Abstract
The aryl hydrocarbon receptor (AHR) is a pivotal chemical sensor that transduces extrinsic and intrinsic signals into cellular responses. AHR was originally thought to be involved in not only drug metabolism but also carcinogenic and toxicological responses against environmental contaminants, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons. However, recent studies demonstrate that the AHR plays multiple intrinsic roles in host defense and homeostasis as well, including immunity, stem cell maintenance, and cell differentiation, upon binding with an increasing number of newly defined dietary, cellular, and microbe-derived ligands. In addition, AHR is a convergence point for several signaling cascades, which may be involved in the diverse diseases caused by binding of the persistent ligand TCDD with extremely high affinity to AHR. A comprehensive understanding of physiological and pathological processes initiated by endogenous AHR agonists and antagonists may allow for the therapeutic regulation of AHR activity. Thus, the AHR can be a valuable diagnostic marker and therapeutic target for human diseases.
Keywords: bHLH-PAS transcription factor, immunity, microbes, P450, TCDD
Introduction: A Short History of TCDD and AHR Research
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a toxic byproduct of various industrial, combustion, and natural processes that is now a persistent and ubiquitous environmental contaminant in air, water, soil, and food. TCDD exerts a broad spectrum of toxic effects even at very low concentrations. Lethal doses of TCDD cause slow death as a result of a wasting syndrome, while reproductive and developmental effects, tumor promotion, immune suppression, and skin lesions called chloracne are observed at nonlethal doses [53]. TCDD is probably best known as a contaminant in Agent Orange, a mixture of N-butyl esters of 2,4-dichlorophenoxyacetic (2,4-D) and 2,4,5-trichlorophenoxyacetic (2,4,5-T) acids widely used as defoliants by the United States during the war in Vietnam (1962–1971) [88]. In 1968, a mass food poisoning termed “Yusho” occurred in western Japan, and the causal agent of “Yusho” was thought to be polychlorinated dibenzofurans (PCDFs) [65]. In 1976, a severe industrial accident occurred in Seveso, Italy, and up to kilogram quantities of TCDD and additional TCDD-like chemicals were dispersed into the air, with adverse health consequences for the local population [30]. In 2004, Viktor Yushchenko, who was the president of Ukraine at that time and had been exposed to TCDD at a single oral dose 5 million-fold higher than the accepted daily exposure for the general population, became a widely recognized victim of TCDD poisoning [81]. Since the summer of 2013, more than 100 rusty barrels have been unearthed from land in Okinawa City that was formerly part of Kadena Air Base. Tests on the barrels’ contents have revealed ingredients of Vietnam-era defoliants, including 2,4-D, 2,4,5-T, and TCDD [60].
The aryl hydrocarbon receptor (AHR) is traditionally defined as a ligand-dependent transcription factor involved in the biotransformation and carcinogenic/teratogenic effects of environmental toxins, such as TCDD and polycyclic aromatic hydrocarbons (PAHs). The AHR was first identified in the mid-1970s as the cytoplasmic receptor that binds TCDD with an extremely high affinity [72], and it was further revealed that PAHs also bind to the receptor followed by induction of drug-metabolizing P450 (CYP1A1) [25]. In the first few decades after its discovery, AHR studies focused largely on the responses to xenobiotics.
In 1991, Bradfield el al. [10] reported a partial N-terminal 27 amino acid sequence of the purified fragment of mouse AHR, and cDNA clones for the Ahr were soon isolated independently by two research groups [12, 20] using oligonucleotide probes synthesized according to the determined N-terminal partial sequence. At the same time, Reyes et al. isolated a cDNA for AHR Nuclear Translocator (ARNT), a factor required for nuclear translocation of the AHR by the cytogenetic methods [79]. The amino acid sequences of AHR and ARNT revealed the presence of a basic helix-loop-helix (bHLH) motif and Period-ARNT-Single minded homology region (PAS) domains. The isolation of Ahr and Arnt cDNAs and their cognate genes greatly accelerated our understanding of the molecular mechanisms concerning the regulatory functions of the AHR in response to chemicals, including TCDD and 3MC. In 1999, Mimura et al. [58] isolated a cDNA for AhR Repressor (AHRR), a negative regulator of the AHR signaling pathway. In the mid-1990s, Ahr-deficient mice were generated by three independent groups [21, 59, 82] with the homologous recombination method, and experiments using these mutant mice have substantially extended the knowledge about the regulatory functions of the AHR in immunology and physiology as well as pharmacology and toxicology [22].
Information regarding AHR phylogeny in the animal kingdom has expanded rapidly with the recent development and increased accessibility of advanced gene cloning technology. The presence of Ahr homologs in most major groups of animals, including representatives of the bilaterians, cnidarians, and placozoans, provides strong evidence that the original eumetazoa, whose last common ancestor lived approximately 600 million years ago (MYA), possessed an Ahr homolog [27]. The Ahr orthologs in C. elegans [73] and D. melanogaster [19], respectively, contribute to the development of specific neurons and sensory neurons, but they exhibit no ligand binding. Thus, the ligand binding may be a secondary and acquired function of this receptor that arose during vertebrate evolution, and the primary function of the mammalian AHR is probably related to normal development and homeostasis. In fact, AHR has recently been revealed to be implicated in immune responses, stem cell regulation, inflammation, cell differentiation and proliferation, apoptosis, reproduction, and tumor suppression [22]. As these intrinsic functions of the AHR are performed largely in response to endogenous ligands generated from the host cell, diet, or microbiota, AHR is currently considered an environmental chemical senor, connecting external environmental signals to cellular processes. In addition, the AHR is a convergence point of multiple signaling pathways that may contribute to the pathogenesis of diseases caused by toxic ligands such as TCDD. In this review, we will summarize and discuss our recent advances in the studies on the regulatory mechanisms and physiological functions of the AHR, with emphasis on immunity, stem cell maintenance, and cell differentiation.
AHR Signaling Pathways
Structure of AHR, ARNT, and AHRR
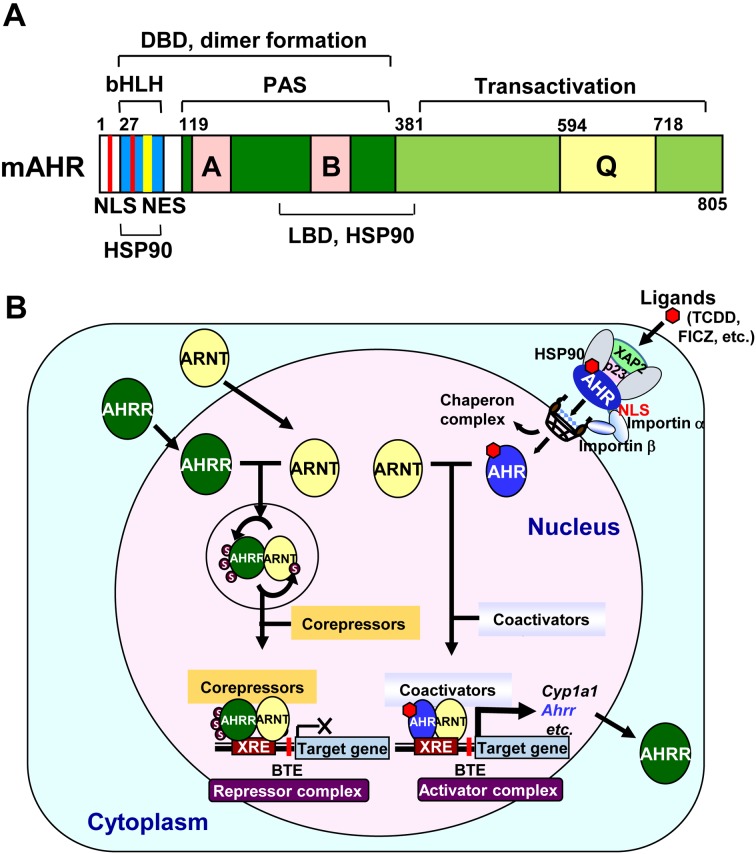
AHR and ARNT are members of a structurally related gene family within the bHLH/PAS superfamily, whose members have critical functions in gene expression networks underlying many essential physiological and developmental processes, particularly those requiring responses to environmental signals [55]. As is already known, the bHLH motif in the N-terminal region is involved in DNA binding and dimerization of proteins. This domain specific for AHR contains both nuclear localization (NLS) and nuclear export signals (NES) for nucleocytoplasmic shuttling (Fig. 1A). In contrast, ARNT contains only an NLS in the bHLH domain for constitutive nuclear localization. The AHR PAS domain consisting of two imperfect repeats, PAS A and PAS B, is considered to be an interactive surface for protein-protein interactions in dimer formation, and the PAS B region is overlapped in part with a minimal ligand-binding domain (LBD) and the binding site for heat shock protein 90 (HSP90), a chaperone that maintains the ligand-binding conformation of AHR. In addition to the PAS B domain, HSP90 interacts with the bHLH region to mask the NLS, resulting in cytoplasmic localization of AHR. The C-terminal segments of AHR and ARNT both contain transcriptional activation domains (TADs), the activities of which are mediated through CBP/p300 and RIP140 coactivators. On the other hand, AHRR is structurally similar to the AHR in the bHLH region, which allows it to dimerize with ARNT and binds Xenobiotic Responsive Element (XRE; 5′-TNGCGTG-3′) [23]. The C-terminal repression domain of the AHRR has three SUMOylation sites, all of which must be SUMOylated for full suppressive activity on the AHR target genes [22].
Fig. 1.
A: A schematic representation of the mouse C57BL/6 AHR. The characterized domains represented are the basic-helix-loop-helix (bHLH), Per-ARNT-Sim (PAS), and transactivation domains. DBD, DNA binding domain; HSP90, HSP90 interaction domain; LBD, ligand binding domain; Q, glutamine-rich transcription activation domain; A and B, weakly homologous repeated regions. Signals for nuclear import (NLS: red) and export (NES: yellow) are shown. B: A schematic model for the transcriptional regulation of the AHR/ARNT activator complex and AHRR/ARNT repressor complex. Unmodified ARNT forms a heterodimer with AHR and recruits coactivators, such as CBP/p300, to form the transcriptional activator complex. Meanwhile, ARNT forms a heterodimer with AHRR, which significantly enhances the SUMOylation of both proteins. SUMOylated AHRR recruits corepressors, such as HDAC4, HDAC5, and ANKRA2, to form the transcriptional repressor complex.
Ligand diversity of AHR
Environmental chemicals such as synthetic PAHs and halogenated PAHs are considered classic AHR ligands [18, 64]. Increasing interest in the intrinsic functions of the AHR has led to the identification of many natural AHR ligands generated by cells and microbiota or derived from dietary sources [37]. 6-Formylindolo[3,2-b]carbazole (FICZ) is an ultraviolet photoproduct of L-tryptophan that can also be generated by other metabolic pathways [86]. Indolo[3,2-b]carbazole (ICZ) is generated with 3,3-diindolylmethane (DIM) from indole-3-carbinol (I3C) under acidic conditions in the stomach [37]. I3C is enzymatically generated from glucobrassicin, an L-tryptophan-derived glucosinolate contained in cruciferous vegetables, such as broccoli and cabbage. Kynurenine (Kyn) is a tryptophan metabolite generated by the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) [37]. Organic extractions from porcine lung tissues led to the discovery of the high-affinity AHR ligand 2-(1’H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) [37]. Both indigo and indirubin are AHR ligands that have been identified in human urine [1]. Some microbe-derived ligands can also bind the AHR and activate AHR signaling; Malassezia, a commensal yeast in human skin, can metabolize tryptophan into AHR ligands including FICZ and ICZ [52], and Lactobacillus converts tryptophan into indole-3-aldehyde (IAld) [98]. Bacterial pigmented virulence factors, such as phenazines and naphthoquinone phthiocol, have recently been shown to bind the AHR [62]. Several naturally occurring marine-derived brominated indoles also bind to the AHR [17]. In addition, a variety of AHR antagonists with heterogenous characteristics have been reported [18, 64].
Transcriptional regulation mediated by the AHR
In the absence of a ligand, AHR exists in a latent state complexed with HSP90, hepatitis B virus X-associated protein 2 (XAP2, also known as AIP or ARA9), and p23 chaperone (p23) in the cytoplasm. Ligand binding results in a conformational change that in turn leads to nuclear translocation of the AHR complex in association with importins. In the nucleus, the translocated AHR exchanges the HSP90 complex for ARNT present there. Thus, the formed AHR/ARNT complex then binds to XRE to initiate transcription of the target genes.
The mechanisms of AHR-mediated transcription are best characterized for the Cyp1a1 gene. In the approximately 1-kb sequence upstream of the Cyp1a1 gene, a cluster of XREs function as an enhancer. Furthermore, a basic transcription element (BTE), a GC box sequence located immediately upstream of the gene, and the transcription factor SP1 that binds to the GC box sequence are required for full Cyp1a1 expression because the binding of the two transcription factors to their cis-elements is synergistic. Chromatin remodeling is initiated by the liganded AHR/ARNT heterodimer binding to XREs in the enhancer region, which leads to increased DNase sensitivity and the appearance of a DNase hypersensitive site within 300 bp upstream of the transcription initiation sites. Studies have shown that when the AHR/ARNT complex binds to the XRE sequences, the heterodimer recruits CBP/p300, NCOA1, NCOA2, NCOA3, and other coactivators, including RIP140, as well as components of the ATP-dependent chromatin remodeling complexes such as BRG-1, p-TEFβ, and RNA elongation factors. In addition, the TRAP/DRIP/ARC/Mediator complex must be recruited to the Cyp1a1 promoter to activate the target gene expression in response to xenobiotic stress. AHR signaling can be downregulated by negative feedback inhibition through an AHRR transcription factor whose expression is activated by the AHR. Newly synthesized AHRR then translocates into the nucleus and forms a heterodimer with ARNT, and this heterodimer competitively binds to the XRE sequences, followed by recruitment of corepressors such as ANKLA2, HDAC4, and HDAC5 (Fig. 1B) [6, 22, 28, 89].
AHR in Immunity
The AHR is an important regulator of the development and function of both innate and adaptive immune cells through its ability to respond to cellular, dietary, and environmental exogenous ligands [99]. The innate immune system represents the first line of defense against infections by microbial pathogens and is mediated primarily by myeloid lineage cells, such as macrophages and neutrophils, while adaptive immune responses are dependent on the activation, differentiation, and clonal expansion of antigen-specific T and B lymphocytes over a period of several days after infection. The AHR is induced in several immune cell types by lipopolysaccharide (LPS) and microbial infection, and the promoters of many genes involved in the immune responses contain multiple XRE sequences [43].
AHR in the innate immune system
Antigen-presenting cells (APCs), such as macrophages and dendritic cells, are important for innate immune defense and for the generation and regulation of adaptive immunity against various pathogens. Upon LPS treatment, the serum levels of interleukin (IL)-6 and IL-1β were significantly elevated in Ahr-deficient mice as compared with those in wild-type mice [45, 83, 93]. Moreover, peritoneal macrophages isolated from Ahr-deficient mice exhibited much higher IL-6 secretion in response to LPS treatment than those from wild-type mice. These results indicate that AHR suppresses the expression of inflammatory cytokines to protect against persistent inflammation. In addition, peritoneal macrophages showed markedly enhanced AHR expression in response to LPS treatment. The AHR-dependent repression of IL-6 production may be caused via two independent mechanisms. First, AHR appears to play a suppressive role by forming a complex with STAT1 and NF-κB in macrophages, leading to the inhibition of Il6 gene promoter activity [45]. In addition, an interaction between the AHR and SP1 in LPS-treated macrophages can inhibit histidine decarboxylase expression and reduce the production of histamine, an important signal molecule involved in the inflammation [54]. In the case of IL-1β secretion, the AHR activates the expression of plasminogen activator inhibitor-2 (PAI-2) [83], an inhibitor of caspase-1 activation that functions in the process of IL-1β secretion [26]. AHR ligands markedly enhanced PAI-2 expression in wild-type macrophages, and transfection of the Ahr-deficient macrophages with PAI-2 restored suppression of IL-1β secretion. Using compound mutant mice lacking both genes for the AHR and ASC, which is an essential for caspase-1 activation in inflammasomes, we demonstrated a functional association between the ASC- and AHR-directed pathways in the regulation of the IL-1β-dependent inflammatory response in vivo [38].
Kimura et al. [44] showed that AHR is critical for the confinement and clearance of Listeria monocytogenes(LM, the food-borne causative pathogen of listeriosis)in vitro and in vivo. Ahr-deficient mice showed high susceptibility to LM infection. The AHR contributes to macrophage survival by inducing the apoptosis inhibitor of macrophages (AIM) and promoting the production of reactive oxygen species (ROS) during LM infection, resulting in enhanced bacterial clearance. p40phox, a member of NADPH oxidase subunits, was also reported to be induced by the AHR [93]. Bessede et al. [7] studied the roles of AHR and tryptophan catabolism in primary LPS responsiveness and in the induction of endotoxin tolerance. They showed that the hypersensitivity to a primary LPS challenge was mitigated by the AHR activity through TDO2-dependent Kyn production. In contrast, endotoxin tolerance required the combined effects of AHR, IDO1, and TGFβ. This protective, LPS-triggered tolerance was not restricted to immunopathology induced by LPS or Gram-negative bacteria, but it also specifically targeted inflammatory cytokine production in a Streptococcus-induced multifocal septic arthritis model. Moura-Alves et al. [62] demonstrated that AHR senses multiple bacterial virulence factors from pulmonary pathogens, including phenazines produced by Pseudomonas aeruginosa and naphthoquinone phthiocol from Mycobacterium tuberculosis. Upon ligand binding, AHR activation regulates cytokine and chemokine production and leads to virulence factor degradation, inducing the expression of drug-metabolizing enzymes such as CYP1A1 and CY1B1. In Ahr-deficient mice, infection with P. aeruginosa induced more aggressive disease characteristics and increased bacterial load compared with in wild-type mice.
AHR in the adaptive immune system
Regulatory T cells (Treg) and Th17 cells are newly identified subpopulations of CD4+ T lymphocytes defined by the expression of FOXP3 and RORγt transcription factors, respectively. Th17 cells secrete pro-inflammatory cytokines such as IL-17 and IL-22, which are implicated in autoimmune conditions including multiple sclerosis and rheumatoid arthritis [53]. The production of TGFβ and IL-10 family anti-inflammatory cytokines prevents the development of autoimmune diseases in part by increasing Treg polarization. The functions of the AHR in CD4+ naïve T cell differentiation have been extensively studied [46, 76, 92]. These studies show a reciprocal relationship between the production of Th17 and Treg cells. While TGFβ1 in combination of IL-6 boosts the differentiation of Th17 cells, TGFβ1 alone induces the differentiation of Treg cells from CD4+ naïve T cells under T cell differentiation conditions. AHR expression is remarkably accelerated in the induced Th17 cells, with approximately 10-fold higher expression than in the induced Treg cells. Ligand-activated AHR binds to STAT1 and STAT5, transcription factors that suppresses Th17 generation, suggesting that the AHR may regulate the generation of Th17 cells by modifying the activity of these two STATs [46]. AHR activation by TCDD induces Treg cells, which in turn suppresses experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, by a TGFβ1-dependent mechanism. Conversely, AHR activation by FICZ interferes with Treg cell differentiation and boosts Th17 cell differentiation, resulting in worsening EAE [76, 77, 92]. The promoter of the Foxp3 gene contains functional XREs, and ChIP analysis revealed that TCDD-activated AHR was recruited to the Foxp3 promoter [76]. TCDD-dependent Foxp3 gene induction may also occur via epigenetic regulation [85]. Thus, the AHR activity is a critical regulator of T cell differentiation, particularly of the balance between Th17 and Treg cells, depending on the presence of specific ligands and the cytokine milieu. Moreover, lack of the AHR specifically in T cells suppresses Th17 cell generation and collagen-induced arthritis development [66].
While both Th1 and Th2 cells are CD4+ helper T cells, there are fundamental differences between these two cell types [29]. Th1 cells mainly secrete interferon (INF)-γ, a cytokine that promotes the differentiation of CD8+ cytotoxic T lymphocytes (CTLs), thereby providing effective cellular immunity against intracellular pathogens. In contrast, Th2 cells produce cytokines such as IL-4 and IL-5, leading to an IgE-mediated humoral immune response against extracellular pathogens. The AHR signaling pathway activated by M50354, an active metabolite of an anti-allergic agent, M50367, skews the Th1/Th2 balance toward Th1 cell dominance, resulting in immunological responses with anti-allergic effects [67]. In addition, type 1 regulatory T cells (Tr1 cells) that produce IL-10 are instrumental in the prevention of tissue inflammation, autoimmunity, and graft-versus-host disease (GVHD). During T cell activation under Tr1-skewing conditions, ligand-activated AHR, induced by IL-27, physically associated in synergy with the master transcription factor c-MAF and transactivated the Il10 and Il21 promoters, resulting in development of Tr1 cells and amelioration of EAE [2]. A recent report by Gagliani et al. [24] suggested that Th17 cells can transdifferentiate into Tr1 cells during resolution of inflammation by a change in signature transcription profile and acquisition of potent regulatory capacity. This study also revealed a role for canonical TGFβ and for the AHR signaling pathways in conversion. These results suggest that Th17 cell phenotype instability and plasticity may present a therapeutic opportunity for treatment of inflammatory diseases.
Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme, conserved throughout the past ~600 MYA [98], that catalyzes the rate limiting step of tryptophan catabolism in the kynurenine pathway [56]. IDO is present in subsets of dendritic cells and is thought to be a central effector of Treg generation from naïve T cells by dendritic cell-T cell interactions based on the following observations [57]: 1) Exposure of bone marrow-derived dendritic cells (BMDCs) to TCDD increases IDO mRNA in an AHR-dependent manner, 2) induced IDO promotes generation of the endogenous AHR agonist kynurenine (Kyn), 3) Kyn directly activates the AHR and induces generation of FOXP3+ Treg cells in an AHR-dependent manner, 4) TGFβ1 upregulates the AHR expression, further potentiating the transcription activation of the XRE-dependent genes by Kyn, and 5) Kyn does not lead to Th17 cell generation. Importantly, the AHR and IDO/TDO are closely interconnected; AHR regulates IDO/TDO expression, and Kyn produced by IDO/TDO is an AHR agonist. Absence of the AHR in BMDCs inhibited Treg development, facilitated Th17 generation from naïve T cells, and promoted naïve T cell proliferation [68].
AHR in gut immunity
On the gut mucosal surfaces, constant integration of dietary and microbial signals is required for the maintenance of intestinal homeostasis [33], and disturbance of this process increases susceptibility to intestinal infections, inflammatory bowel diseases, and inflammation-induced cancers. Innate lymphoid cells (ILCs) and intraepithelial lymphocytes (IELs) are two predominant cell types in the mucosal immune system. ILC populations are classified into three subsets (ILC1, 2, and 3) that serve distinct roles in the immune response [91]. Among them, ILC3 cells, which express the transcription factor RORγt and produce IL-17 and IL-22, induce the postnatal formation of intestinal lymphoid follicles and regulate intestinal homeostasis. These RORγt+ ILCs express AHR, which is required for the postnatal expansion of intestinal RORγt+ ILCs and the formation of intestinal lymphoid follicles [47, 49]. The AHR activity within RORγt+ ILCs can be induced by dietary ligands such as those found in cruciferous vegetables. Production of IL-22 was reduced dramatically in Ahr-deficient mice, reflecting a marked decrease in RORγt+ ILC number that eventually resulted in a high susceptibility to Citrobacter rodentium infection. Direct interaction between RORγt and AHR cooperatively promoted expression of IL-22 [75], a cytokine important for defense against pathogenic intestinal infections.
IELs are an important first line of defense and contribute to both epithelial barrier organization and wound repair. In the gut, IELs consist of distinct subpopulations according to expression of T cell receptor TCRγδ or TCRαβCD8 αα+. Li et al. [50] showed that the AHR is crucial for maintaining the IEL number in the intestine, as AHR deficiency or lack of an AHR ligand compromised the maintenance of IELs and the control of the microbial load and composition, resulting in heightened immune activation and increased vulnerability to epithelial damage. AHR activity can be regulated by dietary-derived compounds, such as those present in cruciferous vegetables, providing a mechanistic link between dietary compounds, the intestinal immune system, and the microbiota.
The vertebrate intestine is colonized by hundreds of distinct species of microorganisms that have mutually beneficial relationships with their host animals. Intestinal microbiota are known to influence the development and balance of the host immune system. Recent reports have shown that segmented filamentous bacteria (SFB) are localized in the terminal ileum and cecum of mice and induce pro-inflammatory Th17 cells [40]. Alternatively, Clostridium clusters IV and XIVa are most abundant in the cecum and proximal colon and are strongly associated with the distribution of Treg cells [3]. Zelante et al. [98] showed that a subset of commensal Lactobacilli utilizes tryptophan (Trp) as an energy source and produces indole-3-aldehyde (IAld) as a metabolite. IAld activated AHR to enhance the expression of IL-22 in ILC3s, which in turn stimulated epithelial cells to produce antimicrobial proteins, including REGIIIγ, lipocalin, and calprotectin. As a result, Candida albicans colonization was reduced, possibly because of its susceptibility to antimicrobial protein-mediated growth inhibition. Qiu et al. [74] showed that AHR expression in innate ILC3s plays a protective role in T cell-mediated experimental colitis by suppressing pathogenic Th17 cells, suggesting that the intricate balance between ILCs and Th17 cells is regulated by the AHR and commensal flora. These two studies provide mechanistic insights into functional interactions between commensal microbes and innate lymphoid cells mediated by the AHR [5].
We previously reported that Ahr-deficient mice develop cecal tumors with aberrant accumulation of β-catenin and severe inflammation [42] and that both germ-free (GF)Ahr-deficient mice and compound mutant mice lacking genes for AHR and ASC show markedly reduced tumor development compared to Ahr-deficient mice [38]. AHR-dependent degradation of β-catenin was induced by dietary AHR ligands such as I3C and DIM, which suppressed tumor development in the small intestine of ApcMin/+ mice [42]. A recent report by Park et al. showed that FICZ also promotes β-catenin degradation in crypt cells [71]. From these results, it is suggested that the AHR plays dual roles in tumor suppression in the intestine by acting as a regulator of β-catenin and as an inhibitor of inflammation.
AHR in Stem Cell Maintenance and Cell Differentiation
AHR in hematopoietic stem cell maintenance and differentiation
Several lines of evidence support the suggestion that the AHR is important for hematopoietic stem cell (HSC) regulation and function [84]. Further evidence suggests that AHR expression is necessary for the proper maintenance of quiescence in HSCs and that AHR downregulation allows HSCs to escape from quiescence and subsequently proliferate. Boitano et al. [9] identified a purine derivative, StemRegenin 1 (SR1), which acts as an AHR antagonist to promote expansion of CD34+ cells ex vivo. Culture of HSCs with SR1 led to a 50-fold increase in cells expressing CD34+ and a 17-fold increase in cells retaining compatibility for engraftment in immunodeficient mice. Wagner et al. [94] showed the feasibility and safety of SR1-treated HSCs for transplantation and observed marked expansion of CD34+ cells with absence of graft failure and enhanced hematopoietic recovery. SR1 treatment of isolated CD34 progenitor cells also led to an enriched CD34+CD41low fraction with an increased capacity to generate proplatelet-producing megakaryocytes and platelet-like elements similar to circulating platelets [90]. In addition, Rentas et al. [78] showed that the RNA-binding protein Musashi-2 mediates the posttranscriptional downregulation of the AHR complex components in human HSPCs and progenitor cells, resulting in reduction of the capacity of AHR signaling to expand human HSCs and progenitor cells. Pabst et al. [69] identified a new pyrimidoindole compound, UM729, that cooperates with SR1, a known AHR antagonist, in more efficiently preventing AML cell differentiation. Since UM729 lacks AHR suppressor activity, these results suggest that at least two different pathways contribute to the maintenance of leukemia stem cell activity ex vivo.
In 1995, our group provided the first report implicating the AHR in cell differentiation. Studies using phorbol ester as an inducer revealed that differentiation of promyelocytic HL-60 and erythroblastic HEL cells to monocytes increased the levels of transcriptionally active AHR [31]. Retinoic acid (RA)-induced differentiation of HL-60 depends on the AHR and its ability to downregulate the stem cell factor OCT4 [11]. Further, the AHR directs hematopoietic progenitor cell expansion and differentiation [87]. Several lines of evidence have suggested possible reciprocal suppression in the expression between Ahr and Oct4 genes. Ko et al. [48] showed that interactions between OCT4, NANOG, SOX2, and polycomb group (PcG) proteins at the Ahr promoter lead to repression of the AHR expression in pluripotent embryonic stem (ES) cells, while during differentiation, the AHR expression was activated by reversal of repressive marks in Ahr promoter chromatin, release of the pluripotency factors and PcG proteins, binding of SP factors, establishment of histone marks of open chromatin, and subsequently, recruitment of active RNA polymerase II. On the other hand, Cheng et al. [15] demonstrated that the tryptophan derivative ITE enhanced binding of the AHR to the Oct4 gene promoter to suppress Oct4 transcription. Reduction of ITE levels in cancer cells by tryptophan deprivation or hypoxia led to OCT4 elevation, which was reversed by addition of synthetic ITE. Consequently, synthetic ITE induced the differentiation of stem-like cancer cells and reduced their tumorigenic potential.
Morales-Hernandez et al. [61] showed that differentiation of the pluripotent embryonic teratocarcinoma NTERA-2 with RA produced a marked increase in AHR expression. This increase activated the transcription of Alu retrotransposons located within the Oct4 and Nanog promoters harboring XRE and RNA polymerase III binding sites in the differentiated carcinoma cells. Noncoding RNA transcripts produced from these Alu retrotransposons may repress Oct4 and Nanog expression through the miRNA pathway. Thus, it is suggested that the transcripts produced from AHR-regulated Alu may control the retrotransposons expression of Oct4 and Nanog during differentiation of carcinoma cells. On the other hand, miR-302 is highly expressed in ES cells [35] and has the pivotal role in the maintenance of pluripotency and the cell reprogramming process. Hu et al. [36] found that an anti-allergy drug, tranilast, which is an AHR agonist, promotes miR-302 expression in an XRE-dependent manner. Sustaining miR-302 expression supported pluripotency of mouse ES cells due to increased expression of pluripotency genes, such as Oct4 and Nanog, and reduced expression of differentiation marker genes involving Fgf5 and Gata4. Furthermore, tranilast and other AHR agonists promoted somatic cell reprogramming through AHR-mediated miR-302 expression.
AHR in homeostatic function and TCDD-mediated diseases
Increasing evidence indicates that AHR has physiological functions in maintenance of cellular homeostasis that do not require activation by xenobiotics. Thus, the AHR activation by environmental chemicals, such as TCDD, may be expected to disrupt cell differentiation and homeostasis. In fact, genome-wide expression profiling during differentiation of mouse ES cells into cardiomyocytes revealed that TCDD-driven AHR activation during the early stages maintained the pro-proliferative state of ES cells and inhibited differentiation [95]. In addition, Wang et al. [96] also reported that TCDD exposure during the initial differentiation stages of mouse embryo cells (the period of panmesoderm development) disrupted the TGFβ/BMP and WNT signaling pathways essential for cardiac development. Further, cardiomyocyte contractility was a stage-specific AHR-dependent TCDD target, and TCDD-induced impairment of contractility was successfully reversed by addition of BMP4, WNT3a, or WNT5a during the first 3 days of differentiation in culture. Carreira et al. [13] showed that disruption of AHR signaling during mouse development leads to abnormal cardiac structure and function in the adult mouse and also suggested [14] that the AHR signaling in the developing mammalian heart is crucial for cellular metabolism and cardiac function, as it regulates mitochondrial structure and function. Identification of the critical window of developmental exposure to an environmental toxicant is of extreme importance for our understanding of the health outcome resulting from such an exposure.
The epidermis is maintained by a multipotent stem cell population, located in the bulge region of the basal layer, that gives rise to cells of different fates, including those forming hair follicles, interfollicular epidermis, and associated glands such as sebaceous glands. The proto-oncogene c-MYC, which stimulates stem cell exit from quiescence and transformation to proliferating sebocyte progenitors [8], can divert epidermal stem cells to a sebaceous gland fate at the expense of hair follicles [32]. B lymphocyte maturation protein 1 (Blimp 1) is expressed in a population of cells that act as unipotent precursors of proliferative sebocytes, which in turn differentiate into lipid-containing sebocytes [34]. Blimp 1 represses expression of c-MYC in sebocyte progenitor cells and regulates the size and number of cells within the gland. Chloracne, a severe and persistent skin disease, is the hallmark of TCDD-mediated toxicity [53]. TCDD is known to accumulate persistently in sebum, and we showed that AHR expression in the sebaceous gland co-localized with Blimp 1. Expression of Blimp 1 was induced by the AHR ligand MC and by UVB treatment both in sebocyte (SZ95) and keratinocyte (HaCaT) cell lines, although its expression was AHR/ARNT-dependent but XRE-independent [39]. In addition, TCDD treatment of SZ95 cells led to sebocyte atrophy and increased keratinocyte-like differentiation [41]. Taken together, sustained AHR activation dysregulates sebaceous gland homeostasis, leading to exhaustion of stem/progenitors and apoptosis of sebocytes, as evidenced by the consequences of severe dioxin poisoning [70].
The rapid turnover of the intestinal epithelium (3–5 days) is supported by stem cells located around the base of the crypt. Genetic inducible fate mapping studies have identified two principal epithelial stem cell populations, the LGR5-expressing crypt base columnar cells (CBCs) that facilitate homeostatic self-renewal and cells localized at or near the +4 cell position that mediate injury-induced regeneration [97]. However, the relative functions of these two stem cell populations and their interrelationships are not well understood [4]. Since AHR has important roles in maintenance of cellular homeostasis, it is of interest to investigate its function in intestinal epithelial regeneration. In this context, we showed AHR expression in Paneth cells of the mouse small intestine and ileum [42], while Park et al. showed its localization in LGR5+ CBCs [71]. Paneth cells are interspersed between CBCs and serve as multifunctional guardians of stem cells, both by secreting anti-microbial products and constituting the niche for LGR5+ stem cells [80]. Two recent studies suggested a critical function for immune cells expressing the AHR in crypt homeostasis. ILC3s are crucial for maintaining gastrointestinal integrity and barrier function, and Lindemans et al. [51] found that IL-22, produced by combined AHR and RORγt activation in ILC3s after intestinal injury, promoted intestinal stem cell-mediated epithelial regeneration. Using ex vivo organoid cultures, they showed that ILC3s increase the growth of mouse small intestine organoids in an IL-22-dependent fashion. Chng et al. [16] reported that Cre-mediated deletion of the Ahr gene in CD11c-expressing dendritic cells was associated not only with aberrant mucosal immunity but also altered epithelial morphogenesis. These findings reveal a fundamental mechanism by which the immune cell system is involved in regeneration of the intestinal epithelium. Further investigations into the roles of the AHR in intestinal homeostasis are awaited.
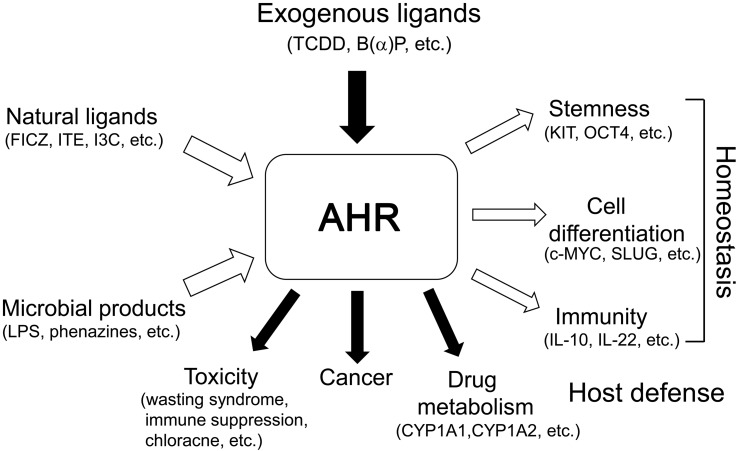
The AHR was first identified by Poland and his colleagues over 40 years ago as a TCDD-binding cytosolic receptor controlling induction of aryl hydrocarbon hydroxylase. However, substantial progress in the studies on the physiological functions of the AHR was not been made until cDNA cloning and elucidation of the primary sequence of the AHR were reported in 1992. Since then, an explosive increase of evidence has been reported indicating that AHR plays multiple physiological roles in host defense and homeostasis, including immunity, stem cell maintenance, and cell differentiation, as a mediator of newly defined dietary, cellular, and microbe-derived ligands (Fig. 2). In addition, AHR interacts with a multitude of diverse signaling pathways in cells, such as those of regulating cell proliferation and the cell cycle, cell morphology, cell adhesion, and cell migration [63]. Thus, AHR is now considered to function as an environmental sensor, connecting external environmental signals to internal cellular responses.
Fig. 2.
The AHR functions as a pivotal sensor connecting external and internal environments. AHR is involved in drug metabolism, carcinogenesis, and toxicities of classical ligands, such as TCDD and polycyclic aromatic hydrocarbons, while it mediates important functions for development, host defense, and homeostasis, including stem cell maintenance, cell differentiation, and immunity, using newly defined ligands from dietary, cellular, and microbe-derived products.
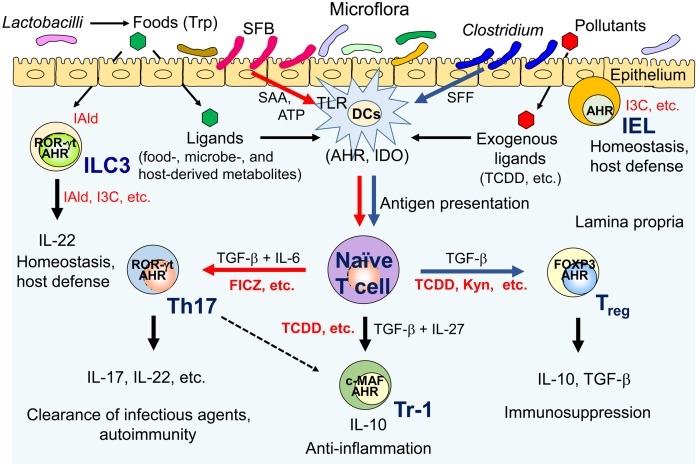
The AHR is an important bHLH-PAS transcription factor not only in innate and adaptive immunity but also in gut immune responses (Fig. 3). AHR activation suppresses the expression of inflammatory cytokines, such as IL-6 and IL-1β, to protect against persistent inflammation. AHR is also involved in LM clearance by inducing AIM and ROS production, in tolerance to LPS shock by interacting with TDO2/IDO1, and in pathogen virulence factor degradation. Importantly, AHR regulates naïve T cell differentiation, thereby controlling the balance of pro-inflammatory Th17 cells and anti-inflammatory Treg cells in a specific cytokine milieus. The AHR also participates in Tr1 cell differentiation in conjunction with c-MAF and mediates Th17 cell transdifferentiation into regulatory T cells during resolution of inflammation. TCDD exposure causes profound suppression of immune activity, mainly due to AHR/IDO1 axis-derived Treg generation and IL-10 expression. In mucosal immune responses, AHR expressed by ILC3s participates in postnatal formation of intestinal lymphoid follicles and gut homeostasis, while AHR in IELs regulates cell number and microbial load control. In the intestines, particular commensal bacteria, SFB, induce pro-inflammatory Th17 cells, while Clostridia associate with Treg cells. Commensal Lactobacilli utilize tryptophan as an energy source and produce an AHR ligand, Iald, as a metabolite, resulting in AHR activation and IL-22 expression in ILC3s. In turn, IL-22 induces anti-microbial proteins that control bacterial infections. Thus, the AHR functions in host defense not only via drug metabolism of external chemicals but also by prevention of bacterial infection at multiple steps in the infection process. Deeper mechanistic insights into the functional association between commensal microbes and immune cells through AHR activation are expected. Furthermore, identification and characterization of dietary, endogenous, and microbe-derived AHR ligands will promote the discovery of novel therapeutics targeting the AHR pathway.
Fig. 3.
Roles of the AHR in gut immunity. A mechanistic link between AHR ligands, the gut immune system, and the microbiota. SFB, segmented filamentous bacteria; SAA, serum amyloid A; SFF, spore-forming fraction; DCs, dendritic cells; ILC3, group 3 innate lymphoid cells; IEL, intraepithelial lymphocytes; IDO, indoleamine 2,3-dioxygenase; TLR, toll-like receptor; Th17, IL-17-producing T helper cells; Treg, regulatory T cells; ROR-γt, retinoic acid receptor-related orphan receptor γt; FOXP3, forkhead box P3; I3C, indole-3-carbinol; Kyn, kynurenine. See text for details.
Another recent discovery is the importance of the AHR in regulation of cell pluripotency and differentiation. The finding indicating that an AHR antagonist SR1 promotes HSCs expansion ex vivo suggests that AHR is necessary for proper maintenance of quiescence in HSCs. Involvement of AHR in maintenance of HSCs was further confirmed by different AHR antagonists and by downregulation of HSP90, a component of the AHR complex. An interesting possibility is reciprocal suppression in the expression of Ahr and Oct4 genes. In addition, AHR-directed transcripts of Alu retrotransposons or miR-302 may regulate the expression of pluripotent factors. The AHR also regulates organogenesis and tissue repair by controlling cell differentiation, such as cardiac and sebocyte differentiation. Cardiomyocyte contractility is a stage-specific AHR-dependent TCDD target, and disruption of AHR signaling during development leads to abnormal cardiac structure and function. Sustained activation of the AHR by TCDD dysregulates sebaceous gland homeostasis and leads to TCDD-induced chloracne. Taken together, a myriad of deficits induced by disruption of AHR signaling implicates this receptor in multiple developmental, physiological, and pathophysiological processes and underscores the therapeutic potential of drugs targeting AHR-associated pathways.
References
- 1.Adachi J., Mori Y., Matsui S., Takigami H., Fujino J., Kitagawa H., Miller C.A., 3rd, Kato T., Saeki K., Matsuda T.2001. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 276: 31475–31478. doi: 10.1074/jbc.C100238200 [DOI] [PubMed] [Google Scholar]
- 2.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K.2010. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11: 854–861. doi: 10.1038/ni.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K.2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341. doi: 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N., van Oudenaarden A., Clevers H.2012. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 11: 452–460. doi: 10.1016/j.stem.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 5.Behnsen J., Raffatellu M.2013. Keeping the peace: aryl hydrocarbon receptor signaling modulates the mucosal microbiota. Immunity 39: 206–207. doi: 10.1016/j.immuni.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 6.Beischlag T.V., Luis Morales J., Hollingshead B.D., Perdew G.H.2008. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 18: 207–250. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M.C., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M.L., Orabona C., Bianchi R., Lanz T.V., Platten M., Della Fazia M.A., Piobbico D., Zelante T., Funakoshi H., Nakamura T., Gilot D., Denison M.S., Guillemin G.J., DuHadaway J.B., Prendergast G.C., Metz R., Geffard M., Boon L., Pirro M., Iorio A., Veyret B., Romani L., Grohmann U., Fallarino F., Puccetti P.2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511: 184–190. doi: 10.1038/nature13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bock K.W.2016. Toward elucidation of dioxin-mediated chloracne and Ah receptor functions. Biochem. Pharmacol. 112: 1–5. doi: 10.1016/j.bcp.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 9.Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., Walker J.R., Flaveny C.A., Perdew G.H., Denison M.S., Schultz P.G., Cooke M.P.2010. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329: 1345–1348. doi: 10.1126/science.1191536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradfield C.A., Glover E., Poland A.1991. Purification and N-terminal amino acid sequence of the Ah receptor from the C57BL/6J mouse. Mol. Pharmacol. 39: 13–19. [PubMed] [Google Scholar]
- 11.Bunaciu R.P., Yen A.2011. Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 71: 2371–2380. doi: 10.1158/0008-5472.CAN-10-2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbach K.M., Poland A., Bradfield C.A.1992. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. USA 89: 8185–8189. doi: 10.1073/pnas.89.17.8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreira V.S., Fan Y., Kurita H., Wang Q., Ko C.I., Naticchioni M., Jiang M., Koch S., Zhang X., Biesiada J., Medvedovic M., Xia Y., Rubinstein J., Puga A.2015. Disruption of Ah receptor signaling during mouse development leads to abnormal cardiac structure and function in the adult. PLOS ONE 10: e0142440. doi: 10.1371/journal.pone.0142440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carreira V.S., Fan Y., Wang Q., Zhang X., Kurita H., Ko C.I., Naticchioni M., Jiang M., Koch S., Medvedovic M., Xia Y., Rubinstein J., Puga A.2015. Ah receptor signaling controls the expression of cardiac development and homeostasis genes. Toxicol. Sci. 147: 425–435. doi: 10.1093/toxsci/kfv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J., Li W., Kang B., Zhou Y., Song J., Dan S., Yang Y., Zhang X., Li J., Yin S., Cao H., Yao H., Zhu C., Yi W., Zhao Q., Xu X., Zheng M., Zheng S., Li L., Shen B., Wang Y.J.2015. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat. Commun. 6: 7209. doi: 10.1038/ncomms8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chng S.H., Kundu P., Dominguez-Brauer C., Teo W.L., Kawajiri K., Fujii-Kuriyama Y., Mak T.W., Pettersson S.2016. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci. Rep. 6: 23820. doi: 10.1038/srep23820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGroot D.E., Franks D.G., Higa T., Tanaka J., Hahn M.E., Denison M.S.2015. Naturally occurring marine brominated indoles are aryl hydrocarbon receptor ligands/agonists. Chem. Res. Toxicol. 28: 1176–1185. doi: 10.1021/acs.chemrestox.5b00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denison M.S., Soshilov A.A., He G., DeGroot D.E., Zhao B.2011. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124: 1–22. doi: 10.1093/toxsci/kfr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan D.M., Burgess E.A., Duncan I.1998. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 12: 1290–1303. doi: 10.1101/gad.12.9.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y.1992. cDNA cloning and structure of mouse putative Ah receptor. Biochem. Biophys. Res. Commun. 184: 246–253. doi: 10.1016/0006-291X(92)91185-S [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Salguero P., Pineau T., Hilbert D.M., McPhail T., Lee S.S., Kimura S., Nebert D.W., Rudikoff S., Ward J.M., Gonzalez F.J.1995. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268: 722–726. doi: 10.1126/science.7732381 [DOI] [PubMed] [Google Scholar]
- 22.Fujii-Kuriyama Y., Kawajiri K.2010. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 86: 40–53. doi: 10.2183/pjab.86.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y.1987. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 15: 4179–4191. doi: 10.1093/nar/15.10.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W., de Zoete M.R., Licona-Limón P., Paiva R.S., Ching T., Weaver C., Zi X., Pan X., Fan R., Garmire L.X., Cotton M.J., Drier Y., Bernstein B., Geginat J., Stockinger B., Esplugues E., Huber S., Flavell R.A.2015. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523: 221–225. doi: 10.1038/nature14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenlee W.F., Poland A.1979. Nuclear uptake of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J and DBA/2J mice. Role of the hepatic cytosol receptor protein. J. Biol. Chem. 254: 9814–9821. [PubMed] [Google Scholar]
- 26.Greten F.R., Arkan M.C., Bollrath J., Hsu L.C., Goode J., Miething C., Göktuna S.I., Neuenhahn M., Fierer J., Paxian S., Van Rooijen N., Xu Y., O’Cain T., Jaffee B.B., Busch D.H., Duyster J., Schmid R.M., Eckmann L., Karin M.2007. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130: 918–931. doi: 10.1016/j.cell.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn M.E., Karchner S.I.2012. Structural and functional diversification of AHRs during metazoan evolution. pp. 389–403. In: The AH receptor in biology and toxicology. (Pohjanvirta, R. ed.), John Willey & Sons, New Jersey. [Google Scholar]
- 28.Hankinson O.2012. The AHR/ARNT dimer and transcriptional coactivators. pp. 93–100. In: The AH receptor in biology and toxicology. (Pohjanvirta, R. ed.), Joh Willey & Sons, New Jersey. [Google Scholar]
- 29.Hao N., Whitelaw M.L.2013. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 86: 561–570. doi: 10.1016/j.bcp.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 30.Hay A.1976. Seveso: the aftermath. Nature 263: 538–540. doi: 10.1038/263538a0 [DOI] [Google Scholar]
- 31.Hayashi S., Okabe-Kado J., Honma Y., Kawajiri K.1995. Expression of Ah receptor (TCDD receptor) during human monocytic differentiation. Carcinogenesis 16: 1403–1409. doi: 10.1093/carcin/16.6.1403 [DOI] [PubMed] [Google Scholar]
- 32.Honeycutt K.A., Roop D.R.2004. c-Myc and epidermal stem cell fate determination. J. Dermatol. 31: 368–375. doi: 10.1111/j.1346-8138.2004.tb00687.x [DOI] [PubMed] [Google Scholar]
- 33.Hooper L.V., Macpherson A.J.2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10: 159–169. doi: 10.1038/nri2710 [DOI] [PubMed] [Google Scholar]
- 34.Horsley V., O’Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., Fuchs E.2006. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126: 597–609. doi: 10.1016/j.cell.2006.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houbaviy H.B., Murray M.F., Sharp P.A.2003. Embryonic stem cell-specific MicroRNAs. Dev. Cell 5: 351–358. doi: 10.1016/S1534-5807(03)00227-2 [DOI] [PubMed] [Google Scholar]
- 36.Hu W., Zhao J., Pei G.2013. Activation of aryl hydrocarbon receptor (ahr) by tranilast, an anti-allergy drug, promotes miR-302 expression and cell reprogramming. J. Biol. Chem. 288: 22972–22984. doi: 10.1074/jbc.M113.475624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubbard T.D., Murray I.A., Perdew G.H.2015. Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 43: 1522–1535. doi: 10.1124/dmd.115.064246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikuta T., Kobayashi Y., Kitazawa M., Shiizaki K., Itano N., Noda T., Pettersson S., Poellinger L., Fujii-Kuriyama Y., Taniguchi S., Kawajiri K.2013. ASC-associated inflammation promotes cecal tumorigenesis in aryl hydrocarbon receptor-deficient mice. Carcinogenesis 34: 1620–1627. doi: 10.1093/carcin/bgt083 [DOI] [PubMed] [Google Scholar]
- 39.Ikuta T., Ohba M., Zouboulis C.C., Fujii-Kuriyama Y., Kawajiri K.2010. B lymphocyte-induced maturation protein 1 is a novel target gene of aryl hydrocarbon receptor. J. Dermatol. Sci. 58: 211–216. doi: 10.1016/j.jdermsci.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 40.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R.2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498. doi: 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ju Q., Fimmel S., Hinz N., Stahlmann R., Xia L., Zouboulis C.C.2011. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters sebaceous gland cell differentiation in vitro. Exp. Dermatol. 20: 320–325. doi: 10.1111/j.1600-0625.2010.01204.x [DOI] [PubMed] [Google Scholar]
- 42.Kawajiri K., Kobayashi Y., Ohtake F., Ikuta T., Matsushima Y., Mimura J., Pettersson S., Pollenz R.S., Sakaki T., Hirokawa T., Akiyama T., Kurosumi M., Poellinger L., Kato S., Fujii-Kuriyama Y.2009. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 106: 13481–13486. doi: 10.1073/pnas.0902132106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerkvliet N.I.2009. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem. Pharmacol. 77: 746–760. doi: 10.1016/j.bcp.2008.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura A., Abe H., Tsuruta S., Chiba S., Fujii-Kuriyama Y., Sekiya T., Morita R., Yoshimura A.2014. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 26: 209–220. doi: 10.1093/intimm/dxt067 [DOI] [PubMed] [Google Scholar]
- 45.Kimura A., Naka T., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T.2009. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 206: 2027–2035. doi: 10.1084/jem.20090560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura A., Naka T., Nohara K., Fujii-Kuriyama Y., Kishimoto T.2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. USA 105: 9721–9726. doi: 10.1073/pnas.0804231105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiss E.A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., Diefenbach A.2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334: 1561–1565. doi: 10.1126/science.1214914 [DOI] [PubMed] [Google Scholar]
- 48.Ko C.I., Wang Q., Fan Y., Xia Y., Puga A.2014. Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem Cell Res. (Amst.) 12: 296–308. doi: 10.1016/j.scr.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., Colonna M.2011. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13: 144–151. doi: 10.1038/ni.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., Veldhoen M.2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640. doi: 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 51.Lindemans C.A., Calafiore M., Mertelsmann A.M., O’Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G., Ivanov J.A., Fu Y.Y., Takashima S., Hua G., Martin M.L., O’Rourke K.P., Lo Y.H., Mokry M., Romera-Hernandez M., Cupedo T., Dow L.E., Nieuwenhuis E.E., Shroyer N.F., Liu C., Kolesnick R., van den Brink M.R.M., Hanash A.M.2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564. doi: 10.1038/nature16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magiatis P., Pappas P., Gaitanis G., Mexia N., Melliou E., Galanou M., Vlachos C., Stathopoulou K., Skaltsounis A.L., Marselos M., Velegraki A., Denison M.S., Bassukas I.D.2013. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J. Invest. Dermatol. 133: 2023–2030. doi: 10.1038/jid.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall N.B., Kerkvliet N.I.2010. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 1183: 25–37. doi: 10.1111/j.1749-6632.2009.05125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masuda K., Kimura A., Hanieh H., Nguyen N.T., Nakahama T., Chinen I., Otoyo Y., Murotani T., Yamatodani A., Kishimoto T.2011. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. Int. Immunol. 23: 637–645. doi: 10.1093/intimm/dxr072 [DOI] [PubMed] [Google Scholar]
- 55.McIntosh B.E., Hogenesch J.B., Bradfield C.A.2010. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu. Rev. Physiol. 72: 625–645. doi: 10.1146/annurev-physiol-021909-135922 [DOI] [PubMed] [Google Scholar]
- 56.Mellor A.L., Munn D.H.2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4: 762–774. doi: 10.1038/nri1457 [DOI] [PubMed] [Google Scholar]
- 57.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A.2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185: 3190–3198. doi: 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mimura J., Ema M., Sogawa K., Fujii-Kuriyama Y.1999. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 13: 20–25. doi: 10.1101/gad.13.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T.N., Nakao K., Ema M., Sogawa K., Yasuda M., Katsuki M., Fujii-Kuriyama Y.1997. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2: 645–654. doi: 10.1046/j.1365-2443.1997.1490345.x [DOI] [PubMed] [Google Scholar]
- 60.Mitchel J. http://www.jonmitchellinjapan.com/agent-orange-on-okinawa.html.
- 61.Morales-Hernández A., González-Rico F.J., Román A.C., Rico-Leo E., Alvarez-Barrientos A., Sánchez L., Macia Á., Heras S.R., García-Pérez J.L., Merino J.M., Fernández-Salguero P.M.2016. Alu retrotransposons promote differentiation of human carcinoma cells through the aryl hydrocarbon receptor. Nucleic Acids Res. 44: 4665–4683. doi: 10.1093/nar/gkw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moura-Alves P., Faé K., Houthuys E., Dorhoi A., Kreuchwig A., Furkert J., Barison N., Diehl A., Munder A., Constant P., Skrahina T., Guhlich-Bornhof U., Klemm M., Koehler A.B., Bandermann S., Goosmann C., Mollenkopf H.J., Hurwitz R., Brinkmann V., Fillatreau S., Daffe M., Tümmler B., Kolbe M., Oschkinat H., Krause G., Kaufmann S.H.2014. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512: 387–392. doi: 10.1038/nature13684 [DOI] [PubMed] [Google Scholar]
- 63.Mulero-Navarro S., Fernandez-Salguero P.M.2016. New trends in aryl hydrocarbon receptor biology. Front. Cell Dev. Biol. 4: 45. doi: 10.3389/fcell.2016.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray I.A., Patterson A.D., Perdew G.H.2014. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer 14: 801–814. doi: 10.1038/nrc3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagayama J., Todaka T., Hirakawa H., Hori T., Kajiwara J., Yoshimura T., Furue M.2010. Polychlorinated dibenzofurans as a causal agent of fetal Yusho. Chemosphere 80: 513–518. doi: 10.1016/j.chemosphere.2010.04.062 [DOI] [PubMed] [Google Scholar]
- 66.Nakahama T., Kimura A., Nguyen N.T., Chinen I., Hanieh H., Nohara K., Fujii-Kuriyama Y., Kishimoto T.2011. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 108: 14222–14227. doi: 10.1073/pnas.1111786108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negishi T., Kato Y., Ooneda O., Mimura J., Takada T., Mochizuki H., Yamamoto M., Fujii-Kuriyama Y., Furusako S.2005. Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J. Immunol. 175: 7348–7356. doi: 10.4049/jimmunol.175.11.7348 [DOI] [PubMed] [Google Scholar]
- 68.Nguyen N.T., Kimura A., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T.2010. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 107: 19961–19966. doi: 10.1073/pnas.1014465107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pabst C., Krosl J., Fares I., Boucher G., Ruel R., Marinier A., Lemieux S., Hébert J., Sauvageau G.2014. Identification of small molecules that support human leukemia stem cell activity ex vivo. Nat. Methods 11: 436–442. doi: 10.1038/nmeth.2847 [DOI] [PubMed] [Google Scholar]
- 70.Panteleyev A.A., Bickers D.R.2006. Dioxin-induced chloracne—reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 15: 705–730. doi: 10.1111/j.1600-0625.2006.00476.x [DOI] [PubMed] [Google Scholar]
- 71.Park J.H., Choi A.J., Kim S.J., Cheong S.W., Jeong S.Y.2016. AhR activation by 6-formylindolo[3,2-b]carbazole and 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of mouse intestinal epithelial cells. Environ. Toxicol. Pharmacol. 43: 44–53. doi: 10.1016/j.etap.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 72.Poland A., Glover E., Kende A.S.1976. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 251: 4936–4946. [PubMed] [Google Scholar]
- 73.Powell-Coffman J.A., Bradfield C.A., Wood W.B.1998. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc. Natl. Acad. Sci. USA 95: 2844–2849. doi: 10.1073/pnas.95.6.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu J., Guo X., Chen Z.M., He L., Sonnenberg G.F., Artis D., Fu Y.X., Zhou L.2013. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 39: 386–399. doi: 10.1016/j.immuni.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., Zhou L.2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36: 92–104. doi: 10.1016/j.immuni.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L.2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71. doi: 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 77.Quintana F.J., Murugaiyan G., Farez M.F., Mitsdoerffer M., Tukpah A.M., Burns E.J., Weiner H.L.2010. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 107: 20768–20773. doi: 10.1073/pnas.1009201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rentas S., Holzapfel N.T., Belew M.S., Pratt G.A., Voisin V., Wilhelm B.T., Bader G.D., Yeo G.W., Hope K.J.2016. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature 532: 508–511. doi: 10.1038/nature17665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reyes H., Reisz-Porszasz S., Hankinson O.1992. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256: 1193–1195. doi: 10.1126/science.256.5060.1193 [DOI] [PubMed] [Google Scholar]
- 80.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H.2011. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418. doi: 10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saurat J.H., Kaya G., Saxer-Sekulic N., Pardo B., Becker M., Fontao L., Mottu F., Carraux P., Pham X.C., Barde C., Fontao F., Zennegg M., Schmid P., Schaad O., Descombes P., Sorg O.2012. The cutaneous lesions of dioxin exposure: lessons from the poisoning of Victor Yushchenko. Toxicol. Sci. 125: 310–317. doi: 10.1093/toxsci/kfr223 [DOI] [PubMed] [Google Scholar]
- 82.Schmidt J.V., Su G.H., Reddy J.K., Simon M.C., Bradfield C.A.1996. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 93: 6731–6736. doi: 10.1073/pnas.93.13.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sekine H., Mimura J., Oshima M., Okawa H., Kanno J., Igarashi K., Gonzalez F.J., Ikuta T., Kawajiri K., Fujii-Kuriyama Y.2009. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol. Cell. Biol. 29: 6391–6400. doi: 10.1128/MCB.00337-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh K.P., Casado F.L., Opanashuk L.A., Gasiewicz T.A.2009. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem. Pharmacol. 77: 577–587. doi: 10.1016/j.bcp.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh N.P., Singh U.P., Singh B., Price R.L., Nagarkatti M., Nagarkatti P.S.2011. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE 6: e23522. doi: 10.1371/journal.pone.0023522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smirnova A., Wincent E., Vikström Bergander L., Alsberg T., Bergman J., Rannug A., Rannug U.2016. Evidence for new light-independent pathways for generation of the endogenous Aryl hydrocarbon receptor agonist FICZ. Chem. Res. Toxicol. 29: 75–86. doi: 10.1021/acs.chemrestox.5b00416 [DOI] [PubMed] [Google Scholar]
- 87.Smith B.W., Rozelle S.S., Leung A., Ubellacker J., Parks A., Nah S.K., French D., Gadue P., Monti S., Chui D.H., Steinberg M.H., Frelinger A.L., Michelson A.D., Theberge R., McComb M.E., Costello C.E., Kotton D.N., Mostoslavsky G., Sherr D.H., Murphy G.J.2013. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood 122: 376–385. doi: 10.1182/blood-2012-11-466722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stellman J.M., Stellman S.D., Christian R., Weber T., Tomasallo C.2003. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 422: 681–687. doi: 10.1038/nature01537 [DOI] [PubMed] [Google Scholar]
- 89.Stevens E.A., Mezrich J.D., Bradfield C.A.2009. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127: 299–311. doi: 10.1111/j.1365-2567.2009.03054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strassel C., Brouard N., Mallo L., Receveur N., Mangin P., Eckly A., Bieche I., Tarte K., Gachet C., Lanza F.2016. Aryl hydrocarbon receptor-dependent enrichment of a megakaryocytic precursor with a high potential to produce proplatelets. Blood 127: 2231–2240. doi: 10.1182/blood-2015-09-670208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian J., Feng Y., Fu H., Xie H.Q., Jiang J.X., Zhao B.2015. The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals. Environ. Sci. Technol. 49: 9518–9531. doi: 10.1021/acs.est.5b00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B.2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453: 106–109. doi: 10.1038/nature06881 [DOI] [PubMed] [Google Scholar]
- 93.Wada T., Sunaga H., Ohkawara R., Shimba S.2013. Aryl hydrocarbon receptor modulates NADPH oxidase activity via direct transcriptional regulation of p40phox expression. Mol. Pharmacol. 83: 1133–1140. doi: 10.1124/mol.112.083303 [DOI] [PubMed] [Google Scholar]
- 94.Wagner J.E., Jr, Brunstein C.G., Boitano A.E., DeFor T.E., McKenna D., Sumstad D., Blazar B.R., Tolar J., Le C., Jones J., Cooke M.P., Bleul C.C.2016. Phase I/II trial of stemregenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 18: 144–155. doi: 10.1016/j.stem.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Q., Chen J., Ko C.I., Fan Y., Carreira V., Chen Y., Xia Y., Medvedovic M., Puga A.2013. Disruption of aryl hydrocarbon receptor homeostatic levels during embryonic stem cell differentiation alters expression of homeobox transcription factors that control cardiomyogenesis. Environ. Health Perspect. 121: 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Q., Kurita H., Carreira V., Ko C.I., Fan Y., Zhang X., Biesiada J., Medvedovic M., Puga A.2016. Ah receptor activation by dioxin disrupts activin, BMP, and WNT signals during the early differentiation of mouse embryonic stem cells and inhibits cardiomyocyte functions. Toxicol. Sci. 149: 346–357. doi: 10.1093/toxsci/kfv246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan K.S., Chia L.A., Li X., Ootani A., Su J., Lee J.Y., Su N., Luo Y., Heilshorn S.C., Amieva M.R., Sangiorgi E., Capecchi M.R., Kuo C.J.2012. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 109: 466–471. doi: 10.1073/pnas.1118857109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., Romani L.2013. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39: 372–385. doi: 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 99.Zhou L.2016. AHR function in lymphocytes: emerging concepts. Trends Immunol. 37: 17–31. doi: 10.1016/j.it.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]