Abstract
A new species, Oscheius microvilli n. sp., was found on Chongming Island (Shanghai, China). The new species is morphologically similar to the type strain of Oscheius myriophilus, but can be distinguished from it and other species of Oscheius on the basis of unique morphological characteristics of the bursa as well as male papillae. In this new species, the male bursal papillar formula is 2, 1, 3, 3 with everted tips in the first, fifth, and seventh pairs. The bursal rim is jagged, joins together anterior to the spicules, and is partially extended and decorated with microvilli. The spicules are incompletely separated, and the tail does not extend beyond the bursa. Phylogenetic trees of 18S rDNA and internal transcribed spacer indicate that the new species belongs to the insectivora group of the genus Oscheius; it is most closely related to O. myriophilus, and the two species can be distinguished on the basis of their different body length, morphological features of the bursa, and molecular data. The new species is facultatively associated with a bacterial strain of Serratia. The LC50 of this novel nematode against Galleria mellonella was 69.1 dauer juveniles per milliliter after 48 hr of infection.
Keywords: entomopathogenic nematodes, entomophilic nematodes, insectivora group, new nematode species, Oscheius microvilli n. sp., taxonomy
Entomopathogenic nematodes (EPNs) and other parasitic nematodes are lethal parasites against insects and slugs, and they have been applied as biological approaches to pest management (Poinar, 1979; Wilson et al., 1993; Hazir et al., 2003; Nguyen et al., 2008; Stock et al., 2009; Tamura et al., 2011; Kanzaki et al., 2013; Phan et al., 2014; San-Blas et al., 2016). EPNs in particular have been utilized to control a range of pests for more than half a century in U.S. and European countries, where they have been found to be particularly effective against soil-dwelling insects, plant-boring insects, and unknown pests (Poinar, 1979; Ehlers, 1996; Berry et al., 1997; Hazir, 2003).
EPNs of Steinernematidae and Heterorhabditidae are characterized by carrying specific symbiotic bacterial strains of genera Photorhabdus and Xenorhabdus, respectively, in their intestines, whereas species of Heterorhabditidoides (=Oscheius, family Rhabditidae) carry symbiotic bacterial strains of Serratia in both the intestine and the cuticle (Kaya and Gaugler, 1993; Zhang et al., 2008, 2009, 2012). Phylogenetic trees based on 18S rDNA and internal transcribed spacer (ITS) sequences indicate that species of the genus Oscheius can be divided into three groups, the dolichura group, insectivora group, and Heterorhabditidoides group (Ye et al., 2010; Liu et al., 2012; Zhang et al., 2012). Several EPN species, all symbiotic with bacterial strains of Serratia, have been found in the Heterorhabditidoides group (Heterorhabditidoides (=Oscheius) chongmingensis and H. rugaoensis) and the insectivora group (Oscheius carolinensis) (Zhang et al., 2008; 2009; 2012; Ye et al., 2010; Liu et al., 2012). Other Oscheius species are parasitic to insects, some of them also commonly associated with Serratia (Lephoto et al., 2015; Torrini et al., 2015), although their entomopathogenicity has yet to be examined according to the criteria of EPNs revised by Dillman et al. (2012).
During a survey of EPNs and other potential insect-parasitic nematodes distributed in the Yangtze River Delta Basin in China, we found and identified an Oscheius species, CM19, collected from soil samples taken from Chongming Island, Shanghai. Combined morphological and molecular data indicate this species is a facultatively pathogenic nematode belonging to the insectivora group of the genus Oscheius, described herein as O. microvilli n. sp.
Materials and Methods
Nematode isolation and cultivation:
The new species, O. microvilli n. sp., was collected from Chongming Island, Shanghai, China, in 2014, using the Galleria mellonella baiting method (Bedding and Akhurst, 1975; Zhang et al., 2008). Five last-instar larvae of G. mellonella were each placed in a 250-ml plastic box containing collected soil samples and kept at room temperature (20°C ± 2°C) to attract and trap nematodes from these samples (Stock et al., 1999). Water was replenished regularly to maintain the sample moisture. Larvae of G. mellonella were checked every day, and each dead larva was replaced by a fresh one. After 7 d, dead larvae were thoroughly washed with distilled water and then placed in modified white traps (Kaya and Stock, 1997) until the emergence of dauer/infective juveniles. Fifteen G. mellonella larvae were exposed to approximately 1,500 dauer juveniles in a petri dish (60 × 15 mm) lined with two wet filter papers at 20°C ± 2°C. Hermaphrodites and second-generation adult nematodes were obtained by dissecting last-instar G. mellonella larvae after infection with dauer juveniles 3 to 4 and 5 to 7 d later, respectively (Nguyen and Smart, 1995a).
Morphological observation and preparation of type materials:
For light microscopy, 20 females, 20 males, and 20 infective larvae were randomly selected from different G. mellonella cadavers and treated as follows. All nematode samples were rinsed three times in distilled water to remove impurities and then heat-killed quickly on glass slides in Ringer’s solution at 60°C. The heat-killed nematodes were placed in hot triethanolamine formalin (TAF) fixative (Kaya and Stock, 1997) and transferred to anhydrous glycerin for mounting (Seinhorst, 1959). Examination and measurements were performed using an Axio Imager A1 microscope (Carl Zeiss, Jena, Germany). For examination by scanning electron microscopy (SEM), nematode samples were fixed in 3% glutaraldehyde buffered with 0.1 M phosphate buffer at pH 7.2 for at least 24 hr at 4 to 8°C (Nguyen and Smart, 1995b). Samples were then postfixed with 2% osmium tetroxide solution for 12 hr at 25°C, dehydrated in a graded ethanol series, critical point dried with liquid CO2, mounted on SEM stubs, and coated with gold (Nguyen and Smart, 1995b). Finally, samples were examined using a Hitachi S-3000N scanning electron microscope (Hitachi, Tokyo, Japan).
Molecular and phylogenetic analysis:
DNA extraction and PCR amplification were performed as described by Zhang et al. (2008). Primers used for amplification and sequencing of the ITS and 18S rDNA of the new isolate were reported by Vrain et al. (1992) and Liu et al. (1997), respectively. Primers used for ITS were 5′-TTG ATT ACG TCC CTG CCC TTT-3′(forward) and 5′-TTT CAC TCG CCG TTA CTA AGG-3′ (reverse). Primers used for 18S rDNA were 5′-GGT GAA ACT GCG AAC GGC TCA-3′ (forward) and 5′-CCG GTT CAA GCC ATT GCG ATT-3′ (reverse). PCR products were purified and sequenced by the Beijing Genomics Institute (Shenzhen, China). We performed taxonomic and phylogenetic analyses on the basis of the sequences of the ITS and 18S rDNA of related nematodes, including previously described EPN species, using sequences available from GenBank (Table 1). Sequences were assembled using Sequencing Analysis 3.0 and aligned using ClustalX (Thompson et al., 1997) under the default alignment parameters, followed by manual alignment in MEGA version 5 (Tamura et al., 2011). The program ModelTest 2.0 (Nylander, 2004) was used to find the best fitting substitution model for our molecular dataset. Phylogenetic relationships were inferred using MrBayes 3.0b4 (Huelsenbeck and Ronquist, 2001). Four cold Metropolis-coupled Markov Chains Monte Carlo was run for 10 million generations, with the first 2 million generations discarded as burn-in and one tree retained every 100 generations.
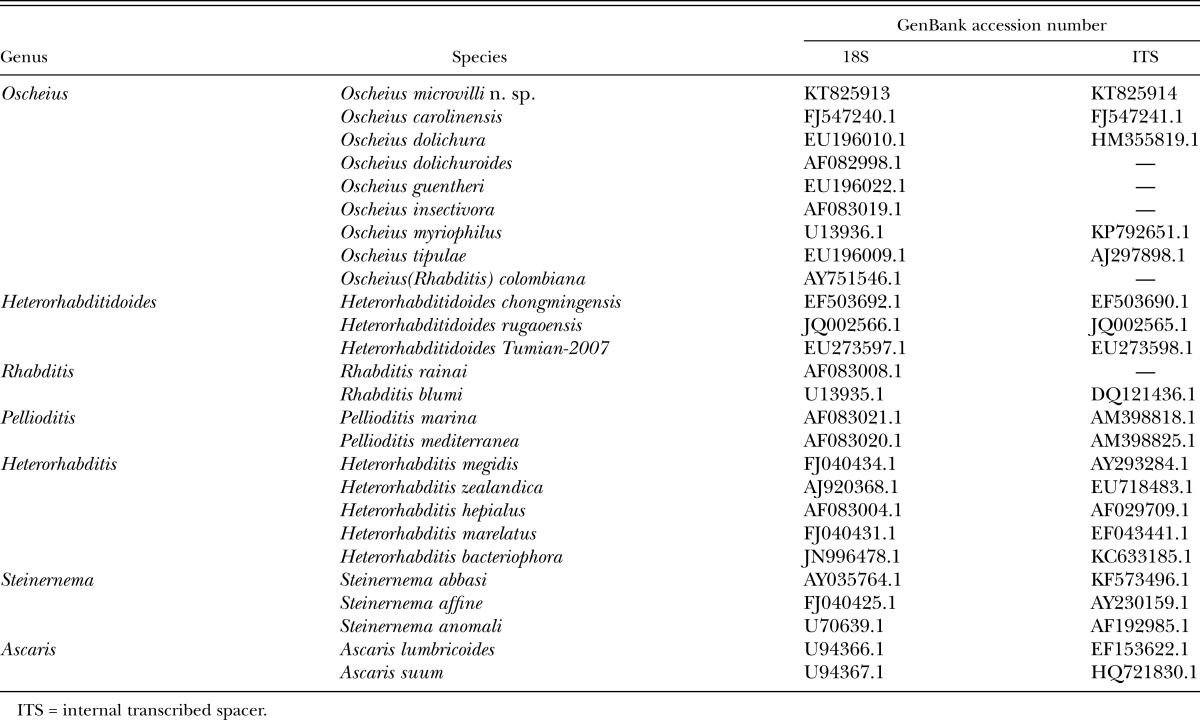
Table 1.
Taxa analyzed in this study.
Isolation and identification of bacterial strains associated with O. microvilli n. sp.
Associated bacterial strains were obtained from dauer/infective larvae of O. microvilli n. sp. by the method of Akhurst (1980). Approximately, 100 surface-sterilized dauer larvae were crushed and then streaked on nutrient-bromothymol blue agar plates (NBTA, nutrient agar, 0.0025% bromothymol blue, and 0.004% triphenyltetrazolium chloride medium) and incubated at 28°C for 48 hr (Akhurst, 1980, Zhang et al., 2008). Bacterial genomic DNA was prepared following the method of Marmur (1961) and PCR amplification of the 16S rRNA gene was performed as described by Xu et al. (2003). Primers used were 5′-AGA GTT TGA TCC TGG CTC AG-3′ (forward) and 5′-TAC CTT GTT ACG ACT TCA CCC CA-3′ (reverse) (Brosius, 1978). PCR products were purified and sequenced by the Beijing Genomics Institute. Identification analysis of 16S rDNA sequences of strain Serratia sp. N19 against all described bacteria type strains was conducted using EzTaxon Server Version 2.1 (Chun et al., 2007).
Pathogenicity of bacterial associate against the insect host:
Bacteria diluent (10 μl) of each of the eight treatment levels was extracted and injected into 20 last-instar G. mellonella larvae, which were incubated at 25°C for 48 hr. Each treatment was performed in triplicate. Larval mortality was recorded every 12 hr.
Pathogenicity of the new nematode against the insect host:
The pathogenicity of O. microvilli n. sp. dauer juveniles against last-instar larvae of G. mellonella was estimated according to previously described methods (Bonifassi et al. 1999). Eight treatment levels (0, 10, 20, 40, 80, 160, 320, and 640 dauer juveniles in 1.2 ml water) were respectively placed onto filter paper in petri dishes (10-cm diameter) with 10 last-instar G. mellonella larvae as targets, and incubated at 25°C for 72 hr. Each treatment was replicated three times. Larval mortality was recorded every 12 hr and determined by an arcsine square root, and then subjected to one-way analysis of variance, followed by Duncan’s multiple range test to compare differences among the three replicates at a 0.05 significance level using the SPSS 20.0 software (IBM, 2011). All of the original data passing these tests were applied to estimate larval mortality means varying with treatment time and concentration of dauer juveniles, using Graphpad prism v.5.01 (2007). We then estimated the lethal concentration required to kill 50% of the infected insects (LC50) with the method Bliss. All statistical analyses were conducted with SPSS v.20.0 (IBM, 2011).
Influence of the associated bacterial strain on host development:
Sterile eggs were acquired by crushing 1,000 mature females in sterile Ringer solution (NaCl 0.9%, w/v) with NaOCl (2.5%, w/v) and NaOH (4%, w/v) during 10 min, according to previously described methods (Sicard et al., 2003). Sterilized eggs were collected and then rinsed twice with sterile Ringer’s solution and transferred to “liver-agar” plates (containing, per liter: 15 g biotrypticase, 5 g biosoyase, 5 g NaCl, 15 g agar, 2 g beer yeast, 100 g calf liver) (Sicard et al., 2003) with 1-d-old bacterial strains (Serratia sp. N19, Escherichia coli OP50), as well as to uninfected liver-agar plates as control, for incubation at 25°C. Mature nematodes (100) of Oscheius microvilli n. sp. were collected and rinsed twice with sterile Ringer’s solution. Nematodes were then disinfected using thimerosal disinfectant for 10 min and then rinsed twice with sterile Ringer’s solution and transferred to liver-agar plates for incubation at 25°C. Each treatment was replicated three times. Nematode development was observed and recorded once every 2 d.
Hybrid mating experiments:
A hybridization experiment between CM19 and Oscheius myriophilus EM435 was conducted to confirm its species status, as the new species shares high sequence conservation with O. myriophilus. Crosses were performed reciprocally, and self-crosses were performed as a control. Each cross was performed with one female and one male, in 15 replicates per cross type. Females and males were identified by development of the genital anlagen. Nematodes were maintained on 1-d-old cultures of E. coli strain OP50 grown on liver-agar plates maintained at 25°C. Five days after setting up crosses, F1 nematodes were collected from plates and counted (Bolla and Boschert, 1993).
Results and Discussion
Oscheius microvilli n. sp.
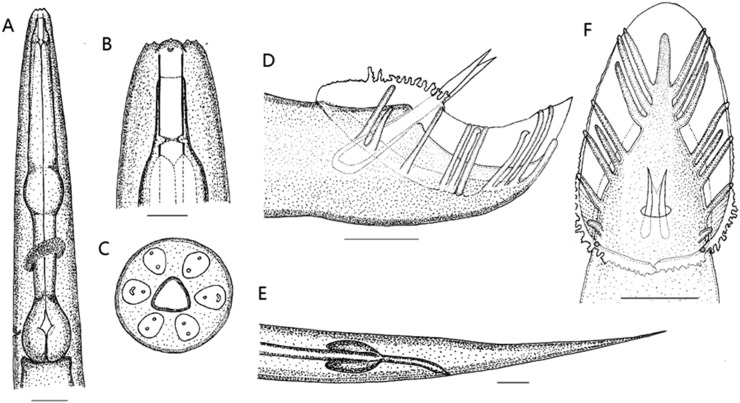
Fig. 1.
Morphology of Oscheius microvilli n. sp. A. Lateral view of pharyngeal region. B. Lateral view of head. C. En face view of head. D. Lateral view of male tail. E. Lateral view of female tail. F. Ventral view of male tail. Scale bars: A and E = 20 μm; B, C, D, and F = 10 μm.
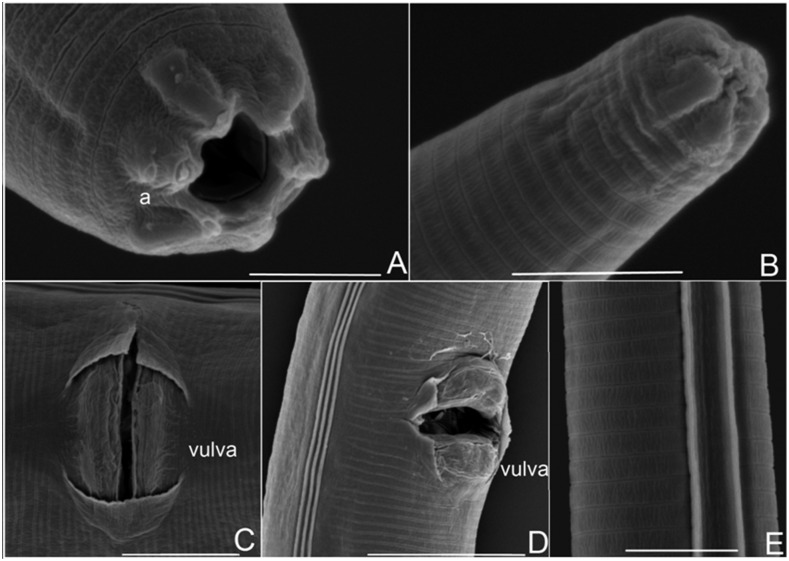
Fig. 3.
Morphology of female nematode and juvenile. A. Head of hermaphroditic female, stoma is triangular. B. Anterior of juvenile, with mouth not yet open. C and D. Vulva pattern of female. Note lateral fields with three longitudinal ridges. E. Two lateral lines of juvenile. Scale bars: A, B, and E = 5 μm; C = 10 μm; D = 20 μm.
Measurements:
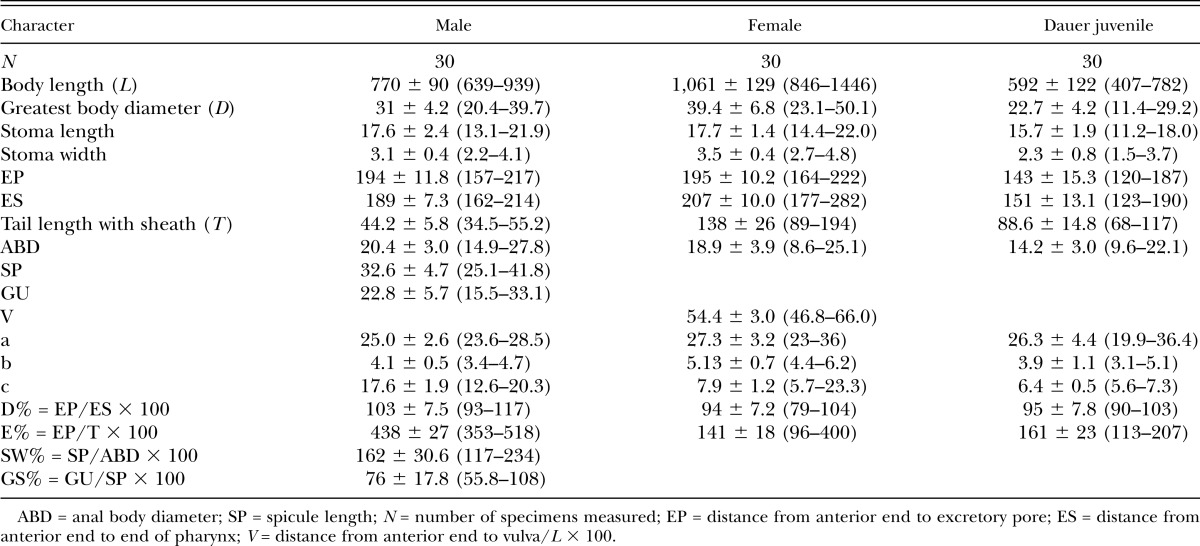
Table 2.
Morphometrics (µm) of Oscheius microvilli n. sp., presented as mean ± standard deviation (range).
Table 4.
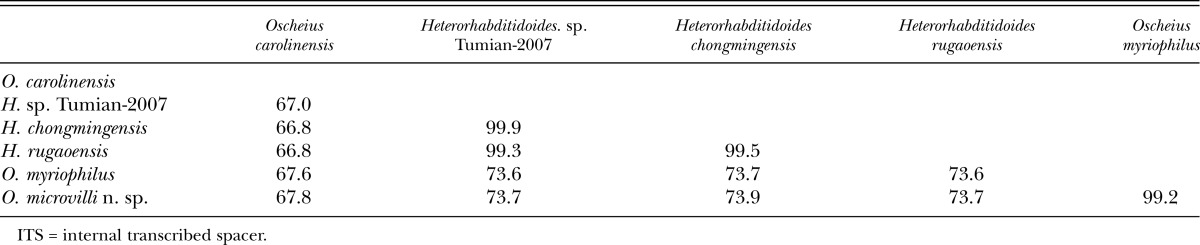
ITS rDNA sequence identity (%) matrix among Oscheius microvilli n. sp. and molecularly close species.
Table 3.
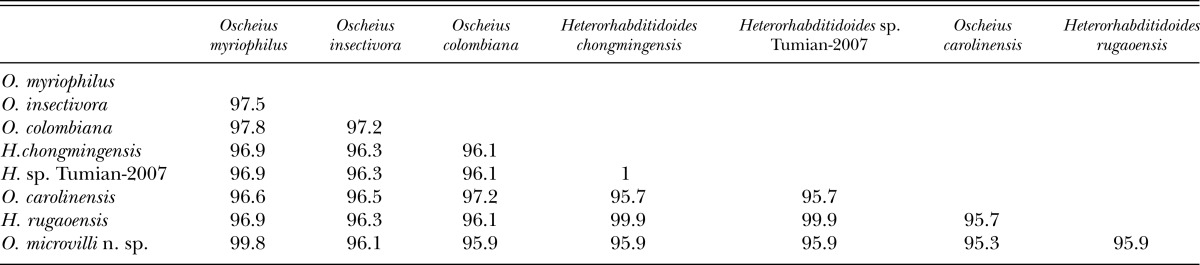
18S rDNA sequence identity (%) matrix among Oscheius microvilli n. sp. and molecularly close species.
Description
Adults:
Body cylindrical, anterior, and posterior ends tapered. Epidermis striated horizontally. Head continuous with neck. Head bulging with six separate, well-developed lips, the two lateral of which have an arcuate amphidial aperture and a bristle-like labial papilla. Other four lips bear one inner labial papilla and one outer labial papilla (Fig. 3A). Stomatal opening triangular in en face view, strongly cuticularized in lateral view. Metarhabdia isomorphic, tubular-shaped, slender. Corpus cylindrical, significantly enlarged to constitute median bulb (Fig. 2B). Isthmus distinct, finer than front portion of pharynx. Nerve ring usually surrounding mid part of isthmus. Basal bulb globose, with distinct valve at pharyngeointestinal junction. Excretory pore opens at level of basal bulb (Fig. 1A). Phasmids distinct.
Fig. 2.
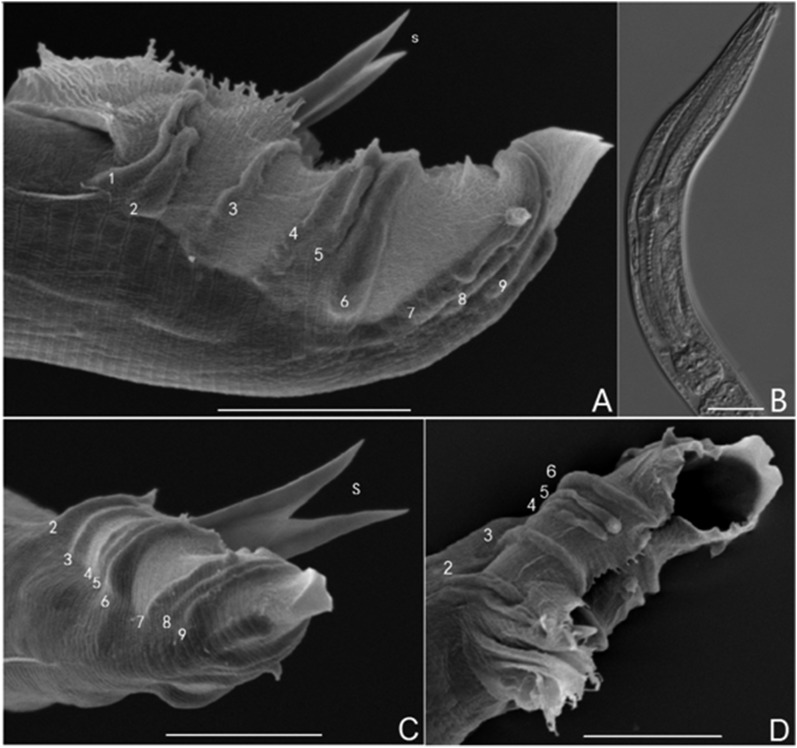
Morphology of male nematode. A, C, D. Various view of male tails with spicules and bursal papillae. A. Papillar formula of the bursa is 2, 1, 3, 3. Tips of the fifth and seventh pairs are everted. The anterior rim of the bursa is jagged, partially extended, and decorated with microvilli. B (DIC). Corpus cylindrical, significantly enlarged to form a median bulb. C. Spicules paired, without complete separation, symmetrical, slightly curved ventrally. Papilla tips of the first, fifth, and seventh pairs are everted. The tail does not extend beyond the bursa. D. The edges of the bursa on both sides tend to join together. Scale bars: A, C, and D = 10 μm; B = 20 μm.
Males:
Tail bent ventrally, J-shaped after killing with 65°C hot water. Males smaller than females in several morphometric dimensions (Table 2). Lateral fields with three longitudinal ridges. Spicules paired, incompletely separated, symmetrical, slightly curved ventrally, with spiculate head. Membrane-like extensions present at part attached to spicule. Gubernaculum dorsoventrally flattened, following contour of spicules. Bursal rim jagged, joined together anterior to spicules, partially extended and decorated with microvilli. Nine pairs of well-developed papillae, distributed in a 2-1-3-3 pattern from anterior to posterior. Papillae P4, P5, and P6 form a group just posterior to cloaca, and P7, P8, and P9 form a separate, postcloacal group. Distance between P1 and P2 less than that between the P2 and P3. P1 papillae well-developed, extended, tips reaching beyond bursal rim. P2 papillae short and stout, not extending beyond bursal rim. P4 papillae are the slimmest of all pairs. Tips of P1, P5, and P7 turned outward (Fig. 2). Bursa peloderan, such that tail does not extend posterior to bursa (Fig. 2C).
Females:
Body C-shaped when killed by heat and fixed with TAF, longer and more robust than that of male (Table 2). Cuticle relatively smooth when observed by light microscopy, finely annulated when observed by SEM. Lateral fields also contain three longitudinal ridges (Fig. 3D). Vulva slightly posterior to midbody, opening with distally swelling bulge, covered by two symmetrical cuticular flaps (Fig. 3C). Many eggs queued in uterus. Anus opening as horizontal arc. Tail longer than anal body width, conoid or swollen postanally, with pointed terminus.
Dauer/infective juveniles:
Body C-shaped when killed by heat (Table 2). Lips and pharynx similar to adult in morphology, except for unopened mouth and inconspicuous lip papillae (Fig. 3B). Amphid aperture further posterior from lip apices than in adults. Some dauer juveniles sheathed. Bodies of unsheathed dauer juveniles annulated, with two longitudinal ridges forming very deep groove in lateral fields (Fig. 3E). Tails long and pointed.
Type host and locality:
The novel nematode species was collected by baiting with G. mellonella larvae from a soil sample taken from Chongming Island, Shanghai, People’s Republic of China. The natural host remains unknown.
Type material and nomenclatural registration:
Holotypes and paratypes (males, females, and dauer juveniles) were deposited in the Animal Resources Laboratory, Department of Zoology, College of Life Sciences, Nanjing Agricultural University, Nanjing, People’s Republic of China. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier: 5468F58B-94BE-444B-968B-2EF7E8CACC2B.
Diagnosis and relationships:
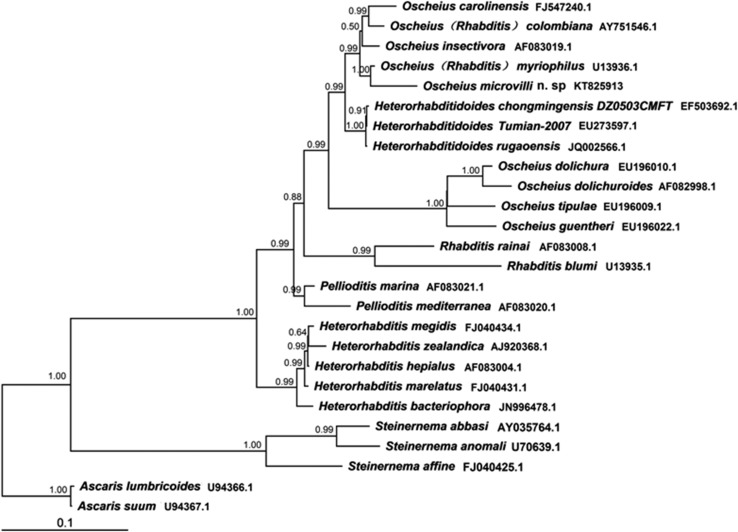
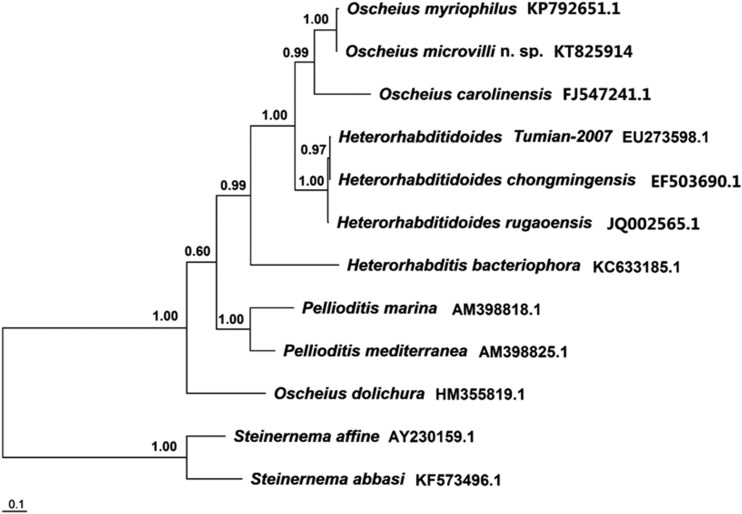
Phylogenetic trees of 18S rDNA and internal transcribed spacer indicate that the new species belongs to the insectivora group of the genus Oscheius; it is most closely related to O. myriophilus (Figs. 4,5). The most apparent differences between O. microvilli n. sp. and the phylogenetically closest known species of Oscheius lie in the unique bursal characteristics of male individuals, the formula and form of bursal papillae (i.e., jagged, partly microvilli-shaped, and joined with the bursal rim), and the well-developed median bulb. The new species has a shorter body length compared with close Oscheius species (Poinar, 1986; Ye et al., 2010; Zhang et al., 2012): the mean length of females of O. microvilli n. sp., O. myriophilus, O. (=Rhabditis) colombiana, and O. carolinensis are 1,061, 1,320, 1,288, and 1,728, respectively (Table 1; Poinar, 1986). Distinguishing O. microvilli n. sp. from O. myriophilus is a longer stoma (relative to body length) and a well-developed, significantly enlarged median bulb (Poinar, 1986). Additional characteristics that distinguish the new species from other species of Oscheius are bursal papillae that are more corrugated, serrated, and longer than in other species; elongation of the bursa, which is joined together in front of the spicules; the decoration of the bursa with many microvilli. Furthermore, the papillar formula of O. microvilli n. sp. bursa is 2, 1, 3, 3, whereas that of other species of Oscheius is 1, 2, 3, 3. The tips of the first, fifth, and seventh pairs are everted, whereas for other species of the genus Oscheius, those of the fifth and eighth pairs are everted. The tail of O. microvilli n. sp. does not extend beyond the bursa, whereas that of other species in the insectivore group of Oscheius protrudes outside of the bursa. In addition, O. microvilli n. sp. is reproductively isolated from O. myriophilus, as indicated by mating tests. Finally, the facultative association with Serrati asp. N19 may be unique to the new species.
Fig. 4.
Phylogenetic relationships of Oscheius microvilli n. sp. and closely related species inferred from 18S rDNA sequence data.
Fig. 5.
Phylogenetic relationships of Oscheius microvilli n. sp. and closely related species inferred from internal transcribed spacer sequence data.
Hybrid mating tests:
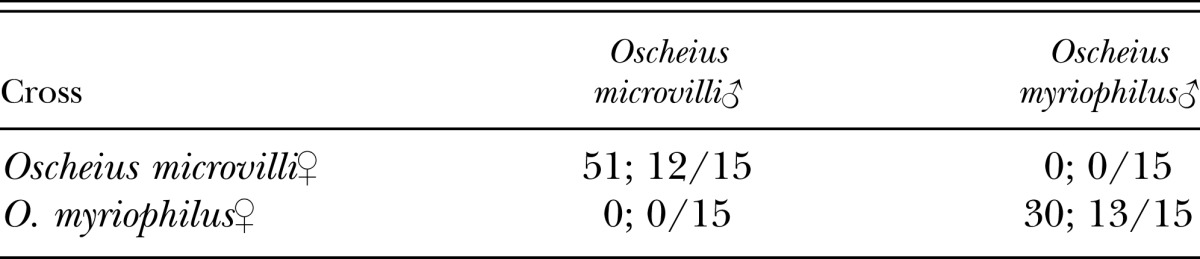
Conspecific crosses of O. microvilli n. sp. (n = 15 crosses) and O. myriophilus (n = 15) were successful (Table 5). The success score of O. microvilli n. sp. conspecific crosses was 12/15 and the average number of nematodes was 51, and those of O. myriophilus were 13/15 and 30, respectively. Hybridization of O. microvilli n. sp. (n = 15 crosses) and O. myriophilus (n = 15) were not successful in any replicates (Table 5). Results thus show that O. microvilli n. sp. and O. myriophilus are reproductively isolated, satisfying the biological species concept.
Table 5.
Results of hybrid mating tests, presented as number of offspring; successful/total crosses performed.
Isolation and identification of the new species’ bacterial associates:
Three bacterial colonies were randomly selected for identification. The identity of 16S rDNA sequences among all of them was 100%, indicating they were of the same bacterial species. Strain N19 (GenBank accession number KT825915) was selected as the representative bacterial strain for further identification. This strain produced a red pigment on NBTA and LB plates. Identification of N19 with respect to all type strains of bacterial species using 16S rRNA gene sequence data, as implemented in EzBioCloud, identified the isolate as a species of Serratia and shares 98.96%, 98.96%, and 98.68% 16S rRNA sequence similarity with its closest relatives S. nematodiphila DZ0503SBS1T (GenBank accession: EU036987), S. marscecens subsp. sakuensis KREDT (AB06168), and S. marscecens subsp. marscecens DSM 30121T (AJ233431), respectively.
Pathogenicity of bacterial associate against the insect host:
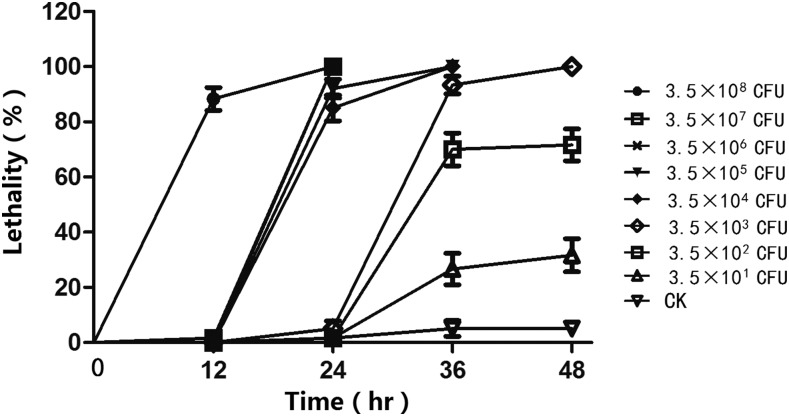
Serratia sp. N19 showed high virulence against G. mellonella. The lethality rates of the last-instar larvae of G. mellonella were positively correlated with treatment concentration of the bacterial strain and time of infection (Fig. 6). The LC50 of Serratia sp. N19 against last-instar larvae within 48 hr of infection was 99 (40.1-198.6) CFU/ml (Fig. 6).
Fig. 6.
Temporal variation in lethality to Galleria mellonella larvae by Serratia sp. N19.
Pathogenicity of the new nematode against the insect host:
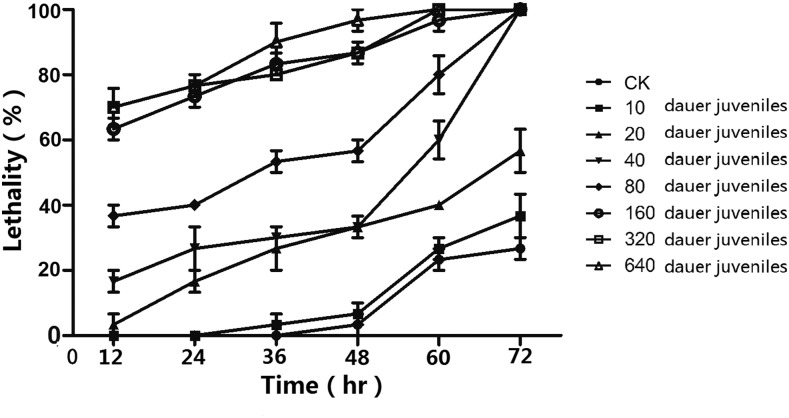
The lethality rates of insect hosts were positively correlated with nematode concentration and time of infection (Fig. 7). About 320 dauer juveniles per milliliter could result in 93.1% larval mortality at 48 hr. The LC50 of the novel nematode species against G. mellonella within 48 hr of infection was 69.1 (41.9–111.9) dauer juveniles per milliliter.
Fig. 7.
Temporal variation in lethality to Galleria mellonella larvae by dauer/infective juveniles of Oscheius microvilli n. sp.
Influence of Serratia sp. N19 on reproductive rate of O. microvilli n. sp.:
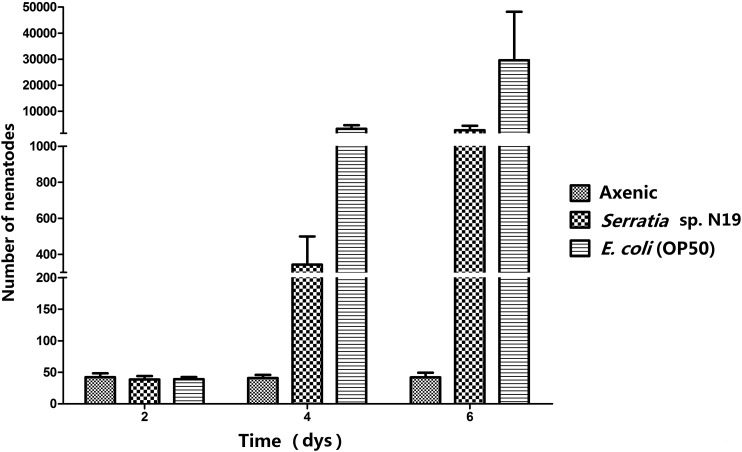
On the second day after egg-laying, the vast majority of living eggs had hatched (Fig. 8). Males and females were observed in the group grown on both bacterial species, whereas nematodes failed to reproduce on axenic control plates. By the fourth day, the number of nematodes carrying E. coli OP50 was greater than that on Serratia sp. N19 and this trend continued through the last (sixth) day of measurements. Results indicate that the development of nematodes carrying E. coli OP50 is faster than nematodes carrying Serratia sp. N19. From the beginning of the fourth day, some dead insects were observed in the group carrying Serratia sp. N19. Differences of nematode growth and development between the three experimental groups may be due to the toxicity and concentration of Serratia sp. N19.
Fig. 8.
Reproductive rate, measured in terms of number of individuals, of Oscheius microvilli n. sp. fed different bacterial strains, following 2 to 6 d of exposure.
Literature Cited
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology. 1980;121:303–309. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Berry RE, Liu J, Groth E. Efficacy and persistence of Heterorhabditis marelatus (Rhabditida: Heterorhabditidae) against root weevils (Coleoptera: Curculionidae) in strawberry. Environmental Entomology. 1997;26:465–470. [Google Scholar]
- Bonifassi E, Fischer-Le Saux M, Boemare N, Lanois A, Laumond C, Smart G. Gnotobiological study of infective juveniles and symbionts of Steinernema scapterisci: A model to clarify the concept of the natural occurrence of monoxenic associations in entomopathogenic nematodes. Journal of Invertebrate Pathology. 1999;74:164–172. doi: 10.1006/jipa.1999.4866. [DOI] [PubMed] [Google Scholar]
- Brosius J, Palmer JL, Kennedy JP, Noller HF. Complete nucleotide sequence of 16S ribosomal RNA gene from Escherichia coli. Proceedings of the National Academy of Sciences of the USA. 1978;75:4810–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. International Journal of Systematic and Evolutionary Microbiology. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- Dillman AR, Chaston JM, Adams BJ, Ciche TA, Goodrich-Blair H, Stock SP, Sternberg PW. An entomopathogenic nematode by any other name. PLoS Pathogens. 2012;8:e1002527. doi: 10.1371/journal.ppat.1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers RU. Current and future use of nematodes in biocontrol: Practice and commercial aspects with regard to regulatory policy issues. Biocontrol Science and Technology. 1996;6:303–316. [Google Scholar]
- Gaugler R, Kaya HK. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- Hazir S, Stock SP, Keskin N. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turkish Journal of Biology. 2003;27:181–202. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Roseler W, Sommer RJ. Pristionchus bucculentus n. sp. (Rhabditida: Diplogastridae) isolated from a shining mushroom beetle (Coleoptera: Scaphidiidae) in Hokkaido, Japan. Journal of Nematology. 2013;45:78–86. [PMC free article] [PubMed] [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. 1997. Techniques in insect nematology. Pp. 281–324 in L. A. Lacey, ed. Manual of techniques in insect pathology. San Diego, CA: Academic Press.
- Lephoto TE, Featherston J, Gray VM. Draft whole-genome sequence of Serratia sp. strain TEL, associated with Oscheius sp. TEL-2014 (Nematoda: Rhabditidae) isolated from a grassland in South Africa. Genome Announcements. 2015;3:e00747–15. doi: 10.1128/genomeA.00747-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Berry RE, Moldenke AF. Phylogenetic relationships of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) inferred from partial 18S rRNA gene sequences. Journal of Invertebrate Pathology. 1997;69:246–252. doi: 10.1006/jipa.1997.4657. [DOI] [PubMed] [Google Scholar]
- Liu QZ, Mracek Z, Zhang LJ, Puza V, Dong LM. Re-description of Oscheius chongmingensis (Zhang et al., 2008) (Nematoda:Rhabditidae) and its entomopathogenicity. Nematology. 2012;14:139–149. [Google Scholar]
- Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. Journal of Molecular Biology. 1961;3:208–218. [Google Scholar]
- Nguyen KB, Půža V, Mráček Z. Steinernema cholashanense n. sp. (Rhabditida, Steinernematidae) a new species of entomopathogenic nematode from the province of Sichuan, Chola Shan Mountains, China. Journal of Invertebrate Pathology. 2008;97:251–64. doi: 10.1016/j.jip.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Smart GC., Jr Morphometrics of infective juveniles of Steinernema species and Heterorhabditis bacteriophora (Nemata: Rhabditida) Journal of Nematology. 1995a;27:206–212. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Smart GC., Jr Scanning electron microscope studies of Steinernema glaseri (Nematoda: Steinernematidae) Nematologica. 1995b;41:183–190. [Google Scholar]
- Poinar GO., Jr . Nematodes for biological control of insects. Boca Rato, FL: CRC Press; 1979. [Google Scholar]
- Poinar GO. Rhabditis myriophilus n sp. (Rhabditidae: Rhabditida), associated with the millipede, Oxidis gracilis (Polydesmida: Diplopoda) Proceedings of the Helminthological Society of Washington. 1986;53:232–236. [Google Scholar]
- San-Blas E, Morales-Montero P, Portillo E, Nermuť J, Půža V. Steinernema goweni n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Zulia State, Venezuela. Zootaxa. 2016;4067:200–214. doi: 10.11646/zootaxa.4067.2.5. [DOI] [PubMed] [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Stock SP, Pryor BM, Kaya HK. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California. USA. Biodiversity and Conservation. 1999;8:535–549. [Google Scholar]
- Stock SP, Rivera-Orduno B, Flores-Lara Y. Heterorhabditis sonorensis n. sp. (Nematoda: Heterorhabditidae), a natural pathogen of the seasonal cicada Diceroprocta ornea (Walker) (Homoptera: Cicadidae) in the Sonoran desert. Journal of Invertebrate Pathology. 2009;100:175–184. doi: 10.1016/j.jip.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony method. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ. Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biological Control. 2011;59:123–129. [Google Scholar]
- Torrini G, Mazza G, Carletti B, Benvenuti C, Roversi PF, Fanelli E, de Luca F, Troccoli A, Tarasco E. Oscheius onirici sp n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an Italian cave. Zootaxa. 2015;3937:533–548. doi: 10.11646/zootaxa.3937.3.6. [DOI] [PubMed] [Google Scholar]
- Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI. Intraspecific rDNA restriction fragment length polymorphisms in the Xiphinema americanum group. Fundamental and Applied Nematology. 1992;15:563–574. [Google Scholar]
- Wilson MJ, Glen DM, George SK. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Science and Technology. 1993;3:503–511. [Google Scholar]
- Xu P, Li WJ, Xu LH, Jiang CL. A microwave-based method for genomic DNA extraction from Actinomycetes. Microbiology (in Chinese) 2003;30:82–84. [Google Scholar]
- Ye W, Torres-Barragan A, Cardoza YJ. Oscheius carolinensis n. sp. (Nematoda: Rhabditidae), a potential entomopathogenic nematode from vermicompost. Nematology. 2010;12:121–135. [Google Scholar]
- Zhang KY, Liu XH, Tan J, Wang Y, Qiao L, Yedid G, Lai R, Gao GF. Heterorhabditidoides rugaoensis n. sp. (Rhabditida: Rhabditidae), a novel highly pathogenic entomopathogenic nematode member of Rhabditidae. Journal of Nematology. 2012;44:348–360. [PMC free article] [PubMed] [Google Scholar]
- Zhang CX, Liu JR, Xu MX, Sun J, Yang SY, An XH, Gao GF, Lin MS, Lai R, He ZY, Wu YD, Zhang KY. Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida:Rhabditidae), a novel member of the entomopathogenic nematodes. Journal of Invertebrate Pathology. 2008;98:153–168. doi: 10.1016/j.jip.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang CX, Yang SY, Xu MX, Sun J, Liu H, Liu JR, Liu H, Kan F, Sun J, Lai R, Zhang KY. A novel species of Serratia, family Enterobacteriaceae: Serratia nematodiphila sp. nov., symbiotically associated with entomopathogenic nematode Heterorhabditidoides chongmingensis (Rhabditida: Rhabditidae) International Journal of Systematic and Evolutionary Microbiology. 2009;59:1603–1608. doi: 10.1099/ijs.0.65718-0. [DOI] [PubMed] [Google Scholar]