Abstract
In order to examine their suitability for studies on coronary atherosclerosis, we evaluated the features of coronary atherosclerotic plaques in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits, a spontaneous animal model for coronary atherosclerosis and myocardial infarction. Coronary segments of the hearts of 187 WHHLMI rabbits (10–29 months old) were sectioned serially and stained histopathologically and immunohistologically. Progression of coronary lesions was prominent in rabbits that had died suddenly. The degree of coronary lesions of females was higher than that of males. Various types of atherosclerotic lesions were observed in the coronary arteries, such as plaques with a large lipid core covered by a thin fibrous cap, fatty streaks, early and advanced fibroatheromas, fibrous lesions, and advanced lesions with calcium accumulation and the vasa vasorum. In rabbits that had died suddenly, the frequencies of fibroatheromas or advanced lesions were higher than those of rabbits euthanized. Matrix metalloproteinase (MMP)-positive macrophages were detected in gaps among endothelial cells at the plaque surface, beneath the fibrous cap of thin-capped fibroatheromas, and at the bottom of the intimal plaques in which the tunica media was attenuated. Immunohistological results suggest that MMP-positive macrophages are involved in the initiation, progression, and destabilization of coronary plaques, in addition to vascular remodeling, even in WHHLMI rabbits. In conclusion, coronary lesions in WHHLMI rabbits resemble human atherosclerotic lesions, and thus, the WHHLMI rabbit is a suitable animal model for studies on human coronary plaques.
Keywords: animal model, coronary atherosclerosis, MMP-positive macrophages, WHHLMI rabbit
Introduction
Coronary atherosclerosis is an important risk factor for coronary heart disease. The rupture of coronary plaques and subsequent formation of thrombi induce sudden cardiac events [1, 9, 15]. These symptoms are called acute coronary syndromes. At many ruptured sites, the fibrous cap covering the large lipid core was found to be thin, and inflammatory cells was found to have infiltrated the thin fibrous cap [18, 32, 35]. At the thin fibrous cap, macrophages express matrix metalloproteinases (MMPs), which hydrolyze collagen fibers [3, 15, 17]. Therefore, MMP-positive coronary lesions are regarded as vulnerable plaques [7, 8, 15, 17]. Furthermore, high MMP levels in plasma have potential as a marker for acute coronary syndromes [7]. Consequently, MMPs are regarded as important factors for assessing the fragility of plaques. Although previous studies reported the expression of MMP in atherosclerotic lesions, few pertinent animal models currently exist for investigating the role of MMP-positive macrophages in coronary plaque growth and destabilization [17]. In order to promote studies on coronary plaque rupture and acute coronary syndromes, it is important to develop pertinent animal models with coronary lesions resembling human coronary plaques.
At Kobe University, we developed the Watanabe heritable hyperlipidemic (WHHL) rabbit [20, 37], which exhibits hypercholesterolemia due to a deficiency of low-density lipoprotein (LDL) receptors [20, 29, 39]. We subsequently developed an improved strain with severe coronary atherosclerosis and myocardial infarction (the WHHLMI rabbit) [25]. Since there are few animal models for coronary plaques, aortic lesions in mice or rabbits have been used in studies on atherosclerosis. We previously demonstrated that the composition of aortic lesions markedly differed from those of coronary lesions in WHHL rabbits [24]. However, no detailed studies about age-dependent changes in coronary lesions of WHHLMI rabbits have been reported until now. It is very important in animal models to clarify these properties.
In the present study, we examined coronary atherosclerotic lesions in WHHLMI rabbits histopathologically and immunohistologically in order to establish whether the WHHLMI rabbit is a suitable animal model for human coronary atherosclerosis.
Materials and Methods
Animals
We used 187 WHHLMI rabbits aged 10–29 months old to examine coronary lesions (Table 1 and Supplemental Table 1). Rabbits were bred at the Institute for Experimental Animals, Kobe University Graduate School of Medicine. Rabbits were housed individually in metal cages (550 mm × 600 mm × 450 mm; in width, depth, and height) with a flat metal floor and fed standard rabbit chow (LRC4, Oriental Yeast Co., Ltd., Tokyo, Japan) at 120 g/day. According to information from the manufacture, the chow contained 3.3% crude fat, 18% crude protein, 8% crude ash, 16% soluble nitrogen free extract, 14% crude fiber, and 0.002% cholesterol. The caloric content was 288 kcal/100 g. Animals were maintained under SPF conditions with a constant temperature (22 ± 2°C), relative humidity (50–60%), ventilation rate (15 cycles/hour), air supply (through a HEPA filter), and lighting cycle (12-h light/dark). This study was approved by the Kobe University Animal Care and Use Committee (approval numbers: P080110R, P100604, and P110512), and all animal experiments were conducted in accordance with the Regulations for Animal Experimentation of Kobe University, the Act on Welfare and Management of Animals (Law No. 105, 1973, revised in 2006), Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain (Notification No. 88, 2006), and Fundamental Guidelines for the Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the Jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology (Notice No. 71, 2006).
Table 1. Background data of WHHLMI rabbits.
| All rabbits examined | Rabbits that died suddenly | Rabbits euthanized | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Sacrificed | Sadden death | Female | Male | Female | Male | ||||||||
| Number of rabbits examined | 113 | 74 | 107 | 80 | 64 | 16 | 49 | 58 | |||||||
| Serum lipid levels (mmol/l) at 6 months old (187 rabbits) | |||||||||||||||
| Total cholesterol | 32.4 ± 0.4 | 31.8 ± 0.6 | P=0.414 | 31.4 ± 0.4 | 33.3 ± 0.5 | P=0.003 | 33.1 ± 0.5 | 34.2 ± 1.4 | P=0.340 | 31.6 ± 0.6 | 31.2 ± 0.6 | P=0.664 | |||
| Triglycerides | 3.2 ± 0.1 | 2.9 ± 0.1 | P=0.0918 | 3.1 ± 0.1 | 3.1 ± 0.1 | P=0.943 | 3.2 ± 0.1 | 2.8 ± 0.2 | P=0.131 | 3.3 ± 0.2 | 3.0 ± 0.2 | P=0.246 | |||
| Serum lipid levels (mmol/l) at 12 months old (156 rabbits) | |||||||||||||||
| Total cholesterol | 25.5 ± 0.5 | 21.2 ± 0.6 | P<0.001 | 23.1± 0.7 | 24.8 ± 0.5 | P=0.049 | 25.4 ± 0.5 | 22.2 ± 0.9 | P=0.006 | 25.5 ± 0.9 | 20.8 ± 0.8 | P<0.001 | |||
| Triglycerides | 3.2 ± 0.1 | 3.1 ± 0.2 | P=0.789 | 3.3 ± 0.2 | 3.1 ± 0.2 | P=0.597 | 3.2 ± 0.2 | 2.9 ± 0.3 | P=0.575 | 3.3 ± 0.2 | 3.2 ± 0.3 | P=0.853 | |||
| Serum lipid levels (mmol/l) at 24 months old (33 rabbits) | |||||||||||||||
| Total cholesterol | 19.2 ± 1.5 | 18.1 ± 1.5 | P=0.688 | 20.9 ± 1.8 | 18.1 ± 1.5 | P=0.246 | 17.7 ± 1.9 | 19.7 ± 2.0 | P=0.5977 | 23.2 ± 1.8 | 15.4 ± 1.9 | P=0.034 | |||
| Triglycerides | 2.9 ± 0.2 | 2.5 ± 0.3 | P=0.388 | 3.0 ± 0.5 | 2.7 ± 0.2 | P=0.437 | 3.1 ± 0.2 | 2.8 ± 0.2 | P=0.894 | 2.8 ± 0.5 | 2.0 ± 0.1 | P=0.257 | |||
| Aortic surface lesion area (%)* | |||||||||||||||
| 10–16 months old | 86 ± 1.4 | 84 ± 2.4 | P=0.579 | 83 ± 1.7 | 88 ± 1.6 | P=0.060 | 89 ± 1.7 | 85 | 83 ± 2.0 | 83 ± 2.5 | P=0.522 | ||||
| 17–22 months old | 88 ± 1.3 | 86 ± 1.4 | P=0.233 | 85 ± 1.4 | 89 ± 1.3 | P=0.025 | 90 ± 1.5 | 88 ± 2.5 | P=0.324 | 85 ± 2.1 | 85 ± 1.8 | P=0.900 | |||
| 23–29 months old | 93 ± 0.8 | 90 ± 2.1 | P=0.081 | 92 ± 2.0 | 93 ± 0.8 | P=0.956 | 93 ± 1.0 | 92 ± 1.0 | P=0.217 | 94 ± 0.6 | 88 ± 4.9 | P=0.242 | |||
Data are presented as the mean ± standard error of the mean. *The aortic surface lesion area (%) was calculated by dividing the lesion area by the area of the lumen surface of the entire aorta. Statistical analyses were performed with the Mann-Whitney U-test.
Preparation of coronary sections
Hearts were excised from rabbits euthanized with an intravenous injection of pentobarbital and from rabbits that had died suddenly (Supplemental Table 1). The hearts of euthanized rabbits were perfused with saline and then 10% buffered neutral formalin solution. The other hearts were immersion-fixed with 10% buffered neutral formalin solution without prior perfusion-fixation. Coronary sections were prepared as reported previously [23]. Fixed hearts were cut into 5 blocks and embedded in paraffin. Each block was sliced at 500-µm intervals, and 20 four-micrometer-thick serial sections were prepared. In the present study, we examined the atherosclerotic lesions of the left circumflex arteries (LCXs). The LCXs of rabbits have a greater diameter and length than those of the left anterior descending artery [4], and atherosclerotic lesions develop more frequently in the LCX than in the left anterior descending artery in WHHLMI rabbits [11, 22, 24].
Histopathological and immunohistological staining of coronary lesions
Sections were stained immunohistologically with monoclonal antibodies: RAM-11 (Dako A/S, Glostrup, Denmark), specific for rabbit macrophages/macrophage-derived foam cells [30]; 1A4 (Dako A/S), specific for the alpha actin of smooth muscle cells (SMCs); CD31 (Dako A/S), specific for arterial endothelial cells; DLH3, specific for oxidized phosphatidylcholine [10]; MMP-1 (Daiichi Fine Chemical Co., Ltd., Takaoka, Toyama, Japan); MMP-9 (Daiichi Fine Chemical Co., Ltd.), and MMP-12 (R&D Systems, Inc., Minneapolis, MN, USA), in addition to Elastic van Gieson and Azan staining. Immunohistological staining was performed using a DAKO EnVision+ kit according to the manufacturer’s instructions and accompanied by a hematoxylin counterstain. In addition, as negative control staining, we used mouse IgG2a, IgG1, IgG2b, and IgM instead of MMP-1, MMP-9, MMP-12, and DLH3, respectively, at a concentration equivalent to that of each monoclonal antibody.
Classification of plaque types
In the present study, we classified coronary lesions observed under light microscope as fatty streaks; fibroatheromas, including early, intermediate, and advanced stages; fibrous lesions; thin-capped fibroatheromas; and advanced lesions showing few cell components, extracellular lipid deposition, and calcium accumulation in reference to the American Heart Association classification [27, 28] and classification by Virmani and co-workers [35]. Many intermediate types of lesions were not classified.
Evaluation of the degree of atherosclerosis
The degree of coronary stenosis was evaluated microscopically as cross-sectional narrowing (%) by dividing the lesion area by the area surrounded by the internal elastic lamina. The extent of aortic atherosclerosis was evaluated macroscopically as a percentage of the surface lesion area by dividing the lesion area on the surface of the aortic lumen by the surface area of the aortic lumen. The evaluation of atherosclerotic lesions was performed someone other than the individual who analyzed coronary data.
Other assays
After more than 12 h of fasting, total cholesterol and triglyceride levels in serum were assayed with dry chemistry using a Fuji Dri-Chem 3500SV (Fuji Film Co., Ltd., Tokyo, Japan) [38].
Statistical analyses
Data are represented as the mean ± standard error of the mean (SEM). Statistical analyses were performed on mean values with Student’s t-test, Welch’s t-test, the Mann-Whitney U-test, or the Tukey-Kramer test and on frequencies with Fisher’s exact probability test or the Mantel-Haenstzel test. A value of P<0.05 was considered to be significant.
Results
Background data of WHHLMI rabbits
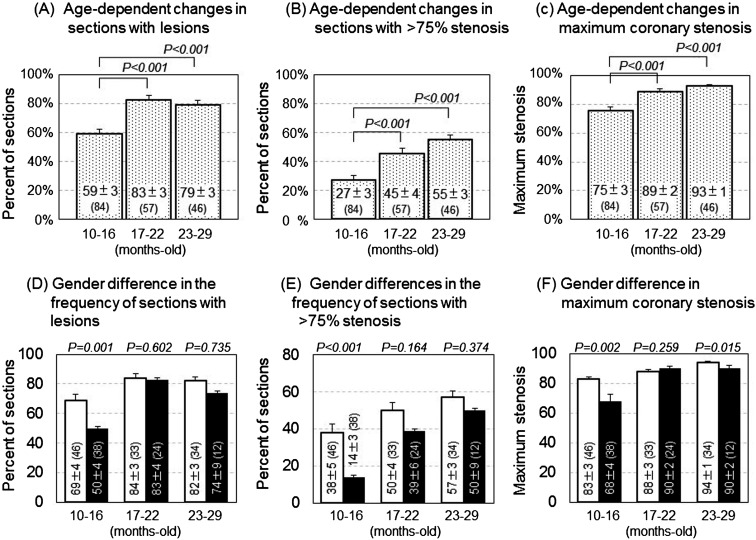
Table 1 shows the background data of the WHHLMI rabbits examined in the present study. Serum cholesterol levels decreased significantly with aging, although there were no significant changes in triglyceride levels. Age-dependent decrease of serum cholesterol levels was previously found to be due to decrease of secretion of very-low-density lipoprotein cholesterol from the liver [21]. Serum cholesterol levels of females were higher than those of males, and those of rabbits that had died suddenly were higher than those of euthanized rabbits. Figure 1 shows age-dependent progression of coronary atherosclerosis. The percentage of sections with lesions (panel A), the percentage of sections with more than 75% stenosis (panel B), and maximum stenosis (panel C) increased significantly with aging. The gender differences were small, except in rabbits aged 10–16 months (panels D–F). In rabbits aged 10–16 months, sudden death was observed in only one male, while 20 females died suddenly (Supplemental Table 1). Since coronary lesions progressed in the sudden death group compared with rabbits euthanized (Supplemental Fig. 1), large gender differences in terms of coronary lesions in rabbits aged 10–16 months were due to the low incidence of sudden death in this age group. There were almost no significant gender differences in the degree of coronary lesions in rabbits that had died suddenly or rabbits that had been sacrificed (Supplemental Table 2) when analyzed independently. The incidence of sudden death increased significantly with aging, rising from 25% (21/84) in rabbits aged 10–16 months to 71.7% (33/46) in rabbits aged 23–29 months (P=0.008), although the age-related increase in the frequency was not statistically significant in analysis using either only females or males when analyzed independently (Supplemental Table 1), and gender differences in the incidence of sudden death were observed in rabbits aged 10–16 months (Supplemental Table 1). In every rabbits that had died suddenly, severe coronary lesions were observed serially, and myocardial lesions were observed in several rabbits. No other findings related to death were observed. These results suggest that these rabbits died from myocardial infarction or acute coronary syndromes.
Fig. 1.
Development of coronary lesions in WHHLMI rabbits including both euthanized rabbits and rabbits that had died suddenly (panels A–C), and gender differences in coronary lesions (panels D–F). Open columns represent euthanized rabbits, and black columns represent rabbits that had died suddenly. A total of 8,092 sections in 5,200 segments from 187 rabbits were observed. Bars represent the standard error. Statistical analyses were performed with the Tukey-Kramer test in multiple comparison tests (panels A–C) and with the Mann-Whitney U-test in comparisons between rabbits euthanized and rabbits that had died suddenly (panels D–F). Values in parentheses represent number of rabbits examined. Bars indicate the standard error of the mean.
Types of coronary lesions observed in WHHLMI rabbits
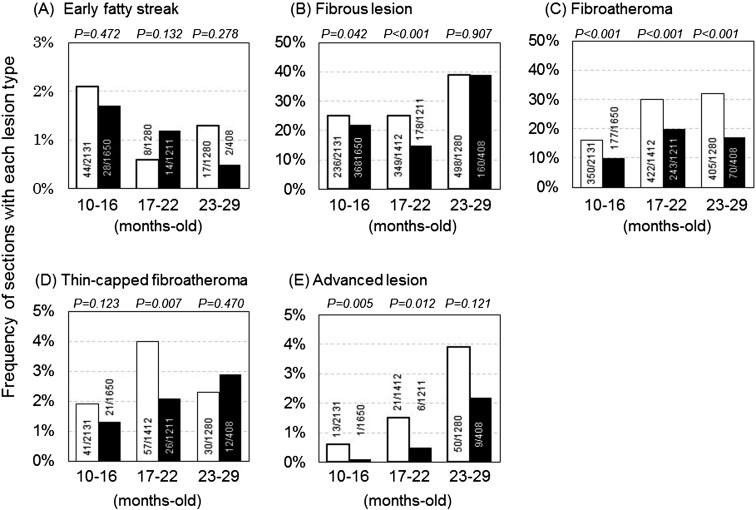
As shown in Fig. 2, various atherosclerotic lesions from early to advanced types were observed in the same coronary arteries of WHHLMI rabbits. Gender differences were observed in the frequencies of fibrous lesion, fibroatheroma, and advanced lesions. These types of lesions increased with aging (Supplemental Fig. 2). Compared with euthanized rabbits, the frequencies of each lesion type other than early fatty streak were high in the rabbits that had died suddenly (Supplemental Fig. 3).
Fig. 2.
Frequency of various types of atherosclerotic lesions in coronary arteries in WHHLMI rabbits. Open columns represent female rabbits, and black columns represent male rabbits. We analyzed 84 rabbits aged 10–16 months old, 57 aged 17–22 months old, and 46 aged 23–29 months old. Frequency was calculated by dividing the number of sections with lesions by the number of sections examined. Statistical analyses were performed with Fisher’s exact probability test.
Morphological features of fatty streaks
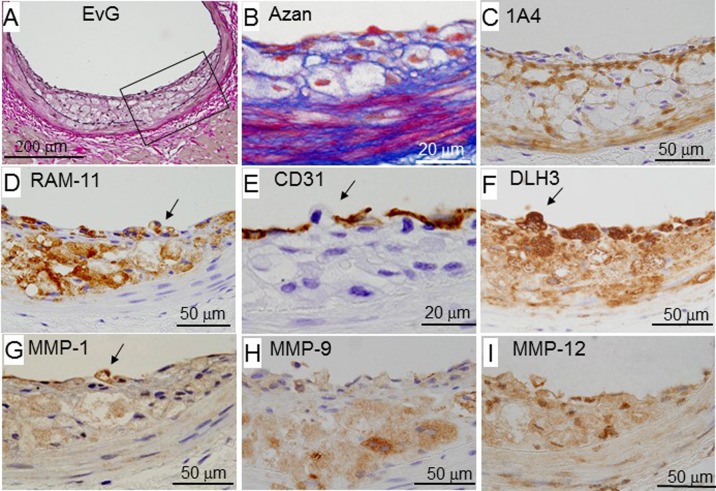
Figure 3 shows a representative fatty streak of WHHLMI rabbits. This lesion was observed at both ends of large plaques or a small focal lesion. Macrophages infiltrated the subendothelial region (panel D). SMCs and fibrous components were detected around macrophages (panels B and C). The internal elastic lamina disappeared in parts infiltrated by macrophages (panel A). A macrophage located in a gap between endothelial cells on the plaque surface (panels D and E) was also positive for MMPs and DLH3-positive oxidized LDL (panels F–H).
Fig. 3.
Representative photomicrographs showing a fatty streak of a male rabbit aged 21 months. Panels A–I show serial sections, and panels B–I are higher magnified views of the square area in panel A. Arrows indicate a macrophage attached to the plaque surface. 1A4 is a monoclonal antibody specific for smooth muscle cell actin. RAM-11 is a monoclonal antibody specific for rabbit monocytes and macrophages [30]. CD31 is a monoclonal antibody specific for arterial endothelial cells. DLH3 is a monoclonal antibody specific for oxidized phosphatidylcholine [10]. EvG, Elastic van Gieson staining.
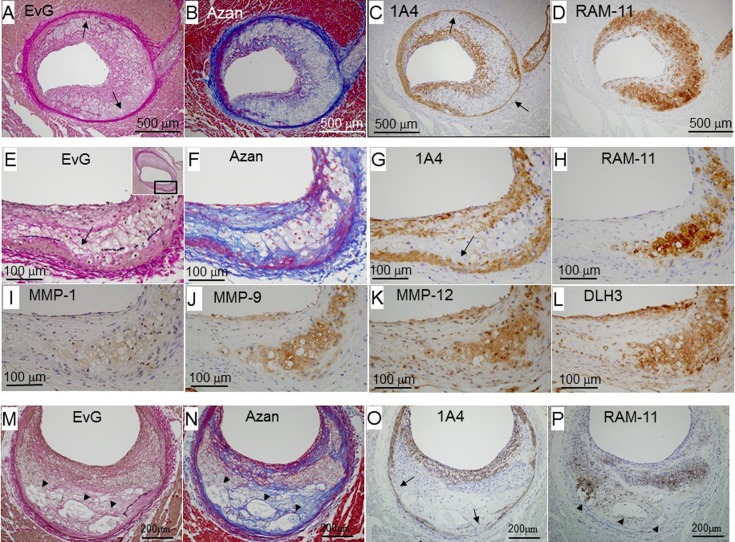
Morphological features of fibroatheromas
Figures 4A–4D show an early fibroatheroma. A large number of macrophages accumulated in the intima (panel D), and layers with SMCs were thicker at the lesion surface (panel C) despite few fibrous components (panel B). These macrophages co-localized with the positive area of MMPs and DLH3-positive oxidized LDL (data not shown). The SMC content of the tunica media partly decreased, and the tunica media was attenuated at the affected site (panel J).
Fig. 4.
Representative photomicrographs of fibroatheromas from early to advanced stages: an early fibroatheroma (female, 19 months old, panels A–D), intermediate lesion between early and advanced fibroatheromas (female, 16 months old, panels E–L), and advanced fibroatheroma (male, 10 months old, panels M–P). Panels A–D, E–L, and M–P are serial sections. Panels F–L are higher magnified views of the square area in panel E. Arrows indicate where the elastic lamina disappeared. Arrowheads indicate necrotic cores. EvG, Elastic van Gieson staining.
Figures 4E–4H show an intermediate lesion between early and advanced fibroatheromas. Foam cells derived from macrophages were detected under the thick fibrous cap, but there was no necrotic core in the plaque. Some macrophages infiltrated the tunica media (panel H), and the internal elastic lamina disappeared (panel E). The SMC content of the tunica media decreased (panel G), and the tunica media protruded outward in the area in which macrophages accumulated (panels E-G). Macrophages and macrophage-derived foam cells collocated with the positive area of MMPs and DLH3-positive oxidized LDL (panels I–L).
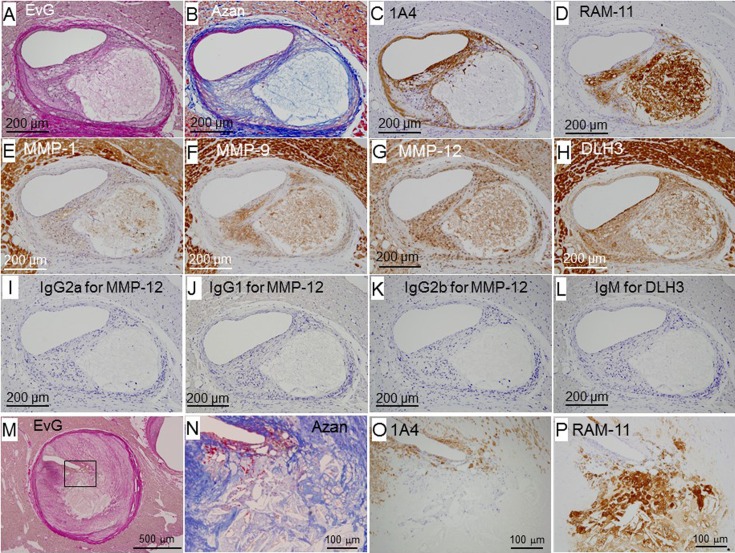
Figures 4M–4P show an advanced fibroatheroma. A fibrous cap was formed at the plaque surface (panels N and O). The fibrous cap covered a lipid core (arrowheads in panels M, N, and P) and clusters of macrophage-derived foam cells (panel P). One lipid core was also positive for RAM-11 (panel P), suggesting that it was derived from the debris of macrophage-derived foam cells. The tunica media was partly attenuated (arrow in panel O).
Morphological features of apparently vulnerable coronary lesions
Figure 5 shows plaques with a large necrotic core containing cholesterol clefts (panels N and P), macrophage-derived foam cells, and macrophage debris covered by a thin fibrous cap (panels A–L, and M–P). In the thin fibrous cap, the density of fibrous components and/or 1A4-positive SMCs was low (panels B, C, N, and O), and MMP-positive macrophages accumulated (panel D). In panel N, a large number of red blood cells were observed under the thin fibrous cap. However, the rupture site was not detected in serial sections.
Fig. 5.
Representative photomicrographs of thin-capped fibroatheromas. Panels A–L (female, 16 months old) and M–P (male, 25 months old) are serial sections. IgG2a (panel I), IgG1 (panel J), IgG2b (panel K), and IgM (panel L) were used instead of MMP-1, MMP-9, MMP-12, and DLH3, respectively, for negative control staining.
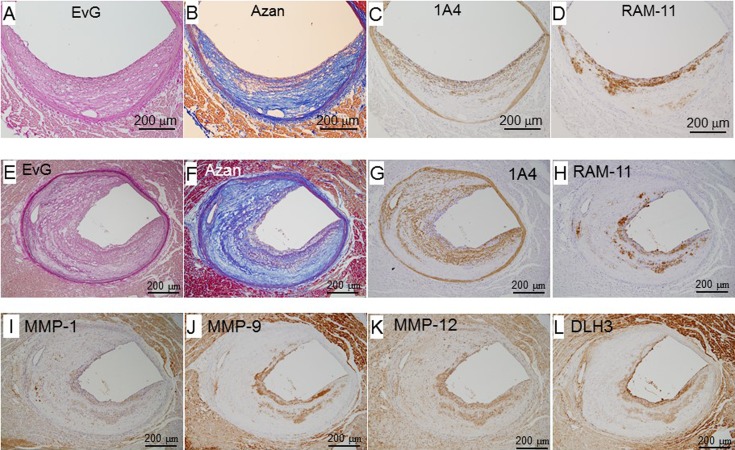
Morphological features of fibrous lesions
Figure 6 shows representative fibrous lesions. One is a mild lesion (panels A–D), and the other is a progressed lesion (panels E–L). These lesions were occupied by fibrous components and 1A4-positive SMCs (panels B, C, F, and G), and macrophages were observed on the plaque surface (panels D and H). These macrophages were positive for MMPs (panels I–K) and DLH3-positive oxidized LDL (panel L). Layers of macrophages (panel H) or small lipid deposits were also located between layers of fibrous components (panels E and F) and/or SMCs (panel G).
Fig. 6.
Representative photomicrographs of fibromuscular lesions. Panels A–D (female, 10 months old) and E–L (female, 16 months old) are serial sections.
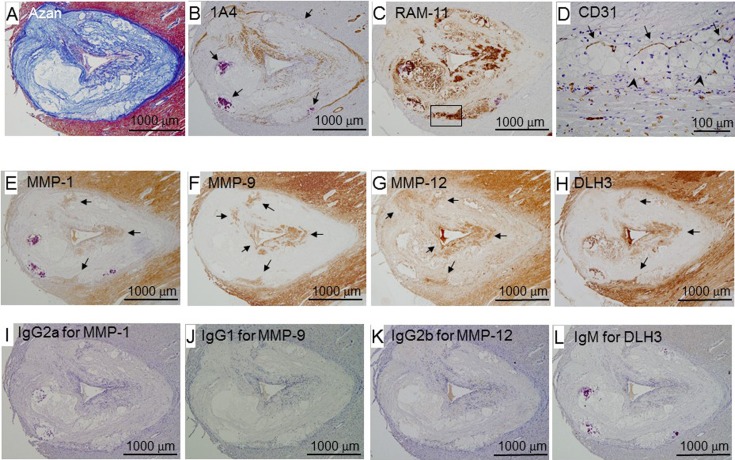
Morphological features of advanced coronary lesions
Figure 7 shows a representative advanced lesion with few cell components, calcium accumulation, extracellular lipid deposits with foam cell debris, cholesterol clefts, and the accumulation of fibrous components (panels A–C). The fibrous cap covered a large lipid core (panel A). Macrophages were detected at the surface and bottom of the intimal lesion (panel C) and co-localized with the positive area of MMPs (panels E–G) and DLH3-positive oxidized LDL (panel H). CD31-positive cells (endothelial cells) were detected (panel D) in the area in which macrophages were located at the bottom of the lesion.
Fig. 7.
Representative photomicrographs of an advanced coronary lesion (female, 19 months old). Panels A–L are serial sections. Panel D is a higher magnified view of the square area in panel C. Arrows in panel B indicate calcium accumulation. In panel D, arrows indicate the vasa vasorum, and arrowheads indicate the internal elastic lamina. In panels E–H, arrows indicate positive cells for MMPs and DLH3. IgG2a, IgG1, IgG2b, and IgM were used instead of MMP-1, MMP-9, MMP-12, and DLH3, respectively, for negative control staining.
Discussion
In WHHLMI rabbits, atherosclerotic lesions were observed in more than half of sections at 10–16 months old and expanded with aging. Progression of coronary lesions was prominent in rabbits that had died suddenly. In addition, the degree of coronary lesions of females was higher than that of males. Several types of lesions develop in the same coronary arteries. This feature of coronary plaques in WHHLMI rabbits resembles human coronary plaques, as reported previously [8, 27, 28, 32,33,34,35]. MMPs were always positive in macrophages in coronary lesions regardless of the lesion type. The localization of MMP-positive macrophages plays a role in the features and growth of coronary plaques in WHHLMI rabbits.
In females, coronary lesions progressed, and the frequencies of fibroatheroma and advanced lesions were higher than those of males. These results are contrary to those in humans. In humans, estrogen plays an important role in prevention of atherosclerosis and decreases serum cholesterol levels [2]. In female rabbits, serum cholesterol levels were higher than those of males (Table 1). These high serum cholesterol levels may influence the progression of coronary atherosclerosis and the high frequency of fibroatheroma and advanced lesions.
Fatty streaks (Fig. 3), early lesions observed in WHHLMI rabbits, may correspond to type II in the AHA classification [27, 28] and intimal xanthoma in Virmany’s classification [35]. In humans, the frequency of fatty streaks was previously reported to increase from 5% in adolescents (16–20 years old) to 17% in mature adults (41–45 years old) [33], suggesting the continuous occurrence of atherosclerotic lesions. Similar to in humans, fatty streaks were observed in all age groups of WHHLMI rabbits. Velican et al. [34] also reported that most coronary lesions in adult humans originated as a pre-existing intimal bed (physiological intimal thickening) due to the proliferation of SMCs. There is no physiological intimal thickening in the coronary arteries of mice and rabbits, which represents a prominent difference in coronary lesions between humans and other animals. Although Virmany et al. [35] speculated that xanthomatous lesions observed in childhood regressed, xanthomatous coronary lesions with severe stenosis were reported in a 4-year-old boy with homozygous familial hypercholesterolemia [36]. The xanthomatous coronary lesions observed in the boy with familial hypercholesterolemia resembled early fibroatheromas in WHHLMI rabbits, as shown in Figs. 4A–4D.
Fibroatheromas (Fig. 4) correspond to types IV and V in the AHA classification [27, 28] and fibrous cap atheromas in Virmany’s classification [35]. Early fibroatheromas (Figs. 4A–4D) were not classified in human coronary plaques. These lesions may be the early stage of fibroatheromas because of the presence of an SMC-rich fibrous cap. After collagen fibers had been synthesized by SMCs at the plaque surface, clusters of macrophage-derived foam cells were covered by a fibrous cap (Figs. 4E–4L); however, few lipid cores or small amount of foam cell debris was observed in the intima. These lesions may proceed to advanced fibroatheromas, which are characterized by the presence of a fibrous cap and necrotic core/foam cell debris surrounded by macrophage-derived foam cells (Figs. 4M–4P). These fibroatheromas were observed in coronary arteries in WHHLMI rabbits, and their frequency increased in an age-dependent manner (Fig. 2C).
Thin-capped fibroatheromas (Fig. 5) correspond to thin fibrous-cap atheromas in Virmany’s classification [35], but are not classified in the AHA classification [27, 28]. In humans, the rupture of coronary plaques was previously reported to frequently occur in thin-capped fibroatheromas [13, 18, 32, 35], and the thickness of the fibrous cap was less than 65 µm in 95% of the ruptured plaques [13]. Therefore, this lesion is considered to be a rupture-prone plaque in humans [3, 13, 15, 18, 32, 35]. MMPs hydrolyze collagen fibers and are involved in the destabilization of atherosclerotic plaques [3, 15]. Although many MMP-positive macrophages were observed in the thin fibrous cap (Figs. 5A–5H), the morphological changes associated with plaque rupture were not observed in WHHLMI rabbits. In addition, Newby [17] reported that plaque rupture reported in mice is not considered to reflect true rupture. In our previous studies, coronary plaques in WHHLMI rabbits were ruptured after provocation of coronary spasms [19] and after treatments with angiotensin II [14]. Therefore, additional factors other than thin-capped fibroatheromas may play important roles in rupture of coronary plaques.
Fibrous lesions (Fig. 6) were observed in most WHHLMI rabbits, and corresponded to type Vc in the AHA classification [27, 28] and pathological intimal thickening or intimal thickening in Virmany’s classification [35]. The accumulation of macrophages on the plaque surface and the lamellar structure, consisting of fibrous components and macrophage-lipid layers, suggest that the infiltration of macrophages is involved in the growth of lesions toward the lumen, in addition to SMC proliferation. Virmany et al. [35] described these lesions as a bed for the development of erosion. However, we did not detect erosion on the surface of fibrous lesions. Unknown additional factors may play important roles in the provocation of erosion.
Advanced lesions (Fig. 7) corresponded to type Vb in the AHA classification [27, 28] and fibrocalcific plaques in Virmany’s classification [35]. Macrophages accumulated at the bottom of plaques and lines of CD-31-positive cells (endothelial cells) were observed at an adjacent area. These results suggest that macrophages at the bottom of plaques infiltrate through the vasa vasorum, participate in attenuating the coronary arterial wall, and are involved in the formation of a lipid core.
MMP-positive macrophages or macrophage-derived foam cells were observed in early to advanced coronary lesions. On the surface of coronary fatty streaks, MMP-positive macrophages were detected in gaps among endothelial cells (Fig. 3). These macrophages may infiltrate the subendothelial region, and MMPs may play a role in passing through the endothelial line, as reported by Duran-Vilaregut et al. [5]. MMP-positive macrophages/macrophage-derived foam cells were observed among fibromuscular layers in plaques (Fig. 6) and plaque surfaces (Figs. 4 and 6). These lesions suggest that the repetition of MMP-positive macrophage infiltration and the covering of macrophage/macrophage-derived foam cell layers by fibromuscular layers is involved in plaque growth. MMP-positive macrophages were also detected at the bottom of intimal lesions, even in fibrous lesions (Fig. 6), in addition to several other types of lesions (Figs. 3, 4, and 7). In areas in which MMP-positive macrophages had infiltrated the tunica media, the internal elastic lamina disappeared, and the tunica media protruded outward. The diameter of coronary segments exhibiting these features was larger than that of proximal segments lacking lesions (data not shown). The present results suggest outward remodeling [26]. In the present study, every macrophage was positive for MMP-1, 9, and 12.
Early fibroatheromas, which contain a large number of macrophages in their plaques, also developed in WHHLMI rabbits (Fig. 4). However, a thick layer of SMCs covered the intimal macrophage pool. Therefore, these plaques appear to be difficult to disrupt. The present results suggest that not every macrophage-rich lesion is vulnerable.
Limitations of the present study
In immunohistological staining, the myocardium was also positive for MMPs and DLH3 (Figs. 5E–5H, Figs. 6I–6L, and Figs. 7E–7H). In negative control staining using corresponding immunoglobulins, there was no positive area (Figs. 5I–5L, and Figs. 7I–7L). Furthermore, the mRNAs and/or proteins of MMPs have been detected in the myocardial cells of rabbits [12] and humans [31]. In addition, Ekmekcioglu et al. [6] reported that oxidized LDL has been shown to be present in human ventricles. Therefore, the specificity of the antibodies for MMPs and DLH3 used in the present study was adequate.
In the aortic lesions of WHHL rabbits, the expression of mRNAs for MMP-9 and the MMP-9 protein was not increased compared with the expression of those in the normal aorta [40]. However, in the present study, the intensity of staining for MMP-9 was very strong in coronary lesions but was negative in coronary arterial walls without lesions (Figs. 6I–6L). The reason for this difference currently remains unknown. The expression of MMPs in coronary lesions may differ from that in aortic lesions.
In conclusion, the morphological features of coronary plaques in WHHLMI rabbits resemble human coronary lesions. Immunohistological results on coronary plaques suggest that MMP-positive macrophages are involved in the initiation, progression, and destabilization of coronary plaques as well as in coronary outward remodeling, even in WHHLMI rabbits. Therefore, the WHHLMI rabbit is a suitable animal model to study coronary atherosclerosis.
Conflict of Interests
The authors declare that no competing interests exist.
Sources of Funding
This study was partly supported by a grant for Research on Biological Resources and Animal Models for Drug Development and Research on Advanced Medical Technology from the Ministry of Health, Labour and Welfare of Japan, a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23300157), and a research grant from Daiichi-Sankyo Pharmaceutical Co., Ltd., Tokyo, Japan.
Supplementary Material
Acknowledgments
We appreciate Miss Haruka Adachi and Miss Miki Oya for their care of WHHLMI rabbits.
References
- 1.Ahmadi A., Leipsic J., Blankstein R., Taylor C., Hecht H., Stone G.W., Narula J.2015. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circ. Res. 117: 99–104. doi: 10.1161/CIRCRESAHA.117.305637 [DOI] [PubMed] [Google Scholar]
- 2.Barton M.2013. Mechanisms and therapy of atherosclerosis and its clinical complications. Curr. Opin. Pharmacol. 13: 149–153. doi: 10.1016/j.coph.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Butoi E., Gan A.M., Tucureanu M.M., Stan D., Macarie R.D., Constantinescu C., Calin M., Simionescu M., Manduteanu I.2016. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys. Acta 1863:(7 Pt A): 1568–1578. doi: 10.1016/j.bbamcr.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Clozel J.P., Lengsfeld H., Kuhn H., Baumgartner H.R.1988. Decreased coronary vascular reserve in Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis 8: 310–320. doi: 10.1161/01.ATV.8.3.310 [DOI] [PubMed] [Google Scholar]
- 5.Duran-Vilaregut J., del Valle J., Manich G., Camins A., Pallàs M., Vilaplana J., Pelegrí C.2011. Role of matrix metalloproteinase-9 (MMP-9) in striatal blood-brain barrier disruption in a 3-nitropropionic acid model of Huntington’s disease. Neuropathol. Appl. Neurobiol. 37: 525–537. doi: 10.1111/j.1365-2990.2010.01157.x [DOI] [PubMed] [Google Scholar]
- 6.Ekmekcioglu C., Mehrabi M.R., Glogar H.D., Jucewicz M., Volf I., Spieckermann P.G.2000. Oxidized low-density lipoprotein is localized in the ventricles of hearts from patients with coronary heart disease. Int. J. Clin. Lab. Res. 30: 133–140. doi: 10.1007/s005990070012 [DOI] [PubMed] [Google Scholar]
- 7.Fiotti N., Altamura N., Orlando C., Simi L., Reimers B., Pascotto P., Zingone B., Pascotto A., Serio M., Guarnieri G., Giansante C.2008. Metalloproteinases-2, -9 and TIMP-1 expression in stable and unstable coronary plaques undergoing PCI. Int. J. Cardiol. 127: 350–357. doi: 10.1016/j.ijcard.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Fuster V., Moreno P.R., Fayad Z.A., Corti R., Badimon J.J.2005. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 46: 937–954. doi: 10.1016/j.jacc.2005.03.074 [DOI] [PubMed] [Google Scholar]
- 9.Fuster V., Stein B., Ambrose J.A., Badimon L., Badimon J.J., Chesebro J.H.1990. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 82:(Suppl): II47–II59. [PubMed] [Google Scholar]
- 10.Itabe H., Takeshima E., Iwasaki H., Kimura J., Yoshida Y., Imanaka T., Takano T.1994. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J. Biol. Chem. 269: 15274–15279. [PubMed] [Google Scholar]
- 11.Ito T., Yamada S., Shiomi M.2004. Progression of coronary atherosclerosis relates to the onset of myocardial infarction in an animal model of spontaneous myocardial infarction (WHHLMI rabbits). Exp. Anim. 53: 339–346. doi: 10.1538/expanim.53.339 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H., Minatoguchi S., Yasuda S., Bao N., Kawamura I., Iwasa M., Yamaki T., Sumi S., Misao Y., Ushikoshi H., Nishigaki K., Takemura G., Fujiwara T., Tabata Y., Fujiwara H.2008. Post-infarct treatment with an erythropoietin-gelatin hydrogel drug delivery system for cardiac repair. Cardiovasc. Res. 79: 611–620. doi: 10.1093/cvr/cvn154 [DOI] [PubMed] [Google Scholar]
- 13.Kojima C., Ino J., Ishii H., Nitta K., Yoshida M.2010. MMP-9 inhibition by ACE inhibitor reduces oxidized LDL-mediated foam-cell formation. J. Atheroscler. Thromb. 17: 97–105. doi: 10.5551/jat.1685 [DOI] [PubMed] [Google Scholar]
- 14.Li S., Wang Y.N., Niimi M., Ning B., Chen Y., Kang D., Waqar A.B., Wang Z., Yu Q., Liu E., Zhang J., Shiomi M., Chen Y.E., Fan J.2016. Angiotensin II Destabilizes Coronary Plaques in Watanabe Heritable Hyperlipidemic Rabbits. Arterioscler. Thromb. Vasc. Biol. 36: 810–816. doi: 10.1161/ATVBAHA.115.306871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P.2008. The molecular mechanisms of the thrombotic complications of atherosclerosis. J. Intern. Med. 263: 517–527. doi: 10.1111/j.1365-2796.2008.01965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matoba T., Sato K., Egashira K.2013. Mouse models of plaque rupture. Curr. Opin. Lipidol. 24: 419–425. doi: 10.1097/MOL.0b013e3283646e4d [DOI] [PubMed] [Google Scholar]
- 17.Newby A.C.2015. Metalloproteinases promote plaque rupture and myocardial infarction: A persuasive concept waiting for clinical translation. Matrix Biol. 44–46: 157–166. doi: 10.1016/j.matbio.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 18.Reith S., Battermann S., Hoffmann R., Marx N., Burgmaier M.2014. Optical coherence tomography derived differences of plaque characteristics in coronary culprit lesions between type 2 diabetic patients with and without acute coronary syndrome. Catheter. Cardiovasc. Interv. 84: 700–707. doi: 10.1002/ccd.25267 [DOI] [PubMed] [Google Scholar]
- 19.Shiomi M., Ishida T., Kobayashi T., Nitta N., Sonoda A., Yamada S., Koike T., Kuniyoshi N., Murata K., Hirata K., Ito T., Libby P.2013. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction-prone strain of the Watanabe heritable hyperlipidemic rabbits. Arterioscler. Thromb. Vasc. Biol. 33: 2518–2523. doi: 10.1161/ATVBAHA.113.301303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiomi M., Ito T.2009. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis 207: 1–7. doi: 10.1016/j.atherosclerosis.2009.03.024 [DOI] [PubMed] [Google Scholar]
- 21.Shiomi M., Ito T., Fujioka T., Tsujita Y.2000. Age-associated decrease in plasma cholesterol and changes in cholesterol metabolism in homozygous Watanabe heritable hyperlipidemic rabbits. Metabolism 49: 552–556. doi: 10.1016/S0026-0495(00)80025-6 [DOI] [PubMed] [Google Scholar]
- 22.Shiomi M., Ito T., Hasegawa M., Yoshida K., Gould K.L.2004. Novel insights into coronary lumen preservation during progression of coronary atherosclerosis in coronary atherosclerosis-prone rabbits. Coron. Artery Dis. 15: 419–426. doi: 10.1097/00019501-200411000-00009 [DOI] [PubMed] [Google Scholar]
- 23.Shiomi M., Ito T., Shiraishi M., Watanabe Y.1992. Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis 96: 43–52. doi: 10.1016/0021-9150(92)90036-G [DOI] [PubMed] [Google Scholar]
- 24.Shiomi M., Ito T., Tsukada T., Yata T., Ueda M.1994. Cell compositions of coronary and aortic atherosclerotic lesions in WHHL rabbits differ. An immunohistochemical study. Arterioscler. Thromb. 14: 931–937. doi: 10.1161/01.ATV.14.6.931 [DOI] [PubMed] [Google Scholar]
- 25.Shiomi M., Ito T., Yamada S., Kawashima S., Fan J.2003. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler. Thromb. Vasc. Biol. 23: 1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50 [DOI] [PubMed] [Google Scholar]
- 26.Shiomi M., Yamada S., Matsukawa A., Itabe H., Ito T.2008. Invasion of atheromatous plaques into tunica media causes coronary outward remodeling in WHHLMI rabbits. Atherosclerosis 198: 287–293. doi: 10.1016/j.atherosclerosis.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 27.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Jr, Rosenfeld M.E., Schwartz C.J., Wagner W.D., Wissler R.W.1995. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374. doi: 10.1161/01.CIR.92.5.1355 [DOI] [PubMed] [Google Scholar]
- 28.Stary H.C., Chandler A.B., Glagov S., Guyton J.R., Insull W., Jr, Rosenfeld M.E., Schaffer S.A., Schwartz C.J., Wagner W.D., Wissler R.W.1994. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 89: 2462–2478. doi: 10.1161/01.CIR.89.5.2462 [DOI] [PubMed] [Google Scholar]
- 29.Tanzawa K., Shimada Y., Kuroda M., Tsujita Y., Arai M., Watanabe H.1980. WHHL-rabbit: a low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Lett. 118: 81–84. doi: 10.1016/0014-5793(80)81223-3 [DOI] [PubMed] [Google Scholar]
- 30.Tsukada T., Rosenfeld M., Ross R., Gown A.M.1986. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis 6: 601–613. doi: 10.1161/01.ATV.6.6.601 [DOI] [PubMed] [Google Scholar]
- 31.Turner N.A., Warburton P., O’Regan D.J., Ball S.G., Porter K.E.2010. Modulatory effect of interleukin-1α on expression of structural matrix proteins, MMPs and TIMPs in human cardiac myofibroblasts: role of p38 MAP kinase. Matrix Biol. 29: 613–620. doi: 10.1016/j.matbio.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Wal A.C., Becker A.E., van der Loos C.M., Tigges A.J., Das P.K.1994. Fibrous and lipid-rich atherosclerotic plaques are part of interchangeable morphologies related to inflammation: a concept. Coron. Artery Dis. 5: 463–469. [PubMed] [Google Scholar]
- 33.Velican C., Velican D.1983. Progression of coronary atherosclerosis from adolescents to mature adults. Atherosclerosis 47: 131–144. doi: 10.1016/0021-9150(83)90150-8 [DOI] [PubMed] [Google Scholar]
- 34.Velican D., Velican C.1980. Atherosclerotic involvement of the coronary arteries of adolescents and young adults. Atherosclerosis 36: 449–460. doi: 10.1016/0021-9150(80)90238-5 [DOI] [PubMed] [Google Scholar]
- 35.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M.2000. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20: 1262–1275. doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T., Tanaka K., Yanai N.1968. Essential familial hypercholesteremic xanthomatosis--an autopsy case with special reference to the pathogensis of cardiovascular lipidosis. Acta Pathol. Jpn. 18: 319–331. [PubMed] [Google Scholar]
- 37.Watanabe Y.1980. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis 36: 261–268. doi: 10.1016/0021-9150(80)90234-8 [DOI] [PubMed] [Google Scholar]
- 38.Yamada S., Ito T., Tamura T., Shiomi M.2004. Age-related changes in serum/plasma biochemical parameters of WHHLMI rabbits. Exp. Anim. 53: 159–163. doi: 10.1538/expanim.53.159 [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T., Bishop R.W., Brown M.S., Goldstein J.L., Russell D.W.1986. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science 232: 1230–1237. doi: 10.1126/science.3010466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Koike T., Kitajima S., Liu E., Morimoto M., Shiomi M., Hatakeyama K., Asada Y., Wang K.Y., Sasaguri Y., Watanabe T., Fan J.2008. Temporal and quantitative analysis of expression of metalloproteinases (MMPs) and their endogenous inhibitors in atherosclerotic lesions. Histol. Histopathol. 23: 1503–1516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.