Abstract
The Bioartificial Renal Epithelial Cell System (BRECS), is a cell-based device to treat acute kidney injury through renal cell therapy from an extracorporeal circuit. To enable widespread implementation of cell therapy, the BRECS was designed to be cryopreserved as a complete device, cryostored, cryoshipped to an end-use site, thawed as a complete device, and employed in a therapeutic extracorporeal hemofiltration circuit. This strategy overcomes storage and distribution issues that have been previous barriers to cell therapy. Previous BRECS housings produced by Computer Numerical Control (CNC) machining, a slow process taking hours to produce one bioreactor, was also prohibitively expensive (>$600/CNC-BRECS); major obstacles to mass production. The goal of this study was to produce a BRECS to be mass produced by injection molding (IM-BRECS), decreasing cost (<$20/unit) and improving manufacturing speed (hundreds of units/hr), while maintaining the same cell therapy function as the previous CNC-BRECS, first evaluated through prototypes produced by stereolithography (SLA-BRECS). The finalized IM-BRECS design had a significantly lower fill volume (10 mL), mass (49 g) and footprint (8.5 cm×8.5 cm×1.5 cm), and was demonstrated to outperform the previous BRECS designs with respect to heat transfer, significantly improving control of cooling during cryopreservation and reducing thaw times during warming. During in vitro culture, IM-BRECS performed similarly to previous CNC-BRECS with respect to cell metabolic activity (lactate production, oxygen consumption and glutathione metabolism) and amount of cells supported.
Keywords: cell therapy, renal cell, progenitor, extracorporeal, bioreactor, Acute Kidney Injury, Acute Renal Failure, cell-based device, tissue engineering, rapid prototyping
Introduction
Cell therapy is an emerging translational medicine approach to treat disease states arising from a lack of a specific, vital cell population. In acute kidney injury (AKI), the disease state arises due to damage and resulting necrosis of renal proximal tubule cells.1 Conventional therapy of AKI focuses on removal of the insult, such as treatment with antibiotics in the case of bacteremia, followed by the treatment of uremia by providing small solute clearance, through hemofiltration. Conventional therapy fails to address the metabolic, endocrine, and organ system cross-talk functions of the kidney that are diminished due to the cellular damage and loss in the disease state. Renal cell therapy, where applied exogenous cells supplement missing endogenous cell function, in conjunction with a hemofiltration platform, is a novel approach to address the shortcomings of conventional therapy to treat AKI. Renal epithelial cell therapy has already shown clinical promise.2, 3 In a phase II clinical trial, a renal assist device (RAD) containing human cells was shown to have a beneficial impact on survival in acute renal failure (ARF) patients with multiple organ failure (MOF) without safety concerns.
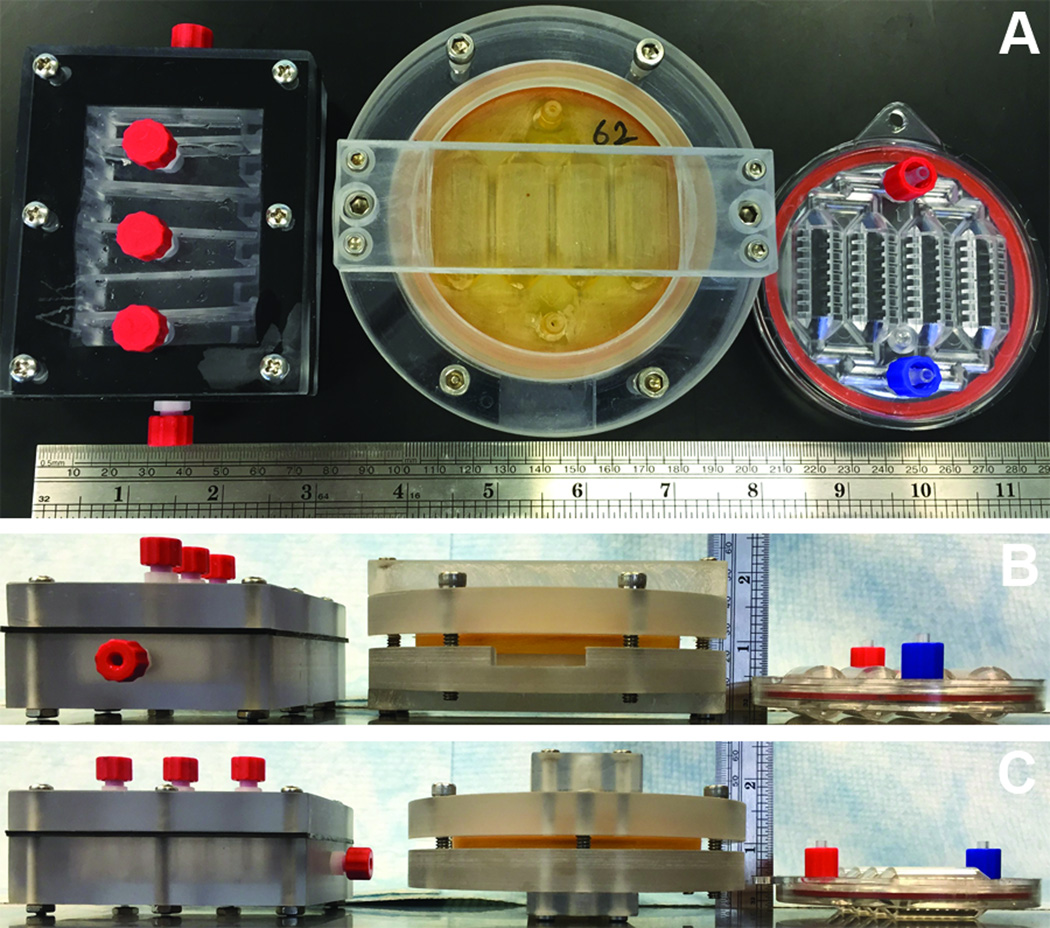
Currently in regenerative medicine, cell, tissue, and organ replacements are being created in the lab. However, it is the lack of technologies to preserve and distribute these complex biological systems, including cell-based devices, that has remained a critical issue to the best clinical use of these resources to extend human life.4 The Bioartificial Renal Epithelial Cell System (BRECS) is a cell-based device that was developed to enable distribution for potential therapeutic use via cryopreservation.5 An initial design of the BRECS made by Computer Numerical Control (CNC) machining (herein referred to as a CNC-BRECS), a perfusion bioreactor was able to support adult progenitor derived renal cells cultivated on porous disks, which could be cryopreserved as a complete device, thawed as a complete device, and employed in a therapeutic extracorporeal hemofiltration circuit.5 The capability of cryopreservation, along with the use of an allogenic cell source in an immuno-isolated extracorporeal circuit, enables widespread availability of cell-based devices for on-demand therapy of acute disease states; however the initial CNC-BRECS (Figure 1 left) was not conducive to mass production. Herein, the design and testing of BRECS rapid prototypes created by stereolithography (SLA-BRECS, Figure 1 middle) are presented, to arrive at a finalized injection molded (IM-BRECS, Figure 1 right) design which overcomes hurdles associated with mass production and distribution for clinical translation of cell therapy.
Figure 1.
CNC-BRECS (left), SLA-BRECS (middle), and IM-BRECS (right) for mass production shown from the top (A), front (B) and side (C), with a ruler for scale.
Materials and Methods
New BRECS Design and Simulation
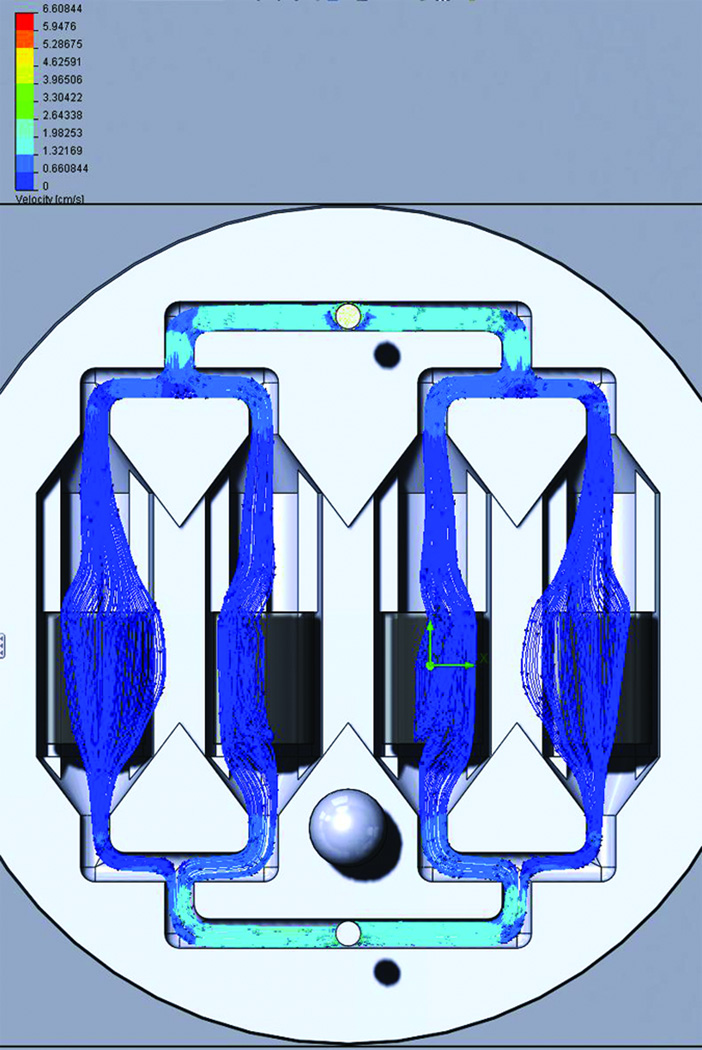
New designs of the BRECS for potential mass production were drafted in SolidWorks (Dassault Systems, Concord, MA), a computer aided design (CAD) software, with design criteria which included 1) a smaller footprint 2) smaller volume 3) thinner walls to allow for better heat transfer 4) maintaining or improving cell dose through the capacity to accommodate porous disks providing surface area for cell attachment 5) improvement of flow path through the device, avoiding stagnation points, recirculation zones and potential air entrapment 6) snap feature for sterile assembly of BRECS, affording patency at pressures approaching 1,000 mmHg 7) being amenable to both rapid fabrication techniques such as stereolithography (SLA) and to injection molding (IM) for mass production. These designs were analyzed by Computational Flow Dynamics (CFD) simulations using finite element analysis (FEA) within SolidWorks' Flow Simulation software: FloXpress (Dassault Systems, Concord, MA). CFD has previously been used in simulation driven product development for medical devices and bioreactors.6–9 Briefly, for each BRECS design, a flow path was defined, based on the assembly of the housing, inlet and outlet. The flow path was converted into a mesh with individual nodes for FEA (Figure 2). Mesh refinement in area of confined or restricted flow was used to ensure accurate flow data for these regions. Mesh was validated using geometry check and mesh quality check function. After mesh setup, initial conditions and boundary conditions were specified in a steady state, homogenous, laminar flow model. The inlet boundary condition was set to the intended flow rate (10mL/min or 50mL/min), and the outlet boundary condition was ambient pressure, along with a no-slip wall boundary condition. Water's fluid parameters were used to simulate cell culture media perfusion during in vitro cell growth conditions, and also to simulate ultrafiltrate perfusion during ex vivo extracorporeal circuit therapy. Porous cell culture disks were modelled as porous, isotropic media. After simulation, flow profile throughout the BRECS was analyzed for flow heterogeneity, areas of recirculation, and stagnation points, hallmarks of poor fluid dynamics for homogenous nutrient delivery. Promising BRECS designs without stagnation points or areas of recirculation were chosen for rapid prototyping (Figure 2).
Figure 2.
Representative Computational Fluid Dynamics (CFD) data for prospective SLA-BRECS designs at a simulated flow rate of cell culture media at 10mL/min. Flow lines show flow from inlet to outlet passing relatively uniformly through porous disks for cell attachment housed within the interior of 4 flow channels, without areas of stagnation or recirculation.
Stereolithographic rapid prototyping of BRECS (SLA-BRECS)
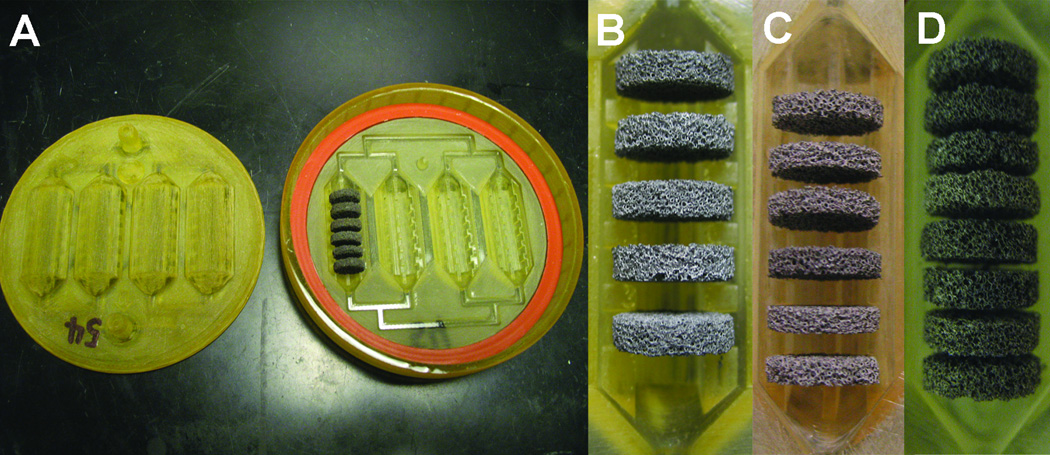
Rapid prototypes of BRECS designs for evaluation in vitro as well as in ex vivo models of acute and chronic renal failure were fabricated using SLA with RenShape® SL 7870 (Hunstman®, Woodlands, TX) or Watershed® XC 11122 resin (DSM Somos®, Elgin, IL). These resins fit a critical list of specifications including: good biocompatibility, translucence to enable flow visualization in the device, and the ability to be sterilized. Rapid prototype SLA-BRECS were fabricated in two pieces: a top and a bottom (produced by Eagle Design and Technology, Inc., Zealand Michigan), which were clamped together using an external clamp ring (made by ARL Service, LLC, Clarkson, MI), and a water-tight seal was achieved by using an annular gasket made out of medical grade silicone (McMaster-Carr, Aurora, IL). The snap closure feature (introduced into IM-BRECS below) was omitted from these prototypes due to potential issues with a snap seal due to small "build steps" from the layer-by layer approach of SLA fabrication. Various designs holding different numbers of 2mm or 2.5mm thick porous disks were produced for evaluation (Figure 3).
Figure 3.
SLA-BRECS (A) consisting of top and bottom housing pieces, and a silicone gasket to make a liquid tight seal when the two pieces are assembled with an external clamp force exerted by a clamp ring (not shown). A porous disk column consisting of 5, 2.5mm thick disks (B) is shown from a SLA BRECS containing a total of 20 disks. A porous disk column consisting of 6, 2mm thick disks (C) is shown from a SLA BRECS containing a total of 24 disks. Lastly, a porous disk column consisting of 8, 2mm thick disks (D) is shown from a SLA BRECS containing a total of 32 disks. Alternative disk arrangements (B–D) are not shown at the same scale.
Injection Molded BRECS (IM-BRECS) production
After initial tests with SLA-BRECS prototypes, an optimal design was selected for injection mold fabrication utilizing medical grade polycarbonate. Injection mold tools were designed to produce the specified IM-BRECS in two pieces, where the top piece had an inlet and outlet which was completed with red and blue finger snap luer lock rings (Value Plastics, Fort Collins, CO). Similar to SLA-BRECS, IM-BRECS also utilized annular gaskets made out of medical grade silicone. IM-BRECS were designed with a tamper-proof seal, created by complimentary snap-fit features in the top and bottom pieces, outside of the gasket seal. To aid the process of snapping together top and bottom IM-BRECS pieces, a custom press with a torque lever arm was fabricated to generate the snapping force. The snap feature determines the internal pressure that the BRECS can withstand without physical compromise. A minimum design criteria was set at over 600 mmHg burst pressure, since maximum pressures in therapeutic extracorporeal circuits are 400 to 500 mmHg.
BRECS Quality Control
Upon receipt, quality control caliper measurements, patency/flow testing, and integrity testing, up to a positive pressure of 600 mmHg, was used to verify that rapid prototype SLA-BRECS or IM-BRECS were fabricated to specifications. Practical handling characteristics of each BRECS type were assessed including: sterilization using an established method (autoclave sterilization, ethylene oxide, gamma sterilization, or with Minncare (Minntech, Minneapolis, MN) cold sterilant), uniform flow-profile during perfusion (1% trypan blue dye flow visualization assessed by time-lapse photography), the ability to remove air bubbles from the internal volume, and component integrity up to a 3 month culture period (photographic documentation).
REC isolation and culture
Primary human renal epithelial cells (REC) were isolated from human kidneys unsuitable for transplantation due to anatomic or fibrotic defects (procured from the National Disease Research Interchange, NDRI), following an established method.10 For all tissues used, provided by NDRI, written informed consent was obtained according to Institutional Review Board (IRB) #5 at the University of Pennsylvania. Cells were cultured in Ultra MDCK media (Lonza, Walkersville, MD) supplemented with 1/2× the manufacturer’s recommended dilution for insulin, transferrin, ethanolamine and selenium supplement (ITES, Lonza), 60ng/mL epidermal growth factor (EGF, #E1257 Sigma-Aldrich; St. Louis, MO), 10−9M triiodothyronine (T3, #T6397, Sigma-Aldrich, St Louis, MO) and antibiotic-antimycotic (15240-062, Invitrogen, Carlsbad, CA). Cells were passaged upon 70–80% confluence, and either seeded on carbon disks or frozen for future seeding. This process called expanded propagation (EP) was carried out using a recently developed culture system10.
REC seeding of extracellular matrix coated carbon disks
REC were seeded onto porous disks utilizing either of 2 methods: a two-side static seeding process or a seeding process where the disks were submerged in a concentrated cell solution. For the two-sided method, 75µL of cell suspension in Ultra MDCK media containing 106 cells was applied drop-wise onto one side of a disk followed by 1.5 hour incubation at 37°C. The disk was then flipped over, cell-side down, and a second identical drop-wise cell loading was applied to the exposed side. Following an additional 1.5 hr incubation, disks were loaded into a sterilized BRECS within a biosafety cabinet.
For the submersion method, dry porous disks were added directly to a concentrated cell solution and mixed well. Cell covered disks were loaded into BRECS and incubated for 2hrs, followed by rotating the BRECS and incubation for an additional 2 hrs to allow for cell attachment.
Maintenance of REC on carbon disks in SLA-BRECS or IM-BRECS
BRECS were placed in a continuous recirculation circuit with 250 mL of supplemented media in a 500 mL sterile perfusion bottle. Custom tubing sets for BRECS consisted of PharMed BPT double-stop pump tubing (Cole-Parmer, Vernon Hills, IL) and silicone tubing (Cole-Parmer). A Masterflex peristaltic pump (Cole-Parmer) was used to maintain the BRECS at a constant perfusion of between 10 to 100 mL/min. Media changes on the BRECS were performed twice per week.
Metabolic activity assessment
Metabolic activity was evaluated using lactate production, oxygen consumption rates (OCR) and glutathione degradation. Concentrations of produced lactate were determined using a colorimetric assay.11, 12 Oxygen measurements were performed using oxygen sensitive PSt3 patches (PreSens, Regensburg, Germany) either set up in a recirculating, closed, oxygen impermeable circuit, where oxygen concentration was continuously logged or with inlet and outlet measurements, where oxygen consumption was determined in real-time. To determine average OCR during recirculation, a linear regression approximation of slope was used. Glutathione metabolism was determined by measuring the rate of degradation of exogenously added glutathione. Supplemented Ultra MDCK media (70mL) containing an initial concentration of 20µM GSH (#G6257 Sigma-Aldrich) was recirculated through BRECS for 1 hour at 37°C. Samples were collected at initial baseline (t = 0) and at 60 minutes for determination of GSH degradation rates. Samples were analyzed for GSH by the method of Tietze.13
Cell viability on porous disks
Cell viability was assessed by adding 1 µg/mL fluorescein diacetate and propidium iodide to individual disks in well plates. Living cells and dead cells were visualized immediately with a Zeiss Axiovert 200 inverted fluorescence microscope (Carl Zeiss, Inc. Thornwood, NY) equipped with corresponding filter sets, and micrographs were obtained using Zeiss AxioCam MRm and ICc1 cameras (Carl Zeiss, Inc.).
Cell quantification by DNA content
Quantification of total cellular DNA in BRECS was performed using a custom protocol after live/dead stains. For specified BRECS, each individual porous disk from SLA-BRECS was placed in a 2.5 mL DNase, RNase free centrifuge tube, including cell debris from the live/dead imaging plates. Using a solution of 1× SDS and 2µL Proteinase K per mL, 350µL was pipetted into each centrifuge tube, and mixed well to isolate DNA. Centrifuge tubes were centrifuged at 300 × g for 1 min, and stored in an Ultralow Freezer at −80°C for later DNA quantification. Upon thaw, samples were mixed well and incubated overnight at 60°C. DNA solution was removed from porous disks by centrifugation. Other BRECS were batch processed for DNA, where all disks from one BRECS were pooled together and were processed in 50mL conical tubes, following the same steps.
Total DNA per porous disk was determined using the FluoReporter Blue quantification kit as per the manufacturer’s instructions (Molecular Probes, Eugene, OR). Standard curve was constructed by using the DNA extracted from parallel dish cultures with known cell numbers by manual trypsinized cell counts.
Temperature Measurements in SLA-BRECS or IM-BRECS
One acellular SLA-BRECS was prepared with four thermocouples, potted in place with epoxy, to measure temperatures during potential cryopreservation and thaw protocols. Thermocouple placement locations were chosen based on theoretical minimum and maximum temperatures expected to be experienced by porous disks. An acellular IM-BRECS was also prepared with 4 thermocouples in a similar configuration. Temperatures were logged for these SLA-BRECS and IM-BRECS during cryopreservation and thaw processes using a National Instruments (Austin, TX) NI 9211 module in a USB High Speed Carrier.
SLA-BRECS and IM-BRECS Cryopreservation
SLA-BRECS or IM-BRECS were flushed and incubated with room temperature HTS-Purge Solution (# 637112 BioLife Solutions, Inc., Bothell, WA) for 2 minutes. HTS-Purge was then replaced with cryopreservation buffer, CryoStor 10 (CS10, #640222 BioLife Solutions) at 4°C, and the inlet and outlet were capped with sterile, Nylon, male luer integral lock ring plugs (Value Plastics, Fort Collins, CO) to prepare HREC BRECS for cryostorage. A CryoMed controlled rate freezer (Thermo Scientific, Waltham, MA) was utilized to freeze complete, individual SLA-BRECS or IM-BRECS. Briefly, this protocol consisted of a cooling rate of −1°C/min sample temperature to −4°C, followed by a rapid cooling phase during phase change, −25°C/min chamber temperature to −40°C. The chamber was then warmed 10°C/min to −14°C. After phase change, a cooling rate of −1°C/min sample temperature to −40°C was used. The last rapid cooling step was −10°C/min sample temperature to −90°C. BRECS were then directly transferred into the gas phase of a liquid nitrogen tank for cryostorage.
SLA-BRECS and IM-BRECS Thaw
Cryopreserved SLA-BRECS or IM-BRECS were thawed in a 37°C water bath until all internal ice was melted. This time was recorded as a metric for heat transfer and efficiency of warming. Post-thaw, BRECS were removed from the heating bath, and the exterior of the BRECS was dried, and decontaminated with 70% ethanol. Within a biosafety cabinet, male luer integral lock ring plugs on the inlet and outlet were removed, and a single pass perfusion circuit was installed to perfuse BRECS with 37°C media for complete removal of the cryopreservation buffer. The BRECS were then switched to a recirculation circuit with Ultra MDCK media with supplements for the duration of the study.
During cryopreservation and thaw, BRECS were inspected for adequate mechanical stability during freeze and thaw process. Validation of the hermetic seal during thaw in a water bath was conducted by inspecting the water bath for phenol-red containing media during the thaw process.
Statistics
Data is presented as averages ± standard deviation (SD) where applicable. Comparisons between groups were made using Student’s t test assuming equal variance. Significance was set at p<0.05.
Results
BRECS design and simulation
An earlier CNC-BRECS design (Figure 1, left) had a fill volume of 30 mL, a maximum external housing dimension of 11 cm, and internal dimensions of 9 cm × 7.5 cm × 3 cm (length × width × height)5. The new BRECS designs to be fabricated by SLA (Figure 1, middle) were optimized for heat transfer to maximize control of the cryopreservation and reconstitution processes, with a smaller fill volume (10 mL), increased portability (maximum external dimension of 8.5 cm) and internal dimensions with a higher aspect ratio (diameter = 8.5 cm, maximum height = 1.5 cm). Having a higher aspect ratio (maximum dimension to minimum dimension) and a higher surface area to volume ratio, theoretically would allow for better heat transfer.
In CFD simulations, the designs that exhibited the most homogenous flow profiles and fewest stagnation points or recirculation zones were those that utilized bifurcating flows as fluid flows from the inlet and merging flows to the outlet of the BRECS, with cylindrical flow channels to house porous disks and conical flow development zones (Figure 2).
Rapid Prototype SLA-BRECS
SLA-BRECS were fabricated with two different resins, and 3 different designs (Figure 3). A previous version of the BRECS utilized 20 porous disks (each 2.5 mm thick); therefore the target number of disks to be housed in the SLA-BRECS was 20. Use of bifurcating flow required an even number of channels, and therefore a design with 4 channels, each holding 5 disks spaced approximately 2 mm apart, was chosen for the initial fabrication (Figure 3 B).
Later SLA-BRECS designs included variants in disk spacing, and total number of porous disks housed within the SLA-BRECS. A design holding 24 porous disks (each 2 mm thick) with equivalent total surface area to the initial design with 20 disks (2.5 mm thick) was fabricated (Figure 3C). A final design keeping the overall dimensions of the SLA-BRECS the same, but decreasing the spacing between disks to 1 mm, allowed for a total capacity of 32 disks (2 mm thick). This spacing is shown in Figure 3D.
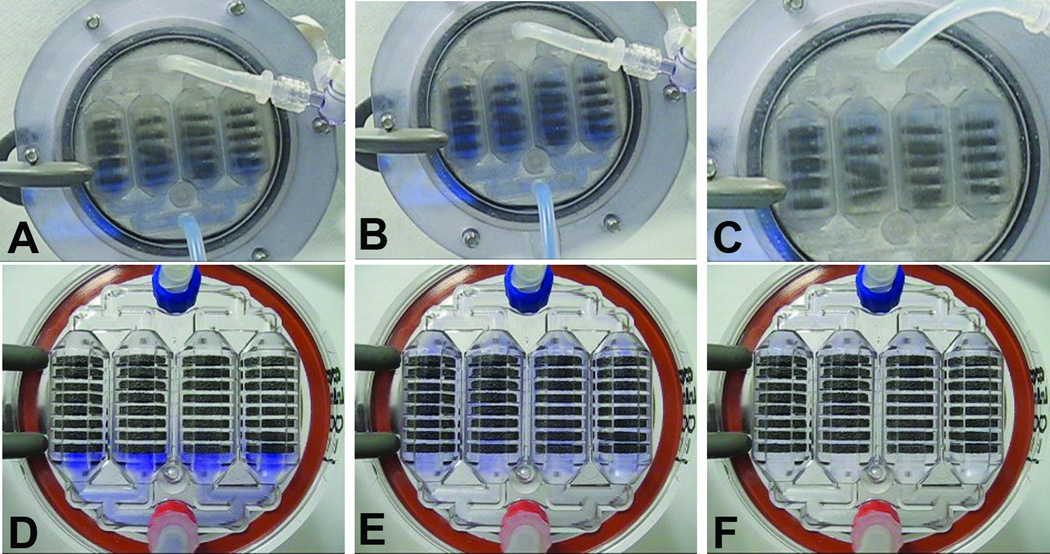
Fabricated SLA-BRECS were tested through quality control measures, and all units were found to be patent, capable of maintaining flow, and maintained integrity during application of up to 600 mmHg of positive pressure. Flow visualization studies, using dilute trypan blue flowing through porous disk loaded SLA-BRECS, demonstrated strong correlation with flow simulation results, suggesting homogenous flow with few detectable stagnation points or recirculation zones (Figure 4 A, B & C). At flow rates tested, no significant pressure drop was measured across the SLA-BRECS, demonstrating that incorporation of the BRECS in an extracorporeal circuit would not restrict flow.
Figure 4.
SLA-BRECS and IM-BRECS shown assembled and during flow visualization with a blue dye at a flow rate of 10 mL/min. Dye injection along with time-lapse photography allowed for the analysis of flow dynamics within the SLA-BRECS during initial inlet flow distribution (A), SLA-BRECS filling (B), and outlet clearance (C), and in IM-BRECS during inlet flow (D), filled (E) and clearing (F). Note that the SLA-BRECS had some issues holding porous disks tightly in the correct place due to manufacturing limitations afforded by SLA. Porous disks were more tightly held in the correct place by IM-BRECS.
The first rapid prototypes produced made of RenShape® SL 7870 SLA resin were successfully sterilized using gamma sterilization. During perfusion within an incubator at 37°C, the RenShape® SL 7870 SLA-BRECS deformed due to the pressure and temperature over time, leading to wall displacement up to 0.5 cm, and therefore did not offer the desired structural integrity at culture conditions.
A second SLA resin material, Watershed® XC 11122, was used to produce SLA-BRECS, which were successfully sterilized with either gamma sterilization or cold sterilant (Minncare, Minntech, Minneapolis, MN). Cold sterilization was preferred, as gamma sterilization induced a color change that made the SLA-BRECS more opaque, and more difficult to visualize interior fluid flow. Perfusion at culture conditions resulted in only minor deformation, and with appropriate support structures built into a clamp-ring, SLA-BRECS were found to have adequate structural integrity over the 3 month period tested. All subsequent SLA-BRECS design variants were fabricated with Watershed® XC 11122, including SLA-BRECS accommodating 20 disks (2.5 mm thick), 24 disks (2 mm thick), or 32 disks (2 mm thick).
Plastic deformation was not a concern for BRECS made by injection molding with polycarbonate, as the heat deflection and glass transition temperature of polycarbonate is much higher than these SLA resins, and therefore no deformation results during 37°C media perfusion.
REC metabolic activity in SLA-BRECS
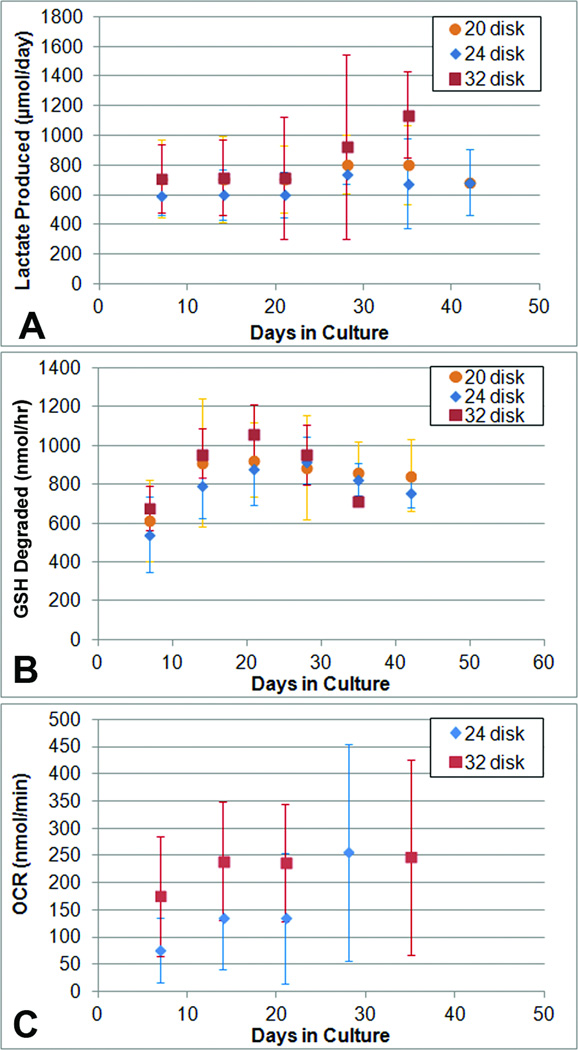
When perfused at 10 mL/min, SLA-BRECS with 20 cell-seeded disks (2.5 mm thick) had similar metabolic activity to previous BRECS designs with 20 disks (2.5 mm thick) when perfused at 30 mL/min5, as assessed by lactate production, oxygen consumption, and glutathione degradation (Figure 5). A plateau in metabolic activity was normally reached within one to two weeks in culture, and were in general maintained for up to 6 weeks. The lactate production rate in SLA-BRECS after 2 weeks of in vitro culture was ~700 µmol/day, which was consistent with a cell number approaching 108 cells (Figure 5A), as 106 cells were found to consume ~7 µmol/day. Glutathione (GSH) degradation by these SLA-BRECS demonstrated consistent REC specific function, with degradation rates of ~800 nmol/hr (Figure 5B). Oxygen Consumption Rates (OCR) of ~180 nmol /min were also suggestive of a cell number approaching 108 cells when a rate of 2.29 nmol /min per 1 × 106 cells was assumed (Figure 5C).14
Figure 5.
Metabolic activity measurements: lactate production (A), glutathione metabolism (B), and oxygen consumption rate (C) for SLA-BRECS with 20, 24 and 32 disks. Data presented as averages ± standard deviation, for each SLA type at 1 week (day 7), 2 weeks (day 14), 3 weeks (day 21), 4 weeks (day 28) and 5 weeks (day 35).
To ensure performance was not strongly flow rate dependent, this SLA-BRECS design was tested at higher perfusion rates between 30 to 100 ml/min. Similar metabolic activity was demonstrated for all metabolic parameters and flow rates tested. At a flow rate of 30 mL/min, lactate production ranged: 450 – 1095 µmol/day, GSH degradation: 977 – 1204 nmol/hr. For a flow rate of 50 mL/min, lactate production: 258 – 642 µmol/day, GSH degradation: 956 – 1105 nmol/hr. For a flow rate of 100 mL/min, lactate production: 381 – 1257 µmol/day, GSH degradation: 827 – 1236 nmol/hr.
SLA-BRECS with 32 disks (2 mm thick) were found to perform at least as well as SLA-BRECS accommodating 24 disks (2 mm thick) and SLA-BRECS with 20 disks (2.5 mm thick) which had the same total surface area and therefore performed similarly, when multiple metabolic parameters were evaluated (Figure 4A, 4B, 4C). At 2 weeks, 20 disk SLA-BRECS had an average lactate production rate of 707 µmol/day, compared with 716 µmol/day for 32 disk SLA-BRECS. This difference was more pronounced at 5 weeks, where 20 disk SLA-BRECS had an average lactate production rate of 804 µmol/day, compared with 1139 µmol/day for 32 disk SLA-BRECS. Similarly for glutathione metabolism at 2 weeks, 20 disk SLA-BRECS metabolized 913 nmol/hr, whereas 32 disk SLA-BRECS metabolized 959 nmol/hr. At 3 weeks, this was more pronounced: 928 nmol/hr (20 disk) and 1064 nmol/hr (32 disk). Average OCR for 24 disk SLA-BRECS at 2 weeks was 136 nmol/min, compared to 240 nmol/min in 32 disk SLA-BRECS.
Viability and total cell quantification in SLA-BRECS
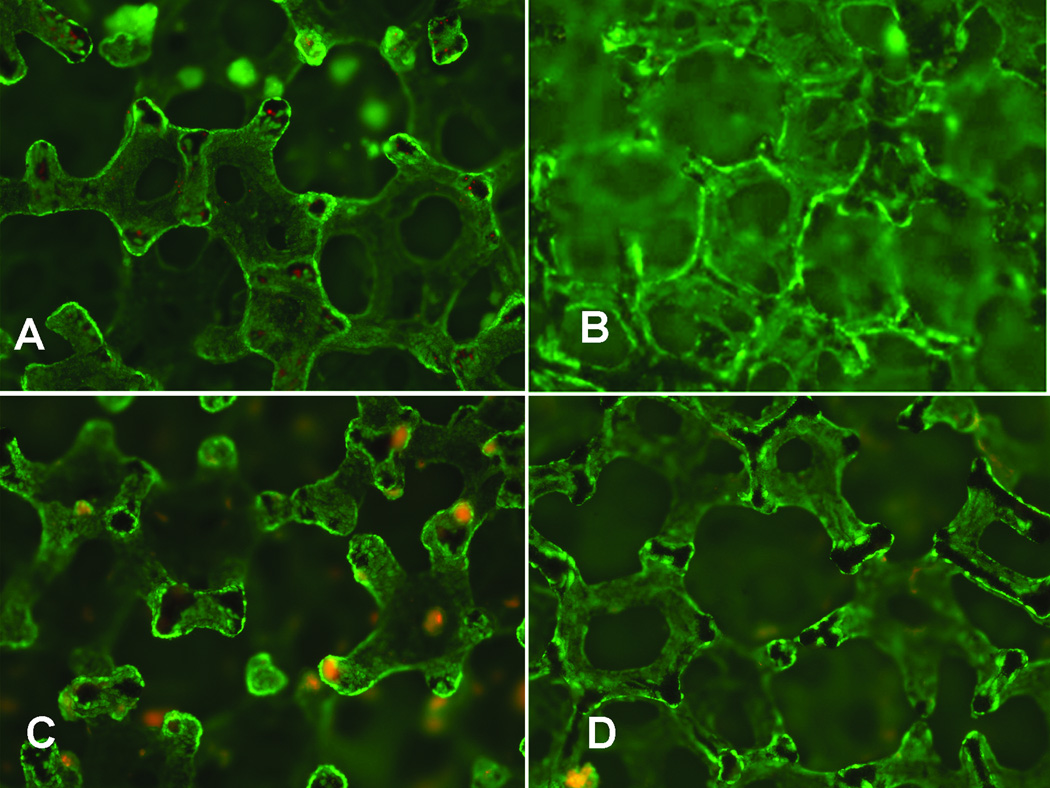
A viability stain of REC grown on porous disks within SLA-BRECS (Figure 6A) demonstrated a dense population of living cells, positive for fluorescein diacetate (viable cells fluoresce green), with relatively few dead cells (propidium iodide positive cells fluoresce red). The total number of cells supported by SLA-BRECS, measured using FluoReporter Blue to quantify DNA, demonstrated an average of 4.13 ± 3.49 ×107 cells in SLA-BRECS with 20 disks (2.5 mm thick), 5.90 ± 2.53 ×107 cells in SLA-BRECS with 24 disks (2 mm thick), and 7.59 ± 1.61 ×107 cells in SLA-BRECS with 32 disks (2 mm thick).
Figure 6.
Cell viability of REC grown on porous disks within SLA-BRECS prior to cryopreservation (A), SLA-BRECS after thaw (B), IM-BRECS prior to cryopreservation (C), and IM-BRECS after thaw (D), where living cells were stained with fluorescein diacetate (green), and dead cells were stained with propidium iodide (red).
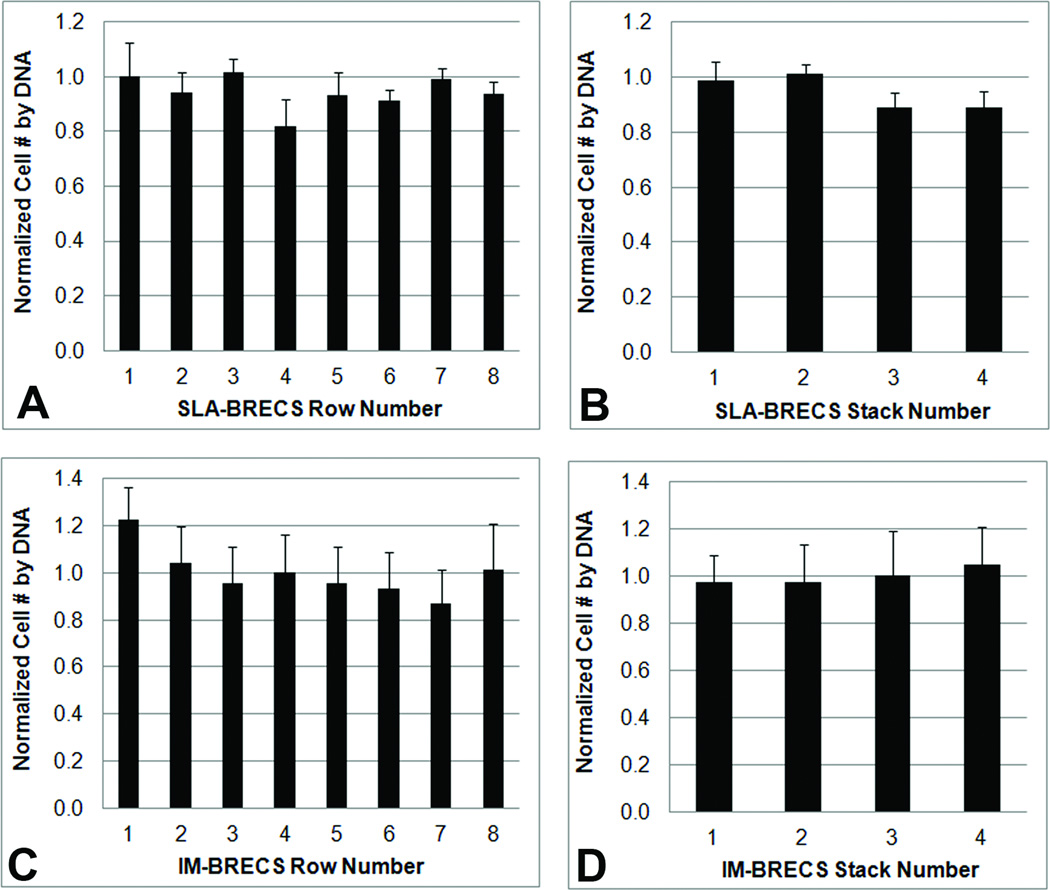
Analysis of the distribution of cells throughout the SLA-BRECS with 32 disks (2 mm thick) by DNA analysis demonstrated that cell number from disk to disk was consistent (Figure 7 A&B).
Figure 7.
Cell number distribution on porous disks within SLA-BRECS (A&B) and IM-BRECS (C&D) as estimated by DNA isolation and quantification. Similar to the homogenous flow distribution demonstrated by dye visualization, cells were found to be homogenously distributed across porous disks, with little variation when the disks were analyzed by their arrangement in rows (A & C, row 1= closest to inlet, row 8 = closest to the outlet) or stacks (B & D, stack 1= left most, stack 4= right most).
SLA-BRECS cryopreservation, storage, and cell viability upon thaw
SLA-BRECS have been cryopreserved in the liquid nitrogen vapor phase for up to 3 months with successful reconstitution as suggested by metabolic data: lactate production rate, OCR and glutathione metabolism, both pre and post cryopreservation. Furthermore, this data is supported by live/dead cell viability stains (Figure 6B) and DNA quantification.
Mass Produced IM-BRECS
Mass produced IM-BRECS made of medical grade polycarbonate had a weight without cell-covered disks of 49 g, with cell-covered disks: 52 g, and total fluid-filled mass of 62 g. This was significantly lower mass than the previous CNC-BRECS, which had a mass of ~240 g without cell-cover disks and was over 270 g when fluid filled.
Flow Visualization in IM-BRECS
Similar to the testing described for SLA-BRECS, Trypan Blue was used to visualize the flow through porous disk loaded IM-BRECS, which was recorded by time-lapse photography. The clear case of the IM-BRECS was especially amenable to visualization, demonstrating very homogenous flow with few detectable stagnation points or recirculation zones (Figure 4 D,E & F).
REC metabolic activity in IM-BRECS
IM-BRECS with 32 REC-covered disks (2 mm thick) had similar cellular metabolic activity to SLA-BRECS with 32 REC-covered disks (2 mm thick), and therefore were no statistically significant differences for lactate production, glutathione metabolism or oxygen consumption rate, at any time point. Metabolic activity plateau occurred within one to two weeks in culture, and IM-BRECS were maintained for up to 6 weeks, with an average lactate consumption of ~890 µmol/day, average glutathione metabolism of ~950 nmol/hr.
Viability and total cell quantification in IM-BRECS
Cell viability staining in IM-BRECS demonstrated a high concentration of viable cells with few dead cells on porous disks (Figure 6C). The total number of cells supported by IM-BRECS was 9.22 ± 2.72 ×107 cells, as determined by DNA quantification (Table 1). Analysis of cell distribution on disks showed good homogeneity, with only a slight, but not statistically significant trend toward more cells on the first disk of each flow channel and slightly fewer cells in row 7 (Figure 7 C&D).
Table 1.
Cell number estimation by DNA quantification
| Design | Estimated Cell Number |
|---|---|
| SLA-BRECS: 20 disk (2.5 mm thick) | 4.13 ± 3.49 × 107 (N=14) |
| SLA-BRECS: 24 disk (2 mm thick) | 5.90 ± 2.53 × 107(N=8) |
| SLA-BRECS: 32 disk (2 mm thick) | 7.59 ± 1.61 × 107 (N=6) |
| IM-BRECS: 32 disk (2 mm thick) | 9.22 ± 2.72 × 107 (N=19) |
Temperature measurements in SLA-BRECS and IM-BRECS during cooling for cryopreservation
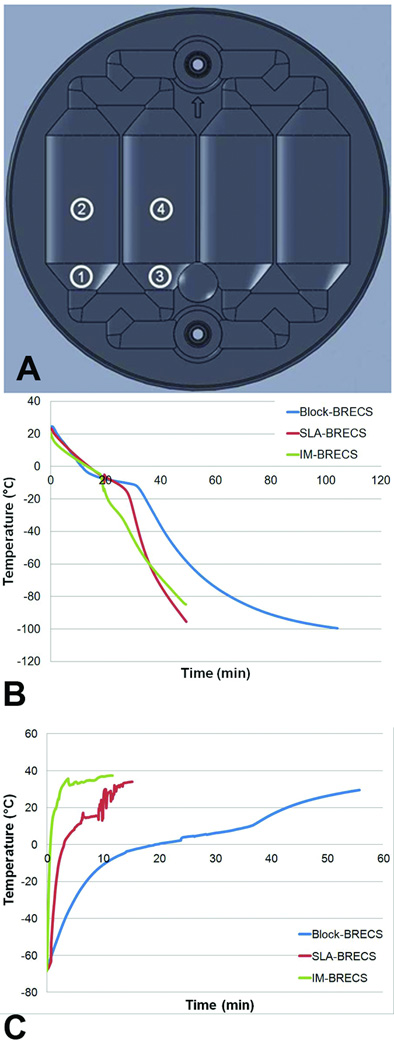
Temperatures were measured by four thermocouples, placed as shown in Figure 8A: 1) outside channel, start of conical region, 2) midpoint of outside channel 3) inside channel, start of conical region, 4) midpoint of inside channel, with thermocouple 4 having the greatest thermal inertia. Temperature profiles within the CNC-BRECS design previously reported5 when cooled directly in the vapor phase of liquid nitrogen, and SLA-BRECS and IM-BRECS during cryopreservation in a controller rate freezer, are shown in Figure 8B. In brief, IM-BRECS and SLA-BRECS cooled to cryogenic temperature (−80°C) in significantly less time (46 ± 5 and 42 ± 4 minutes respectively) than CNC-BRECS (65 ± 6* minutes). This was a key significant improvement as the CNC-BRECS, even when inserted directly in the vapor phase of liquid nitrogen, which is the most extreme cooling condition available to test, was unable to cool at a rate of −1°C/min, a widely accepted ideal cooling rate during cryopreservation. Both SLA and IM-BRECS were able to reach this ideal cooling rate in moderate controlled rate cooling programs. The phase change during CNC-BRECS cryopreservation was pronounced and elongated, which is demonstrated by the flattening of the temperature profile in Figure 8B.
Figure 8.
Thermocouple measurement locations (A). CNC-BRECS, SLA-BRECS, and IM-BRECS temperature measurements at thermocouple 4 during cryopreservation (B) and during thaw in a 37°C water bath (C).
Due to the design improvement for better heat transfer, the SLA-BRECS allowed for cooling rates approaching ideal cryopreservation with a controlled rate freezer, as well as decreased times required for phase transition compared to the CNC-BRECS. The polycarbonate material of the IM-BRECS further improved heat transfer characteristics, allowing for tighter control of the temperature profile during controlled rate freezer cooling along with a shorter phase transition time.
Heat transfer was further tested for quantification of design improvement; each design was cooled by placing a room temperature device directly in a −80°C Freezer. Time to reach −60°C was 32.2 ± 1.1, 43.4 ± 0.5 and 94.5 ± 0.4 minutes, for IM, SLA and CNC-BRECS respectively, demonstrating significantly increased heat transfer, and reduced cooling times.
Temperature measurements in SLA-BRECS and IM-BRECS during warming for thaw
Thaw of CNC-BRECS5, the SLA-BRECS, and IM-BRECS in a 37°C water bath, is shown in Figure 8C. Time to the complete melting of ice (confirmed by visual inspection) within BRECS was reduced in IM-BRECS (5.0 ± 0.3 minutes, n=3) compared to SLA-BRECS (9.3 ± 1.9 minutes, n=3), which were both significantly reduced compared to CNC-BRECS (37.6 ± 1.8 minutes, n=3). Furthermore, a cryopreserved CNC-BRECS thawed in a 37°C water bath, required up to 1hr for all internal temperatures measured to fully reach physiological temperature. The design of the SLA-BRECS allowed for greatly improved physiological temperature warm-up times, with the internal temperature reaching 37°C, in under 15 minutes. The material properties of polycarbonate used in IM-BRECS allowed for even better heat transfer, which enabled reaching physiological temperature within 10 minutes.
IM-BRECS cryopreservation, storage, and cell viability upon thaw
IM-BRECS have been cryopreserved in the liquid nitrogen vapor phase for up to 3 months with successful reconstitution as suggested by metabolic data: lactate production rate, OCR and glutathione metabolism, both pre and post cryopreservation. Furthermore, this data is supported by live/dead cell viability stains (Figure 6D) and DNA quantification.
Discussion
The initial CNC-BRECS design was previously reported, demonstrating the ability to culture and cryopreserve cells in the same device.5 In brief, SLA-BRECS and IM-BRECS utilized the same basic design concept as the previous cryopreservable BRECS: a perfusion bioreactor, constituted by a top and a bottom piece forming a housing, sealed by a gasket, accommodating porous disks for cell attachment. BRECS therapy is built on a proven approach using an immuno-isolated, extracorporeal blood circuit to deliver renal epithelial cell products to patients with acute kidney injury (AKI). Previous clinical studies with a RAD (that utilized similar cells as used in the BRECS) suggested that if patients with ARF and MOF can be treated with renal cell therapy, survival outcomes were improved. Long term survival outcome improvement was likely due to the innate ability of renal epithelium to regenerate, and repair the kidney.2, 3, 15
SLA-BRECS rapid prototypes allowed for the quick evaluation of a number of complex BRECS designs including SLA-BRECS with 20 disks (2.5 mm thick), 24 disks (2 mm thick), and 32 disks (2 mm thick), each with different internal configurations to determine the design to be mass produced by IM. Prior to fabrication of prototypes, designs were evaluated in silico. CAD and simulation based design cycles have been previously shown to be time and cost effective, when used in the design of medical devices6, 7 and bioreactors.8, 9 The use of SolidWorks to create the 3-D CAD data for SLA-BRECS was chosen because it allowed for design and simulation, as well as easy conversion into other formats for distribution to rapid prototype sub-contractors. Good correlation was shown between simulations and bench top testing of SLA rapid prototypes, as near plug flow was achieved through CAD design and simulation and validated by flow visualization in SLA-BRECS.
The first SLA-BRECS rapid prototypes produced, made of RenShape® SL 7870, deformed during perfusion culture at 37°C. Internal positive pressure from perfusion at incubator temperature over weeks of culture time, lead to SLA-BRECS wall displacement, likely due to the resin's relatively low heat deflection temperature (48°C). Because this resin did not offer the desired structural integrity at culture conditions for this application, a second SLA resin material, Watershed® XC 11122, was used with a slightly higher heat deflection temperature (49 to 55°C). In addition, support crossbars were built into the clamp-ring, which was being used to keep the two halves of the SLA-BRECS together. This approach provided adequate mechanical support to prevent deformation over an incubator culture period of 3 months. This support was also adequate to maintain structural stability during cryopreservation, cryostorage at gas phase liquid nitrogen temperatures, and during thaw.
SLA-BRECS with 20 disks (2.5 mm thick) and SLA-BRECS with 24 disks (2 mm disks) theoretically should be able to support the same number cells due to their equivalent surface area for cell growth. However, these studies demonstrated that SLA BRECS with 24 disks (2 mm thick) perform better, likely due to better delivery of oxygen and nutrients to the interior of the thinner porous disks, or due to greater cell seeding efficiency. SLA-BRECS with 32 disks (2 mm thick) increased the available cell growth surface area and effectively increased cell dose. Growth of adequate cell numbers to achieve a therapeutic impact (~108 cells) was allowed due to disks' high surface area, and high porosity (80–90%) which allowed for sufficient, homogeneous delivery of oxygen and nutrients to maintain the cells on disks.
Due to the finalized design identified through SLA-BRECS rapid prototypes, the mass production of IM-BRECS was enabled through injection molding with the tools developed. The resulting IM-BRECS with 32 disks (2 mm thick) performed similarly, if not better than the SLA-BRECS design with 32 disks (2 mm thick). Furthermore, similar to the previously reported BRECS5, the SLA-BRECS and IM-BRECS were both able to be cryopreserved for cryostorage, and then thawed and maintained at 37°C. This process was accompanied by an average cell retention and viability of over 90%.
The finalized IM-BRECS design can be rapidly mass produced, providing an all-in-one solution to several challenging manufacturing, storage, distribution and therapeutic use challenges for cell therapy. Mass production and quicker heating and cooling, while maintaining similar cellular metabolic output are characteristics of the IM-BRECS which are substantial improvements over SLA-BRECS, and the CNC-BRECS previously reported by our group.5
Allogenic cell sourcing from human kidneys unsuitable for transplant,10 combined with the ability to cryopreserve, store and ship cell-devices for future thaw, followed by therapeutic application via an immuno-isolated extracorporeal circuit, enables the ability to maintain a clinically relevant supply of devices. This capability makes both emergent and acute use of the BRECS technology feasible.
Lastly, the devices described in this report, were recently used in the pre-clinical testing of the BRECS for the treatment of AKI caused by septic shock in an established porcine model.16 Results from this porcine model suggest that BRECS cell therapy delivered from an extracorporeal circuit exhibited therapeutic efficacy with improved cardiovascular performance and prolonged survival time compared with acellular controls.17
Acknowledgments
Sources of Funding: This work was supported by the U.S. Army Medical Research and Materiel Command (http://mrmc.amedd.army.mil/), Contract W81XWH-05-2-0010 and W81XWH-10-2-0137, and the Small Business Innovation Research (SBIR) (https://www.sbir.gov/) program of the National Institutes of Health (NIH) (http://www.nih.gov/), Grants NIDDK R44 DK074289 and NIDDK R43 DK082050.
We acknowledge the provision of human kidneys, used to isolate renal epithelial cells, by the National Disease Research Interchange (NDRI) (http://ndriresource.org/) with support by grant number 5 U42 RR006042 from NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the U.S. Army Medical Research and Materiel Command.
Conflict of Interest: HDH is a shareholder of Innovative BioTherapies, Inc. CJP, AJW and DAB are employees of Innovative BioTherapies.
Mark Neitzke and Tyson Delandsheer were instrumental in CAD, and physical production of prototypes and injection molded parts. Tyson Delandsheer, Charu DeWitt, Miller Tsai, and Min Wang provided valuable technical support toward the findings in these studies.
References
- 1.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. Distal tubular injury. Am J Physiol. 1998;275:F623–F631. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 2.Humes HD, Weitzel WF, Bartlett RH, et al. Initial clinical results of the bioartificial kidney containing human cells in icu patients with acute renal failure. Kidney international. 2004;66:1578–1588. doi: 10.1111/j.1523-1755.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 3.Tumlin J, Wali R, Williams W, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: The missing link in the regenerative medicine supply chain. Rejuvenation Res. 2006;9:279–291. doi: 10.1089/rej.2006.9.279. [DOI] [PubMed] [Google Scholar]
- 5.Buffington D, Pino C, Chen L, Westover A, Hageman G, Humes H. Bioartificial renal epithelial cell system (brecs): A compact, cryopreservable extracorporeal renal replacement device. Cell Medicine. 2012;4:33–43. doi: 10.3727/215517912X653328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtas AR, Wood HG, Allaire PE, McDaniel JC, Day SW, Olsen DB. Computational fluid dynamics modeling of impeller designs for the heartquest left ventricular assist device. ASAIO J. 2002;48:552–561. doi: 10.1097/00002480-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Fill B, Gartner M, Johnson G, Horner M, Ma J. Computational fluid flow and mass transfer of a functionally integrated pediatric pump-oxygenator configuration. ASAIO J. 2008;54:214–219. doi: 10.1097/MAT.0b013e3181648d80. [DOI] [PubMed] [Google Scholar]
- 8.Hutmacher DW, Singh H. Computational fluid dynamics for improved bioreactor design and 3d culture. Trends Biotechnol. 2008;26:166–172. doi: 10.1016/j.tibtech.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Kelly WJ. Using computational fluid dynamics to characterize and improve bioreactor performance. Biotechnol Appl Biochem. 2008;49:225–238. doi: 10.1042/BA20070177. [DOI] [PubMed] [Google Scholar]
- 10.Westover AJ, Buffington DA, Humes HD. Enhanced propagation of adult human renal epithelial progenitor cells to improve cell sourcing for tissue-engineered therapeutic devices for renal diseases. J Tissue Eng Regen Med. 2012;6:589–597. doi: 10.1002/term.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieg AF, Rosenblum LJ, Henry JB. Lactate dehydrogenase isoenzymes a comparison of pyruvate-to-lactate and lactate-to-pyruvate assays. Clin Chem. 1967;13:196–203. [PubMed] [Google Scholar]
- 12.Van Den Hamer CJ, Elias RW. A method for the determination of d(−)-lactic acid. Biochim Biophys Acta. 1958;29:556–562. doi: 10.1016/0006-3002(58)90012-x. [DOI] [PubMed] [Google Scholar]
- 13.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 14.Nikolovski J, Gulari E, Humes HD. Design engineering of a bioartificial renal tubule cell therapy device. Cell transplantation. 1999;8:351–364. doi: 10.1177/096368979900800403. [DOI] [PubMed] [Google Scholar]
- 15.Swann RC, Merrill JP. The clinical course of acute renal failure. Medicine (Baltimore) 1953;32:215–292. doi: 10.1097/00005792-195305000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Humes HD, Buffington DA, Lou L, et al. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Critical care medicine. 2003;31:2421–2428. doi: 10.1097/01.CCM.0000089644.70597.C1. [DOI] [PubMed] [Google Scholar]
- 17.Westover AJ, Buffington DA, Johnston KA, Smith PL, Pino CJ, Humes HD. A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]