Abstract
P-selectin glycoprotein ligand-1 (PSGL-1) has long been studied as an adhesion molecule involved in immune cell trafficking and is recognized as a regulator of many facets of immune responses by myeloid cells. PSGL-1 also regulates T cell migration during homeostasis and inflammatory settings. However, recent findings indicate that PSGL-1 can also negatively regulate T cell function. As T cell differentiation is finely tuned by multiple positive and negative regulatory signals that appropriately scale the magnitude of the immune response, PSGL-1 has emerged as an important checkpoint during this process. Here we summarize what is known regarding PSGL-1 structure and function and highlight how it may act as an immune checkpoint inhibitor in T cells.
Co-Regulation of Migration and T Cell Differentiation
PSGL-1 and many other adhesion receptors contribute to the integration of contextual signals in the inherently mobile cells of the immune system as they migrate into [1] and within [2] the microenvironments in which they become localized in the processes of surveillance for invading microbes. These are highly dynamic, coordinated pathways that promote signaling events that are now appreciated to provide essential cues in the regulation of many facets of innate and adaptive immune responses. Here we provide an overview of the multi-pronged means by which the adhesion receptor, PSGL-1, functions in cell migration and as a regulator of myeloid and T cell responses [3]. Although other adhesion receptors such as LFA-1 can promote costimulation to enhance T cell responses [4], we highlight the emerging role of PSGL-1 as a negative regulator of T cell differentiation both at steady state and during adaptive immune responses.
PSGL-1 Structure
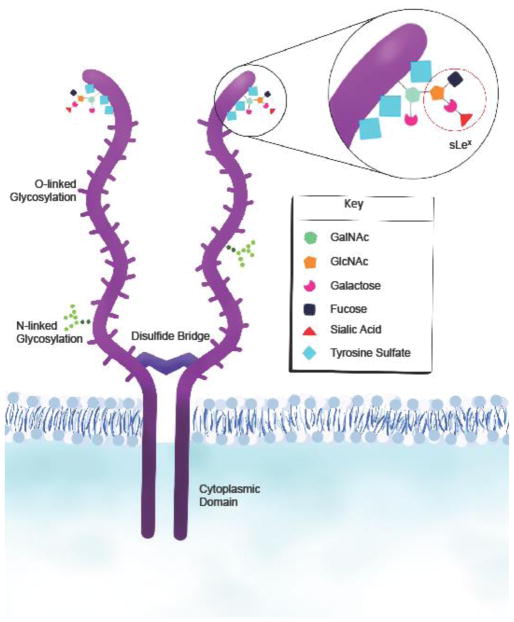
PSGL-1 is a 120kd transmembrane protein that is primarily expressed as a homodimer on lymphoid and myeloid cells, including platelets (Figure 1). PSGL-1 binds P-, E-, and L-selectin through the N-terminus of the extracellular domain [5–7], although with varying affinity [8–12]. Selectin-binding requires appropriate glycosylation that depends upon the sequential addition of carbohydrate moieties by glycosyltransferases that form the predominate sialylated fucosylated O-linked glycan, sialyl Lewis x (sLex), that mediates selectin binding with distinct enzyme requirements for binding each of the selectins (reviewed in [13, 14]). The enzymes needed for selectin binding by PSGL-1 are constitutively expressed by myeloid cells [15], as well as by T cell progenitors [16] and hematopoietic stem cells (HSC) [6, 17]. Although PSGL-1 is expressed on resting T cells, selectin binding capacity is only acquired during the proliferation/differentiation of effector T cells [18, 19]. Binding of P- and L-selectin also requires sulfation of tyrosine residues at the N-terminus which differ in number across species. The core O-glycosylation site of an N-terminal threonine is conserved as is the mucin-like domain, although variable decameric repeats and polymorphisms occur both inter- and intra-species [20, 21]. The transmembrane and cytoplasmic domains are also highly conserved [14]. Thus, despite differences in the extracellular domain of PSGL-1 among species, the overall structure and function appear to be similarly regulated [21].
Figure 1. PSGL-1 structure.
PSGL-1 is expressed as a disulfide-linked homodimer on the surface of cells. The protein contains extracellular, transmembrane, and cytoplasmic domains. The extracellular domain contains branching sites that permit O- and N-linked glycosylation and terminal sites where further postranslational modifications fine-tune counter-receptor binding availability. Myeloid cells constitutively express the enzymes that induce selectin binding whereas T cells induce these enzymes during T cell activation. Abbreviations: P-selectin glycoprotein ligand-1, PSGL-1; sialyl Lewis x, sLex; N-Acetylgalactosamine, GalNAc; N-acetylglucosamine, GlcNAc.
PSGL-1 and Cell Migration
PSGL-1 was first identified to regulate the rolling/tethering of neutrophils on activated endothelium through P-selectin binding in vitro. Additional studies validated its function in regulating the migration of macrophages/monocytes, plasma B cells, dendritic cells, and T cells by selectin engagement [22] (reviewed in [13, 14]) and through characterization of the first Selplg−/− mouse [23]. The physiological relevance of PSGL-1 was shown by its requirement for neutrophil migration into inflamed peritoneum [24], for recruitment of CD8+ T cells into the inflamed colon [25], and of CD4+ T cells into responding lymph nodes [26], intestinal lamina propria [27] and inflamed retina [28], underscoring a fundamental role in regulating immune cell recruitment to sites of inflammation (Figure 2). PSGL-1 has also been shown to regulate localization of macrophages, dendritic cells, and B cells in the lamina propria at steady state [22]. Additionally, PSGL-1 mediates adhesion of neutrophils and monocytes to P-selectin expressed on platelets that become bound to inflamed endothelium. These interactions promote targeted extravasation into tissues by eliciting chemokine secretion by platelets as well as inflammatory mediator production by neutrophils [29]. Platelets also utilize PSGL-1 to adhere to vasculature [30], suggesting that it is important for formation of cellular complexes that function in pathogen clearance. For example, PSGL-1/P-selectin interactions are critical for neutrophil recruitment and host defense against Salmonella typhimurium [31]. However, microbes can also escape immune responses by targeting selectin-binding by PSGL-1. Recent evidence shows that sialic acid binding toxin staphylococcal superantigen-like 5 (SSL5) secreted by Staphylococcal aureus binds to sLex expressed on PSGL-1 by neutrophils, thereby inhibiting neutrophil activation and recruitment [32, 33]. Other studies indicate that endogenous mechanisms can also modulate PSGL-1 function. Siglec 5, another sialic acid binding protein expressed by most hematopoietic cells, colocalizes with cell surface PSGL-1 and in soluble form inhibits leukocyte rolling on P- and E- selectin [34]. The association of PSGL-1 with the proteolytic enzyme, ADAM8, causes cleavage of extracellular PSGL-1 and blocks neutrophil rolling [35], whereas association of PSGL-1 with ADAM28 in the decamer repeat domain enhances binding to P-selectin [36]. This suggests that modulation of selectin-binding by PSGL-1 may tightly regulate recruitment of immune cells into sites of inflammation.
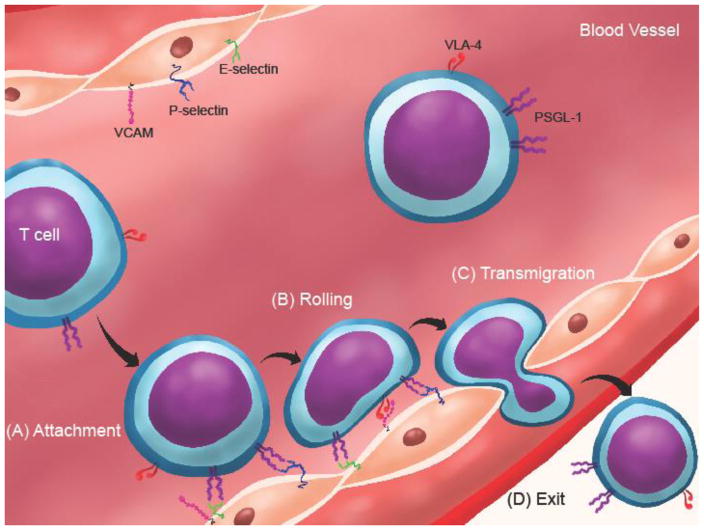
Figure 2. T cells utilize receptor-ligand binding interactions to enter sites of inflammation.
(A) Effector T cells circulating in blood at high velocities can attach to activated endothelium through PSGL-1 (on T cell) and P- or E-selectin (on endothelium) binding to initiate rolling and the extravasation cascade. (B) VLA-4 binding to VCAM support T cell rolling and firm adhesion. (C) T cells squeeze through endothelium via transmigration and (D) exit the blood and enter inflammatory sites where they can engage other molecules that can bind PSGL-1. Abbreviations: PSGL-1, P-selectin glycoprotein ligand-1; VCAM, vascular cell adhesion molecule; P-selectin, platelet selectin; E-selectin, endothelial selectin; VLA-4, α4β1 integrin, also known as very late antigen-4.
Migration Independent Functions of PSGL-1 in Myeloid Cells
One of the earliest reports defining PSGL-1 as a potent regulator of cell function identified its role in inhibiting proliferation of human HSC in response to ligation by P-selectin or anti-PSGL-1 antibody in vitro [37]. A regulatory role for PSGL-1 was identified in human DCs, where engagement by P-selectin or anti-PSGL-1 induced the expression of c-Fos, indoleamine 2,3 dioxygenase (IDO), IL-10, and transforming growth factor (TGF)-β genes, which are associated with immune inhibition, and promoted their ability to induce regulatory T cells in vitro [38]. Conventional and plasmacytoid murine DCs from Selplg−/− mice expressed higher levels of MHC class II molecules, CD86, and CD40 in response to TLR-induced maturation. In addition, Selplg−/− conventional DCs displayed a greater capacity to induce the proliferation of CD4+ T cells in vitro when compared with DCs from wild-type mice [38].
Although monocytes/macrophages have been primarily studied in the context of migration, macrophage adherence promotes PSGL-1-dependent, selectin-independent activation of the mammalian Target of Rapamycin (mTOR), suggesting that adherence-induced cytoskeletal rearrangement can elicit PSGL-1’s signaling functions [39]. However, PSGL-1 ligation on a human monocyte line was also shown to induce mTOR signals [40], indicating that ligand binding can induce the mTOR pathway. Binding of monocytes to P-selectin enhanced secretion of the chemokine CCL2 (also known as MCP-1) and tumor necrosis factor (TNF)-α in response to stimulation [41]. These findings show that PSGL-1 plays a positive role in activation of these cells that is related to adhesion. Importantly, neutrophil adhesion is upregulated by PSGL-1 ligation in the absence of chemokines [42], and thus may signal through similar pathways in monocytes/macrophages.
PSGL-1 has now been shown to have novel functions in microbial infections. PSGL-1 contributes to control of Streptoccus pneumoniae by promoting phagocytosis by neutrophils through binding of the capsular polysaccharide and cell wall autolysin, LytA [43]. In contrast, Enterovirus 71 has been found to utilize the selectin-binding domain of PSGL-1 as a primary receptor to infect leukocytes, which supports viral replication [44]. These examples demonstrate that PSGL-1 can be directly targeted by bacteria and viruses to promote or inhibit leukocyte responses.
Signaling by PSGL-1
In addition to regulating migration of leukocytes and T cells into sites of inflammation, PSGL-1 functions as a cell signaling transmembrane receptor. Much of what is known about signaling mechanisms that can be engaged by PSGL-1 comes from studies of neutrophils. In the absence of selectin binding, PSGL-1 localizes along with integrins in lipid rafts that are distributed over the surface of circulating leukocytes [45]. Upon adhesion to the endothelium, leukocytes adopt a polarized shape in response to chemokine gradients regulated by Rho GTPases [46]. PSGL-1 and other adhesion receptors such as CD44 move to the uropod through cytoskeletal rearrangement that depends on interactions with the ERM (Ezrin, Radixin, Moesin) proteins ezrin and moesin [47]. In neutrophils, signaling is concentrated in the uropod [48]. Since the selectin binding motifs and the intracellular domains of PSGL-1 are conserved in myeloid cells and lymphocytes, and PSGL-1 localizes in the uropod of T cells, it is likely that the functions of PSGL-1 induced by selectin binding during migration are shared.
Although the cytoplasmic domain has no identifiable signaling domains, ezrin and moesin [48–50], which anchor PSGL-1 to the actin cytoskeleton, act as adaptors that facilitate intracellular signaling. Src family kinases are activated by selectin binding by PSGL-1 [42, 51], which may then lead to phosphorylation of an immunoreceptor tyrosine-based activating motif (ITAM)-like domain in moesin similar to what has been described downstream of the B cell receptor [52]. Tyrosine residues present in the ITAM-like sequence of Moesin and Ezrin were necessary for PSGL-1 interaction with and activation of the tyrosine kinase Syk [53]. Signaling through PSGL-1 has also been shown to promote Syk-dependent [54] and Src-dependent [51] rolling of neutrophils on endothelium and activates β2-integrin mediated attachment to ICAM-1 [55], which is also regulated by Src kinases [42]. Src-dependent β1-integrin binding to ICAM has also been described downstream of PSGL-1 involving the P85 subunit of PI3-kinase in a complex with Rho-GDI2 [56]. Additional ITAM-containing adaptor proteins that regulate Src-dependent Syk-activation in response to PSGL-1 ligation on neutrophils via E-selectin include DAP12 and FcRγ [51], although the nature of their interaction with PSGL-1 remains unclear.
Signaling activated by antibody ligation of PSGL-1 on neutrophils induces the phosphorylation of multiple proteins including extracellular-signal related kinase (ERK)1 and ERK2 [57], demonstrating that PSGL-1 has the potential to regulate essential cellular functions. In support of this concept, activation of mTOR was also described as downstream of PSGL-1 signaling in macrophages and induced the expression of the Rho-associated kinase-1 (ROCK-1), which suggests potential signaling through the phosphoinositide 3 kinase (PI3K) pathway [39]. Similarly on Jurkat T cells, ligation of PSGL-1 by antibody has been shown to induce β1 integrin clustering that is dependent on PI3K activation [58]. Ligation of PSGL-1 also induces cytokine secretion by macrophages, DCs, and T cells suggesting a positive role in immune regulation [38, 57, 59–61].
Although both signaling through Syk and cytokine production have been demonstrated for T cells, the main Syk family kinase in T cells is Zap70 [59, 62, 63]. It is not known whether Zap70 interacts with the ITAM-like sequence in ERM proteins and lead to signal transduction in T cells downstream of PSGL-1. For T cell receptor (TCR) signaling, ezrin is needed for proper Zap70 recruitment to the immune synapse, although after initial recruitment into the synapse, ezrin and moesin move to the uropod of the cell [64–66]. PSGL-1 also becomes excluded from the immune synapse on T cells, and then localizes to the uropod, suggesting that PSGL-1 would not influence TCR signaling. However, we found reduced TCR signaling via the downstream ERK and AKT pathways when PSGL-1 was cross-linked with antibody at the time of TCR stimulation [67] (Figure 3, Key Figure). While it is possible that crosslinking PSGL-1 prevents optimal formation of the synapse, additional studies on the localization of PSGL-1 in T cells are needed to resolve the intracellular binding-partners and mechanisms of signaling.
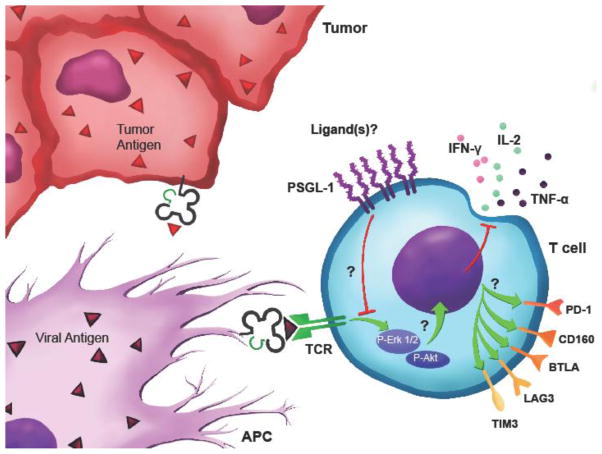
Key Figure 3. PSGL-1 is an inhibitory checkpoint that facilitates T cell exhaustion.
T cells stimulated by tumor or viral antigens while engaging PSGL-1 leads to dampened TCR signaling through ERK and AKT dephosphorylation. Dysfunctional T cells display loss of cytokine production, reduced survival, and upregulation of inhibitory receptors that promote their exhaustion. Abbreviations: PSGL-1, P-selectin glycoprotein ligand-1; TCR, T cell receptor; APC, antigen presenting cell; ERK, extracellular signal regulated kinase; AKT, also known as protein kinase B; IFN-γ, interferon gamma; IL-2, interleukin-2; TNF-α, tumor necrosis factor-α; PD-1, programmed death-1; BTLA, B and T lymphocyte attenuator; LAG3, lymphocyte-activation gene-3; TIM3, T-cell immunoglobulin and mucin-domain containing-3.
Since PSGL-1 removal from the synapse has been shown to be dependent on moesin and loss of both ezrin and moesin lead to reduced T cell activation, including reduced calcium flux, nuclear factor of activated T cells (NFAT) activation and IL-2 production, one can speculate that PSGL-1 may control the relative amount of Zap70 that is available to signal through the TCR by recruiting ERM proteins away from the immune synapse [64, 65]. In the absence of PSGL-1, greater availability of Zap70 could lead to enhanced TCR signaling. However, although Syk is present in T cells and Syk and Zap-70 are homologues, they can have different functions. For example, T cells from Lupus patients show enhanced Syk signaling in response to TCR engagement, in lieu of Zap70 [68]. Thus, Selplg−/− T cells may allow additional Syk to preferentially become engaged upon TCR signaling, where under normal circumstances, Syk is kept out of the synapse by PSGL-1. It would be interesting to determine if there are differences in PSGL-1 expression or function in T cells from Lupus patients. Nevertheless, further studies will be needed to determine whether Zap70 and/or Syk can be regulated by PSGL-1 downstream of the T cell receptor.
Functions of PSGL-1 in T cells
Naïve T Cells
PSGL-1 is expressed at high levels on all T cells. However, naïve T cells and resting effector and central memory T cells express PSGL-1 lacking the terminal glycosylation required for selectin binding [13]. Studies of Selplg−/− mice found that P-selectin and functional PSGL-1 were involved in the migration of early T lymphocyte progenitors to the thymus but no differences in the frequencies and cellularity of single positive or double positive T cell subsets were observed [16]. PSGL-1 is required for mature T cell egress from the thymus and downregulation of selectin binding capacity occurs prior to their exit [69]. In the periphery of Selplg−/− mice, overall T cell cellularity in lymphoid organs was normal; however, naïve CD4+ and CD8+ T cells were significantly reduced in the blood [70]. Selplg−/− naïve CD8+ T cells had delayed entry and egress from lymph nodes, although this effect was not reported for Selplg−/− naïve CD4+ T cells (Figure 4A). Even though naïve T cells were retained in lymph nodes, cellularity in Selplg−/− lymph nodes was not increased indicating that perhaps Selplg−/− naïve T cells have differences in turnover and/or survival. Indeed, Selplg−/− CD8+ T cells were shown to be hyper-responsive to high concentrations of the homeostatic cytokines IL-2, IL-4, and IL-15 in vitro but this was not observed for Selplg−/− CD4+ T cells [70]. Selplg−/− CD8+ T cells also displayed greater turnover at steady state, suggesting that they could increase in number over time; however this increase was not observed in aged Selplg−/− mice [67]. Thus, it is conceivable that naïve Selplg−/− CD8+ T cells are more susceptible to apoptosis in vivo.
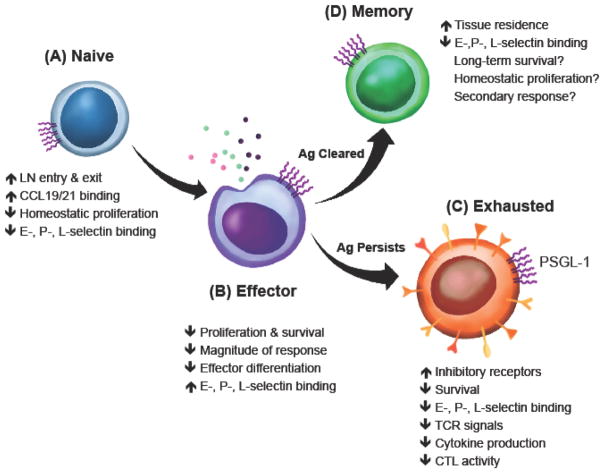
Figure 4. T cell subsets are regulated by PSGL-1.
(A) Naïve T cells cells can utilize PSGL-1 to enter and exit lymph nodes. In lymph nodes, PSGL-1 on naïve T cells binds the chemokines CCL-19 and -21 produced by stroma cells that can support their migration and survival. (B) Naïve T cells transition to effector T cells where PSGL-1 acts to restrain effector proliferation and differentiation limiting the magnitude of their response. (C) Antigen persistence leads to PSGL-1 signaling that reduces T cell survival, increases inhibitory receptor expression, and dampens TCR signals and cytokine production to promote T cell exhaustion. (D) After antigen is cleared, PSGL-1 restrains memory T cell formation and facilitates memory T cell homing to lymphoid and non-lymphoid tissues. Abbreviations: P-selectin glycoprotein ligand-1, PSGL-1; CCL19, chemokine ligand 19; CCL21, chemokine ligand 21; E-selectin, endothelial selectin; P-selectin, platelet selectin; L-selectin, leukocyte selectin; TCR, T cell receptor; CTL, cytotoxic T lymphocyte.
The possibility that ligands other than selectins regulate functions of PSGL-1 on T cells was revealed by the finding that the skin homing chemokine, CCL27 [71], and the lymphoid tissue chemokines, CCL19 and CCL21 [72] bind to PSGL-1. CCL27 binds to the N-terminus of human PSGL-1 and this binding is dependent upon sulfation, but not the terminal glycosylation required for selectin binding. Although CCL27 binds to recombinant or cell surface PSGL-1 and can regulate chemotaxis of a pre-B cell line transfected with the CCL27 ligand, CCR10, it is not known whether this interaction has functional significance in vivo [71]. An unexpected role for PSGL-1 in selectin-independent T cell migration to lymph nodes was discovered in short-term homing studies that identified a reduced capacity for localization of Selplg−/− compared to Selplg+/+ resting T cells [72].
A function in regulation of chemotaxis to CCL19 and CCL21 was defined in vitro, and could be blocked by the anti-PSGL-1 antibody 4RA10 [30], which also inhibits binding of P-selectin by PSGL-1 on leukocytes [73]. Moreover, T cell chemotaxis in response to CCL21 was facilitated by the absence of the N-glucosaminyltransferase C2GlcNAct-I, which is necessary for selectin binding by PSGL-1, suggesting independence of terminal glycosylation. The chemokine binding motif of human PSGL-1 was identified in a study that investigated binding of CCL19 to the N-terminus of either CCR7 or PSGL-1, which confirmed overlapping binding sites [74]. CCL19/CCL21 binding by PSGL-1 on naïve T cells [72] could regulate migration via high endothelial venules, although the extent to which this occurs physiologically is not known. In addition, it remains to be determined whether this is a mechanism by which T cells can present these chemokines to other T cells for engagement by either CCR7 or PSGL-1, or whether binding can transduce signals that directly regulate T cell function. Since CCL19 and CCL21 are primarily secreted by fibroblastic reticular cells in the T cell areas of lymphoid organs [75], their binding to PSGL-1 may function in the spatial positioning of T cells as well as their directional motility in lymphoid tissues. Furthermore, PSGL-1 and CCR7 co-receptor signals may cooperate to regulate naïve T cell homeostasis.
Effector T Cells
Acquisition of the form of PSGL-1 that supports engagement of P-selectin and E-selectin on activated endothelium facilitates effector T cell entry into inflammatory sites [11]. The importance of this interaction in guiding effector T cells was demonstrated using PSGL-1 blocking antibodies which reduced Th1 recruitment in a delayed-type hypersensitivity model [76], and CD8+ T cell recruitment in brain vessels of patients with multiple sclerosis [77]. Even though all T cells express PSGL-1, not all effectors acquire the selectin-binding form. For example, Th1 and Th2 cells upregulate and express similar levels of PSGL-1 in vitro, but only Th1 cells can engage P-selectin [76]. Selplg−/− Th1 cells failed to infiltrate the colon in a DSS colitis model whereas Selplg−/− Th17 cells effectively migrated without PSGL-1 expression, indicating that T cell subsets can utilize posttranslational modifications on PSGL-1 to further fine tune effector T cell homing [78]. Furthermore, in sites of inflammation, the majority of CD4+ cells can exhibit selectin-binding capacity [79, 80]. Thus, terminal glycosylation of PSGL-1 on T cells may delineate more terminally differentiated effectors that are driven to migrate to sites of inflammation where they promote resolution of infections. However, it is clear that selectin binding on T cells can be dynamically regulated. For example, in a model of contact sensitivity, Tregs were also found to engage P-selectin on inflamed endothelium via PSGL-1 but this capacity was lost upon subsequent challenge [81]. Although CD4+ and CD8+ T cell effectors acquire selectin binding capacity permitting PSGL-1 engagement and recruitment to inflammatory sites [82], they can then lose glycosylation enzymatic activity as a response resolves, suggesting that inflammatory processes can control selectin-binding by PSGL-1 on T cells (Figure 4B).
Although contributions of PSGL-1 to effector T cell recruitment facilitate adaptive responses, negative regulatory functions have been demonstrated in studies of Selplg−/− mice. Selplg−/− T cells were shown to have increased proliferation and re-expression of the full-length or intracellular domain of PSGL-1 suppressed proliferation but not when Selplg−/− T cells only re-expressed the extracellular/transmembrane PSGL-1 domain [83]. This finding highlights the requirement for signaling via the cytoplasmic tail of PSGL-1 for its function in regulating T cell proliferation. Adoptive transfer of naïve T cells into Rag−/− mice showed that Selplg−/− T cells were more potent in promoting colitis [83]. Selplg−/− mice also developed more severe experimental autoimmune encephalomyelitis (EAE) [84], which suggest that PSGL-1 not only regulates T cell proliferation but also their activation. PSGL-1 expression in Tregs can also regulate effector T cell activity. Although normal frequencies of CD25+FoxP3+ regulatory T cells with comparable surface phenotypes were found in naïve Selplg−/− mice, Selplg−/− Tregs failed to modulate MOG peptide-specific T cell motility, T cell-DC contact, and T cell clustering in EAE [84]. In rheumatoid arthritis and systemic lupus erythematosus patients, engaging PSGL-1 on monocyte derived DCs resulted in Treg generation [85]. These findings indicate that PSGL-1 expression on Tregs supports their suppressive capacity to inhibit effector T cells. Interestingly, one report showed that Selplg−/− mice had increased numbers of T cell effectors producing IFN-γ, IL-4, and IL-17 in skin, corresponding to Th1, Th2, and Th17 populations, but no differences in Tregs [86]. This spontaneous autoimmune phenotype was not reported in other studies [23, 67, 70] indicating that potential microbiota and/or strain differences may modulate effector responses in Selplg−/− animals.
In contrast to other effector T cells, follicular T helper (Tfh) cells that regulate B cells downregulate PSGL-1 during their differentiation and responses in the follicles [87]. B cells express very low levels of PSGL-1, and do not upregulate expression or selectin binding in response to activation [72, 88]. In contrast, long-lived plasma cells upregulate PSGL-1 expression that preferentially binds E-selectin but not P-selectin [89]. PSGL-1 can be downregulated by the Bcl6 and Ascl2 transcription factors that instruct a Tfh program in CD4+ T cells [87, 90]. Tfh cells are characterized by upregulation of PD-1 and CXCR5, and the downregulation of PSGL-1 is thought to facilitate Tfh entry into B cell follicles to support the development of antibody responses [87, 91–93], although the underlying mechanisms are unknown.
Exhausted T Cells
T cell exhaustion occurs in the settings of chronic viral infections and cancers. Antigen-specific T cells that differentiate in hosts where antigen is not eliminated leads to tonic TCR stimulation that sustains expression of inhibitory receptors including PD-1, CTLA-4, LAG-3, 2B4, and several others [94]. Exhausted T cells progressively lose effector function that facilitates antigen persistence [95]. The role of adhesion molecules in the establishment of T cell exhaustion has not been thoroughly investigated. During persistent viral infections in humans and mice, the prolonged systemic inflammation may shape T cell exhaustion fates by engaging PSGL-1 and other adhesion molecules that are highly expressed on antigen-specific CD4+ and CD8+ T cells. We found that virus-specific CD8+ T cells upregulated PSGL-1 in infected tissues after chronic LCMV Cl13 infection and that the majority of CD8+ T cells expressed PSGL-1 that was not terminally glycosylated, leaving the molecule available to engage other ligands independently of selectin binding. The negative regulatory function of PSGL-1 was revealed in Selplg−/− mice only after infection with LCMV Cl13 [67]. These mice had an accumulation of virus-specific CD4+ and CD8+ T cells in lymphoid and non-lymphoid tissues due to enhanced survival. In addition, virus-specific T cells from Selplg−/− infected mice had reduced expression of PD-1 and other inhibitory receptors and retained effector function that resulted in virus control. Greater than 50% mortality was observed in Selplg−/− infected mice, highlighting the regulatory function of PSGL-1 in viral clearance and viral-induced immunopathology. Additionally, PSGL-1-deficiency was associated with improved anti-tumor T cell responses and recruitment in melanoma tumors from Pten−/−Cdkn2a−/−BrafVE600E+ Yumm1.5 cells [96]. No differences in Tregs were observed after Cl13 infection or injection of Yumm1.5 melanoma cells. Furthermore, activated Selplg−/− CD8+ OT-I T cells were more effective at delaying B16-OVA melanoma growth. This is in contrast to reports where B16 tumors were shown to grow more effectively in Selplg−/− mice, with a key difference being that one study transferred activated effector T cells to tumor bearing WT mice while the other study examined B16 growth in Selplg−/− hosts [97].
Mechanistically, we found that engaging PSGL-1 with anti-PSGL-1 antibody (clone 4RA10) paired with TCR stimulation resulted in ERK and AKT early dephosphorylation, indicating that PSGL-1 signaling can extinguish TCR signaling events [67]. These findings are consistent with inhibitory receptors like PD-1 mediating T cell exhaustion through dampening TCR signals [98, 99], however how PSGL-1 signaling contributes to this is unknown (Figure 4C). Furthermore, antibody-mediated PSGL-1 ligation on exhausted WT CD8+ T cells together with TCR stimulation ex vivo or in vivo, upregulated PD-1, LAG3 and TIM-3 levels, consistent with a role in promoting T cell dysfunction (Figure 3). No differences were observed after selectin blockade, supporting the notion that other ligands can engage PSGL-1 to mediate T cell exhaustion [67]. Transcription programs can alter the state of T cell exhaustion. The levels of Eomes and T-bet for example, can alter the extent of terminal differentiation in T cells during chronic viral infection [100, 101]. T-bet can repress PD-1 expression [102] whereas the transcription factor NFAT can directly regulate inhibitory receptor expression [103]. Here we found that PSGL-1 expression impacted T cell differentiation as Selplg−/− CD8+ T cells had a transcription program that favored effector differentiation and not exhaustion.
Memory T Cells
PSGL-1 can regulate the migration of resting and activated memory T cells. Like naïve T cells, resting central memory CD4+ and CD8+ T cells were shown to engage CCL19 and CCL21 via PSGL-1 leading to an increased chemotactic response [72]. Furthermore, PSGL-1 can also facilitate rolling of a subset of central memory T cells in bone marrow microvessels [104], implying glycosylation-dependent recruitment. In human lungs, the non-selectin binding form of PSGL-1 was highly expressed on resident memory T cells poised to confer protective responses [105]. Furthermore, PSGL-1 can be modified via a carbohydrate epitope through fucosyltransferase VII activity into the cutaneous lymphocyte-associated antigen (CLA) that regulates tissue-specific homing of memory T cells [106]. Resident T cells in the lung and skin express high levels of PSGL-1, but only those in the skin are CLA+ [105–108]. In contrast, effector memory cells can engage P-selectin to migrate into lymph nodes undergoing a response [26]. In secondary responses, PSGL-1 on memory CD8+ T cells becomes functionally glycosylated to engage selectins and is required for their early recruitment to inflamed tissues [109]. Increased numbers of virus-specific CD8+ T cells were generated in Selplg−/− mice after acute LCMV Armstrong infection [70], indicating that PSGL-1 can limit memory T cell generation in this model (Figure 4D). While PSGL-1 remains highly expressed on memory T cells, few cells maintain the capacity for selectin binding, supporting the concept that this function is primarily important for the recruitment of effector T cells [110] and requires inflammatory processes for maintenance. More studies are necessary to determine whether PSGL-1 expression and signaling impact secondary T cell responses and how these signals shape their differentiation and function in lymphoid and non-lymphoid tissues.
Concluding Remarks
Collectively, studies demonstrate that PSGL-1 is a dynamically regulated molecule on T cells and myeloid cells that can engage ligands that shape their responses during homeostasis and in disease settings. While it is clear that PSGL-1-dependent migration into tissues engages multiple signaling pathways that are fundamental to cell survival and function, it has only recently become evident that PSGL-1 can also modulate T cell receptor signals as an immune checkpoint regulator. In view of this role, identification of PSGL-1 signaling pathways in T cells could lead to new therapeutic targets that augment immune function, such as in cancer and chronic viral infections, or dampen immune function, such as in autoimmunity (‘see Outstanding Questions’).
Outstanding Questions Box.
What are the interacting partners that bind the intracellular and extracellular domains of PSGL-1 in responding T cells? What are the effects of ligands such as chemokines (CCL19, 21, 27), selectins on endothelial cells, L-selectin on myeloid cells, and others?
By what mechanisms does PSGL-1 signaling modulate inhibitory receptor expression and ligand engagement in T cells?
How does PSGL-1 crosslinking impact proximal T cell receptor signaling pathways and downstream targets to modulate survival and function?
How is PSGL-1 localized in the immunological synapse during priming and antigen recognition in vivo and does this impact T cell differentiation and function?
What signaling mechanisms regulate transcription programs that modulate PSGL-1 downregulation in CD4+ T cells?
How is PSGL-1 expressed and which post-translational modifications are present in human exhausted T cells?
Can PSGL-1 blockade with a neutralizing or antagonizing antibody reverse T cell exhaustion? Can it synergize with existing checkpoint blockade approaches in mouse and humans?
PSGL-1 is only one of the many adhesion molecules that decorate the surface of immune cells and instruct them to home to tissues where they can exert their effector functions. T cells depend on adhesion molecule expression to traffic through secondary lymphoid and non-lymphoid tissues during steady state and inflammatory conditions. During T cell migration, PSGL-1 can engage various known and unknown ligands that can modulate their differentiation state within tissue microenvironments. PSGL-1 expression, glycosylation, sialylation, fucosylation, and sulfation add to the complexity by which PSGL-1 can engage various ligands. It is well documented that tissue microenvironments throughout the body change with respect to nutrient and oxygen availability, antigen load, inhibitory ligand expression, cytokine and chemokine concentrations, and the cell types they encounter. T cells must adapt to these changing environments and PSGL-1 serves as a checkpoint that can be engaged to deliver regulatory signals as they migrate through these diverse sites. It is therefore important to consider that in addition to their migratory properties, adhesion molecules like PSGL-1, can be engaged to fine-tune immune cells at every stage of the immune response.
Trends Box.
PSGL-1 is an adhesion molecule expressed by T cells and many other hematopoietic cells. PSGL-1 binds to members of the selectin family, but this binding depends on post-translational modifications that are cell-type and context dependent.
Naïve, effector, and memory T cell migration can be regulated by PSGL-1.
PSGL-1-deficiency results in enhanced proliferation of naïve T cells to homeostatic cytokines, and increased effector cytokine production by activated T cells.
PSGL-1 downregulation is important for Tfh migration in germinal centers.
In chronic viral infection and melanoma tumor models, PSGL-1 promotes T cell exhaustion.
Ligating PSGL-1 during antigen stimulation decreases TCR signals, cell survival and function and increases inhibitory receptor expression.
Acknowledgments
This work was supported by grants R01 AI06895, R21 CA209627, and the Melanoma Research Foundation 322189 to LMB, and the NIH Institutional Research and Academic Career Development Award (IRACDA) K12 GM068524 and the UCSD Chancellor’s Postdoctoral fellowship to RT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 3.Carlow DA, et al. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230(1):75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 4.Graf B, et al. LFA-1-mediated T cell costimulation through increased localization of TCR/class II complexes to the central supramolecular activation cluster and exclusion of CD45 from the immunological synapse. J Immunol. 2007;179(3):1616–24. doi: 10.4049/jimmunol.179.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz DJ, et al. Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin. J Cell Biol. 1997;137(2):509–19. doi: 10.1083/jcb.137.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spertini O, et al. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol. 1996;135(2):523–31. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia L, et al. N-terminal residues in murine P-selectin glycoprotein ligand-1 required for binding to murine P-selectin. Blood. 2003;101(2):552–9. doi: 10.1182/blood-2001-11-0036. [DOI] [PubMed] [Google Scholar]
- 8.Asa D, et al. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270(19):11662–70. doi: 10.1074/jbc.270.19.11662. [DOI] [PubMed] [Google Scholar]
- 9.Guyer DA, et al. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood. 1996;88(7):2415–21. [PubMed] [Google Scholar]
- 10.Martinez M, et al. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280(7):5378–90. doi: 10.1074/jbc.M410899200. [DOI] [PubMed] [Google Scholar]
- 11.Sako D, et al. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83(2):323–31. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 12.Tu L, et al. L-selectin binds to P-selectin glycoprotein ligand-1 on leukocytes: interactions between the lectin, epidermal growth factor, and consensus repeat domains of the selectins determine ligand binding specificity. J Immunol. 1996;157(9):3995–4004. [PubMed] [Google Scholar]
- 13.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4(5):325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 14.Zarbock A, et al. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86(5):1119–24. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer JD, et al. Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: cutaneous lymphocyte antigen. Biochem Biophys Res Commun. 2001;285(3):577–87. doi: 10.1006/bbrc.2001.5230. [DOI] [PubMed] [Google Scholar]
- 16.Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–34. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 17.Katayama Y, et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102(6):2060–7. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 18.Wagers AJ, Kansas GS. Potent induction of alpha(1,3)-fucosyltransferase VII in activated CD4+ T cells by TGF-beta 1 through a p38 mitogen-activated protein kinase-dependent pathway. J Immunol. 2000;165(9):5011–6. doi: 10.4049/jimmunol.165.9.5011. [DOI] [PubMed] [Google Scholar]
- 19.Carlow DA, et al. Inducing P-selectin ligand formation in CD8 T cells: IL-2 and IL-12 are active in vitro but not required in vivo. J Immunol. 2005;174(7):3959–66. doi: 10.4049/jimmunol.174.7.3959. [DOI] [PubMed] [Google Scholar]
- 20.Afshar-Kharghan V, et al. Human polymorphism of P-selectin glycoprotein ligand 1 attributable to variable numbers of tandem decameric repeats in the mucinlike region. Blood. 2001;97(10):3306–7. doi: 10.1182/blood.v97.10.3306. [DOI] [PubMed] [Google Scholar]
- 21.Baisse B, et al. Evolutionary conservation of P-selectin glycoprotein ligand-1 primary structure and function. BMC Evol Biol. 2007;7:166. doi: 10.1186/1471-2148-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunez-Andrade N, et al. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol. 2011;224(2):212–21. doi: 10.1002/path.2850. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190(12):1769–82. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges E, et al. The P-selectin glycoprotein ligand-1 is important for recruitment of neutrophils into inflamed mouse peritoneum. Blood. 1997;90(5):1934–42. [PubMed] [Google Scholar]
- 25.Asaduzzaman M, et al. P-selectin and P-selectin glycoprotein ligand 1 mediate rolling of activated CD8+ T cells in inflamed colonic venules. J Investig Med. 2009;57(7):765–8. doi: 10.2310/JIM.0b013e3181b918fb. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Fontecha A, et al. CD40L+ CD4+ memory T cells migrate in a CD62P-dependent fashion into reactive lymph nodes and license dendritic cells for T cell priming. J Exp Med. 2008;205(11):2561–74. doi: 10.1084/jem.20081212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad W, et al. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198(3):369–77. doi: 10.1084/jem.20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, et al. Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J Immunol. 2004;172(5):3215–24. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- 29.Zuchtriegel G, et al. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016;14(5):e1002459. doi: 10.1371/journal.pbio.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frenette PS, et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med. 2000;191(8):1413–22. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kum WW, et al. Lack of functional P-selectin ligand exacerbates Salmonella serovar typhimurium infection. J Immunol. 2009;182(10):6550–61. doi: 10.4049/jimmunol.0802536. [DOI] [PubMed] [Google Scholar]
- 32.Bestebroer J, et al. Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood. 2009;113(2):328–37. doi: 10.1182/blood-2008-04-153882. [DOI] [PubMed] [Google Scholar]
- 33.Walenkamp AM, et al. Staphylococcal SSL5 binding to human leukemia cells inhibits cell adhesion to endothelial cells and platelets. Cell Oncol. 2010;32(1–2):1–10. doi: 10.3233/CLO-2009-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepin M, et al. Soluble Siglec-5 associates to PSGL-1 and displays anti-inflammatory activity. Sci Rep. 2016;6:37953. doi: 10.1038/srep37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez-Luis M, et al. The metalloprotease ADAM8 is associated with and regulates the function of the adhesion receptor PSGL-1 through ERM proteins. Eur J Immunol. 2011;41(12):3436–42. doi: 10.1002/eji.201141764. [DOI] [PubMed] [Google Scholar]
- 36.Shimoda M, et al. Binding of ADAM28 to P-selectin glycoprotein ligand-1 enhances P-selectin-mediated leukocyte adhesion to endothelial cells. J Biol Chem. 2007;282(35):25864–74. doi: 10.1074/jbc.M702414200. [DOI] [PubMed] [Google Scholar]
- 37.Levesque JP, et al. PSGL-1-mediated adhesion of human hematopoietic progenitors to P-selectin results in suppression of hematopoiesis. Immunity. 1999;11(3):369–78. doi: 10.1016/s1074-7613(00)80112-0. [DOI] [PubMed] [Google Scholar]
- 38.Urzainqui A, et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179(11):7457–65. doi: 10.4049/jimmunol.179.11.7457. [DOI] [PubMed] [Google Scholar]
- 39.Fox R, et al. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J. 2007;26(2):505–15. doi: 10.1038/sj.emboj.7601522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahoney TS, et al. Cell adhesion regulates gene expression at translational checkpoints in human myeloid leukocytes. Proc Natl Acad Sci U S A. 2001;98(18):10284–9. doi: 10.1073/pnas.181201398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weyrich AS, et al. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95(5):2297–303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu T, et al. P-selectin cross-links PSGL-1 and enhances neutrophil adhesion to fibrinogen and ICAM-1 in a Src kinase-dependent, but GPCR-independent mechanism. Cell Adh Migr. 2007;1(3):115–23. doi: 10.4161/cam.1.3.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos-Sevillano E, et al. PSGL-1 on Leukocytes is a Critical Component of the Host Immune Response against Invasive Pneumococcal Disease. PLoS Pathog. 2016;12(3):e1005500. doi: 10.1371/journal.ppat.1005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura Y, et al. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med. 2009;15(7):794–7. doi: 10.1038/nm.1961. [DOI] [PubMed] [Google Scholar]
- 45.Shao B, et al. O-glycans direct selectin ligands to lipid rafts on leukocytes. Proc Natl Acad Sci U S A. 2015;112(28):8661–6. doi: 10.1073/pnas.1507712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18(3):501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10(5):353–9. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 48.Alonso-Lebrero JL, et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95(7):2413–9. [PubMed] [Google Scholar]
- 49.Wang HB, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8(8):882–92. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 50.Schaff UY, et al. SLIC-1/sorting nexin 20: a novel sorting nexin that directs subcellular distribution of PSGL-1. Eur J Immunol. 2008;38(2):550–64. doi: 10.1002/eji.200737777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarbock A, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205(10):2339–47. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozsnyay Z, et al. Membrane-bound ezrin is involved in B-cell receptor-mediated signaling: potential role of an ITAM-like ezrin motif. Immunol Lett. 1996;54(2–3):163–9. doi: 10.1016/s0165-2478(96)02667-3. [DOI] [PubMed] [Google Scholar]
- 53.Urzainqui A, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17(4):401–12. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 54.Abbal C, et al. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108(10):3352–9. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- 55.Blanks JE, et al. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur J Immunol. 1998;28(2):433–43. doi: 10.1002/(SICI)1521-4141(199802)28:02<433::AID-IMMU433>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 56.Luo J, et al. p85-RhoGDI2, a novel complex, is required for PSGL-1-induced beta1 integrin-mediated lymphocyte adhesion to VCAM-1. Int J Biochem Cell Biol. 2013;45(12):2764–73. doi: 10.1016/j.biocel.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Hidari KI, et al. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272(45):28750–6. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- 58.Luo J, et al. PI3K is involved in beta1 integrin clustering by PSGL-1 and promotes beta1 integrin-mediated Jurkat cell adhesion to fibronectin. Mol Cell Biochem. 2014;385(1–2):287–95. doi: 10.1007/s11010-013-1837-x. [DOI] [PubMed] [Google Scholar]
- 59.Ba XQ, et al. Engagement of PSGL-1 upregulates CSF-1 transcription via a mechanism that may involve Syk. Cell Immunol. 2005;237(1):1–6. doi: 10.1016/j.cellimm.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Celi A, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91(19):8767–71. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damle NK, et al. GMP-140 (P-selectin/CD62) binds to chronically stimulated but not resting CD4+ T lymphocytes and regulates their production of proinflammatory cytokines. Eur J Immunol. 1992;22(7):1789–93. doi: 10.1002/eji.1830220718. [DOI] [PubMed] [Google Scholar]
- 62.Chu DH, et al. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–80. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 63.Chan AC, et al. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71(4):649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 64.Shaffer MH, et al. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182(2):1021–32. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ilani T, et al. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179(4):733–46. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serrador JM, et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol. 2002;32(6):1560–6. doi: 10.1002/1521-4141(200206)32:6<1560::AID-IMMU1560>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 67.Tinoco R, et al. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity. 2016;44(5):1190–203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnan S, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181(11):8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gossens K, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206(4):761–78. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veerman KM, et al. PSGL-1 regulates the migration and proliferation of CD8(+) T cells under homeostatic conditions. J Immunol. 2012;188(4):1638–46. doi: 10.4049/jimmunol.1103026. [DOI] [PubMed] [Google Scholar]
- 71.Hirata T, et al. Human P-selectin glycoprotein ligand-1 (PSGL-1) interacts with the skin-associated chemokine CCL27 via sulfated tyrosines at the PSGL-1 amino terminus. J Biol Chem. 2004;279(50):51775–82. doi: 10.1074/jbc.M409868200. [DOI] [PubMed] [Google Scholar]
- 72.Veerman KM, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8(5):532–9. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- 73.Thatte A, et al. Binding of function-blocking mAbs to mouse and human P-selectin glycoprotein ligand-1 peptides with and without tyrosine sulfation. J Leukoc Biol. 2002;72(3):470–7. [PubMed] [Google Scholar]
- 74.Veldkamp CT, et al. Solution Structure of CCL19 and Identification of Overlapping CCR7 and PSGL-1 Binding Sites. Biochemistry. 2015;54(27):4163–6. doi: 10.1021/acs.biochem.5b00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015;36(1):30–9. doi: 10.1016/j.it.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Borges E, et al. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185(3):573–8. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Battistini L, et al. CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood. 2003;101(12):4775–82. doi: 10.1182/blood-2002-10-3309. [DOI] [PubMed] [Google Scholar]
- 78.Brown JB, et al. P-selectin glycoprotein ligand-1 is needed for sequential recruitment of T-helper 1 (Th1) and local generation of Th17 T cells in dextran sodium sulfate (DSS) colitis. Inflamm Bowel Dis. 2012;18(2):323–32. doi: 10.1002/ibd.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kretschmer U, et al. Expression of selectin ligands on murine effector and IL-10-producing CD4+ T cells from non-infected and infected tissues. Eur J Immunol. 2004;34(11):3070–81. doi: 10.1002/eji.200424972. [DOI] [PubMed] [Google Scholar]
- 80.Syrbe U, et al. Microenvironment-dependent requirement of STAT4 for the induction of P-selectin ligands and effector cytokines on CD4+ T cells in healthy and parasite-infected mice. J Immunol. 2006;177(11):7673–9. doi: 10.4049/jimmunol.177.11.7673. [DOI] [PubMed] [Google Scholar]
- 81.Abeynaike LD, et al. Regulatory T cells dynamically regulate selectin ligand function during multiple challenge contact hypersensitivity. J Immunol. 2014;193(10):4934–44. doi: 10.4049/jimmunol.1400641. [DOI] [PubMed] [Google Scholar]
- 82.Xie H, et al. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189(11):1765–76. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumoto M, et al. P-selectin glycoprotein ligand-1 negatively regulates T-cell immune responses. J Immunol. 2009;183(11):7204–11. doi: 10.4049/jimmunol.0902173. [DOI] [PubMed] [Google Scholar]
- 84.Angiari S, et al. Regulatory T cells suppress the late phase of the immune response in lymph nodes through P-selectin glycoprotein ligand-1. J Immunol. 2013;191(11):5489–500. doi: 10.4049/jimmunol.1301235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Estrada-Capetillo L, et al. Induction of Th17 lymphocytes and Treg cells by monocyte-derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Dev Immunol. 2013;2013:584303. doi: 10.1155/2013/584303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Frias A, et al. Development of an autoimmune syndrome affecting the skin and internal organs in P-selectin glycoprotein ligand 1 leukocyte receptor-deficient mice. Arthritis Rheumatol. 2014;66(11):3178–89. doi: 10.1002/art.38808. [DOI] [PubMed] [Google Scholar]
- 87.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185(1):313–26. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snapp KR, et al. A novel P-selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood. 1998;91(1):154–64. [PubMed] [Google Scholar]
- 89.Underhill GH, et al. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules. Blood. 2002;99(8):2905–12. doi: 10.1182/blood.v99.8.2905. [DOI] [PubMed] [Google Scholar]
- 90.Liu X, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507(7493):513–8. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 92.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–17. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35(4):633–46. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44(5):1052–68. doi: 10.1016/j.immuni.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Meeth K, et al. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 2016;29(5):590–7. doi: 10.1111/pcmr.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaoka T, et al. The roles of P- and E-selectins and P-selectin glycoprotein ligand-1 in primary and metastatic mouse melanomas. J Dermatol Sci. 2011;64(2):99–107. doi: 10.1016/j.jdermsci.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 99.Chemnitz JM, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 100.Buggert M, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10(7):e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338(6111):1220–5. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12(7):663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez GJ, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42(2):265–78. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22(2):259–70. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 105.Purwar R, et al. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fuhlbrigge RC, et al. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 107.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176(7):4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 108.Ni Z, Walcheck B. Cutaneous lymphocyte-associated antigen (CLA) T cells up-regulate P-selectin ligand expression upon their activation. Clin Immunol. 2009;133(2):257–64. doi: 10.1016/j.clim.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest. 2014;124(3):1013–26. doi: 10.1172/JCI72039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lord GM, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106(10):3432–9. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]