells were characterized by the accumulation of dark granular material, condensed vesiculate cytoplasm, increased tannin-like depositions within the vacuoles, and general loss of membrane integrity of the tonoplast. In most cases, there was also a condensation of the chromatin within the nucleus, disruption of the nuclear membrane, and degeneration of cell organelles (e.g. mitochondria, endoplasmic reticulum). Our results corroborated other TEM studies performed in alfalfa roots infected with P. penetrans, where the occurrence of such cellular changes was also observed (Townshend et al., 1989).
The typical dark phenotype of infected root tissues depends on nematode infection and has been long linked to the presence of phenolic compounds and tissue oxidation, as a mechanism of plant response to Pratylenchus spp. (Pitcher et al., 1960; Townshend and Stobbs, 1981; Baldridge et al., 1998; Vaganan et al., 2014). Invasion by Pratylenchus spp. can trigger multiple signaling pathways during penetration and establishment of the nematodes in plants. A network of differential genes within different molecular pathways has been identified at different time points after nematode infection in roots (Backiyarani et al., 2015; Yu et al., 2015; Kaliyappan et al., 2016) and shoots (Zhu et al., 2014) of infected plants by P. coffeae, showing a dynamic expression of defense genes and products of the phenylpropanoid pathway of plant secondary metabolism (Backiyarani et al., 2014; Zhu et al., 2014; Yu et al., 2015). Although the molecular mechanisms of Pratylenchus spp. infection and disease establishment are still poorly understood, the pace and range of gene expression in resistant cultivars seem to be highly accelerated and enhanced when compared to susceptible cultivars of the same plant species (Backiarani et al., 2014; Backiarani et al., 2015; Yu et al., 2015).
The deposition of tannins is often observed as a defense compound produced by the plant against plant pathogens (Lattanzio et al., 2006). The occurrence of tannin-like deposits in the lily roots infected with P. penetrans was mainly observed in cells adjacent to other cells fed upon or in direct contact with the nematode. Previous studies suggested that cells adjacent to the nematode could be affected by components of nematode secretions, which penetrated adjacent cell walls through plasmodesmata (Zunke, 1990a). However, this can be seen as a defense mechanism of the plant in order to resist or block the progression of the nematode. Establishment and development of P. penetrans within the lily root tissues suggest that this species is able to tolerate or overcome the potential damage or toxic effects generated by the presence of such compounds or other defense mechanisms of the plant. Similar to other plant-parasitic nematodes, Pratylenchus spp. harbor a suite of putative-secreted proteins that are often considered key effector molecules for successful parasitism (Haegeman et al., 2011; Nicol et al., 2012; Burke et al. 2015; Vieira et al., 2015; Fosu-Nyarko et al., 2016), including a noteworthy number of genes potentially involved in protection from the host defenses, such as reactive oxygen species (Vieira et al., 2015; Fosu-Nyarko et al., 2016). A myriad of pioneer putative-secreted proteins has been also found within the transcriptomes of several Pratylenchus species (Haegeman et al., 2011; Nicol et al., 2012; Vieira et al., 2015; Fosu-Nyarko et al., 2016), which may encompass a direct protection of the nematode, or be involved in the suppression of defense molecular pathways of the host. However, the role and functionality of such nematode effector proteins are yet to be shown, as well as their effective involvement in protection from and promotion of root-lesion disease.
Progression of P. penetrans into the endodermis and ultimately to the vascular cylinder of roots seemed to be limited in lilies, as endodermis cells collapsed and presented a high accumulation of tannin-like deposits and necrosis. Contrary to other more specialized nematodes, such as cyst (Globodera and Heterodera spp.) and root-knot nematodes (Meloidogyne spp.) that promote their own feeding site (syncytia or giant cells, respectively) in the vascular cylinder of the roots (Rodiuc et al., 2014), Pratylenchus spp. are not able to become clearly established in the vascular cylinder of roots. This type of response observed in infected lily roots followed a similar pattern observed in some other plants, including alfalfa (Thomason et al., 1976), apple (Pitcher et al., 1960), carrot and celery (Townshend, 1963a), or snap and lima beans (Thomason et al., 1976). The endodermis in these plants appears to create a physical barrier that protects and prevents nematode migration into the root vascular cylinder (Thomason et al., 1976). Nevertheless, invasion of the stele or damage of the vascular vessels has been demonstrated in a few cases, such as P. penetrans in cabbage (Acedo and Rohde, 1971) and strawberry roots (Townshend, 1963b), and in corn roots infected by P. brachyurus and P. zeae (Olowe and Corbet, 1976).
The present study characterizes for the first time cytological features of lily plants, and in particular L. longiflorum, infected by P. penetrans. We took advantage of an in vitro system established to grow lily plants, which proved to be a suitable model for following this nematode–plant interaction in detail. The appropriate development of lily plants in vitro allowed for the direct observation of the plant development, as well as nematode infection and root-lesion disease development. This system has the potential to be a useful tool for a fast screening and validation of new cultivars or genetically modified plants against P. penetrans, or other pathogens able to parasitize lilies.
Literature Cited
- Acedo JR, Rhode RA. Histochemical root pathology of Brassica oleracea capitata L. infected by Pratylenchus penetrans (Cobb) Filipjev and Schuurmans Stekhoven (Nematoda: Tylenchidae) Journal of Nematology. 1971;3:62–68. [PMC free article] [PubMed] [Google Scholar]
- Acosta N, Malek RB. Symptomatology and histopathology of soybean roots infected by Pratylenchus scribneri and P. alleni. Journal of Nematology. 1981;13:6–12. [PMC free article] [PubMed] [Google Scholar]
- Backiyarani S, Uma S, Arunkumar G, Saraswathi MS, Sundararaju P. Differentially expressed genes in incompatible interactions of Pratylenchus coffeae with Musa using suppression subtractive hybridization. Physiological and Molecular Plant Pathology. 2014;86:11–18. [Google Scholar]
- Backiyarani S, Uma S, Nithya S, Chandrasekar A, Saraswathi MS, Thangavelu R, Mayilvaganan M, Sundararaju P, Singh NK. Genome-wide analysis and differential expression of chitinases in banana against root lesion nematode (Pratylenchus coffeae) and Eumusa leaf spot (Mycosphaerella eumusae) pathogens. Applied Biochemical Biotechnology. 2015;175:3585–3598. doi: 10.1007/s12010-015-1528-z. [DOI] [PubMed] [Google Scholar]
- Bakhsaie M, Khosravi S, Azadi P, Bagheri H, van Tuyl JM. Biotechnological advances in Lilium. Plant Cell Reports. 2016;35:1799–1826. doi: 10.1007/s00299-016-2017-8. [DOI] [PubMed] [Google Scholar]
- Baldridge GD, O’Neill NR, Samac DA. Alfalfa (Medicago sativa L.) resistance to the root-lesion nematode, Pratylenchus penetrans: Defense-response gene mRNA and isoflavonoid phytoalexin levels in roots. Plant Molecular Biology. 1998;38:999–1010. doi: 10.1023/a:1006182908528. [DOI] [PubMed] [Google Scholar]
- Burke M, Scholl EH, Bird DM, Schaff JE, Coleman S, Crowell R, Diener S, Gordon O, Graham S, Wang X, Windham E, Wright GM, Opperman CH. The plant parasite Pratylenchus coffeae carries a minimal nematode genome. Nematology. 2015;17:621–37. [Google Scholar]
- Byrd DW, Kirkpatrick T, Barker KR. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology. 1983;15:142–143. [PMC free article] [PubMed] [Google Scholar]
- Castillo P, Vovlas N. 2007. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. Nematology Monographs and Perspectives, vol. 6. Pp. 529.
- Castillo P, Vovlas N, Jimenez-Diaz RM. Pathogenicity and histopathology of Pratylenchus thornei populations on selected chickpea genotypes. Plant Pathology. 1998;47:370–376. [Google Scholar]
- Devi N, Ponnuswami V, Sundararaju P, Van den Bergh I, Kavino M. Histopathological changes in banana roots caused by Pratylenchus coffeae, Meloidogyne incognita and Radopholus similis, and identification of RAPD markers associated with P. coffeae resistance. Acta Horticulturae. 2009;828:283–290. [Google Scholar]
- Fosu-Nyarko J, Jones MGK. Advances in understanding the molecular mechanisms of root lesion nematode host interactions. Annual Review of Phytopathology. 2016;54:253–278. doi: 10.1146/annurev-phyto-080615-100257. [DOI] [PubMed] [Google Scholar]
- Fosu-Nyarko J, Tan CHJ-A, Gill R, Agrez VG, Rao U, Jones MGK. De novo analysis of the transcriptome of Pratylenchus zeae to identify transcripts for proteins required for structural integrity, sensation, locomotion and parasitism. Molecular Plant Pathology. 2016;17:532–552. doi: 10.1111/mpp.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Joseph S, Gheysen G. Analysis of the transcriptome of the root lesion nematode Pratylenchus coffeae generated by 454 sequencing technology. Molecular and Biochemical Parasitology. 2011;178:7–14. doi: 10.1016/j.molbiopara.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Han H, Han S, Kim Y. Anatomical and biochemical changes of corn roots infected with Pratylenchus vulnus. Korean Journal of Applied Entomology. 1995;34:112–119. [Google Scholar]
- Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, Perry RN. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology. 2013;14:946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyappan R, Viswanathan S, Suthanthiram B, Subbaraya U, Somasundram SM, Muthu M. 2016. Evolutionary expansion of WRKY gene family in banana and its expression profile during the infection of root lesion nematode, Pratylenchus penetrans. PlosOne 11:e0162013. [DOI] [PMC free article] [PubMed]
- Kamo K, Han BH. Biolistic-mediated transformation of Lilium longiflorum cv. Nellie White. HortScience. 2008;43:1864–1869. [Google Scholar]
- Kang Y-I, Joung HY, Goo DH, Choi YJ, Choi MP, An HR, Ko J-Y, Choi K-J, Lee KH, Hong KW. A survey on cut flower cultivar trends and horticultural status of lilies (Lilium hybrids) in South Korea. HortTechnology. 2013;23:629–634. [Google Scholar]
- Kathiresan T, Mehta UK. Penetration, multiplication and histopathological response of Pratylenchus zeae in resistant and susceptible sugarcane clones. International Journal of Nematology. 2002;12:189–196. [Google Scholar]
- Kurppa S, Vrain TC. Penetration and feeding behaviour of Pratylenchus penetrans in strawberry roots. Revue de Nématologie. 1985;8:273–276. [Google Scholar]
- Lattanzio V, Lattanzio VMT, Cardinali A. 2006. Role of phenolics in the resistance mechanisms of plant against fungal pathogens and insects. Pp. 23–67 in F. Imperato, ed. Phytochemistry: Advances in research, research signpost. Kerala, India: Fort P.O., Trivandrum-695 023.
- Linsell KJ, Riley IT, Davies KA, Oldach KH. Characterization of resistance to Pratylenchus thornei (nematoda) in wheat (Triticum aestivum): Attraction, penetration, motility, and reproduction. Phytopathology. 2014;104:174–187. doi: 10.1094/PHYTO-12-12-0345-R. [DOI] [PubMed] [Google Scholar]
- Maas PWT, Mantel P, Boontjes J. Root lesion nematode, Pratylenchus penetrans, in ‘Fire King’ lilies: Attack and control with aldicarb. Journal of Plant Pathology. 1978;84:217–225. [Google Scholar]
- Mountain WB, Patrick ZA. The peach replant problem in Ontario. VII. The pathogenicity of Pratylenchus penetrans (Cobb, 1917) Filip. & Stek. Canadian Journal of Botany. 1959;37:459–470. [Google Scholar]
- Nicol P, Gill R, Fosu-Nyarko J, Jones MGK. De novo analysis and functional classification of the transcriptome of the root lesion nematode, Pratylenchus thornei, after 454 GS FLX sequencing. International Journal of Parasitology. 2012;42:225–37. doi: 10.1016/j.ijpara.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Ogiga IR, Estey RH. Penetration and colonisation of Brassica rapa and Zea mays root tissues by Pratylenchus penetrans. Phytoprotection. 1975;56:22–30. [Google Scholar]
- Olowet T, Corbett DCM. Aspects of the biology of Pratylenchus brachyurus and P. zeae. Nematologica. 1976;22:202–211. [Google Scholar]
- Oyekan PO, Blake CD, Mitchell JE. Histopathology of pea roots axenically infected by Pratylenchus penetrans. Journal of Nematology. 1972;4:32–35. [PMC free article] [PubMed] [Google Scholar]
- Pinochet J. Histopathology of the root lesion nematode, Pratylenchus coffeae, on plantains, Musa AAB. Nematologica. 1978;24:331–240. [Google Scholar]
- Pitcher RS, Patrick ZA, Mountain WB. Studies on the host-parasite relations of Pratylenchus penetrans (Cobb) to apple seedlings. I. Pathogenicity under sterile conditions. Nematologica. 1960;5:309–314. [Google Scholar]
- Rebois RV, Huettel RN. Population dynamics, root penetration, and feeding behavior of Pratylenchus agilis in monoxenic root cultures of corn, tomato and soybean. Journal of Nematology. 1986;18:392–397. [PMC free article] [PubMed] [Google Scholar]
- Rodiuc N, Vieira P, Banora M, de Almeida Engler J. On the track of transfer cells formation by specialized plant-parasitic nematodes. Frontiers in Plant Science. 2014;5:1–14. doi: 10.3389/fpls.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde RA. Lesion nematode injury to carrot. Phytopathology. 1963;53:886–887. [Google Scholar]
- Thomason IJ, Rich JR, O’Melia FC. Pathology and histopathology of Pratylenchus scribneri infecting snap bean and lima bean. Journal of Nematology. 1976;8:347–352. [PMC free article] [PubMed] [Google Scholar]
- Townshend JL. The pathogenicity of Pratylenchus penetrans to celery. Canadian Journal of Plant Science. 1963a;43:70–74. [Google Scholar]
- Townshend RA. The pathogenicity of Pratylenchus penetrans to strawberry. Canadian Journal of Plant Science. 1963b;43:75–78. [Google Scholar]
- Townshend JL, Stobbs L. Histopathology and histochemistry of lesions caused by Pratylenchus penetrans in roots of forage legumes. Canadian Journal of Plant Pathology. 1981;3:123–128. [Google Scholar]
- Townshend JL, Stobbs L, Carter R. Ultrastructural pathology of cells affected by Pratylenchus penetrans in alfalfa roots. Journal of Nematology. 1989;21:530–539. [PMC free article] [PubMed] [Google Scholar]
- Vaganan MM, Ravi I, Nandakumar A, Sarumathi S, Sundararaju P, Mustaffa MM. Phenylpropanoide enzymes, phenolic polymers and metabolites as chemical defenses to infection of Pratylenchus coffeae in roots of resistant and susceptible bananas (Musa spp.) Indian Journal of Experimental Biology. 2014;52:252–260. [PubMed] [Google Scholar]
- Vieira P, Eves-van den Akker S, Verma R, Wantoch S, Eisenback JD, Kamo K. The Pratylenchus penetrans transcriptome as a source for the development of alternative control strategies: Mining for putative genes involved in parasitism and evaluation of in planta RNAi. PlosOne. 2015;10:e0144674. doi: 10.1371/journal.pone.0144674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerdahl BB, Giraud D, Etter S, Riddle LJ, Radewald JD, Anderson CA, Darso J. Management options for Pratylenchus penetrans in Easter lily. Journal of Nematology. 2003;35:443–449. [PMC free article] [PubMed] [Google Scholar]
- Westerdahl BB, Giraud D, Radewald JD, Anderson CA, Darso J. Management of Pratylenchus penetrans on oriental lilies with drip and foliar-applied nematicides. Journal of Nematology. 1993;25:758–767. [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Zeng L, Yan Z, Liu T, Sun K, Zhu T, Zhu A. Identification of ramie genes in response to Pratylenchus coffeae infection challenge by digital gene expression analysis. International Journal of Molecular Sciences. 2015;16:21989–22007. doi: 10.3390/ijms160921989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Tang S, Tang Q, Liu T. Genome-wide transcriptional changes of ramie (Boehmeria nivea L. Gaud) in response to root-lesion nematode infection. Gene. 2014;552:67–74. doi: 10.1016/j.gene.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Zunke U. Observations on the invasion and endoparasitic behavior of the root lesion nematode Pratylenchus penetrans. Journal of Nematology. 1990a;22:309–320. [PMC free article] [PubMed] [Google Scholar]
- Zunke U. Ectoparasitic feeding behaviour of the root lesion nematode, Pratylenchus penetrans, on root hairs of different host plants. Revue de Nématologie. 1990b;13:331–337. [Google Scholar]