Abstract
A high-fat, low–carbohydrate diet (KD) or calorie restriction in the form of every-other-day fasting (EODF) results in ketone body metabolism with an increasing β-hydroxybutyrate (βOHB) level. Previous studies have supported that a KD and EODF have a neuroprotective effect. However, the βOHB levels in the cerebrospinal fluid (CSF) resulting from a KD and EODF remain unknown. The aim of this study was to detect βOHB levels in rats fed a KD, EODF diet, and every-other-day ketogenic diet (EODKD) and to compare the serum βOHB level with the CSF βOHB level. Twenty-four male Sprague-Dawley rats were randomly divided into KD, EODF, EODKD, and standard diet (SD) groups. A customized food with a ratio of carbohydrates to fats of 1:4 was used in the KD and EODKD groups. The βOHB level was measured using ELISA kits in 200 µl serum and 100 µl CSF samples for each rat after feeding for 2 weeks. The KD, EODF, and EODKD resulted in a significant increase in βOHB levels in both the serum and CSF. The βOHB levels in the EODKD group were the highest. The CSF βOHB level was, on average, 69% of the serum βOHB level. There was a positive correlation between the overall βOHB levels in serum and that in cerebrospinal fluid. This study demonstrated that the KD, EODF, and EODKD resulted in ketone body metabolism, as the βOHB levels increased significantly compared with those resulting from the standard diet. Our results suggested that the serum βOHB level was an indicator of the CSF βOHB level, and that the EODKD was an effective diet to enhance ketogenic metabolism.
Keywords: βOHB concentration, CSF, ketone dody metabolism, serum
Introduction
A high-fat, low-carbohydrate diet or a form of calorie restriction, every-other-day-fasting (EODF), resulted in ketone body metabolism and an increase in β-hydroxybutyrate (βOHB) level in blood. Calorie restriction has been long recognized to extend the lifespan and resilience to diseases of aging [13]. Many animal studies have confirmed the effectiveness of calorie restriction treatment on many major diseases, such as cardiovascular diseases, diabetes, cancers, stroke, and a variety of nervous system degeneration diseases [1, 6, 14]. A ketogenic diet (KD) ameliorated neurological disorders, such as Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, traumatic brain injury [8, 16, 17, 20]; sleep disorders, brain tumors, autism, multiple sclerosis [15, 18]; and spinal cord injury [9], and has been successfully used for treatment drug-resistant epilepsy in children. Some studies showed that better control of seizures with a higher βOHB level was achieved with an every-other-day ketogenic diet (EDOKD) [4].
βOHB, a major component of ketone bodies, is a by-products of fatty acid oxidation in the liver during fasting or consumption of a KD and is carried by several monocarboxylic acid transporters (MCTs) across the blood-brain barrier. MCT transfer molecules are upregulated in the brain when the plasma levels of ketones are elevated [11]. However, the relationship between serum and CSF βOHB levels remains largely unknown under various diets affecting ketone body metabolism.
The objective of the present study was to measure βOHB levels under a standard diet, KD, EODF diet, and EODKD and compare βOHB levels among diets and between the serum and CSF.
Experimental Section
Experimental animals
Twenty-four male 8-week-old Sprague-Dawley rats (290–310 g) were used in this study. After 3 days adaptive feeding, rats were randomly divided into four groups fed the KD, EODF diet, EODKD, and standard diet (SD), respectively. All rats were housed in standard plastic cages (20 × 10 × 10 inches) in a conventional environment with control of the temperature (22 ± 2°C), relative humidity (55% ± 5%), and light/dark cycle (12/12-h light/dark cycle). Body weight was monitored daily. The rats were provided by the Experimental Animal Center of Southern Medical University, and all procedures in this study were conducted in accordance with a protocol approved by the Ethics Committee for Animal Experiments of Southern Medical University.
Diets
Standard diet food was provided by the Experimental Animal Center of Southern Medical University. The KD food was a solid ketogenic sesame cookie with a 1:4 ratio of carbohydrates to fats (Shenzhen Zeneca Inc., Shenzhen, China). The cookie is one of the ketogenic foods used in clinics to treat children with drug-resistant epilepsy. Essential nutrients of the standard and ketogenic diet foods are listed in Table 1.
Table 1. Basic nutrient content.
| Project (per 100 g) | Basic feed | Ketogenic feed |
|---|---|---|
| Energy | 1,254 kJ | 2,263 kJ |
| Protein | 19 g | 5.5 g |
| Fat | 3.3 g | 50.5 g |
| Carbohydrates | 41.2 g | 23.6 g |
| Dietary fiber | 4.9 g | 16.1 g |
| Natrium | 290 mg | 135 mg |
Feeding regimen
All animals were fed with ad libitum supply of water. The animals in the SD group had ad libitum access to standard food, while rats in the KD group had ad libitum access to ketogenic food. In the EODF group, there was no access to food (fasting) during the first 24 h, but the animals had ad libitum access to standard food on the 2nd day, 4th day, 6th day, and so on. This schedule of alternate fasting and feeding days was carried out for two weeks. In the EODKD group, rats were fed with the same schedule as the EODF group but were given the same food as the KD group.
Collection of cerebrospinal fluid and serum
Cerebrospinal fluid was collected according to the method described by Yang et al. [22]. Each rat was anesthetized using a small animal anesthesia machine (Matrix VIP 3000, Midmark Animal Health, Versailles, OH, USA) with 4% isoflurane for anesthesia induction and 2% to 3% isoflurane for anesthesia maintenance. Each rat’s head was fixed onto a stereotaxic frame and kept straight down using an adjustable nose clip. A transverse incision (2 cm) was cut at the midpoint between the ears. Muscles close to the base of the skull were separated bluntly using forceps and a hemostat. No muscle tissue was cut in order to reduce intraoperative bleeding. Muscle layers attached to the neck and the skull base were scratched bluntly until exposure of the atlanto-occipital fascia. A 30 G needle was inserted into the atlanto-occipital fascia under the foramen magnum with an angle of 20° to 30° and depth about 2 to 3 mm (not exceeding the length of the needle bevel). A 1 ml syringe was then used to slowly draw 100 µl of clear cerebrospinal fluid.
Then, an incision was made in the middle abdomen to expose the abdominal cavity. The internal organs were separated with two pieces of gauzes. The abdominal veins were identified and dissociated. A 5 ml syringe was then used to slowly draw 2 ml of blood from the vein. The blood sample was left at room temperature for 2 h and then centrifuged at 1,000 g at 4°C for 20 min to obtain a 200 µl serum sample.
βOHB concentration
In this study, the βOHB level was tested using an ELISA (Rat β-OHB ELISA Kit, Cusabio, Wuhan, China). The βOHB concentration was calculated by regression analysis of a standard curve according to the instructions of the manufacturer.
Data analysis
Statistical analyses were carried out using Statistica 7.1 (Statsoft, Inc., Tulsa, OK, USA). Nonparametric analysis was used, as the data exhibited a non-normal distribution. Serum βOHB levels were compared with CSF βOHB levels using the Wilcoxon Matched Pairs test. Body weight and βOHB levels in serum and CSF were tested among groups using Kruskal-Wallis ANOVA and between groups using the Mann-Whitney U test. Correlation between the serum and cerebrospinal fluid β-hydroxybutyrate levels was tested using Spearman rank-order correlation. The significance level was set at P<0.05.
Results
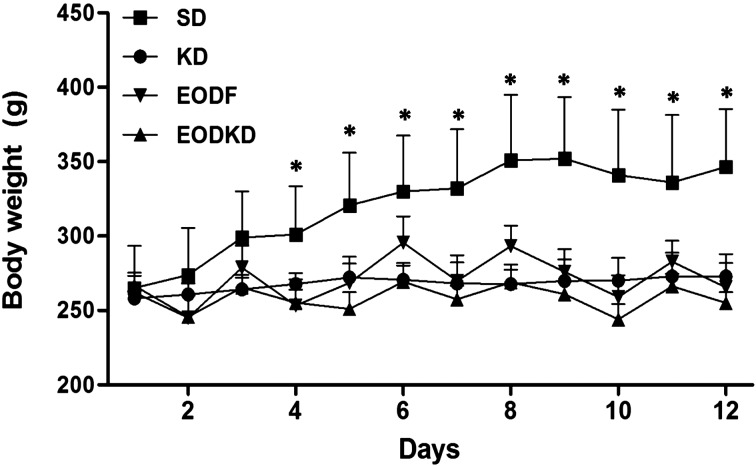
Overall, the body weights in the experimental groups were almost unchanged during the 2-week of feeding except for fluctuation with fasting in the EODF and EODKD groups. In contrast, body weight increased steadily in the SD group and was significantly higher than those of the three experimental groups beginning on the 4th day of the study (Fig. 1).
Fig. 1.
The body weight of rats fed the SD, KD, EODF diet, and EODKD. The body weight in the SD group increased steadily and was significantly higher than those of the three experimental groups beginning on the 4th day. Means marked with an asterisk are significantly different (P<0.05) those of the KD, EODF, and EODKD groups. There were six rats in each group. Differences were tested using Kruskal-Wallis ANOVA and the Mann-Whitney U test.
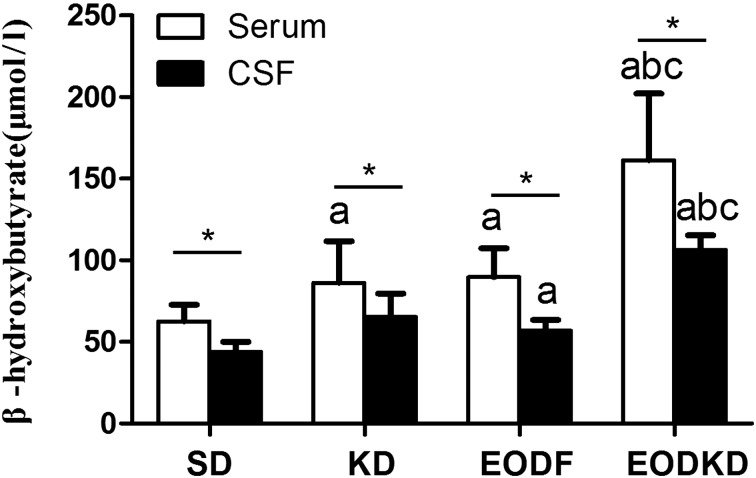
The serum βOHB levels were 86 ± 25 µmol/l, 90 ± 18 µmol/l, 161 ± 41 µmol/l and 62 ± 10 µmol/l in the KD, EODF, EOFKD, and SD groups, respectively. The KD and EODF diet both resulted in obviously increased of βOHB levels. The βOHB level in the EODKD group was significantly higher than those of the other groups, while the SD group had a significantly lower βOHB level compared with any of three diet interventions. There was no significant difference in the βOHB level between the KD and EODF groups (Fig. 2).
The CSF βOHB levels were 65 ± 14 µmol/l, 56.8 ± 6.7 µmol/l, 106 ± 9 µmol/l, and 44 ± 6 µmol/l in the KD, EODF, EOFKD, and SD groups, respectively. Similar to the serum βOHB level, the CSF βOHB level was highest in the EODKD group, while it was lowest in the SD group. There was no difference in CSF βOHB level between the SD and KD groups or between the KD and EODF groups, but the CSF βOHB level was significantly higher in the EODF group than in the SD group (Fig. 2).
Fig. 2.
Concentrations of β-hydroxybutyrate in serum and cerebrospinal fluid of rats fed the SD, KD, EODF diet and EODKD. The letters a, b, and c indicate the SD, KD, and EODF groups, respectively. Means marked with letters (a, b, c) are significantly different (P<0.05). An asterisk indicates a significant difference between the serum and CSF (P<0.05). There were six rats in each group. Differences among groups were tested using Kruskal-Wallis ANOVA and the Mann-Whitney U test, while differences within groups were tested using the Wilcoxon Matched Pairs test.
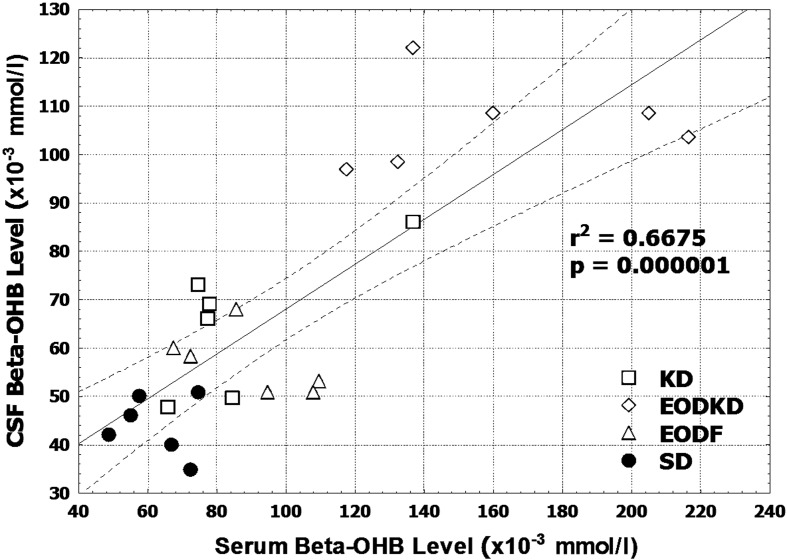
The CSF βOHB level was consistently lower than the serum βOHB level regardless of the diet intervention. The differences in βOHB level between the serum and CSF were 21 µmol/l (24%), 34 µmol/l (38%), 55 µmol/l (34%), and 18 µmol/l (29%) in the KD, EODF, EOFKD, and SD groups, respectively (Fig. 2). The CSF βOHB level was, on average, 69% of the serum βOHB level, with the correlation with the serum βOHB level being positive in the total data (n=24, r=0.817, P<0.01) (Fig. 3). But no significant correlation was observed in the KD, EODF, EOFKD, or SD groups, respectively (P=0.117, 0.207, 0.843, and 0.788).
Fig. 3.
Correlation between the serum and cerebrospinal fluid β-hydroxybutyrate levels in rats fed the SD, KD, EODF diet, and EODKD. There were six rats in each group. Correlation was tested using Spearman rank-order correlation.
Discussion
This study is unique in that it is, to our knowledge, the first time that βOHB levels have been compared in vivo under ketone body metabolism with different dietary intervention. Three different dietary interventions (KD, EODF, and EODKD) were included to map βOHB levels in both blood and cerebrospinal fluid and to pave a foundation for examination of ketone body metabolism.
In order to identify βOHB accurately, we adopted an ELISA to measure the βOHB concentration with a resolution of 7.8 mmol/ml, which enabled more sensitive differentiation of the change in βOHB. The levels of βOHB in serum were 62 µmol/l and 86 µmol/l in the SD and KD groups, respectively, while the measured values were lower compared with those in a previous report [19]. Thus, in order to reconfirm the level of βOHB in serum, we measured the level of blood ketones in the same rat blood samples with ketone strips and by ELISA and found that the level was higher when measured with ketone strips and that the level of ketone bodies in serum was similar to the level reported in the recent studies [3, 5]. As ketone body concentration testing with ketone strips indicated the total level of blood ketone bodies not the βOHB level in serum, we tested βOHB in present study by ELISA which has higher sensitivity. The limited volume of CSF samples also made the blood ketone body strips impractical in our study.
In ketone body metabolism, βOHB, the main component of ketone bodies, is carried by several MCTs across the blood-brain barrier [2, 11] and used as an energy source by the brain and spinal cord during ketone body metabolism. Therefore, the CSF βOHB level may be affected by MCT upregulation or inhibition, and a high level of plasma ketones may increase the protein expression of MCTs [2]. Iriki et al. [7] found that the CSF βOHB level was 13% to 28% of the serum βOHB level in calves that received intraruminal administered of butyrate (11–44 g), while the value was 22% in the control. In the present study, the CSF βOHB levels of the rats given ad libitum access to the standard diet and those given ad libitum access to the ketogenic diet were both 76% of the serum βOHB level, while those of the rats subjected to the EODF diet and the rats subjected to EODKD were 62% and 66% of the serum βOHB level, respectively. These percentages were higher than the ratios reported in previous studies [7, 10]. This inconsistency may due to the longer hyperketonemia period in this study. Hyperketonemia was found to upregulate MCT transfer molecules and increase βOHB transport from serum to CSF [11]. Both studies [7, 10, 11] suggested that the difference in βOHB level between serum and CSF seems to be related to the blood-brain barrier, as there was an obvious difference even in the standard diet in the present study. The present study further suggested that the difference was associated every-other-day fasting (EODF or EODKD).
Ketone body metabolism has neuroprotective effects on many neurodegenerative diseases and acute neurotrauma models [12, 18] and has been applied to treatment of childhood epilepsies that are resistant to anticonvulsant medications. The present study identified positive correlation between blood and CSF βOHB levels in all samples but showed no correlation in each individual group. The reason for this might be the lack of a sufficient number of samples in each group, and further study is needed with a higher number of samples to confirm the correlation between blood and CSF βOHB levels.
White et al. [21] found that IV infusions of hypertonic saline/βOHB are possible and lead to increased plasma and CSF βOHB levels in healthy rats and that increases in brain levels of βOHB are dependent on plasma concentrations, but the study showed the effect of exogenous βOHB on the plasma and CSF βOHB levels and just observed the results after 6 h. In the present study, we used different diets to evaluate the effect of endogenous ketone body metabolism on the plasma and CSF βOHB levels after two weeks and observed that there was a significant rise in βOHB level with the three diet interventions compared with the standard diet. Specifically, the βOHB level resulting from the KD intervention was similar to that resulting from the EODF intervention, while the EODKD intervention led to a higher βOHB level, which was approximately 2 times the βOHB level induced by the KD or EODF. Previous studies have shown that a KD and EODF were neuroprotective for acute cervical spinal cord injury in rat models [17, 19, 20]. Interestingly, a pilot clinical study suggested an EODKD for better seizure control [4], supporting the rationale of dose-dependent-to-βOHB-level neuroprotection under ketone body metabolism. The present study examined βOHB levels among diet interventions and shed light on ketone body metabolism by examining the serum and CSF βOHB levels. It also showed that body weight changed slightly during the 2-week diet interventions with EODF, the KD, and the EODKD and was lower than that with the standard diet. Previous studies observed a steady increase in body weight over time after spinal cord injury (SCI) with a KD or EODF,slightly less than or close to the body weight with the standard diet [8]. There are several limitations of our study. On the one hand, we add not measure acetoacetate levels in plasma or protein expression of MCT in the brain. On the other hand, the sample size was too small in each group to observe correlationbetween blood and CSF βOHB levels.
In general,the KD, EODF, and EODKD resulted in ketone body metabolism, as βOHB levels increased significantly compared with the standard diet. The CSF βOHB level was lower than the serum βOHB level, which may have been the result of the blood-brain barrier and diet protocols, such as the every-other-day fasting. Our results suggested that the βOHB level in blood was an indicator of that in CSF and that the EODKD was an effective diet to enhance ketogenic metabolism.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81472084).
References
- 1.Colombo M., Kruhoeffer M., Gregersen S., Agger A., Jeppesen P., Oerntoft T., Hermansen K.2006. Energy restriction prevents the development of type 2 diabetes in Zucker diabetic fatty rats: coordinated patterns of gene expression for energy metabolism in insulin-sensitive tissues and pancreatic islets determined by oligonucleotide microarray analysis. Metabolism 55: 43–52. [DOI] [PubMed] [Google Scholar]
- 2.de Assis A.M., da Silva J.S., Rech A., Longoni A., Nonose Y., Repond C., de Bittencourt Pasquali M.A., Moreira J.C., Souza D.O., Pellerin L.2016. Cerebral ketone body oxidation is facilitated by a high fat diet enriched with advanced glycation end products in normal and diabetic rats. Front. Neurosci. 10: 509. doi: 10.3389/fnins.2016.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douris N., Melman T., Pecherer J.M., Pissios P., Flier J.S., Cantley L.C., Locasale J.W., Maratos-Flier E.2015. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim. Biophys. Acta 1852: 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman A.L., Rubenstein J.E., Kossoff E.H.2013. Intermittent fasting: a “new” historical strategy for controlling seizures? Epilepsy Res. 104: 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland A.M., Kephart W.C., Mumford P.W., Mobley C.B., Lowery R.P., Shake J.J., Patel R.K., Healy J.C., McCullough D.J., Kluess H.A., Huggins K.W., Kavazis A.N., Wilson J.M., Roberts M.D.2016. Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311: R337–R351. doi: 10.1152/ajpregu.00156.2016 [DOI] [PubMed] [Google Scholar]
- 6.Hursting S.D., Lavigne J.A., Berrigan D., Perkins S.N., Barrett J.C.2003. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu. Rev. Med. 54: 131–152. doi: 10.1146/annurev.med.54.101601.152156 [DOI] [PubMed] [Google Scholar]
- 7.Iriki T., Tamura K., Ishii M., Tanaka H., Miyamoto T., Onda K.2009. Concentrations of ketone body and antidiuretic hormone in cerebrospinal fluid in response to the intra-ruminal administration of butyrate in suckling calves. Anim. Sci. J. 80: 655–661. [DOI] [PubMed] [Google Scholar]
- 8.Jeong M.A., Plunet W., Streijger F., Lee J.H., Plemel J.R., Park S., Lam C.K., Liu J., Tetzlaff W.2011. Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J. Neurotrauma 28: 479–492. doi: 10.1089/neu.2010.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J. 2012. Dietary intervention study therapy for acute spinal cord injury. Clin. Basic Orthop. Res. 2012: 75. [Google Scholar]
- 10.Klepper J., Diefenbach S., Kohlschütter A., Voit T.2004. Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome. Prostaglandins Leukot. Essent. Fatty Acids 70: 321–327. doi: 10.1016/j.plefa.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 11.Leino R.L., Gerhart D.Z., Duelli R., Enerson B.E., Drewes L.R.2001. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 38: 519–527. doi: 10.1016/S0197-0186(00)00102-9 [DOI] [PubMed] [Google Scholar]
- 12.Maalouf M., Rho J.M., Mattson M.P.2009. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Brain Res. Rev. 59: 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin B., Mattson M.P., Maudsley S.2006. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res. Rev. 5: 332–353. doi: 10.1016/j.arr.2006.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer T.E., Kovács S.J., Ehsani A.A., Klein S., Holloszy J.O., Fontana L.2006. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 47: 398–402. doi: 10.1016/j.jacc.2005.08.069 [DOI] [PubMed] [Google Scholar]
- 15.Paoli A., Rubini A., Volek J.S., Grimaldi K.A.2013. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 67: 789–796. doi: 10.1038/ejcn.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plunet W.T., Lam C.K., Lee J.H., Liu J., Tetzlaff W.2010. Prophylactic dietary restriction may promote functional recovery and increase lifespan after spinal cord injury. Ann. N. Y. Acad. Sci. 1198:(Suppl 1): E1–E11. doi: 10.1111/j.1749-6632.2010.05564.x [DOI] [PubMed] [Google Scholar]
- 17.Plunet W.T., Streijger F., Lam C.K., Lee J.H., Liu J., Tetzlaff W.2008. Dietary restriction started after spinal cord injury improves functional recovery. Exp. Neurol. 213: 28–35. doi: 10.1016/j.expneurol.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Stafstrom C.E., Rho J.M.2012. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 3: 59. doi: 10.3389/fphar.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streijger F., Plunet W.T., Lee J.H., Liu J., Lam C.K., Park S., Hilton B.J., Fransen B.L., Matheson K.A., Assinck P., Kwon B.K., Tetzlaff W.2013. Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PLoS ONE 8: e78765. doi: 10.1371/journal.pone.0078765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streijger F., Plunet W.T., Plemel J.R., Lam C.K., Liu J., Tetzlaff W.2011. Intermittent fasting in mice does not improve hindlimb motor performance after spinal cord injury. J. Neurotrauma 28: 1051–1061. doi: 10.1089/neu.2010.1715 [DOI] [PubMed] [Google Scholar]
- 21.White H., Venkatesh B., Jones M., Worrall S., Chuah T., Ordonez J.2013. Effect of a hypertonic balanced ketone solution on plasma, CSF and brain beta-hydroxybutyrate levels and acid-base status. Intensive Care Med. 39: 727–733. doi: 10.1007/s00134-012-2790-y [DOI] [PubMed] [Google Scholar]
- 22.Yang Z., Lai G.C., Wang G.B.2011. Method improvement of collecting cerebrospinal fluid of SD rats. Chin. Occup Med. 38: 117–119. [Google Scholar]