Abstract
Importance
As perinatally HIV-infected youth (PHIVY) in the US grow older and more treatment-experienced, clinicians need updated information about the impact of age, CD4 count, viral load (VL), and antiretroviral drug (ARV) use on risks of opportunistic infections (OIs), key clinical events, and mortality in order to understand patient risks and improve care.
Objective
To determine the incidence or first occurrence during follow-up of key clinical events (including CDC-B and CDC-C events) and mortality among PHIVY stratified by age, CD4, and VL/ARV status.
Design
In the PHACS Adolescent Master Protocol (AMP) and IMPAACT P1074 multicenter cohort studies (2007–2015), we estimated event rates during person-time spent in key strata of age (7–12, 13–17, and 18–30 years), CD4 count (<200, 200–499, and ≥500 cells/μL), and VL/ARV status (< or ≥ 400 copies/mL; ARVs or no ARVs).
Setting
41 ambulatory sites in the US, including Puerto Rico.
Participants
1,562 participants in AMP and P1074 were eligible, 1446 PHIVY were included.
Exposure(s) for observational studies
Age, CD4 count, VL, ARV use.
Main outcomes
Clinical event rates stratified by person-time in age, CD4 count, and VL/ARV categories.
Results
During a mean follow-up of 4.9 years, higher incidences of CDC-B events, CDC-C events and mortality were observed as participants aged. Older PHIVY (13–17 and 18–30 year-olds) spent more time with VL ≥400 copies/mL and with CD4 <200/μL compared to 7–12 year-olds (30% and 44% vs. 22% of person-time with VL ≥400 copies/mL; 5% and 18% vs. 2% of person-time with CD4 <200/μL; p<0.01 for each comparison). We observed higher rates of CDC-B events, CDC-C events, bacterial infections, and mortality at lower CD4 counts, as expected. The mortality rate in older PHIVY was 6–12 times that of the general US population. Higher rates of sexually transmitted infections were also observed at lower CD4 counts, after adjusting for age.
Conclusions and relevance
Older PHIVY were at increased risk of viremia, immunosuppression, CDC-B events, CDC-C events, and mortality. Interventions to improve ART adherence and optimize models of care for PHIVY as they age are urgently needed to improve long-term outcomes among PHIVY.
Keywords: Perinatal HIV infection, antiretroviral therapy, HIV viral load, CD4 count, adolescence, youth
INTRODUCTION
Effective interventions to prevent mother-to-child HIV transmission and treat HIV-infected infants and children have shifted the US pediatric HIV epidemic; youth aged ≥13 years now represent the majority of perinatally HIV-infected youth (PHIVY) in the US.1–3 Rates of viremia and immunosuppression have decreased among PHIVY in the US since the implementation of effective combination antiretroviral therapy (cART), but may remain higher for older PHIVY.4,5 Compared to adults, PHIVY experience lower rates of HIV RNA viral load (VL) suppression and higher rates of loss to follow-up.6–8 As youth age and transition to adult care, their risks of opportunistic infections (OI), other serious clinical events, and mortality are not well described.9–14
Understanding the frequency of important clinical events for PHIVY, as well as the consequences of being prescribed cART without a suppressed VL, will provide critical information to design interventions for this group, who are at risk for severe illness, accumulation of resistance mutations, and secondary transmission.15,16 Our objectives were to determine the frequency of viremia and immunosuppression among PHIVY and young adults aged 7–30 years engaged in care in two large national cohort studies and to analyze associations between age, CD4 count, viremia, ARV use, and risks of significant clinical events and mortality.
METHODS
Study Population
We evaluated participants in the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) and the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1074 cohort studies. AMP enrolled 451 PHIVY aged 7–16 years between March 2007 and November 2009 at 15 US sites; follow-up is ongoing.2 P1074 enrolled 1,201 participants (87% PHIVY) with a mean age of 17.4 years (SD 5.4) between April 2009 and June 2013 at 40 US sites; follow-up was completed in June 2014.17 For this analysis, AMP participants were eligible if they had ≥1 visits between March 2007 and April 2015, and ≥1 CD4 and VL recorded after baseline; P1074 participants were eligible if they were PHIVY and had ≥1 chart abstraction with CD4 count and VL data recorded after baseline. AMP baseline was defined as date of study entry; P1074 baseline was defined as 1 year prior to study entry. Simultaneous co-enrollment in AMP and P1074 was not permitted. Based on guidelines, practice patterns, and trial data, we defined cART regimens as one of three mutually exclusive types expected to be suppressive: (1) ≥3 drugs from ≥2 classes, or (2) a protease inhibitor (PI, excluding ritonavir alone) + 1 drug from another class, or (3) ≥3 nucleos(t)ide reverse transcriptase inhibitors.2,18–22 We excluded person-time when patients had VL <400 copies/mL and were not prescribed ARVs and when patients had VL ≥400 copies/mL while being prescribed an ARV regimen other than cART. Although individual patient circumstances may have warranted these approaches, they are not expected to suppress VL and were not standard of care during the study period.2,18–22 Lost to follow-up was defined as stopping data collection for any reason other than death, study completion, or site closure.
Clinical and Laboratory Data
Participant consent and data collection methods have been described previously for both studies.2,17 CD4 counts, VLs, ARV use, and clinical events were abstracted from medical records, and clinical events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 18 by Frontier Science Technology and Research Foundation.23
Outcome Measures
Primary outcomes included mortality and first occurrence of CDC stage C/WHO stage 4 or CDC stage B/WHO stage 3 events during follow-up.24–27 Secondary outcomes included: bacterial pneumonia; serious bacterial infections; presumptive pelvic inflammatory disease (PID); other sexually transmitted infections (STI); pregnancy; mental health and neurodevelopmental conditions; asthma, atopy or allergy conditions; gastrointestinal conditions; cardiac conditions; anemia; pancreatitis or hepatitis; peripheral neuropathy; and metabolic or bone abnormalities, including renal conditions. Presumptive PID and pregnancy were limited to females aged ≥13; other STIs were limited to age ≥13 and stratified by sex due to sex-specific screening practices. Each MedDRA term was assigned a single diagnostic category, except for bacterial pneumonia and PID which were included in CDC-B/WHO-3 events, and also examined separately.
Statistical Analyses
We estimated incidence rates of key clinical events, stratified by the combination of time-varying age (7–12, 13–17, and 18–30 years), CD4 count (<200, 200–499, and ≥500/μL), and VL/ARV status. VL/ARV status was categorized as: 1) suppressive ARVs: VL <400 copies/mL and prescribed any ARVs, 2) non-suppressive cART: VL ≥400 copies/mL and prescribed cART, and 3) no ARVs: VL ≥400 copies/mL and not prescribed any ARVs.
We estimated incidence rates and assessed trends by age, CD4 count, and VL/ARV categories using Poisson regression models, accounting for within-subject correlation with robust standard variance estimators. For selected individual events (STIs, pregnancy and cardiac events), incidence rates by CD4 counts were adjusted for age using inverse probability weighting because person-time contributing to each age category was unevenly distributed by CD4 category.28–33 Only the first occurrence of each event after baseline for each participant was included.
To assign participants to baseline strata and calculate the total person-time contributed by all participants to each stratum, we used linear interpolation between adjacent CD4 and log10-transformed VL readings, and estimated dates when strata thresholds were crossed. The nearest CD4 and VL readings prior to baseline were used when available for interpolation, and last available CD4 count and VL were carried forward until the end of follow-up.
We used weighted repeated measures generalized estimating equation (GEE) models with an independence working correlation, identity link, normal distribution and robust variance estimators to determine the association between: age and person-time with VL ≥400 copies/mL, age and person-time with CD4 <200/μL, and VL/ARV status and person-time with CD4 <200/μL. For these GEE analyses, we additionally sub-divided the older age stratum into 18–21, 22–25, and 26–30 years.
To compare mortality rates between PHIVY and youth in the general US population, we calculated standardized mortality ratios (SMRs) standardized to CDC age, sex and race distributions.34 Confidence intervals (CI) for SMRs were calculated using the Boice-Monson method.
RESULTS
Characteristics of the study population
Of 1,467 PHIVY in AMP and/or P1074, we excluded 18 who did not have the combination of ARV, CD4 count and VL data at any point during follow-up, 1 who spent their entire person-time in the study with VL ≥400 while on a regimen other than cART, and 2 who spent their entire person-time with VL <400 and off ARVs. We excluded 92.3 person-years (PY) (1.4% of overall person-time) while participants were viremic and on a regimen other than cART and 59.4 PY (0.9% of overall person-time) while participants had VL <400 copies/mL while off ARVs, from a total of 247 participants. Table 1 reports additional baseline and follow-up characteristics. Mean age at baseline was 14.6 years, with 52% female participants; 66% identified as black, and 26% identified as Hispanic. Notably, patients who were lost to follow-up differed significantly from retained patients only in baseline age (15.8 vs. 14.4 years).
Table 1.
Characteristics of PHACS AMP and IMPAACT P1074 participants at baseline
| Study | Participants, n (%) |
|---|---|
| AMP | 421 (29%) |
| P1074 | 1,008 (70%) |
| Both | 17 (1%) |
| Demographic characteristics | Total participants (n=1,446) |
| Age at baseline, years, mean (SD) | 14.6 (4.6) |
| Female sex, n (%) | 759 (52%) |
| Year of birth, mean (SD) | 1994 (4.6) |
| Race | |
| Black/African-American, n (%) | 953 (66%) |
| White/other, n (%) | 470 (33%) |
| Not reported, n (%) | 23 (2%) |
| Hispanic ethnicity, n (%) | 370 (26%) |
| Not reported, n (%) | 1 (0%) |
| Clinical characteristics | |
| CD4 count at baseline, cells/μL, mean (SD) | 712 (422) |
| Viral load <400 copies/mL at baseline, n (%) | 934 (65%) |
| Prescribed cART at baseline, n (%) | 1,330 (92%) |
| Prescribed ARVS but not cART at baseline, n (%) | 68 (5%) |
| No ARVs at baseline, n (%) | 48 (3%) |
| CD4 counts per person per year during follow-up, mean (SD) | 3.6 (9.6) |
| Viral loads per person per year during follow-up, mean (SD) | 3.6 (9.6) |
| Total ARV regimens per person, mean (SD) a | 2.4 (1.6) |
| Years of follow-up under study protocols, mean (SD) | 4.9 (1.3) |
| Cumulative loss to follow-up, n (%) | 171 (12%) |
Data are presented as number (%), or mean (SD).
Regimen change was defined as a change in any single drug.
ARV, antiretroviral; cART, combination antiretroviral therapy; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Network; PHACS AMP, Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol; VL, viral load.
Distribution of person-time
Participants contributed 19% of person-time between ages 7–12, 38% between 13–17, and 43% between 18–30; only 2% of total person-time was between ages 26–30 (Table 2). The majority of person-time was spent with CD4 counts ≥500/μL (65% of person-time, compared to 24% at 200–499/μL and 10% at <200/μL), and on suppressive ARVs (66% of person-time, compared to 28% on non-suppressive cART and 7% on no ARVs). Person-years spent in older age strata (ages 13–17 and 18–30), compared to ages 7–12, were more likely to be spent with CD4 counts <200/μL (5% and 18% vs. 2%, p<0.001 for each pair-wise comparison). For older PHIVY (age ≥18), more person-time was also spent with VL ≥400 copies/mL (either non-suppressive cART or no ARVs, 30% and 44% vs. 22% of person-time, p<0.001 for both). When the older age stratum was further divided into 18–21, 22–25, and 26–30 years, proportions of time spent being viremic and with low CD4 counts remained substantially higher for each sub-stratum compared to younger ages (Table 3). Use of non-suppressive cART was not associated with having CD4 <200/μL, compared to use of no ARVs (25% vs. 24%, p=0.72).
Table 2.
Distribution of person-time stratified by age, CD4 count and viral load and antiretroviral status
| Distribution of person-time during follow-up | Participants, n (%) a | Person-time, years (%) b |
|---|---|---|
| Age (years) | ||
| 7–12 | 504 (35) | 1,243 (19) |
| 13–17 | 921 (64) | 2,459 (38) |
| 18–30 | 967 (67) | 2,846 (43) |
| 18–21 | 871 (60) | 1,887 (29) |
| 22–25 | 428 (30) | 806 (12) |
| 26–30 | 87 (6) | 153 (2) |
| CD4 count (cells/μL) | ||
| ≥500 | 1,211 (84) | 4,286 (65) |
| 200–499 | 850 (59) | 1,597 (24) |
| <200 | 318 (22) | 664 (10) |
| VL/ARV status | ||
| Suppressive ARVs | 1,328 (92) | 4,292 (66) |
| Non-suppressive cART | 941 (65) | 1,810 (28) |
| No ARVs | 346 (24) | 445 (7) |
Number of participants contributing person-time towards a given stratum.
Participants may contribute person-time to more than one stratum.
ARV, antiretroviral; cART, combination antiretroviral therapy; VL, viral load.
Table 3.
Person-time: Association between age, CD4 count and viral load and antiretroviral status
| CD4 count, cells/μL a | Viral load / ARV status b | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| <200 | 200–499 | ≥500 | Suppressive ARVs | Non-suppressive cART | No ARVs | |
| Age, years | ||||||
| 7–12 | 19.2 (2%) | 139.8 (11%) | 1084.1 (87%) | 958.6 (77%) | 239.5 (19%) | 45.1 (4%) |
| 13–17 | 129.3 (5%) | 558.9 (23%) | 1,770.4 (72%) | 1,726.1 (70%) | 587.3 (24%) | 145.2 (6%) |
| 18–30 | 515.7 (18%) | 898.5 (32%) | 1,431.7 (50%) | 1,607.3(56%) | 983.6 (35%) | 255.0 (9%) |
| 18–21 | 276.7 (15%) | 592.4 (31%) | 1,018.1 (54%) | 1,087.0 (58%) | 648.6 (34%) | 151.6 (8%) |
| 22–25 | 202.3 (25%) | 263.0 (33%) | 340.8 (42%) | 426.2 (53%) | 291.0 (36%) | 89.1 (11%) |
| 26–30 | 36.6 (24%) | 43.1 (28%) | 72.8 (48%) | 94.2 (62%) | 44.1 (29%) | 14.3 (9%) |
| Viral load/ARV status c | ||||||
| Suppressive ARVs | 95.4 (2%) | 699.2 (16%) | 3,497.4 (81%) | |||
| Non-suppressive cART | 460.4 (25%) | 704.7 (39%) | 645.3 (36%) | |||
| No ARVs | 108.5 (24%) | 193.3 (43%) | 143.5 (32%) | |||
Row percents are presented.
Proportion of person-time with CD4 count <200 cells/μL according to age: Relative difference (95% confidence interval): 13–17 vs. 7–12 years: 4% (2 – 5%); 18–21 vs. 7–12 years: 13% (10 – 16%); 22–25 vs. 7–12 years: 24% (19 – 28%); 26–30 vs. 7–12 years: 22% (13 – 31%); p<0.001 for all comparisons.
Proportion of person-time with VL ≥400 copies/mL according to age: Relative difference (95% confidence interval): 13–17 vs. 7–12 years: 7% (3 – 11%); 18–21 vs. 7–12 years: 20% (15 – 24%); 22–25 vs. 7–12 years: 24% (18 – 30%); 26–30 vs. 7–12 years: 15% (5 – 25%); p<0.01 for all comparisons. The median frequency of HIV RNA measurements during follow-up for subjects while aged 7–12, 13–17, 18–21, 22–25, and 26–30 years was every 3.6, 3.5, 3.9, 4.3, and 4.3 months, respectively.
Proportion of person-time with CD4 count <200 cells/μL according to VL/ARV status: Relative difference (95% confidence interval): suppressive ARVs vs. no ARVs: −22% (−28 – −17%), p<0.001, non-suppressive cART vs. no ARVs: 1% (−5 – 7%), p=0.72. (Similar results were seen for the outcome of CD4 count <500/μL.)
ARV, antiretroviral; cART, combination antiretroviral therapy
Mortality
Overall, there were 29 deaths (0.4/100PY) (eTables 1–4). Seventy-nine percent of deaths occurred at CD4 <200/μL (3.5/100PY). Eighty-three percent of deaths occurred while VL was ≥400 copies/mL, with mortality rates of 0.9/100PY for non-suppressive cART and 1.6/100PY for no ARVs (p<0.001 for trend). All but one death (97%) occurred in 18–30 year olds (1.0/100PY, p<0.001 for trend). PHIVY between ages 15–19 and 20–29 had 5.6-fold (95%CI: 2.8–11.1) and 12.3-fold (95%CI: 8.0–18.9) higher mortality rates than youth of the same age in the US general population, respectively.34
First events during follow-up
CDC-C/WHO-4 events
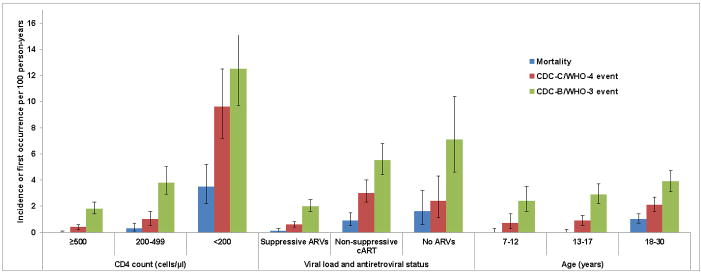
There were 86 CDC-C/WHO-4 events (1.4/100PY; eTable 5). Higher rates of CDC-C/WHO-4 events were observed at lower CD4 counts (<200/μL: 9.6/100PY; 200–499/μL: 1.0/100PY; ≥500/μL: 0.4/100PY; p<0.001 for trend). Of 17 events that occurred at CD4 counts ≥500/μL (20% of total), only 2 were OIs (pulmonary tuberculosis and ocular toxoplasmosis); others were HIV-related kidney and cardiac disease. Higher rates of CDC-C/WHO-4 events occurred with higher VL (suppressive ARVs: 0.6/100PY; non-suppressive cART: 3.0/100PY; no ARVs: 2.4/100PY; p<0.001 for trend). Higher rates of CDC-C/WHO-4 events were also observed with older age (7–12: 0.7/100PY; 13–17: 0.9/100PY; 18–30: 2.1/100PY; p<0.001 for trend).
CDC B/WHO-3 events
There were 193 CDC-B/WHO-3 events (3.2/100PY; Figures 1 and 2; eTable 6). Higher rates of CDC-B/WHO-3 events were observed at lower CD4 counts (<200/μL: 12.5/100PY; 200–499/μL: 3.8/100PY; ≥500/μL: 1.8/100PY; p<0.001 for trend). CDC-B/WHO-3 events were also more common at higher VL (suppressive ARVs: 2.0/100PY; non-suppressive cART: 5.5/100PY; no ARVs: 7.1/100PY; p<0.001 for trend). These event rates also increased as participants aged (7–12: 2.4/100PY; 13–17: 2.9/100PY; 18–30: 3.9/100PY; p=0.01 for trend). For bacterial pneumonia (CDC-B/WHO-3 event) and serious bacterial infections, higher event rates were also observed at lower CD4 counts and higher viral loads, but no trends were observed by age (eTables 7 and 8).
Figure 1.
Incidence of mortality and first occurrence of CDC-C/WHO-4 and CDC-B/WHO-3 events by CD4 count, viral load and antiretroviral status and age in AMP and P1074
VL: viral load; ARV: antiretroviral; cART: combination antiretroviral therapy
Exact Poisson 95% confidence intervals are presented in the error bars.
Figure 2.
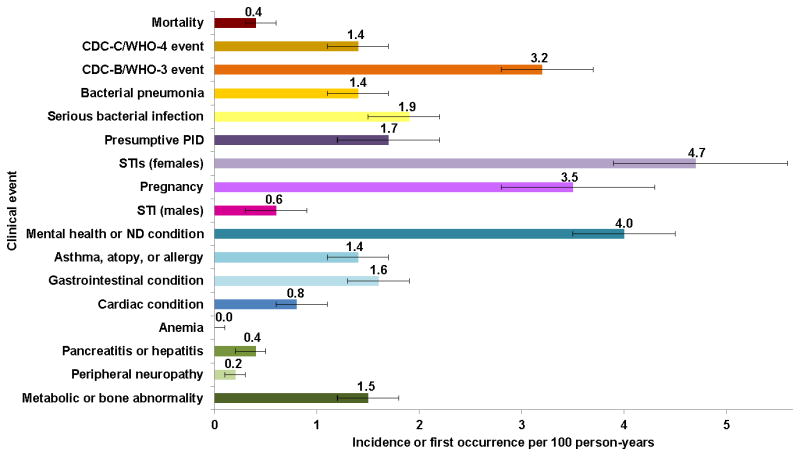
Incidence of mortality and first occurrence of all clinical outcomes in AMP and P1074
PID: pelvic inflammatory disease; STI: sexually transmitted infection; ND: neurodevelopmental
Exact Poisson 95% confidence intervals are presented in the error bars.
Reproductive system events
In female participants, higher rates of presumptive PID, other STIs, and pregnancies were observed with older age and with VL >400 copies/mL (eTables 9–11); higher rates of STIs (not including PID) and pregnancy were observed with lower CD4 counts. After adjusting for age, the association between increasing rates of pregnancies and lower CD4 counts no longer reached significance (<200/μL: 4.5/100PY; 200–499/μL: 4.8/100PY; ≥500/μL: 2.8/100PY; p=0.18); however, the trend of increased rates of first female STI at lower CD4 counts remained significant (<200/μL: 8.1/100PY; 200–499/μL: 5.9/100PY; ≥500/μL: 3.1/100PY; p<0.001). First STIs were infrequently reported in males (eTable 12).
Other clinical events
Mental health and neurodevelopmental conditions were among the most frequent conditions (4.0/100PY); no trends were observed by age, CD4 count, or VL/ARV strata (eTable 13). For asthma, atopy or allergy conditions, no trends were observed by age, CD4 count or VL/ARV strata (eTable 14). For gastrointestinal conditions, rates increased with lower CD4 counts (p<0.001); however, no trends were observed by age or VL/ARV strata (eTable 15). Higher rates of first non-AIDS-defining cardiac events were observed at lower CD4 counts (eTable 16), although this trend was no longer significant after adjusting for age (<200/μL: 2.1/100PY; 200–499/μL: 1.0/100PY; ≥500/μL: 0.7/100PY; p=0.08). Events potentially attributed to antiretroviral toxicity such as anemia (0.0/100PY), pancreatitis or hepatitis (0.4/100PY) and peripheral neuropathy (0.2/100PY) were among the least frequently reported events (eTables 17–19). Rates of first metabolic or bone abnormalities (1.5/100PY) showed no difference by age or CD4 count, but trended towards lower rates in patients with VL >400 copies/mL (suppressive ARVs: 1.7/100PY; non-suppressive cART: 1.1/100PY; no ARVs: 0.7/100PY; p=0.04) (eTable 20).
DISCUSSION
We analyzed rates of clinical events and mortality during 6,548 person-years of follow-up from 1,446 PHIVY aged 7–30 years in the AMP and P1074 cohort studies, stratified by time-updated age, CD4 count, and VL/ARV status. There were three key findings from this work. First, older youth were at highest risk for viremia, low CD4 counts, and serious clinical events, including mortality, CDC-C/WHO-4, and CDC-B/WHO-3 events. Viral load monitoring remained consistent across age ranges, suggesting ongoing engagement in care; high overall rates of viremia in participants aged 18–30 years are likely therefore due to suboptimal medication adherence or acceptance, or accumulated HIV viral resistance. Older PHIVY had greater early exposure to mono- or dual-regimens compared to younger PHIVY; the lack of viral suppression among those PHIVY prescribed cART is more likely related to poor medication adherence or acceptance, as resistance to newer ARVs, such as integrase inhibitors, is uncommon.35 Older youth also spent more time with CD4 counts <200/μL. Data from PHIVY aged 6–17 suggest that having CD4 counts <200/μL is associated with lower quality of life, psychiatric symptoms, and poor cognitive, academic, and social functioning, and data from adults suggest high risks of OIs and death.36,37 Our findings are consistent with a growing literature outlining the challenges to adhering to medications for PHIVY in adolescence, which intensify as PHIVY reach early adulthood38–43
Second, we observed relatively few clinical events and deaths during the follow-up period, during which cART was standard of care. These results add to prior reports of clinical events in AMP and P1074 by stratifying clinical event rates by time-updated age, CD4, and VL/ARV status.1,17 Our results are similar to other cohort studies of PHIVY in the US, UK and Ireland.9,13,44 For example, our observed incidence rates of mortality (0.4/100 PY), CDC-C/WHO-4 events (1.4/100 PY), CDC-B/WHO-3 events (3.2/100 PY), and bacterial pneumonia (1.4/100 PY) were comparable to those reported in the PACTG 219c cohort in 2006, 10 years into the cART-era (mortality: 0.5–0.8/100 PY; CDC-C: 1.5/100 PY; CDC-B: 5.0/100 PY; bacterial pneumonia 2.2/100 PY).5,12,44 However, the mortality rate in older adolescent (ages 15–19) and young adult (ages 20–29) PHIVY remained 6 and 12 times that of the US general population, respectively, after accounting for age, sex, and race. STIs among female participants and mental health and neurodevelopmental diagnoses were among the most commonly documented conditions. Higher STI rates (excluding PID) among female participants were associated with lower CD4 counts after adjusting for age. This finding has been previously reported, as well as the association of lower CD4 with herpes simplex virus reactivation and lack of human papilloma virus clearance.28–30,45–49 These data suggest a potential biological effect of immunosuppression; additionally, more frequent risk behavior among patients incompletely adherent to cART may contribute to higher STI rates.8,30 Pregnancy rates overall (3.5/100PY) and at older ages (13–17 years: 2.0/100PY and 18–30 years: 4.9/100PY) were similar to the general population (range for 15–29 years: 3.95–16.30/100PY).50 Pregnancy rates were higher among those with lower CD4 counts, although the strength of this relationship decreased after age adjustment. Complications potentially related to long-term antiretroviral therapy, including anemia, pancreatitis, hepatitis, peripheral neuropathy, and metabolic and bone abnormalities, were documented infrequently, likely reflecting use of less toxic ARVs over time.35,51
Finally, our person-time results add a valuable dimension to the literature base of cross-sectional and longitudinal studies on viremia in PHIVY. In the AMP and P1074 cohorts described here, 66% of person-time was spent with VL <400 copies/mL, similar to that observed in the HIV Research Network (63% of PHIVY aged 12–21 with VL <400 copies/mL from 2009–2012). However, viral suppression is higher than reported in the Adolescent Trials Network (ATN, 37% of PHIVY with VL below the lower level of detection) and in a recent meta-analysis (pooled North America estimate of youth with suppressed VL: 53% (range: 28–75%) of youth aged 12–24 years from 1990–2013).4,8,52 While lower suppression rates in the meta-analysis cohorts may reflect the inclusion of non-perinatally HIV-infected youth, lower suppression rates for PHIVY in the ATN cohort may reflect older age in that cohort (17.9 years versus 14.6 years). In contrast to data from adults, we found that having VL ≥400 copies/mL while being prescribed cART did not improve immunosuppression compared to having VL ≥400 copies/mL while taking no ARVs at all.53–61 The 35% of person-time spent with VL ≥400 copies/mL in our study raises critical concerns not only for individual patient outcomes, but also for the HIV epidemic among US youth.30,35,62 Youth who are viremic despite being engaged in care are more likely to have resistant virus and to report lower condom use than non-viremic youth, and thus are at risk of secondary horizontal or vertical transmission.63–65
This analysis of PHIVY in two large cohort studies had several limitations. First, the smallest proportion of person-time (19%) was spent at ages 7–12; nevertheless, we had adequate power for all statistical comparisons. Next, data collection protocols in the two cohorts may have impacted incidence rate estimates for specific events: 1) because we analyzed first events, we could not identify “recurrent bacterial pneumonia,” potentially underestimating CDC-C events; 2) STI screening was not performed at study-specific intervals but per local care practice; 3) care sought by participants at non-study healthcare facilities was recorded only if reported to study staff. Additionally, P1074 recorded only events deemed “clinically significant” by healthcare providers, whereas AMP recorded all events; we addressed this limitation by including only events that met the reporting threshold for both studies (e.g. laboratory-value diagnoses were excluded). Furthermore, PHIVY least engaged in care and at highest risk for adverse outcomes may not have been included. Conversely, to permit consistent coding of events between the AMP and P1074 studies, we excluded events prior to baseline, which may have led to overestimation of some first event rates. Finally, adherence data were not included in the analysis, because adherence measures were collected only in one study (AMP). Despite these counterbalancing possibilities, this analysis provides the most recent, detailed data available about clinical risks in US PHIVY over time. This information will be critical information for US policy makers as research and programmatic funding shifts for pediatric HIV in the US.66
In summary, we find that serious clinical events, including OIs and death, are rare in PHIVY receiving suppressive ART, but viremia, lower CD4 counts, and rates of serious clinical events and mortality increase throughout adolescence and young adulthood. Interventions to improve ART adherence and optimize models of care as perinatally HIV-infected youth age are urgently needed to improve long-term outcomes among this growing and vulnerable population.
Supplementary Material
Acknowledgments
PHACS
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigators: Kenneth Rich, Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2015, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx-Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Boston Children’s Hospital: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, James Oleske; St. Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Juliana Darrow, Emily Barr, Paul Harding; University of Miami: Gwendolyn Scott, Grace Alvarez, Anai Cuadra.
IMPAACT
We thank the children and families for their participation in P1074 and the individuals and institutions involved in the conduct of P1074, as follows: New Jersey Medical School: Arry Dieudonne, MD, Linda Bettica, LPN, Anthony Scolpino, BA, James Oleske MD, MPH; UCLA–Los Angeles/Brazil AIDS Consortium: Yvonne Bryson, MD, Michele Carter, RN, Jaime Deville, MD, Karin Nielsen, MD; Texas Children’s Hospital: Michelle Del Rey, RN, Chivon McMullen-Jackson, BSN, RN, ADN, William Shearer, MD, PhD, Mary Paul, MD; Lurie Children’s Hospital of Chicago: Ram Yogev, MD, Margaret Ann Sanders, MPH, Ruth Williams, RN, Lynn Heald, PNP; Columbia University Medical Center (staff not specified); University of Miami Pediatric Perinatal HIV/AIDS: Gwendolyn Scott, MD, Charles Mitchell, MD, Claudia Florez, MD, Grace Alvarez, MD; University of California, San Diego, Mother-Child-Adolescent Program: Stephen Spector, MD, Rolando Viani, MD, MTP, Kimberly Norris, RN, BSN, Lisa Stangl, RN, NP; Duke University Medical Center: John Swetnam, Margaret Donnelly, PA-C, Joan Wilson, RN, MSN, Sunita Patil, PhD; Metropolitan Hospital: Mahrukh Bamji, MD, Indu Pathak, MD, Savita Manwanim, MD, Ekta Patel, MD; Boston Children’s Hospital: Sandra Burchett, MD, MS, Nancy Karthas, RN, MS, CPNP, Catherine Kneut, RN, MS, CPNP, Charlotte Mao, MD, MPH; Boston Medical Center Pediatric HIV Program: Ellen Cooper, MD, Diana Clarke, PharmD, Debra McLaud, RN, Pablo Leitz, MPH; New York University, New York: Aditya Kaul, MD, Nagamah Deygoo, MS, William Borkowsky, MD, Siham Akleh, RN; Jacobi Medical Center, Bronx: Michael Rosenberg, MD, Joanna Dobroszycki, MD, Karen Kassen, RN, BSN, Marlene Burey, NP; Children’s National Medical Center, Washington, DC: Steven Zeichner, MD, PhD, Connie Trexler, RN; Seattle Children’s Hospital: Ann Melvin, MD, MPH, Gloria Bowen, MA, Amanda Robson Nuss, BS, Carrie Pettler, MPH; University of South Florida– Tampa: Carina Rodriguez, MD, Patricia Emmanuel, MD, Denise Casey, RN, Alicia Marion, ARNP; San Juan City Hospital: Nicolas Rosario-Matos, MD, Wanda Marrero-Figueroa, BSN-RN, Carlos Ortega, BA, Lizbeth Fabregas, BS, MS; SUNY Stony Brook: Sharon Nachman, MD, Denise Ferraro, FNP, Erin Infanzon, Michele Kelly, NP; Children’s Hospital of Michigan: Chokechai Rongkavilit, MD, Ayanna Walters, RN, Eric McGrath, MD; Howard University, Washington DC: Sohail Rana, MD, Chandni Parikh, PNP, Caroline Reed, FNP, Patricia Houston, MS; Harbor UCLA Medical Center: Margaret Keller, MD, Michael Bolaris, MD, Judy Hayes, RN, Yolanda Gonzalez, RN; University of Southern California School of Medicine–Los Angeles County: Eva Operskalski, PhD, MBA, James Homans, MD, MPH, LaShonda Spencer, MD, Andrea Kovacs, MD; University of Florida Health Science Center: Mobeen Rathore, MD, Nizar Maraqa, MD, Saniyyah Mahmoudi, MSN, ARNP, Tabetha Gayton, PhD, ARNP; University of Colorado Denver: Hannah Bernath, MPH, Kerry Hahn, CCRP, Jennifer Dunn, MS, RN, FNP, Jennifer Englund, BS; South Florida Children’s Diagnostic and Treatment Center Fort Lauderdale: Ana Puga, MD, Amy Inman, Zulma Eysallenne, RN, James Blood, MSW; Strong Memorial Hospital, University of Rochester Medical Center: Geoffrey Weinberg, MD, Barbra Murante, MS, RN, PNP; Rush University Cook County Hospital, Chicago: Kenneth Boyer, MD, Jamie Martinez, MD, James McAuley, MD, Maureen Haak; Children’s Hospital of Los Angeles: Nancy Flores, Diane Tucker, MSN, Julie McAvoy, MPH, Marvin Belzer, MD; University of California San Francisco: Diane Wara, MD, Theodore Ruel, MD, Mica Muskat, NP, Nicole Tilton, NP; Johns Hopkins University Baltimore: Allison Agwu, MD, ScM, Thuy Anderson, RN, BSN, Aleisha Collinson-Streng, RN, BSN, ACRN, Kaye Park, MPH; Miller Children’s Hospital: Audra Deveikis, MD, Jagmohan Batra, MD, Tempe Chen, MD, David Michalik, DO; University of Maryland Baltimore: Douglas Watson, MD, Maria Johnson, DDS, Susan Lovelace, MSN, Corinda Hilyard; Tulane University New Orleans: Russell Van Dyke, MD, Margarita Silio, MD, Thomas Alchediak, MD, Sheila Bradford, RN; University of Alabama, Birmingham: Dorothy Shaw, BA, Sharan Robbins, BA, MAE, Newana Beatty, CCRC, Marilyn Crain, MD, MPH; The Children’s Hospital of Philadelphia: Carol Vincent, PhD, CRNP, Richard Rutstein, MD, Steven Douglas, MD, Sheri McDougall, MSHed, CCRC; Bronx-Lebanon Hospital: Anna Marie Emeh, MD, Mary Elizabeth Vachon, MPH, Levi Cherian, Murli Purswani, MD, FAAP; St Jude’s Children’s Hospital: Katherine Knapp, MD, Patricia Flynn, MD, Judy Glenn, LPN, Thomas Wride, MS; University of Puerto Rico Pediatric HIV/AIDS Research Program: Irma Febo, MD, Ruth Santos-Otero, RN, MPH, Maritza Cruz-Rodriguez, BA; Western New England Maternal Pediatric Adolescent AIDS: Katherine Luzuriaga, MD, Christina Hermos, MD, Jesica Pagano-Therrien, CPNP, Donna Picard BSN, RN, BC.
CEPAC
The authors gratefully acknowledge Simone Frank and Alex Bulteel for assistance with manuscript preparation, as well as Dr. Kenneth Freedberg and the Cost-effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric research team in the Medical Practice Evaluation Center at Massachusetts General Hospital for providing feedback on study design and interpretation.
Dr. Patel and Mr. Karalius had full access to all data reported in this manuscript
FUNDING SOURCES
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104).
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group was provided by the National Institute of Allergy and Infectious Diseases of the NIH under (grants UM1AI068632 [IMPAACT Leadership and Operations Center], UM1AI068616 [IMPAACT Statistical and Data Management Center], and UM1AI106716 [IMPAACT Laboratory Center]), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health.
This research was also funded by the National Institute of Allergy and Infectious Diseases (T32 AI007433 (AMN), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD079214 (ALC, GRS, KP)) and the Charles Hood Foundation Child Health Research Award (ALC, AMN, KP).
Footnotes
Cohort Registration:
Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) (http://www.phacsstudy.org); NCT01418014
International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1074 (http://impaactnetwork.org); NCT01061164
AUTHOR ROLES
All authors contributed substantively to this manuscript in the following ways: Study design (AMN, KP, RVD, ALA, GRS III, ALC), data analysis (KP, BK), interpretation of results (all authors), drafting the manuscript (AMN, KP, BK, ALC), critical revision of the manuscript (all authors) and final approval of submitted version (all authors).
Conflicts of Interest and Financial Disclosures
The authors have no conflicts of interest or financial disclosures.
Role of the funding sources
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services or other funders. The funding sources had no role in study design; collection, analysis, or interpretation of data; manuscript preparation; or decision to submit the paper for publication.
References
- 1.Krogstad P, Patel K, Karalius B, et al. Incomplete immune reconstitution despite virologic suppression in HIV-1 infected children and adolescents. AIDS. 2015;29(6):683–93. doi: 10.1097/QAD.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57(2):165–73. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Accessed January 17, 2016];HIV Surveillance Report. 2015 27 http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2016. [Google Scholar]
- 4.Agwu AL, Fleishman JA, Rutstein R, Korthuis PT, Gebo K. Changes in advanced immunosuppression and detectable HIV viremia among perinatally HIV-infected youth in the multisite United States HIV Research Network. J Pediatric Infect Dis Soc. 2013;2(3):215–23. doi: 10.1093/jpids/pit008. [DOI] [PubMed] [Google Scholar]
- 5.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDs. 2014;28(3):128–35. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryscavage P, Anderson EJ, Sutton SH, Reddy S, Taiwo B. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58(2):193–7. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 8.Kahana SY, Fernandez MI, Wilson PA, et al. Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally HIV-infected youth linked to care in the United States. J Acquir Immune Defic Syndr. 2015;68(2):169–77. doi: 10.1097/QAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachman SA, Chernoff M, Gona P, et al. Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Arch Pediatr Adolesc Med. 2009;163(2):164–71. doi: 10.1001/archpedi.163.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agwu AL, Siberry GK, Ellen J, et al. Predictors of highly active antiretroviral therapy utilization for behaviorally HIV-1-infected youth: impact of adult versus pediatric clinical care site. J Adolesc Health. 2012;50(5):471–7. doi: 10.1016/j.jadohealth.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dollfus C, Le Chenadec J, Faye A, et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clin Infect Dis. 2010;51(2):214–24. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- 12.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 13.Nesheim SR, Hardnett F, Wheeling JT, et al. Incidence of opportunistic illness before and after initiation of highly active antiretroviral therapy in children. Pediatr Infect Dis J. 2013;32(10):1089–95. doi: 10.1097/INF.0b013e31829ee893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry SA, Gebo KA, Rutstein RM, et al. Trends in hospitalizations among children and young adults with perinatally acquired HIV. Pediatr Infect Dis J. 2014;33(5):488–94. doi: 10.1097/INF.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young J, Psichogiou M, Meyer L, et al. CD4 cell count and the risk of AIDS or death in HIV-infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9(3):e1001194. doi: 10.1371/journal.pmed.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoufaly A, Cozzi-Lepri A, Reekie J, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One. 2014;9(1):e87160. doi: 10.1371/journal.pone.0087160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirani G, Williams PL, Chernoff M, et al. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin Infect Dis. 2015;61(12):1850–61. doi: 10.1093/cid/civ687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panel on antiretroviral therapy and medical management of HIV-infected children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Accessed January 13, 2017];2006 2008:2009. https://aidsinfo.nih.gov/guidelines/archive/pediatric-guidelines/ [Google Scholar]
- 19.Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14(7):572–80. doi: 10.1016/S1473-3099(14)70736-4. [DOI] [PubMed] [Google Scholar]
- 20.Mondi A, Fabbiani M, Ciccarelli N, et al. Efficacy and safety of treatment simplification to atazanavir/ritonavir + lamivudine in HIV-infected patients with virological suppression: 144 week follow-up of the AtLaS pilot study. J Antimicrob Chemother. 2015;70(6):1843–9. doi: 10.1093/jac/dkv037. [DOI] [PubMed] [Google Scholar]
- 21.Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):785–92. doi: 10.1016/S1473-3099(15)00096-1. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Molina JA, Rubio R, Rivero A, et al. Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy (atazanavir/ritonavir + two nucleos(t)ides) in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT study) J Antimicrob Chemother. 2017;72(1):246–53. doi: 10.1093/jac/dkw379. [DOI] [PubMed] [Google Scholar]
- 23.MedDRA introductory guide version 17.1. McLean, VA: Maintenance and Support Services Organization (MSSO); 2014. [Google Scholar]
- 24.2014 Revised classification system for HIV infection. United States. Centers for Disease Control and Prevention; [Accessed January 17, 2017]. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6303a1.htm?s_cid=rr6303a1_e. [Google Scholar]
- 25.1994 Revised classification system for HIV infection in children less than 13 years of age. Centers for Disease Control and Prevention; [Accessed January 17, 2017]. http://www.cdc.gov/mmwr/pdf/rr/rr4312.pdf. [Google Scholar]
- 26.1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Centers for Disease Control and Prevention; [Accessed January 17, 2017]. http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. [Google Scholar]
- 27.2005 Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. World Health Organization; [Accessed January 17, 2017]. http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. [Google Scholar]
- 28.Camacho-Gonzalez AF, Chernoff MC, Williams PL, et al. Sexually transmitted infections in youth with controlled and uncontrolled human immunodeficiency virus infection. J Pediatric Infect Dis Soc. 2016 doi: 10.1093/jpids/piw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownstein PS, Gillespie SE, Leong T, Chahroudi A, Chakraborty R, Camacho-Gonzalez AF. The association of uncontrolled HIV infection and other sexually transmitted infections in metropolitan Atlanta youth. Pediatr Infect Dis J. 2015;34(5):e119–24. doi: 10.1097/INF.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 30.Mellins CA, Tassiopoulos K, Malee K, et al. Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS patient care and STDs. 2011;25(7):413–22. doi: 10.1089/apc.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agwu AL, Jang SS, Korthuis PT, Araneta MR, Gebo KA. Pregnancy incidence and outcomes in vertically and behaviorally HIV-infected youth. JAMA. 2011;305(5):468–70. doi: 10.1001/jama.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazenby GB, Mmeje O, Fisher BM, et al. Antiretroviral resistance and pregnancy characteristics of women with perinatal and nonperinatal HIV infection. Infect Dis Obstet Gynecol. 2016;2016:4897501. doi: 10.1155/2016/4897501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597. doi: 10.7448/IAS.16.1.18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Vital Statistics Reports. 2. Vol. 64. Centers for Disease Control and Prevention; 2013. [Accessed January 03, 2017]. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. [Google Scholar]
- 35.Van Dyke RB, Patel K, Kagan RM, et al. Antiretroviral drug resistance among children and youth in the United States with perinatal HIV. Clin Infect Dis. 2016;63(1):133–7. doi: 10.1093/cid/ciw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166(6):528–35. doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May MT, Vehreschild JJ, Trickey A, et al. Mortality according to CD4 count at start of combination antiretroviral therapy among HIV-infected patients followed for up to 15 Years after start of treatment: Collaborative Cohort Study. Clin Infect Dis. 2016;62(12):1571–7. doi: 10.1093/cid/ciw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearlstein SL, Mellins CA, Dolezal C, et al. Youth in transition: life skills among perinatally HIV-infected and HIV-exposed adolescents. J Pediatr Psychol. 2014;39(3):294–305. doi: 10.1093/jpepsy/jst077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussen SA, Chahroudi A, Boylan A, Camacho-Gonzalez AF, Hackett S, Chakraborty R. Transition of youth living with HIV from pediatric to adult-oriented healthcare: a review of the literature. Future Virol. 2015;9(10):921–9. doi: 10.2217/fvl.14.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–71. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 41.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansom SL, Anderson JE, Farnham PG, et al. Updated estimates of healthcare utilization and costs among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2006;41(4):521–6. doi: 10.1097/01.qai.0000191286.70331.7b. [DOI] [PubMed] [Google Scholar]
- 43.Kacanek D, Angelidou K, Williams PL, Chernoff M, Gadow KD, Nachman S. Psychiatric symptoms and antiretroviral nonadherence in US youth with perinatal HIV: a longitudinal study. AIDS. 2015;29(10):1227–37. doi: 10.1097/QAD.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ylitalo N, Brogly S, Hughes MD, et al. Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med. 2006;160(8):778–87. doi: 10.1001/archpedi.160.8.778. [DOI] [PubMed] [Google Scholar]
- 45.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 46.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis. 1998;178(6):1616–22. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 47.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151(12):1148–57. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 48.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184(6):682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 49.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 50.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60(7):1–21. [PubMed] [Google Scholar]
- 51.Eckard AR, Fowler SL, Haston JC, Dixon TC. Curr HIV/AIDS Rep. 2016. Complications of treatment in youth with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–56. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi T, Wei W, Amin K, Kazanjian P. Effect of maintaining highly active antiretroviral therapy on AIDS events among patients with late-stage HIV infection and inadequate response to therapy. Clin Infect Dis. 2006;42(6):878–84. doi: 10.1086/500210. [DOI] [PubMed] [Google Scholar]
- 54.Miller V, Sabin CA, Phillips AN, et al. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS. 2000;14(14):2129–36. doi: 10.1097/00002030-200009290-00009. [DOI] [PubMed] [Google Scholar]
- 55.Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4(11):1026–31. [PubMed] [Google Scholar]
- 56.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 57.Barron Y, Cole SR, Greenblatt RM, et al. Effect of discontinuing antiretroviral therapy on survival of women initiated on highly active antiretroviral therapy. AIDS. 2004;18(11):1579–84. doi: 10.1097/01.aids.0000131359.37210.1f. [DOI] [PubMed] [Google Scholar]
- 58.McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS. 1999;13(13):1687–95. doi: 10.1097/00002030-199909100-00012. [DOI] [PubMed] [Google Scholar]
- 59.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 60.d’Arminio Monforte A, Sabin CA, Phillips A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165(4):416–23. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 61.Fairlie L, Karalius B, Patel K, et al. CD4+ and viral load outcomes of antiretroviral therapy switch strategies after virologic failure of combination antiretroviral therapy in perinatally HIV-infected youth in the United States. AIDS. 2015;29(16):2109–19. doi: 10.1097/QAD.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usitalo A, Leister E, Tassiopoulos K, et al. Relationship between viral load and self-report measures of medication adherence among youth with perinatal HIV infection. AIDS Care. 2013 doi: 10.1080/09540121.2013.802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tassiopoulos K, Moscicki AB, Mellins C, et al. Sexual risk behavior among youth with perinatal HIV infection in the United States: predictors and implications for intervention development. Clin Infect Dis. 2013;56(2):283–90. doi: 10.1093/cid/cis816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agwu AL, Bethel J, Hightow-Weidman LB, et al. Substantial multiclass transmitted drug resistance and drug-relevant polymorphisms among treatment-naive behaviorally HIV-infected youth. AIDS patient care and STDs. 2012;26(4):193–6. doi: 10.1089/apc.2011.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viani RM, Peralta L, Aldrovandi G, et al. Prevalence of primary HIV-1 drug resistance among recently infected adolescents: a multicenter adolescent medicine trials network for HIV/AIDS interventions study. J Infect Dis. 2006;194(11):1505–9. doi: 10.1086/508749. [DOI] [PubMed] [Google Scholar]
- 66.Mofenson LM, Cotton MF. The challenges of success: adolescents with perinatal HIV infection. J Int AIDS Soc. 2013;16:18650. doi: 10.7448/IAS.16.1.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.