Abstract
Meloidogyne enterolobii is one of the most important root-knot nematode in tropical regions, due to its ability to overcome resistance mechanisms of a number of host plants. The lack of new and safe active ingredients against this nematode has restricted control alternatives for growers. Egg-parasitic fungi have been considered as potential candidates for the development of bionematicides. In tissue culture plates, Pochonia chlamydosporia (var. catenulata and chlamydosporia) and Purpureocillium lilacinum strains were screened for their ability to infect eggs of the root-knot nematode M. enterolobii on water-agar surfaces. Reduction in the hatching of J2 varied from 13% to 84%, depending on strain. The more efficacious strains reduced hatchability of J2 by 57% to 84% when compared to untreated eggs, but average reductions were only 37% to 55% when the same strains were applied to egg masses. Combinations of fungal isolates (one of each species) did not increase the control efficacy in vitro. In experiments in which 10,000 nematode eggs were inoculated per plant, reductions in the number of eggs after 12 months were seen in three of four treatments in banana plants, reaching 34% for P. chlamydosporia var. catenulata. No significant reductions were seen in tomato plants after 3 mon. In another experiment with tomato plants using either P. chlamydosporia var. catenulata or P. lilacinum, the number of eggs was reduced by 34% and 44%, respectively, when initial infestation level was low (500 nematode eggs per plant), but tested strains were not effective under a moderate infestation level (5,000 eggs per plant). Under all infestation levels tested in this work, gall and egg mass indexes (MI) did not differ from the untreated controls, bringing concerns related to the practical adoption of this control strategy by farmers. In our opinion, if the fungi P. chlamydosporia and P. lilacinum are to be used as biocontrol tools toward M. entorolobii, they should focus on agricultural settings with low soil infestation levels and within an IPM approach.
Keywords: biological control, Pochonia chlamydosporia, Purpureocillium lilacinum, Meloidogyne enterolobii, nematophagous fungi
Meloidogyne enterolobii Yang and Eisenback (=M. mayaguensis) is one of the most destructive species of root-knot nematodes (RKN). First collected in Asia (Yang and Eisenback, 1983), M. enterolobii has been reported in both native (Lima et al., 2005) and cultivated areas in different countries, causing damages to different crops, such as tomato, pepper (Carneiro et al., 2006), and perennial plants, including banana and several other fruit crops (Carneiro et al., 2001; Xu et al., 2004; Almeida and Santos, 2011; Freitas et al., 2016). The ability of this nematode to infect a number of plants and overcome resistance mechanisms of hosts has been one of the major drawbacks for the management of field populations (Prot, 1984; Fargette, 1987; Carneiro et al., 2006; Cantu et al., 2009; Kiewnick et al., 2009). Chemical nematicides and cultural practices have been adopted to mitigate pest populations, but these have not always provided a long-term suppression at economically feasible costs (Gomes et al., 2010). Additionally, market phaseout of some chemical nematicides and the difficulty in the discovery and development of new, safe active ingredients by the chemical industry have restricted product alternatives for growers.
Biological control is considered an environmentally friendly strategy against RKN (Mankau, 1980; Sikora, 1992; Kerry, 2000; Eapen et al., 2005; Dong and Zhang, 2006; Sun et al., 2006). The egg-parasitic fungi Pochonia chlamydosporia (formerly Verticillium chlamydosporium) and Purpureocillium lilacinum (formerly Paecilomyces lilacinus) are among the most studied biological agents aiming at nematode management (Hidalgo-Díaz et al., 2000; Atkins et al., 2003; Khan et al., 2006; Anastasiades et al., 2008; Moosavi et al., 2010; Carneiro et al., 2011). Both species are ubiquitous, saprobic filamentous fungi, commonly isolated from soils, root surfaces, and some invertebrates, being naturally distributed in agricultural soils across Brazil (Tigano-Milani et al., 1993; Arevalo et al., 2009). Characteristics such as their ability to successfully colonize the rhizosphere and mass production feasibility (De Leij and Kerry, 1991; Kerry and Hidalgo-Díaz, 2004; Rumbos and Kiewnick, 2006) make these species potential candidates for the development of bionematicides.
A great number of strains can be assessed using in vitro methodologies under well-controlled conditions (Chen et al., 1996; Chen and Chen, 2003; Moosavi et al., 2010; Mensin et al., 2012; Luambano et al., 2015). Although in vitro studies seldom represent the real plant–nematode–fungus interaction in the rhizosphere (Dong and Zhang, 2006), laboratory experiments may also help to understand the fungus mode of action and pathogen–host interactions. Little is known, for example, about the interactions between more than one egg-parasitic fungus targeting the same host or how the parasitism develops in egg masses. Indeed, additional studies on egg parasitism and rhizosphere colonization by the fungus under controlled conditions are needed for better field recommendations (Sikora, 1992; Kerry, 2000; Verdejo-Lucas et al., 2003; Dong and Zhang, 2006).
The aims of the present study were to (i) select pathogenic strains of P. chlamydosporia and P. lilacinum against M. enterolobii eggs under in vitro conditions, (ii) determine the ability of selected strains to decrease J2 hatchability and the interaction between strains targeting the same host in laboratory bioassays, (iii) evaluate the potential of selected strains to reduce nematode populations on tomato and banana plants under greenhouse conditions.
Materials and Methods
Origin of fungal isolates:
All fungal strains were isolated from nematode eggs or soil samples (Tables 1,2) and kept in liquid nitrogen (−196°C) at the Invertebrate-Associated Fungal Collection, at EMBRAPA Genetic Resources and Biotechnology, Brasilia, Brazil. Strains belonged to species/varieties P. chlamydosporia var. catenulata, P. chlamydosporia var. chlamydosporia and P. lilacinum and were identified through morphological characteristics of the colony and reproductive cells (Samson, 1974; Zare et al., 2001; Luangsa-Ard et al., 2011).
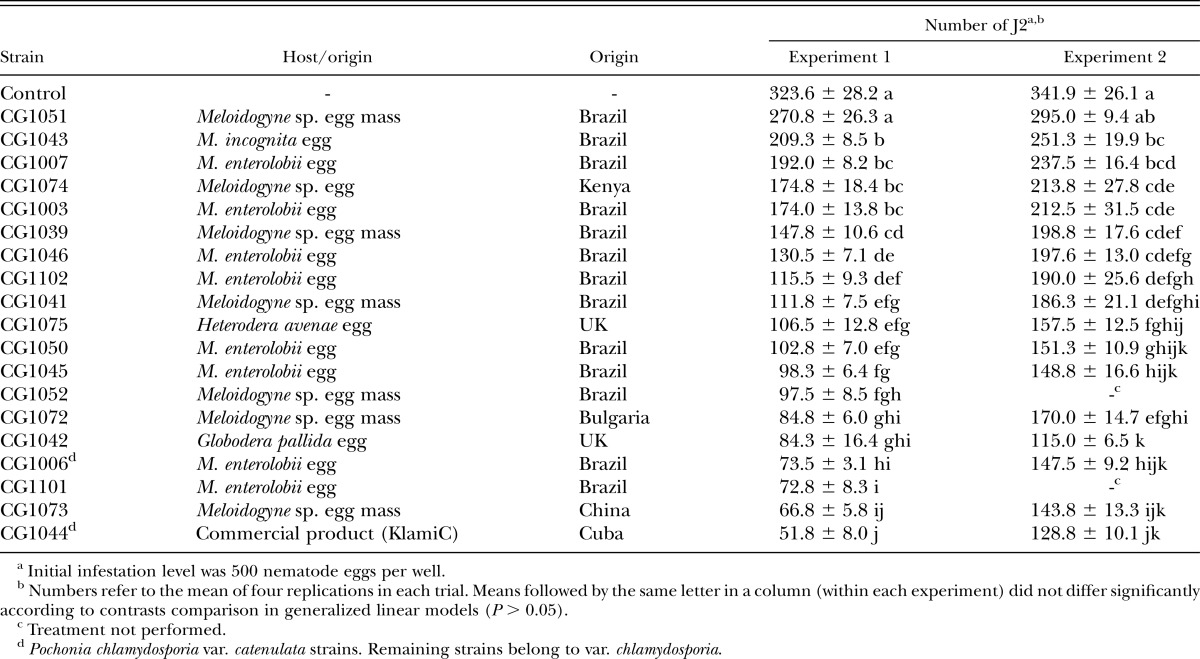
Table 1.
Number (mean ± SE) of second-stage juveniles (J2) hatched per well from Meloidogyne enterolobii eggs treated with different strains of Pochonia chlamydosporia (var. catenulata or chlamydosporia).
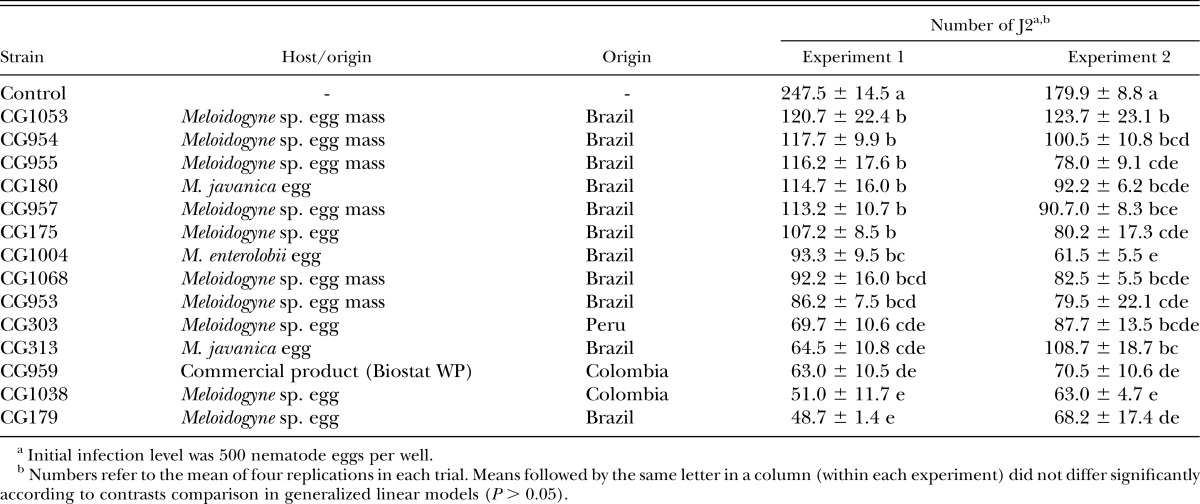
Table 2.
Number (mean ± SE) of second-stage juveniles (J2) hatched per well from Meloidogyne enterolobii eggs treated with different strains of Purpureocillium lilacinum.
Preparation of nematode and fungal inocula for in vitro assays:
Nematode population was originally collected from guava roots in a highly infested area in Northeastern, Brazil, and identification was carried out using esterase phenotyping (Carneiro and Almeida, 2001). Egg suspensions for in vitro and in vivo assays were prepared from tomato plants kept in a greenhouse and previously infected with M. enterolobii. Four-month-old infected roots were washed in running tap water and ground in a sodium hypochlorite solution (NaOCl) (0.5%) for 60 sec, with the aid of a blender. The suspension was passed through an overlaid set of sieves of 20, 100, and 500 meshes, and eggs retained in the last sieve were resuspended in distilled water and washed in a saccharose solution (30%). All washing procedure took less than 5 min and no damage in egg membrane was observed under the microscope (×200 of magnification). For greenhouse assays, eggs and J2 were extracted using Hussey and Barker’s (1973) methodology, with a blender instead of manual agitation and 0.5% NaOCl. The number of eggs + J2 was evaluated using a Peter’s slide and a light microscope at ×100 magnification. The number of eggs containing J1 and J2 were also scored separately. Average percentages for eggs, eggs with J1 and J2, used in nematode inocula in in vitro bioassays were 76.3% ± 2.98%, 13.0% ± 1.35%, and 10.7% ± 3.93%, respectively.
Fungal strains were grown on Potato Dextrose Agar medium (PDA; Acumedia, Lansing, MI) + streptomycin (0.5 g/L) for an 18- to 21-d period at 26°C ± 0.5°C in darkness. Cells (spores and mycelia) from cultures were scraped using a spatula and suspended in distilled water + Tween 80 (0.05% v/v). Suspensions were homogenized in a vortex for 60 sec and filtered through a cloth to remove mycelial fragments. Conidia and chlamydospores of P. chlamydosporia and conidia of P. lilacinum were counted in a Neubauer chamber and suspension diluted to a final concentration of 1 × 106 spores/ml.
In vitro parasitism of M. enterolobii eggs by strains of P. chlamydosporia and P. lilacinum:
Nineteen strains of P. chlamydosporia and 14 strains of P. lilacinum were tested against M. enterolobii eggs, including the active ingredient of the commercial products KlamiC (Centro Nacional de Sanidad Agropecuaria, La Habana, Cuba; Hernández and Hidalgo-Diaz, 2008) and Biostat WP (Laverlam S.A., Cali, Colombia; Tranier et al., 2014). The bioassays were performed in 12-well plates and each well was filled with 3 ml of water agar (1.5% v/v). A 100-µl drop of the nematode egg suspension (around 500 eggs) was placed in the well and air-dried in a laminar flow. Then, for each fungal strain being assessed, a 150-µl drop of spore suspension covered all eggs on the water-agar surface. Distilled water + Tween 80 (0.05% v/v) was used as a control treatment. After being air-dried, plates were covered with the lid and incubated at 25°C ± 0.5°C in darkness. A moistened filter paper was placed in the inner side of the lid in order to keep relative humidity >95%. Fifteen days after inoculation, wells were gently rinsed with 1 ml of distilled water and the agar scraped with a glass rod; this was repeated two more times. Suspensions were collected from the wells and the number of J2 determined in a Peter chamber. Unhatched eggs were also counted in suspensions from wells of the control treatments to determine the percentage of total nematodes recovered in the washing procedure. Experiments with each fungal species were completely randomized and four replicates (wells) were performed per treatment (strain). Both experiments (fungal species) were repeated twice on different dates.
In vitro activity of selected strains of P. chlamydosporia and P. lilacinum against M. enterolobii egg masses:
Strains selected in the previous assay (CG1006 and CG1044 of P. chlamydosporia var. catenulata; CG1042 and CG1101 of P. chlamydosporia var. chlamydosporia; and CG179, CG1038, and CG959 of P. lilacinum) were tested against egg masses of M. enterolobii. First, egg masses were collected directly from nematode-infected tomato roots using a thin needle and forceps under a stereoscope. Groups of five egg masses, homogenous in size, were placed on a water-agar surface in wells. Fungal inoculation and spore concentration were the same as described previously. Distilled water + Tween 80 (0.05% v/v) was used as a control treatment. After being air-dried, plates were covered and incubated at 25°C ± 0.5°C in darkness. Well-washing procedure after 15 d of inoculation was the same as previously described. Experiments with egg masses were completely randomized and four replicates were performed per strain. Each experiment was repeated twice on different dates.
In vitro interactions between strains of P. chlamydosporia var. catenulata and P. lilacinum applied to M. enterolobii eggs:
The combination of the fungal species P. chlamydosporia var. catenulata (CG1006 and CG1044) and P. lilacinum (CG179 and CG1038) was evaluated in vitro using M. enterolobii eggs. Final spore suspensions, containing combinations of one strain from each species, was 1 × 106 spores/ml, half from each fungal species. Treatments with a single species at the full concentration were also included, and distilled water + Tween 80 (0.05% v/v) was used as a control treatment. The methodology and experimental conditions were the same as already described. The experiment was carried out in a completely randomized design with four replicates and was repeated twice on different dates.
Efficacy of selected strains of P. chlamydosporia and P. lilacinum against M. enterolobii on potted tomato and banana plants under greenhouse conditions:
For the greenhouse studies, the selected strains CG1006 and CG1044 of P. chlamydosporia var. catenulata, the commercial product Rizotec of P. chlamydosporia var. chlamydosporia (Rizoflora Biotecnologia S.A., Viçosa, Brazil; Bettiol et al., 2012), and CG179 of P. lilacinum were tested. Spore production was carried out through a two-stage fermentation process. First, three plugs (3 mm in diameter) from PDA cultures were placed in 250-ml Erlenmeyer flasks with 200 ml of autoclaved rice broth (30 g of rice cooked for 10 min in 1 l of distilled water) and incubated at 25°C ± 1°C in an orbital shaker (150 rpm) for 3 d. For the second stage, rice grains were soaked in distilled water and, after removal of excess water, autoclaved for 20 min in polyethylene bags (100 g rice per bag). Each bag was inoculated with 15 ml of the fungal broth, and then incubated at 25°C ± 0.5°C in darkness for 10 and 21 d for P. lilacinum and P. chlamydosporia, respectively. The colonized rice was washed in distilled water + Tween 80 (0.05% v/v), and suspensions were homogenized and diluted as described previously. Spore viability of the commercial product Rizotec was assessed on PDA by counting the number of colony-forming units per gram of product after 5 d on incubation (25°C ± 1°C and darkness). The product was suspended in distilled water to give the same final spore concentration.
Tomato (cv. Santa Clara) and banana (cv. Terra) seedlings, approximately 10 to 15 cm in height, were transferred to 3- or 5-l plastic bags (hereafter referred to as pots), respectively, containing an autoclaved mix of soil (50%), sand (25%), and organic compost (25%). Prior to planting, pots with soil mixture were previously inoculated with 5 × 104 spores/g of soil by drenching 15 ml of fungal suspension in a 15-cm-deep hole made to receive the seedling. An egg suspension of M. enterolobii (10,000 eggs per pot) was applied in three holes around plants, 15 d after seedling transfer and fungal inoculation. Controls were inoculated only with egg suspensions in distilled water + Tween 80 (0.05% v/v). Additionally, a separate experiment was carried out to evaluate the control efficacy of the strains CG1006 (P. chlamydosporia var. catenulata) and CG179 (P. lilacinum) in tomato plants exposed to moderate (5,000 eggs per pot) and low (500 eggs per pot) infestation levels. Materials and methods were the same as described above. Plants were watered periodically and kept under greenhouse conditions for 3 and 12 mon after nematode inoculation in tomato and banana plants, respectively. Nine replicates (plants) were used in each experiment in a completely randomized design.
Roots were washed under running tap water to remove soil and other debris, and then fresh weight was scored. The root galling index (GI) and MI were determined based on a 0 to 5 scale proposed by de Hartman and Sasser (1985), as follows: 0 = absence; 1 = 1 to 2; 2 = 3 to 10; 3 = 11 to 30; 4 = 31 to 100, and 5 = more than 100 galls or egg masses per plant. Then, eggs were extracted using Hussey and Barker’s (1973) methodology, using a blender instead of manual agitation and 1% NaOCl. The number of eggs per gram of root was determined. GI and MI were not evaluated in the banana experiment because root galls and egg masses were not clearly visible on the thick black roots of this plant.
The presence of propagules of each fungal strain in roots (endophytic behavior) of both plants was also determined at the end of the experimental period. Twenty root sections (2 cm long; 0.2 cm in diameter) were randomly collected and surface sterilized in NaOCl (2% for 30 sec) and rinsed twice in sterile water. Root sections were placed on the surface of a selective medium (De Leij and Kerry, 1991), and presence of growing fungus from sections was scored (presence or absence) and identified under a microscope after a 10-d incubation period at 25°C ± 1°C in darkness.
Statistical analyses:
Datasets on number of J2 were analyzed by generalized linear models and fitted to negative-binomial distribution taking overdispersion into account. Due to the presence of overdispersion in all datasets, a chi-square test based on the residual deviance and degrees of freedom was used to compute estimates and P values, which indicates if the model fits the data. Multiple pairwise comparisons (one-way or two-way analysis of variance) were performed, and means of treatments compared by contrasts (P < 0.05). Analyses were performed using R statistical software environment (R Development Core Team, 2015). Synergistic or antagonistic effect between strains of P. chlamydosporia and P. lilacinum was determined using a procedure adopted by Koppenhofer and Kaya (1997). The expected proportional additive effect for the P. chlamydosporia–P. lilacinum combinations on J2 reduction (ER) was calculated by the formula ER = RPc + RPl(1−RPc), where RPc and RPl are the observed proportional mortalities caused by P. chlamydosporia and P. lilacinum alone, respectively. In our case, RPc and RPl values were divided by two since the strain combinations were prepared at half the concentration of each fungal species. Calculated values from a chi-square test (χ2 = [RComb–ER]2/ER, where RComb is the observed J2 reduction for each P. chlamydosporia–P. lilacinum combination) were compared to the chi-square table value for 1 degree of freedom. When the calculated χ2 value exceeded the table value a possible synergistic or antagonistic effect between the two fungi is expected at 0.05 of probability.
Results
In vitro parasitism of M. enterolobii eggs by strains of P. chlamydosporia and P. lilacinum:
The mean percentage of nematodes (eggs and J2) recovered from each well in the control treatment 15 d post-inoculation was 75.6% ± 3.05% of the initial number of eggs and juveniles used as inocula. Significant interactions between trial replicates and strains of P. chlamydosporia (χ2 = 59.95; df = 19; P ≤ 0.0001) and P. lilacinum (χ2 = 31.23; df = 14; P = 0.0051) were detected and results were presented separately (Tables 1, 2). Significant differences in the number of hatched J2 were seen in both trials for P. chlamydosporia strains (χ2 = 617.67; df = 19; P ≤ 0.0001 and χ2 = 200.88; df = 17; P ≤ 0.0001). Similarly, differences in the number of hatched J2 were also seen for P. lilacinum strains (χ2 = 206.58; df = 14; P ≤ 0.0001 and χ2 = 78.63; df = 14; P ≤ 0.0001). When compared to the control treatment, the percent reduction in J2 hatching varied from 13.7% to 84% for P. chlamydosporia, and 31.2% to 80.3% for P. lilacinum strains. Microscopic observations of egg shrinkage and mycelial growth, both external and internal to egg shell, were seen for both species, but mainly for P. chlamydosporia treatments.
In vitro activity of selected strains of P. chlamydosporia and P. lilacinum against M. enterolobii egg masses:
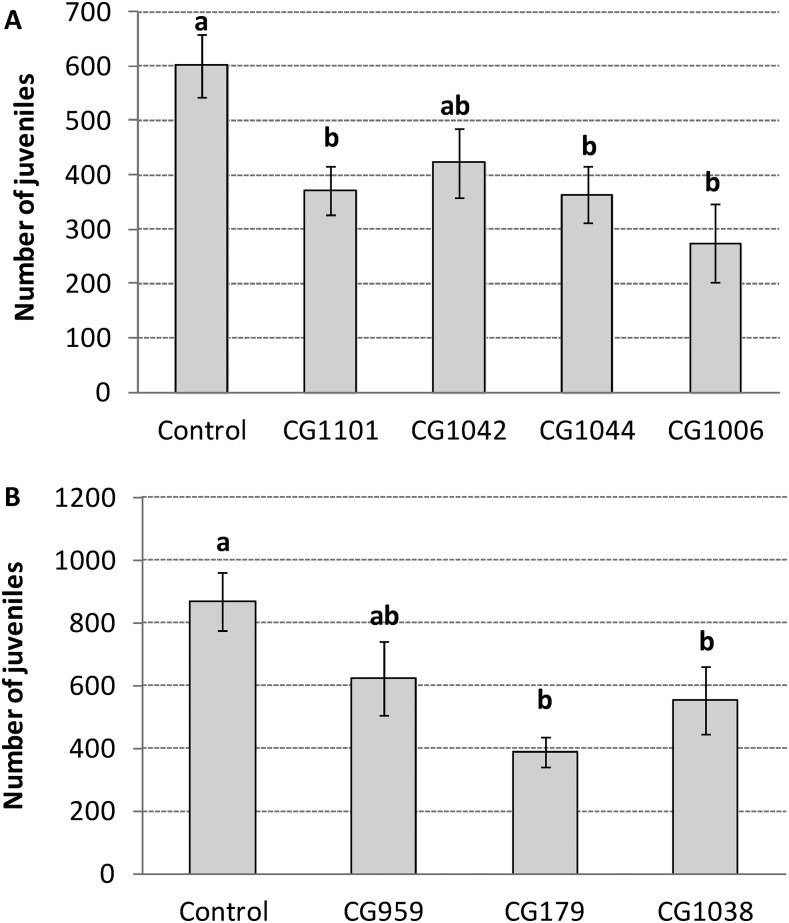
No significant interaction between trial replicates and strains of P. chlamydosporia was seen (χ2 = 6.12; df = 4; P = 0.1903) and data were combined. A significant difference was observed in the experiment with selected P. chlamydosporia strains (χ2 = 15.25; df = 4; P = 0.0042). J2 hatchability was reduced by 54.3% and 39.6% for strains CG1006 and CG1044, respectively, when compared to the control treatment (Fig. 1A). Likewise, no significant interaction between trial replicates and strains of P. lilacinum was seen (χ2 = 7.27; df = 3; P = 0.0636) and data were combined. Differences were also observed in the experiment with selected P. lilacinum strains (χ2 = 16.38; df = 3; P = 0.0009). Strains CG179 and CG1038 showed 55.2% and 36.6% of reduction in J2 hatchability, respectively, when compared to the control treatment (Fig. 1B).
Fig. 1.
Mean number (±SE) of second-stage juveniles (J2) hatched from Meloidogyne enterolobii egg masses treated with strains of the following: A. Pochonia chlamydosporia and B. Purpureocillium lilacinum. Means followed by the same letter on bars did not differ significantly according to contrasts comparison in generalized linear models (P > 0.05).
In vitro interactions between strains of P. chlamydosporia var. catenulata and P. lilacinum applied to M. enterolobii eggs:
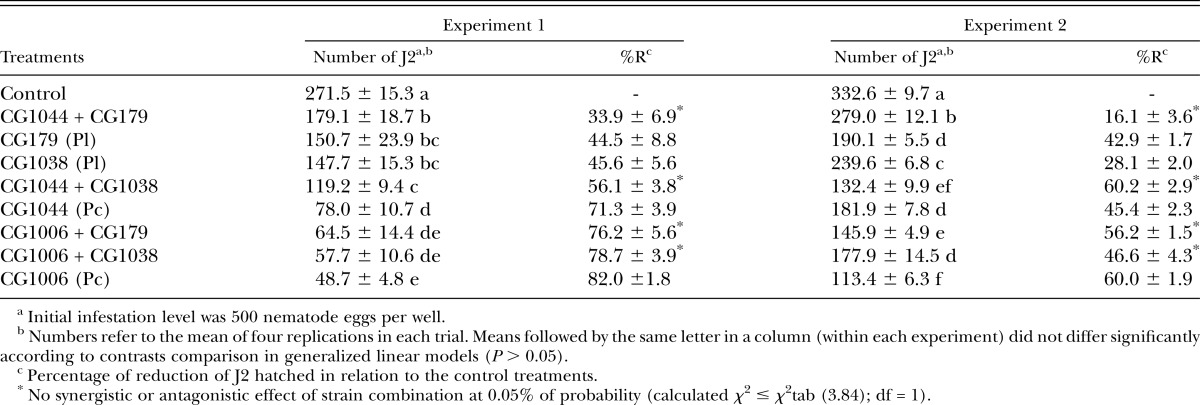
A significant interaction between trial replicates and treatments (χ2 = 58.90; df = 8; P ≤ 0.0001) was observed and results were presented separately (Table 3). The mean percentages of nematodes (eggs and J2) retrieved from each well in the control treatment 15 d post-inoculation were 87.0% ± 0.16% and 92.4% ± 0.40% of the initial egg number used for trials 1 and 2, respectively. Significant differences in the number of J2 hatched from eggs were detected in both trials (χ2 = 237.04; df = 8; P ≤ 0.0001 and χ2 = 555.51; df = 8; P ≤ 0.0001). When used alone, P. chlamydosporia var. catenulata strains showed greater parasitism than did P. lilacinum strains. Combinations of fungal species (one strain from each species) did not increase or decrease the control efficacy. Antagonistic or synergistic effects were not detected for all combinations since calculated χ2 values (between 0.007 and 0.213; df = 1) were lower than χ2tab values.
Table 3.
Number (mean ± SE) of second-stage juveniles (J2) hatched per well from Meloidogyne enterolobii eggs treated with one or more strains of Pochonia chlamydosporia var. catenulata (Pc) and Purpureocillium lilacinum (Pl).
Efficacy of selected strains of P. chlamydosporia and P. lilacinum against M. enterolobii eggs on potted tomato and banana plants under greenhouse conditions:
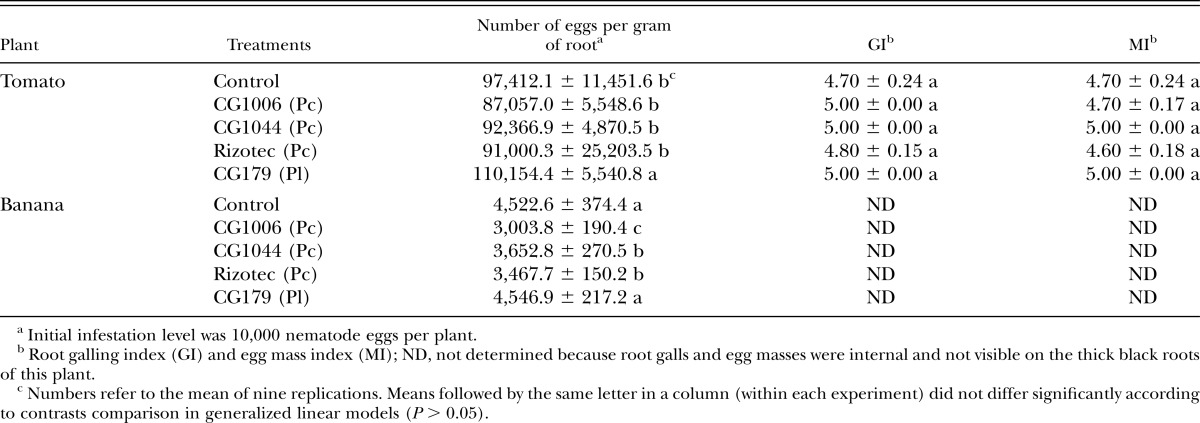
Neither P. chlamydosporia nor P. lilacinum were associated to tomato or banana roots as endophytes, although the methodology used in our study was not aimed to detect colonization of soil or external root surfaces by the tested fungi. The number of eggs per gram of root of tomato plants did not differ among P. chlamydosporia strains and the control treatment after 3 mon and ranged from 87,057 to 97,412 (Table 4). A marginal increase in egg numbers was seen in P. lilacinum CG179-treated plants compared to untreated plants (χ2 = 10.52; df = 4; P = 0.032). Tomato plants in all treatments clearly showed symptoms of severe nematode infection, characterized by limited plant growth, yellowish leaves, and galled and rotten root system. The severe infection was clearly shown by the high GI and egg MI, which were similar among treatments (P > 0.98) and ranged from 4.7 to 5.0 and 4.6 to 5.0, respectively.
Table 4.
Effect of Pochonia chlamydosporia var. catenulata (Pc) and Purpureocillium lilacinum (Pl) strains on Meloidogyne enterolobii population (mean ± SE) in potted tomato and banana plants under greenhouse conditions.
Banana plants cv. Terra were good hosts to M. enterolobii and presented high nematode reproduction, although their growth pattern was not altered. Significant differences in egg and J2 nematode population were seen in banana roots after 12 mon, varying from 3,004 to 4,547 eggs per gram of root (χ2 = 92.65; df = 4; P ≤ 0.0001). The strains CG1006 and CG1044 (P. chlamydosporia var. catenulata) and the commercial product Rizotec (P. chlamydosporia var. chlamydosporia) caused significant reductions of 33.1%, 19.3%, and 19% in nematode eggs compared to control plants, respectively (Table 4).
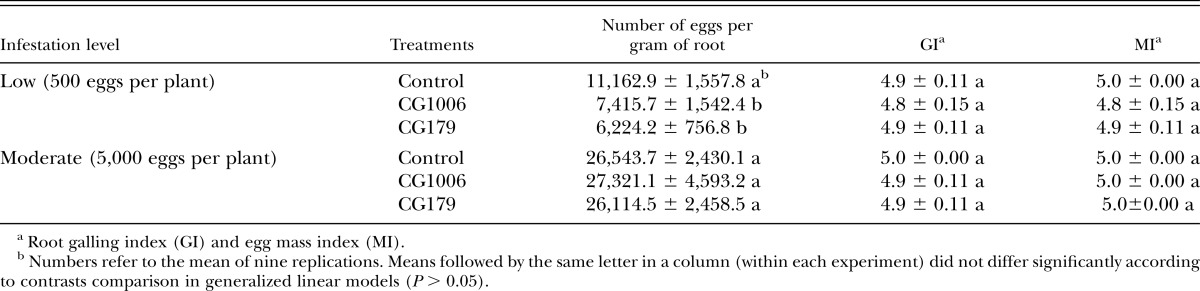
A significant interaction was seen between strain and nematode inoculum concentration in tomato plants (χ2 = 12.904; df = 2; P = 0.0016). The increase of the inoculum concentration (from 500 to 5,000 eggs per plant) increased the number of eggs per gram of root (χ2 = 295.92; df = 1; P ≤ 0.0001) after this 3-mon-long experiment. Under the low inoculum level (500 eggs per plant), strains CG1006 and CG179 reduced the egg numbers per gram of root by 33.6% and 44.2%, respectively (Table 5), and significant differences were seen in egg numbers among treatments (χ2 = 21.45; df = 2; P ≤ 0.0001). Under moderate inoculum level (5,000 eggs per plant), no reductions in the number of eggs per gram of root were observed compared to the control treatment (χ2 = 0.21; df = 2; P = 0.9015) (Table 5). Likewise, GI and MI did not differ among treatments, regardless of the number of eggs used as inoculum (P > 0.854). Short roots and clear signs of rotten roots in tomato plants were observed for both concentrations of the inoculum.
Table 5.
Effect of Pochonia chlamydosporia var. catenulata (CG1006) and Purpureocillium lilacinum (CG179) strains on Meloidogyne enterolobii population (mean ± SE) in low and moderate nematode infestation of potted tomato plants under greenhouse conditions.
Discussion
In the present study, we showed the ability of different P. chlamydosporia (var. catenulata and chlamydosporia) and P. lilacinum strains in reducing J2 hatchability of individual M. enterolobii eggs by direct contact under laboratory conditions. Moreover, our data suggest that eggs within the gelatinous matrix may be less susceptible than individual eggs to infection by both fungal species. Finally, we detected a decrease in the numbers of eggs per gram of root on tomato plants treated with both fungal species when compared to untreated plants under lower levels of nematode infection.
The great genetic variability of invertebrate pathogenic fungi has been largely explored in the development of biopesticides against insects and mites. The importance of screening strains with high biological activity against different RKN species has also been reported (Hidalgo-Díaz et al., 2000; Eapen et al., 2005; Dallemole-Giaretta et al., 2012; Mensin et al., 2012). Egg parasitism is the main mode of action of P. chlamydosporia and P. lilacinum against plant-pathogenic and animal-parasitic nematodes (Chen and Chen, 2003; Sun et al., 2006; Carvalho et al., 2010; Moosavi et al., 2010). Different parameters have been used to assess the parasitism of nematode eggs or egg masses by these pathogens. Although the presence of fungal structures on egg surface and morphological changes in egg shells may indicate parasitism, egg hatchability after exposure to the pathogen gives a better indication of the potential of biological control agents. Water agar has been successfully used for the in vitro assessment of fungal activity on egg hatchability (Chen et al., 1996; Eapen et al., 2005; Moosavi et al., 2010). However, the use of disc cultures on water-agar surface as inoculum represents an important source of nutrients that is unavailable in the soil and that might enhance fungal growth and overestimate its activity. We adopted an in vitro assay with tissue culture plates, which utilized conidia and/or chlamydospores without a nutrient source and provided a simple and precise method for screening potential fungal strains on individualized nematode eggs. Both mechanical and NaOCl protocols have been deployed in in vitro experiments aiming separation of eggs from the gelatinous matrix, although the later is much less time consuming and allowed us to screen a greater number of strains. Additionally, the water agar in the wells kept favorable moisture conditions for embryo development and allowed the contact of germinating fungal spores in standardized concentration against the eggs or egg masses.
Despite methodological differences, parasitism levels on individual eggs observed in our study were similar to some other in vitro studies with Meloidogyne using P. chlamydosporia or P. lilacinum (Moosavi et al., 2010; Aminuzzaman et al., 2013). In our study, reductions from 67% to 78% and 69% to 73% in egg hatchability were observed for selected strains of P. chlamydosporia and P. lilacinum, respectively. On the other hand, the average reductions in egg hatchability when the same strains were used on egg masses were not greater than 55%. These results suggest that the gelatinous matrix provided some degree of protection to eggs against microbial invasion as previously reported by Orion et al. (2001). Although some microorganisms are capable of colonizing the gelatinous matrix, including P. chlamydosporia and P. lilacinum (Dunn et al., 1982; Meyer and Wergin, 1998), a likely delay in microbial invasion may allow eggs to reach developmental stages that are less susceptible to fungal infections. Indeed, eggs in earlier embryonic stages are reported to be more successfully infected by nematophagous fungi (Chen and Chen, 2003; Khan et al., 2006).
Additive or synergic effects by the combination of two or more nematode pathogens have been described (Dube and Smart Jr., 1987; Anastasiades et al., 2008). In our in vitro study, no additive or synergistic effects were detected when selected strains of P. chlamydosporia were used simultaneously with strains of P. lilacinum. The performance of P. chlamydosporia deployed alone (full dose) was the same as the mixture of two strains, one from each species (half dose each). In general, P. lilacinum strains were less effective than P. chlamydosporia strains in reducing egg hatching. It has been shown that nematode eggs precolonized by a saprophytic or weakly parasitic fungus may reduce the infection by other nematophagous fungi (Chen and Chen 2003). Even though strain CG1044 (P. chlamydosporia var. catenulata) promoted significantly lower egg hatchability than its mixture with strain CG179 (P. lilacinum), no antagonistic effect was seen.
Despite considerable nematode egg parasitism caused by some P. chlamydosporia and P. lilacinum strains in lab assays, in general their efficacy in reducing root infection and nematode reproduction in potted plants was very low. None of the strains significantly reduced the galling or egg mass indexes, nor even the number of eggs per gram of root in tomato plants under either moderate or high nematode infestation levels (5,000 and 10,000 eggs per pot, respectively). In contrast, other studies under greenhouse and field conditions showed considerable suppression of RKN populations by both fungal species (Kerry and Hidalgo-Díaz, 2004; Siddiqui and Akhtar, 2009; Aminuzzaman et al., 2013; Bontempo et al., 2014). In a study with P. lilacinum carried out by Cabanillas and Barker (1989), the fungal inoculum level and the application time were shown to have impact on Meloidogyne control. Other parameters such as the type of infective propagules used (conidia, chlamydospores, or mycelia) and soil characteristics may also have influenced the level of nematode suppression. Plant species may also influence the efficacy of the fungus as a biological control agent. The low efficacy of some nematophagous fungal strains against RKN on tomato roots, which produce large galls as a result of nematode infection compared with other hosts, has been already reported (Stirling et al., 1979; Bourne et al., 1996). It is worth mentioning that under lower nematode infestations (500 eggs per plant), a moderate decrease in the egg numbers was seen for both species when compared to untreated tomato plants. Therefore, results suggested that use of nematophaghous fungi as a stand-alone approach would not be advisable, but both fungi could be used as part of an integrated management program for M. enterolobii in tomato crops when the nematode population is low.
This is the first report of suppression of M. enterolobii eggs by P. chlamydosporia on banana plants. Decreases in the number of eggs per root were seen in plants treated with all three P. chlamydosporia strains tested in our greenhouse experiments. Moreover, the high susceptibility of banana plants to M. enterolobii and the development of internal egg masses on roots may have hampered egg parasitism by the fungus. Females and eggs embedded in the roots may isolate egg masses from the fungal attack, since the later are confined to the rhizosphere, as reported by Bourne et al. (1996). The high number of eggs per root in all the treatments was remarkable; nonetheless, plant development apparently was not compromised indicating tolerance of banana plants to M. enterolobii. In cases where no signs of plant debilitation by nematodes are apparent, huge populations may develop, and control strategies based on microbial products are no longer effective.
In conclusion, our results indicate that adoption of selected strains of nematophagous fungi for management of M. enterolobii populations may be possible under some circumstances within an IPM approach, as long as infestation levels in soil are low.
Literature Cited
- Almeida EJ, Santos JM. Ocorrência de Meloidogyne enterolobii Yang & Eisenback, no município de Uberlândia, Minas Gerais, Brasil. Bioscience Journal. 2011;27:877–878. [Google Scholar]
- Aminuzzaman FM, Xie HY, Duan WJ, Sun BD, Liu XZ. Isolation of nematophagous fungi from eggs and females of Meloidogyne spp. and evaluation of their biological control potential. Biocontrol Science and Technology. 2013;23:170–182. [Google Scholar]
- Anastasiades IA, Giannakou IO, Prophetou-Athanasiadou DA, Gowen SR. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Protection. 2008;27:352–361. [Google Scholar]
- Arevalo J, Hidalgo-Díaz L, Martins I, Souza JF, Castro JMC, Carneiro RMDG, Tigano MS. Cultural and morphological characterization of Pochonia clamydosporia and Lecanicillium psalliotae isolated from Meloidogyne mayaguensis eggs in Brazil. Tropical Plant Pathology. 2009;34:158–163. [Google Scholar]
- Atkins SD, Hidalgo-Diaz L, Kalisz H, Mauchline TH, Hirsch PR, Kerry BR. Development of a new management strategy for the control of root-knot nematodes (Meloidogyne spp.) in organic vegetable production. Pest Management Science. 2003;59:183–189. doi: 10.1002/ps.603. [DOI] [PubMed] [Google Scholar]
- Bettiol W, Morandi MAB, Pinto ZV, Paula Júnior TJ, Corrêa EB, Moura AB, Lucon CMM, Costa JCB, Bezerra JL. 2012. Produtos comerciais à base de agentes de biocontrole de doenças de plantas. Documentos 88, Jaguariúna: Embrapa Meio Ambiente.
- Bontempo AF, Fernandes RH, Lopes J, Freitas LG, Lopes EA. Pochonia chlamydosporia controls Meloidogyne incognita on carrot. Australasian Plant Pathology. 2014;43:421–424. [Google Scholar]
- Bourne JM, Kerry BR, De Leij FAAM. The importance of the host plant on the interaction between root-knot nematodes Meloidogyne spp. and the nematophagous fungus, Verticillium chlamydosporium Goddard. Biocontrol Science and Technology. 1996;6:539–548. [Google Scholar]
- Cabanillas E, Barker KR. Impact of Paecilomyces lilacinus inoculum level and application time on control of Meloidogyne incognita on tomato. Journal of Nematology. 1989;21:115–120. [PMC free article] [PubMed] [Google Scholar]
- Cantu RR, Wilcken SRS, Rosa JMO, Goto R. Reação de porta-enxertos de tomateiros a Meloidogyne mayaguensis. Summa Phytopathologica. 2009;35:124–126. [Google Scholar]
- Carneiro RMDG, Almeida MRA. Técnica de eletroforese usada no estudo de enzimas dos nematoides de galhas para identificação de espécies. Nematologia Brasileira. 2001;25:35–44. [Google Scholar]
- Carneiro RMDG, Almeida MRA, Braga RS, Almeida CA, Gioria R. Primeiro registro de Meloidogyne mayaguensis parasitando plantas de pimentão e tomate resistentes à meloidoginose no Estado de São Paulo. Nematologia Brasileira. 2006;30:81–86. [Google Scholar]
- Carneiro RMDG, Hidalgo-Díaz L, Martins I, Silva KFAS, Souza MG, Tigano MS. Effect of nematophagous fungi on reproduction of Meloidogyne enterolobii on guava (Psidium guajava L.) plants. Nematology. 2011;13:721–728. [Google Scholar]
- Carneiro RMDG, Moreira WA, Almeida MRA, Gomes ACMM. Primeiro registro de Meloidogyne mayaguensis em goiabeira no Brasil. Nematologia Brasileira. 2001;25:223–228. [Google Scholar]
- Carvalho RO, Araújo JV, Braga FR, Araújo JM, Alves CDF. Ovicidal activity of Pochonia chlamydosporia and Paecilomyces lilacinus on Toxocara canis eggs. Veterinary Parasitology. 2010;169:123–127. doi: 10.1016/j.vetpar.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Chen SY, Chen FJ. Fungal parasitism of Heterodera glycines eggs as influenced by egg age and pre-colonization of cysts by other fungi. Journal of Nematology. 2003;35:271–277. [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Dickson DW, Mitchell DJ. Pathogenicity of fungi to eggs of Heterodera glycines. Journal of Nematology. 1996;28:148–158. [PMC free article] [PubMed] [Google Scholar]
- Dallemole-Giaretta R, Freitas LG, Lopes EA, Pereira OL, Zooca RJF, Ferraz S. Screening of Pochonia chlamydosporia Brazilian isolates as biocontrol agents of Meloidogyne javanica. Crop Protection. 2012;42:102–107. [Google Scholar]
- De Leij FAAM, Kerry BR. The nematophagous fungus, Verticillium chlamydosporium Goddard, as a biological control agent for Meloidogyne arenaria (Neal) Chitwood. Révue de Nematologie. 1991;14:157–194. [Google Scholar]
- Dong LQ, Zhang KQ. Microbial control of plant-parasitic nematodes: A five-party interaction. Plant Soil. 2006;288:31–45. [Google Scholar]
- Dube B, Smart GC., Jr Biological control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. Journal of Nematology. 1987;19:222–227. [PMC free article] [PubMed] [Google Scholar]
- Dunn MT, Sayre RM, Carrell A, Wergin WP. Colonization of nematode eggs by Paecilomyces lilacinus (Thom) Samson as observed with scanning electron microscope. Scanning Electron Microscopy. 1982;3:1351–1357. [Google Scholar]
- Eapen SJ, Beena B, Ramana KV. Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. Journal of Invertebrate Pathology. 2005;88:218–225. doi: 10.1016/j.jip.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Fargette M. Use of esterase phenotype in the taxonomy of the genus Meloidogyne. 2. Esterase phenotypes observed in West African populations and their characterization. Révue de Nematologie. 1987;10:45–56. [Google Scholar]
- Freitas VM, Silva JGP, Gomes CB, Castro JMC, Correa VR, Carneiro RMDG. Host status of selected cultivated fruit crops to Meloidogyne enterolobii. European Journal of Plant Pathology doi:10.1007/s10658-016-1090-8. 2016 [Google Scholar]
- Gomes VM, Souza RM, Corrêa FM, Dolinski C. Management of Meloidogyne mayaguensis in commercial guava orchards with chemical fertilization and organic amendments. Nematologia Brasileira. 2010;34:23–29. [Google Scholar]
- Hartman KM, Sasser JN. 1985. Identification of Meloidogyne species on the basis of differential host test and perineal-pattern morphology. Pp. 69–77 in K. R. Barker, C. C. Carter, J. N. Sasser, eds. Advanced treatise on Meloidogyne, Methodology, vol. II. Raleigh, NC: North Carolina State University.
- Hernández MA, Hidalgo-Diaz L. KlamiC®: Bionematicida agrícola producido a partir del hongo Pochonia chlamydosporia var. catenulata. Revista de Protección Vegetal. 2008;23:131–134. [Google Scholar]
- Hidalgo-Díaz L, Bourne JM, Kerry BR, Rodríguez MG. Nematophagous Verticillium spp. in soils infested with Meloidogyne spp. in Cuba: Isolation and screening. International Journal of Pest Management. 2000;46(4):277–284. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease. 1973;57:1025–1028. [Google Scholar]
- Khan A, Williams KL, Nevalainem HKM. Infection of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum. BioControl. 2006;51:659–678. [Google Scholar]
- Kerry BR. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annual Review of Phytopathology. 2000;38:423–41. doi: 10.1146/annurev.phyto.38.1.423. [DOI] [PubMed] [Google Scholar]
- Kerry BR, Hidalgo-Díaz L. Application of Pochonia chlamydosporia in the integrated control of root-knot nematodes on organically grown vegetable crops in Cuba. Multitrophic Interactions in the Integrated Control. 2004;27:123–126. [Google Scholar]
- Kiewnick S, Dessimoz M, Franck L. Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. Journal of Nematology. 2009;41:134–139. [PMC free article] [PubMed] [Google Scholar]
- Koppenhofer AM, Kaya HK. Additive and synergistic interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biological Control. 1997;8:131–137. [Google Scholar]
- Lima IM, Souza RM, Silva CP, Carneiro RMDG. Meloidogyne spp. from preserved areas of Atlantic Forest in the State of Rio de Janeiro, Brazil. Nematologia Brasileira. 2005;20:31–38. [Google Scholar]
- Luambano ND, Manzanilla-López RH, Kimenju JW, Powers SJ, Narla RD, Wanjohi WJ, Kerry BR. Effect of temperature, pH, carbon and nitrogen ratios on the parasitic activity of Pochonia chlamydosporia on Meloidogyne incognita. Biological Control. 2015;80:23–29. [Google Scholar]
- Luangsa-Ard J, Houbraken J, van Doorn T, Hong SB, Borman AM, Hywel-Jones NL, Samson RA. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiology Letters. 2011;321:141–149. doi: 10.1111/j.1574-6968.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Mankau R. Biological control of nematode pests by natural enemies. Annual Review of Phytopathology. 1980;18:415–440. [Google Scholar]
- Meyer SLF, Wergin WP. Colonization of soybean cyst nematode females, cysts, and gelatinous matrices by the fungus Verticillium lecanii. Journal of Nematology. 1998;30:436–450. [PMC free article] [PubMed] [Google Scholar]
- Mensin S, Soytong K, Mcgovern RJ, To-Anun C. Selection of efficient nematophagous fungi against root-knot nematodes in the highland cultivated area. Journal of Agricultural Technology. 2012;8:2259–2272. [Google Scholar]
- Moosavi M, Zare R, Zamanizadeh H, Fatemy S. Pathogenicity of Pochonia species on eggs of Meloidogyne javanica. Journal of Invertebrate Pathology. 2010;104:125–133. doi: 10.1016/j.jip.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Orion D, Kritzman G, Meyer SLF, Erbe EF, Chitwood DJ. A role of the gelatinous matrix in the resistance of root-knot nematode (Meloidogyne spp.) eggs to microorganisms. Journal of Nematology. 2001;33:203–207. [PMC free article] [PubMed] [Google Scholar]
- Prot JC. A naturally occurring resistance breaking biotype of Meloidogyne arenaria on tomato: Reproduction and pathogenicity on tomato cultivars Roma and Rossol. Révue de Nematologie. 1984;7:23–28. [Google Scholar]
- R Development Core Team, version 3.1.1 2015. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org.
- Rumbos CI, Kiewnick S. Effect of plant species on persistence of Paecilomyces lilacinus strain 251 in soil and on root colonization by the fungus. Plant and Soil. 2006;283:25–31. [Google Scholar]
- Samson RA. Paecilomyces and some allied Hyphomycetes. Studies in Mycology. 1974;6:1–119. [Google Scholar]
- Siddiqui ZA, Akhtar MS. Effects of antagonistic fungi and plant growth-promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita. Australasian Plant Pathology. 2009;38:22–28. [Google Scholar]
- Sikora RA. Management of the antagonistic potential in agricultural ecosystems for de biological control of plant parasitic nematodes. Annual Review of Phytopathology. 1992;30:245–270. [Google Scholar]
- Stirling GR, McKenry MV, Mankau R. Biological control of root-knot nematodes (Meloidogyne spp.) on peach. Phytopathology. 1979;69:806–809. [Google Scholar]
- Sun MH, Gao L, Shi YX, Li BJ, Liu XZ. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. Journal of Invertebrate Pathology. 2006;93:22–28. doi: 10.1016/j.jip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Taylor DT, Sasser JN. Biology, identification and control of rootknot nematodes (Meloidogyne species) North Carolina, NC: North Carolina State University Graphics; 1978. [Google Scholar]
- Tigano-Milani MS, Faria MR, Martins I, Lecuona RE. Ocorrência natural de Beauveria bassiana (Bals.) Vuill., Metarhizium anisopliae (Metsch.) Sorok. e Paecilomyces sp. em solos de diferentes regiões do Brasil. Anais da Sociedade Entomológica do Brasil. 1993;22:391–393. [Google Scholar]
- Tranier MS, Pognant-Gros J, Quiroz RC, González CNA, Mateille T, Roussos S. Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Brazilian Archives of Biology and Technology. 2014;57:831–841. [Google Scholar]
- Verdejo-Lucas S, Sorribas FJ, Ornat C, Galeano M. Evaluation Pochonia chlamydosporia in a double-cropping system of lettuce and tomato in plastic house infested with Meloidogyne javanica. Plant Pathology. 2003;52:521–528. [Google Scholar]
- Xu J, Liu P, Meng Q, Long H. Characterization of Meloidogyne species from China using isozyme, phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. European Journal of Plant Pathology. 2004;110:309–315. [Google Scholar]
- Yang B, Eisenback JD. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. Journal of Nematology. 1983;15:381–391. [PMC free article] [PubMed] [Google Scholar]
- Zare R, Gams W, Evans HC. A revision of Verticillium section Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwigia. 2001;73:1–86. [Google Scholar]