Summary
UV radiation is a major environmental risk factor for the development of melanoma by causing DNA damage and mutations. Resistance to UV damage is largely determined by the capacity of melanocytes to respond to UV injury by repairing mutagenic photolesions. The nucleotide excision repair (NER) pathway is the major mechanism by which cells correct UV photodamage. This multi-step process involves the basic steps of damage recognition, isolation, localized strand unwinding, assembly of a repair complex, excision of the damage-containing strand 3′ and 5′ to the photolesion, synthesis of a sequence-appropriate replacement strand and finally ligation to restore continuity of genomic DNA. In melanocytes, the efficiency of NER is regulated by several hormonal pathways including the melanocortin and endothelin signaling pathways. Elucidating molecular mechanisms by which melanocyte DNA repair is regulated offers the possibility of developing novel melanoma-preventive strategies to reduce UV mutagenesis, especially in UV-sensitive melanoma-prone individuals.
Keywords: UV photodamage, nucleotide excision repair, melanocortin signaling, endothelin receptor signaling, mutagenesis
Melanoma
We and others have been interested in the mechanisms by which melanocytes regulate genomic stability through UV resistance and DNA repair because of the clear implications to melanoma, a malignancy whose incidence has been increasing for decades. Whereas one in every 1,500 Americans developed melanoma in the 1930’s, now almost one in fifty are affected by the disease)(Eggermont et al., 2014). According to the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program, over 75,000 Americans were diagnosed with melanoma and the disease killed over 10,000 in 2016 alone. Though it accounts for less than 10% of all skin cancers, melanoma is responsible for over three quarters of all deaths by skin cancer (Shenenberger, 2012). It is likely that the increasing incidence of melanoma is multifactorial involving an aging population, better detection and awareness and more occupational and recreational UV exposure. Melanoma incidence peaks in the fifth decade of life, but it can afflict patients of any age, even children. In fact, it is the second most common cancer in young adults between the ages of 15 and 29. Overall, it is estimated that over a million Americans alive today will be diagnosed with melanoma at some point in their lives (Geller et al., 2013). Although significant therapeutic advances have been made over the last decade by targeted therapy against the MAP kinase cascade and by immune checkpoint blockade, the majority of patients with advanced melanoma still die of their disease. Therefore, there is a great unmet need to understand the molecular mechanisms of melanoma susceptibility and to develop rational preventive strategies to reduce incidence of disease, particularly in individuals with an accumulation of identified risk factors.
Ultraviolet Radiation (UV) and its effects on DNA
Abundant epidemiologic and molecular data identify UV as a major environmental risk factor for melanoma (Berwick et al., 2014). Many melanomas derive from nevi on sun-exposed areas of the body (Dodd et al., 2007; Sober, 1987; Valiukeviciene et al., 2007), melanoma incidence correlates with living in UV-rich climates (Lee and Scotto, 1993) and lifetime risk of melanoma increases with exposure to UV through tanning bed use (Schulman and Fisher, 2009; Weinstock and Fisher, 2010). A UV-melanoma link is strengthened by molecular studies that support the concept that UV exposure drives melanomagenesis. Melanoma carries a high somatic mutational burden and the overwhelming majority of mutations are characteristic “UV-signature” transitional mutations in adjacent pyrimidines (Hodis et al., 2012; Lawrence et al., 2013). Moreover, UV-signature mutational burden correlates with melanoma tumor progression (Shain et al., 2015), suggesting that UV drives many stages of melanoma development.
UV causes two main types of DNA injury. First, UV damages DNA through the production of free radicals and subsequent oxidative injury to nucleotide bases. Second, UV promotes covalent changes between pyrimidines directly through absorption of UV energy by cytosine and thymine. UV-induced DNA damage interferes with transcription, replication and genomic stability. Oxidative injury is recognized and repaired by the base excision repair pathway in which lesion-specific glycosylases recognize and remove damaged bases from the DNA backbone to generate apurinic sites that are subsequently filled in by polymerases using the undamaged sister strand as a template. While oxidative lesions can be mutagenic and have been implicated in melanoma development and progression, we will focus our discussion on covalent UV photolesions and their repair since transitional pyrimidine mutations caused by covalent UV-induced pyrimidine photolesions represent the most common somatic mutations in melanoma (Hodis et al., 2012; Lawrence et al., 2013) and correlate with disease progression (Shain et al., 2015).
Through direct absorption of UV energy, the C5/C6 bond in pyrimidines (cytosines and thymines) is vulnerable to disruption. When this bond breaks between adjacent pyrimidines, abnormal bonds may form between the two bases to form cyclopyrimidine dimers (CPDs) or [6,4]-photoproducts (6-4PPs). Each of these covalent lesions distorts the double helix, interferes with transcription and replication and promotes base mispairing and mutagenesis. Since UV photodamage affects adjacent pyrimidines, its causality in mutagenesis can be determined by the presence of “UV signature” dipyrimidine transitional mutations (e.g. CC-to-TT) throughout the genome and in cancer-relevant genes. Tumors that are driven by UV mutagenesis such as malignant melanoma of the skin, squamous cell carcinoma of the skin and basal cell carcinoma of the skin are rich in UV signature mutations (Wikonkal and Brash, 1999).
In addition to the initial CPD burden that occurs from UV photon-induced nucleotide changes in DNA, CPDs may continue to accumulate in melanocytes several hours after UV exposure ends. The mechanism responsible for “dark photodimer” formation was recently elucidated by Brash and colleagues and involves melanin as a redox energy transfer moiety. Thus, melanin acts as a substrate for UV-induced reactive oxygen and nitrogen species, and its chemiexcitation creates a quantum triplet state with the equivalent energy of a UV photon and the ability to break the C5/C6 bond in adjacent pyrimidines to yield CPDs (Premi et al., 2015). Indeed, a large percentage of total UV-induced CPDs may develop through melanin chemiexcitation and delayed CPD formation. The discovery of dark photodimers is an important insight into melanocyte UV damage and resistance. Because many of the regulatory mechanisms that optimize melanocyte DNA repair may themselves be stimulated by UV (and therefore activated some time after UV exposure), they may be particularly relevant to the repair of dark photodimers in melanocytes.
Nucleotide Excision Repair (NER) and Xeroderma Pigmentosum (XP)
Nucleotide excision repair (NER) is the process by which cells repair helical-distorting DNA damage including CPDs and 6-4PPs. NER-targeted lesions, which promote physical deformities in the double helix, are detected either when they interfere with transcription (through stalling of RNA polymerase) which initates transcription-coupled NER (TC-NER), or when they are identified by damage sensing proteins that initiate global genomic NER (GG-NER). Although TC-NER and GG-NER differ in damage recognition, subsequent steps converge into one repair pathway (Fig 1). NER involves the action of over 30 proteins that process damage in a sequential series of reactions that can be summarized as follows: 1) DNA damage recognition, 2) recruitment of structural repair proteins, 3) DNA unwinding, 4) assembly of a pre-incision complex, 5) 3′-5′ dual strand incision, 6) removal of a short oligonucleotide harboring the photodamage, 7) gap-filling using the sister strand as a template and finally 8) DNA ligation (Scharer, 2013). NER is a damage-repairing mechanism of high fidelity that restores DNA to its pre-injured state and in this way is critical for resistance of melanocytes to UV-induced carcinogenesis.
Fig. 1. Overview of nucleotide excision repair (NER).
UV photodamage is repaired by the NER pathway, which can be subdivided into global genome NER (GG-NER) and transcription-coupled NER (TC-NER). GG-NER occurs anywhere in the genome and is initiated by recognition of a distorted double helix, as occurs with UV photoproduct formation. In GG-NER, XPC, stabilized in complex with RAD23B and enabled to recognize CPDs by UV-damage-binding protein 2 (DDB2), identifies sites of damage, binding to the strand opposite the photolesion. In contrast, TC-NER is initiated in actively transcribed genes by stalling of RNA polymerase at the photolesion and involves the CSA, CSB and XAB2 cofactors. TC-NER, which probably accounts for no more than 10% of total NER, differs from GG-NER only through initial lesion recognition; the pathways merge downstream into a common NER mechanism beginning with pre-incision complex formation. In both GG-NER and TC-NER, TFIIH (a multi-complex protein containing the XPB and XPD helicases as well as 8 other subunits) is recruited to the site of damage. Using its XPB and XPD helicase components, TFIIH unwinds the DNA around the photolesion, initiating strand separation to enable the recruitment of other NER factors to form a “pre-incision complex”. XPA, replication protein A (RPA), XPG, XPF and ERCC1 are each recruited to the complex, and at some point, initiation factors exit the region. Next, there is incision of the photolesion-containing strand some distance away from the DNA lesion. Strand incision is accomplished by XPF-ERCC1 working in complex to cleave the strand 5′ to the damage and by XPG 3′ to the damage. Evidence suggests that 5′ strand cleavage may actually occur before 3′ strand cleavage and in fact, DNA polymerase may begin filling in the gap from the 5′ side before the 3′ incision step. After 5′ and 3′ strand incision, a 24-32mer oligonucleotide harboring the photolesion is generated that is removed (associated with TFIIH) from chromatin. The resultant gap is then filled in by DNA polymerases together with PCNA, RFC and RPA using the undamaged sister strand to ensure fidelity of repair. Finally DNA ligation is achieved by DNA ligases I, III or XRCC1 to complete the process.
The importance of NER in melanoma resistance is best illustrated by considering the natural history of xeroderma pigmentosum (XP), a rare UV hypersensitivity syndrome caused by homozygous defects in any of several genes associated with the NER pathway (XPA through XPG and DNA polymerase eta (POLH) (Giordano et al., 2016). XP typically manifests very early in life, the defining characteristics are sunlight-induced changes in cutaneous pigmentation and dry parchment-like skin. During childhood, pigment changes, telangiectasias and atrophy occur on UV-exposed skin. XP patients with defects in complementation groups A, B, D and G have hypersensitivity to UV with UV-induced inflammation (sunburns) occuring after minimal sun exposure, whereas groups C, E and variant do not. In the most serious cases, pre-malignant lesions such as actinic keratosis and fully malignant UV-induced cancers develop with great frequency. XP patients are up to 2000 times more likely to develop melanoma and typically far earlier in life than melanomas develop in the general (non-XP) population (Bradford et al., 2011). As with sporadic melanomas, XP-associated melanomas frequently demonstrate “UV signature mutations” (Daya-Grosjean, 2008), clearly indicating the importance of UV in their causality and of NER in resistance against melanomas. Indeed, evidence is accumulating that inherited polymorphisms in NER genes that affect DNA repair are an important melanoma risk factor in the general population (Li et al., 2013; Xu et al., 2015). Please see the following excellent recent reviews for more detail about NER (DiGiovanna and Kraemer, 2012; Marteijn et al., 2014; Sancar et al., 2004; Scharer, 2013; Wood, 2010).
Melanocyte interactions in the skin
Through cellular dendritic projections, epidermal melanocytes interact with up to thirty or more keratinocytes, fibroblasts and other skin cells (e.g. immune cells) in what has been termed the “epidermal unit” (Nordlund, 2007). There is abundant evidence to suggest a rich intercellular communication between cell types within the epidermal unit that regulates skin homeostasis and responses to UV injury (Cario-Andre et al., 2000; Gilchrest et al., 1996; Jimbow et al., 1991; Kawaguchi et al., 2001; Paus et al., 1999; Schiller et al., 2004; Scholzen et al., 2000; Virador et al., 2002). Keratinocytes, which make up the great majority of cells in the epidermis, influence melanocyte physiology by contact-dependent interactions and by contact-independent means via secretion of growth factors and regulators that act in a paracrine manner on melanocytes through specific ligand-receptor interactions (Imokawa et al., 1997; Imokawa et al., 1992; Jamal and Schneider, 2002; Kadekaro et al., 2005; Rousseau et al., 2007). Although NER can be augmented by cell-autonomous pathways such as autophagy (Qiang et al., 2016), we will focus our discussions on the regulation of melanocytic NER by the melanocortin and endothelin hormonal signaling pathways.
Melanocortin 1 receptor (MC1R)
The melanocortin 1 receptor (MC1R) is a Gs protein-coupled receptor located in the melanocyte extracellular membrane (recently reviewed (Wolf Horrell et al., 2016)). Spanning the membrane seven times, the MC1R transmits ligand-receptor interactions to the cytoplasm through its association with adenylyl cyclase and production of the second messenger cAMP (Garcia-Borron et al., 2014). MC1R signaling regulates many aspects of melanocyte growth and differentiation. MC1R-directed increases in cAMP stimulate melanin production (Abdel-Malek et al., 2000; D'Orazio et al., 2006), increase cellular antioxidant levels (Kadekaro et al., 2003), protect melanocytes against apoptosis (McGill et al., 2002), stimulate growth (Suzuki et al., 1999) and augment the efficiency of NER (Abdel-Malek et al., 2009; Bohm et al., 2005; Hauser et al., 2006; Jarrett et al., 2014; Jarrett et al., 2015; Kadekaro et al., 2010; Song et al., 2009; Swope et al., 2014). Loss-of-function polymorphisms in MC1R are common, particularly in fair-skinned and sun-sensitive individuals (Valverde et al., 1995). Because MC1R stimulates the production of UV-protective eumelanin in the skin, MC1R defects result in an under-melanized epidermis which allows more UV radiation to penetrate the skin (D'Orazio et al., 2006; Suzuki et al., 1999). MC1R signaling defects also favor the production of pheomelanin, which blocks UV radiation much less effectively than eumelanin, promotes free radical formation (Hill et al., 1997) and contributes to melanomagenesis (Mitra et al., 2012). A less eumelanized phenotype may have been selected in populations that migrated out of UV-rich equatorial regions to more polar climates in order to maximize UV-mediated vitamin D production and avoid rickets (Jablonski and Chaplin, 2013). These same MC1R polymorphisms, however, are carried by millions of individuals now living in UV-rich locations and clearly increase lifetime melanoma risk (Box et al., 2001; Kennedy et al., 2001; Pasquali et al., 2015; Valverde et al., 1996).
We and others have reported that MC1R defects also impair NER and melanocyte genomic stability. The integrity of the melanocyte genome is diminished when MC1R signaling is sub-optimal because NER is blunted (Hauser et al., 2006; Jarrett et al., 2014; Jarrett et al., 2015; Swope et al., 2014). Thus, MC1R signaling problems result in an undermelanized epidermis permissive of UV entry and impaired melanocyte NER. MC1R-defective individuals are at higher risk of melanoma because their epidermal melanocytes accumulate more UV mutations over time. Indeed, Robles-Spinoze and coworkers recently reported that melanomas isolated from individuals harboring germline loss-of-function MC1R variants had 42% more somatic mutations than melanomas from MC1R-intact persons and that the overwhelming majority of these mutations were UV signature changes (Robles-Espinoza et al., 2016). Therefore, because MC1R signaling protects melanocytes from UV damage in a number of ways (Rouzaud et al., 2005; Scott et al., 2002), inherited MC1R defects clearly increase melanoma susceptibility (Kadekaro et al., 2010; Song et al., 2009).
MC1R (cAMP) signaling and NER
The melanocortin signaling axis promotes melanocyte genomic stability by positioning the cell to better cope with UV damage (Kadekaro et al., 2010). MC1R-mediated production of eumelanin is accomplished largely through transcriptional upregulation of pigment enzyme gene expression through CREB (cAMP responsive binding element) and MITF (microphthalmia) (Busca and Ballotti, 2000). As a result, there is a delay of several hours between MC1R-mediated cAMP increases and increased eumelanin production (Yamaguchi and Hearing, 2009). MC1R signaling also promotes expression of key DNA repair proteins in a transcription-dependent manner. For example, MITF loss, which mimics blunted MC1R signaling, reduced the expression of many DNA repair factors including XPA, RPA, DNA ligase I, DNA polymerase delta (Pol δ) (Strub et al., 2011) and XPAB1 (April and Barsh, 2006). These studies suggest that MC1R and MITF are needed to maintain optimal levels of a variety of DNA repair factors in melanocytes. In contrast, MC1R-enhanced DNA repair appears to involve both delayed and more immediate mechanisms, allowing melanocytes to augment their repair capacity in a much quicker and responsive manner after UV exposure than pigment up-regulation.
Elegant work from the Abdel-Malek lab first demonstrated that the melanocortin signaling axis controls melanocyte DNA repair (Kadekaro et al., 2005). Her group showed that UV exposure activated the DNA damage sensors ataxia telangiectasia mutated (ATM) and Rad3 related (ATR), ATM, and DNA-PK (Kadekaro et al., 2012; Swope et al., 2014) and that melanocortin-enhanced DNA repair was influenced by increased levels of XPC and H2AX, potentially promoting the formation of DNA-repair complexes (Swope et al., 2014). Other components of the UV DNA damage repair response also are impacted by MC1R and include the NR4As superfamily of nuclear receptors which are recruited to sites of nuclear DNA damage together with XPC and DDB (Smith et al., 2008).
Our group discovered a molecular mechanism by which melanocytes require as little as 15-30 minutes of cAMP stimulation to augment NER (Jarrett et al., 2014), linking MC1R/cAMP signaling to NER through the ATR protein (Fig. 2) (Jarrett and D'Orazio, 2016). ATR is a non-dispensable Ser/Thr kinase in the same family as ATM and DNA-dependent protein kinase (DNA-PK). ATR is essential for cell survival; without it, cells undergo “mitotic catastrophe” during replication. ATR is important for damage signaling (Cimprich and Cortez, 2008), replication integrity (Cliby et al., 1998) and genomic maintenance and repair (Auclair et al., 2008). We determined that the critical molecular event linking MC1R signaling to enhanced NER in melanocytes is a non-canonical activation of ATR via phosphorylation by cAMP-dependent kinase (protein kinase A; PKA) (Jarrett et al., 2014). When cAMP levels are induced either through melanocortin signaling or pharmacologically via adenylyl cyclase activation (e.g. by forskolin) or phosphodiesterase inhibition (e.g. by rolipram), PKA is stimulated to phosphorylate ATR on Serine 435 (S435). However, instead of activating ATR to stop proliferation by Chk1 phosphorylation and cell cycle checkpoint blockade (ATR’s canonical role in cell damage responses), PKA-mediated phosphorylation of ATR on S435 promotes binding of ATR with the key NER factor XPA and together, ATR-pS435 and XPA co-localize with UV photodamage in the nucleus (Jarrett et al., 2014). More recently, we identified A kinase anchoring protein 12 (AKAP12) as the critical molecular scaffold that facilitates PKA-mediated ATR phosphorylation on S435 (Jarrett et al., 2016). Like other AKAPs, AKAP12 is a bipartite protein able to bind the regulatory subunits of PKA through a conserved amphipathic protein interaction domain. It also binds relevant PKA-target proteins (in this case ATR) thereby scaffolding the PKA-ATR interaction. Mutating either AKAP12’s PKA binding sequence or AKAP12’s ATR binding region prevents PKA-mediated ATR phosphorylation and NER enhancement. As a result, there is persistence of UV photodamage in the genome and higher rates of UV mutagenesis (Jarrett et al., 2016).
Fig. 2. MC1R-mediated NER augmentation by the AKAP12-ATR-XPA axis.
In melanocytes, enhancement of NER by cAMP is dependent on a damage-dependent cytoplasmic interaction between ATR and PKA, scaffolded by AKAP12. If cAMP levels have been stimulated either through MC1R-agonist interactions or pharmacologically, cAMP-dependent protein kinase (PKA) is activated. Like other A-kinase anchoring proteins, AKAP12 is a bipartite molecule that brings together PKA with its appropriate phosphorylation targets (in this case ATR). PKA then phosphorylates ATR on S435 to stimulate ATR’s interaction with XPA in the nucleus. Traffic of ATR-pS435 to the nucleus happens in the context of AKAP12 binding, and nuclear translocation of the AKAP12-ATR-p435S complex is dependent on ATR’s phosphorylation of AKAP12 on the S732 residue. Together, ATR, AKAP12 and XPA localize to sites of UV photodamage in chromatin. cAMP accelerates NER by enhancing 5′ strand incision, a molecular event mediated by XPF-ERCC1. In this way, MC1R/cAMP signaling improves cellular ability to repair UV photodamage, resulting in lowered rates of UV mutagenesis and improved genomic stability.
We have also gained insights into the mechanism by which the PKA-ATR-XPA pathway enhances NER. XPA is known to interact with a variety of NER factors, including TFIIH, RPA, XPC-RAD23B, DDB2, ERCC1-XPF and PCNA (Bunick et al., 2006; Gilljam et al., 2012; Li et al., 1994; Nocentini et al., 1997; Park et al., 1995; Wakasugi et al., 2009; You et al., 2003). We measured XPA’s interactions with potential NER binding partners in UV-irradiated melanocytes treated either with vehicle control or with forskolin to raise cAMP levels. Whereas XPA’s interactions with XPB, XPC and XPD were unaffected by cAMP stimulation, it’s binding to XPF, ERCC1 and RPA were increased as determined by co-immunoprecipitation (Jarrett et al., 2016). Since XPF and ERCC1 function together to carry out the 5′ incision step of NER, we reasoned that cAMP stimulation may enhance the strand incision step 5′ to UV photodamage. To test this, we used conventional “bubble cutting” assays to test cAMP’s effect on 3′ and 5′ strand incision. Whereas, 3′ incision was unaffected by cAMP stimulation, forskolin promoted accelerated and enhanced 5′ strand incision, leading us to conclude that the MC1R-ATR-AKAP12-XPA DNA repair signaling axis augments NER through more efficient strand incision 5′ to sites of photodamage (Jarrett et al., 2016). Many more insights remain to be elucidated in this process, including mechanisms of cytoplasmic transport of AKAP12-ATR-pS435 complex, how the large complex is imported into the nucleus (AKAP12 and ATR both have molecular weights in the 250 kDa range which excludes their nuclear entry by diffusion alone), how the complex associates with XPA once in the nucleus and how it targets UV photolesions.
Endothelin signaling and NER
Melanocyte growth and differentiation are regulated by many more signaling pathways than the melanocortin pathway (Swope and Abdel-Malek, 2016). One of the best characterized is the endothelin signaling axis. Endothelins were originally described as endothelial cell-derived vasoactive peptides (Yanagisawa et al., 1988). There are three endothelins expressed in humans, each encoded by a separate gene (Inoue et al., 1989). Endothelins play a major role in melanocyte development and skin homeostasis. Endothelins stimulate melanocyte proliferation and pigment production (Yada et al., 1991) and are secreted by keratinocytes in a UV-dependent manner (Imokawa et al., 1992; Yohn et al., 1993). Indeed, signaling by endothelins through the endothelin B receptor (ETBR) is necessary for melanocyte development and defects lead to pigment phenotypes (Baynash et al., 1994; Hosoda et al., 1994) due to defective neural crest maturation (Shin et al., 1999). ETBR signaling, which is mediated through intracellular calcium (Kang et al., 1998), regulates melanocyte dendricity (Hara et al., 1995), migration (Horikawa et al., 1995), E-cadherin expression (Jamal and Schneider, 2002), apoptosis (Eberle et al., 2002; Kadekaro et al., 2005), secretion of chemokines (Mangahas et al., 2005) and most recently, DNA repair (von Koschembahr et al., 2015).
Abdel-Malek and co-workers recently found that endothelin 1 (ET1), functioning through ETBR signaling, decreases the burden of UV photoproducts in irradiated melanocytes (von Koschembahr et al., 2015). Pretreating melanocytes with ET1 reduced UV photoproduct load over time, consistent with an augmentation of NER, but also at “time zero” immediately after UV exposure, raising the possibility that ET1 priming may protect cells from accumulating UV photodamage even before repair can occur. Intriguingly, ET1 treatment was independent of MC1R function, since it was not associated with cAMP changes and could protect melanocytes from UV damage even when cells expressed loss-of-function MC1R isoforms (von Koschembahr et al., 2015). Our group independently confirmed that ET1 treatment does not impact cellular levels of ATR-pS435 (Jarrett et al., 2015), suggesting that NER enhancement by endothelin signaling is distinct from that mediated by MC1R signaling and cAMP stimulation. Rather, ET1 activated JNK and p38 signaling pathways and was calcium dependent since ET-1 mediated JNK activation and ATF2 phosphorylation were blocked by the Ca2+ chelator BAPTA. JNK signaling was critical to ET1-mediated melanocyte UV resistance since its inhibition blocked ET1-mediated reductions in CPD’s and sensitized melanocytes to UV-mediated apoptosis. Mechanistically, the authors found that ET1 accelerated clearance of XPC, an NER factor involved in lesion recognition, from foci of photodamage. They interpreted these data to indicate that endothelin signaling improved XPC function to accelerate lesion recognition (von Koschembahr et al., 2015).
Melanoma prevention through enhanced DNA repair
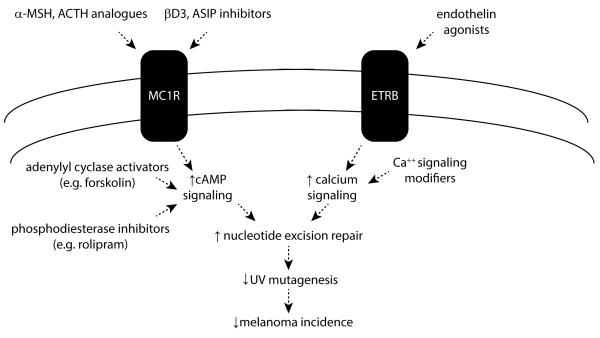
Epidermal melanocytes are long-lived cells at risk of environmental UV exposure because of their position in the skin. While it is surprising that melanocytes wouldn’t always be optimally positioned to efficiently deal with UV DNA damage, we and others have documented that melanocytic NER is inducible by specific molecular pathways including melanocortin and endothelin signaling. Each of these pathways, when intact and activated by appropriate ligands (e.g. α-MSH and ET1, respectively) boosts melanocytic NER above baseline levels. This insight offers potential therapeutic opportunities for melanoma prevention by reducing UV mutagenic burden (Fig 3).
Fig. 3. UV-preventive strategies based on enhancing NER in melanocytes.
Discovering that melanocyte NER can be regulated by the MC1R/cAMP and ETBR/Ca++ signaling pathways introduces the opportunity to reduce UV-mediated mutagenesis and carcinogenesis by enhancing melanocyte genomic stability to reduce UV mutational burden over time. Potential approaches would include targeting the MC1R and ETBR signaling pathways by agonistic mimics, inhibitors of antagonist ligands and downstream induction of intracellular signaling mediators.
Regarding MC1R/cAMP signaling, there are two fundamental ways to achieve enhanced melanocyte NER in the skin. First, if MC1R signaling is intact, it may be possible to target melanocytes by taking advantage of the specificity of melanocortin interactions with MC1R. In the skin, most agree that MC1R expression is limited to melanocytes. Therefore melanocortin analogues (Abdel-Malek et al., 2009; Cho et al., 2005; Ruwe et al., 2009; Yang et al., 2009) would be expected to target melanocytes without much off-target effect. While targeting approaches that limit cAMP induction to melanocytes is important because of the potential for off-target effects, this approach may not benefit individuals harboring loss-of-function mutations in MC1R since signaling through their MC1Rs is blunted. Since these persons are among those of highest risk of melanoma in the general population (Kennedy et al., 2001; Valverde et al., 1996), a broader therapeutic strategy may be needed such as topical application of agents that increase cAMP levels in skin cells either through adenylyl cyclase activation or phosphodiesterase inhibition both of which have been validated in an Mc1r-defective UV-sensitive animal model of the fair-skinned human with respect to pigmentation (Amaro-Ortiz et al., 2013; D'Orazio et al., 2006; Khaled et al., 2010; Spry et al., 2009). Indeed, we have already shown in proof-of-principle pre-clinical experiments that topical application of forskolin enhanced clearance of UV photodamage in the skin (Jarrett et al., 2014).
Another potential strategy for enhancing melanocytic NER, even in MC1R-defective individuals, may be to take advantage of the endothelin signaling axis. If therapies can be developed to target the endothelin pathway in epidermal melanocytes, NER may be enhanced irrespective of MC1R status. Clearly much more research is needed to understand the feasibility of each of these different therapeutic strategies, but the key concept that has emerged from these studies is that it may be possible to reduce UV damage to epidermal melanocytes and lessen melanoma risk by augmenting melanocyte genomic stability by optimizing NER.
Acknowledgements
We are grateful for support from the National Cancer Institute (R01 CA131075), the Melanoma Research Alliance (MRA) and the Regina Drury Endowment for Pediatric Research. We also acknowledge the technical support of the Markey Research Communications Office and NCI Cancer Center Support Grant (P30 CA177558) of the Markey Cancer Center at the University of Kentucky.
Literature Cited
- Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13(Suppl 8):156–62. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekaro AL, Swope V, Haskell-Luevano C, Koikov L, Knittel JJ. alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–44. doi: 10.1111/j.1755-148X.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Amaro-Ortiz A, Vanover JC, Scott TL, D'orazio JA. Pharmacologic induction of epidermal melanin and protection against sunburn in a humanized mouse model. J Vis Exp. 2013 doi: 10.3791/50670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April CS, Barsh GS. Skin layer-specific transcriptional profiles in normal and recessive yellow (Mc1re/Mc1re) mice. Pigment Cell Res. 2006;19:194–205. doi: 10.1111/j.1600-0749.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- Auclair Y, Rouget R, Affar El B, Drobetsky EA. ATR kinase is required for global genomic nucleotide excision repair exclusively during S phase in human cells. Proc Natl Acad Sci U S A. 2008;105:17896–901. doi: 10.1073/pnas.0801585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–85. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Berwick M, Macarthur J, Orlow I, Kanetsky P, Begg CB, Luo L, Reiner A, Sharma A, Armstrong BK, Kricker A, et al. MITF E318K's effect on melanoma risk independent of, but modified by, other risk factors. Pigment Cell Melanoma Res. 2014;27:485–8. doi: 10.1111/pcmr.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Chen W, Stark M, Martin NG, Sturm RA, Hayward NK. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69:765–73. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–76. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick CG, Miller MR, Fuller BE, Fanning E, Chazin WJ. Biochemical and structural domain analysis of xeroderma pigmentosum complementation group C protein. Biochemistry. 2006;45:14965–79. doi: 10.1021/bi061370o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–9. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Cario-Andre M, Pain C, Gall Y, Ginestar J, Nikaido O, Taieb A. Studies on epidermis reconstructed with and without melanocytes: melanocytes prevent sunburn cell formation but not appearance of DNA damaged cells in fair-skinned caucasians. J Invest Dermatol. 2000;115:193–9. doi: 10.1046/j.1523-1747.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- Cho MK, Lee CJ, Lee CH, Li SZ, Lim SK, Baik JH, Lee W. Structure and function of the potent cyclic and linear melanocortin analogues. J Struct Biol. 2005;150:300–8. doi: 10.1016/j.jsb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. Embo J. 1998;17:159–69. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–4. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L. Xeroderma pigmentosum and skin cancer. Adv Exp Med Biol. 2008;637:19–27. doi: 10.1007/978-0-387-09599-8_3. [DOI] [PubMed] [Google Scholar]
- Digiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132:785–96. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AT, Morelli J, Mokrohisky ST, Asdigian N, Byers TE, Crane LA. Melanocytic nevi and sun exposure in a cohort of colorado children: anatomic distribution and site-specific sunburn. Cancer Epidemiol Biomarkers Prev. 2007;16:2136–43. doi: 10.1158/1055-9965.EPI-07-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Fecker LF, Orfanos CE, Geilen CC. Endothelin-1 decreases basic apoptotic rates in human melanoma cell lines. J Invest Dermatol. 2002;119:549–55. doi: 10.1046/j.1523-1747.2002.01848.x. [DOI] [PubMed] [Google Scholar]
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–27. doi: 10.1016/S0140-6736(13)60802-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Abdel-Malek Z, Jimenez-Cervantes C. MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014;27:699–720. doi: 10.1111/pcmr.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, Shaikh W, Miller DR. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172–8. doi: 10.1200/JCO.2012.47.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Gilljam KM, Muller R, Liabakk NB, Otterlei M. Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PLoS One. 2012;7:e49199. doi: 10.1371/journal.pone.0049199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano CN, Yew YW, Spivak G, Lim HW. Understanding photodermatoses associated with defective DNA repair: Syndromes with cancer predisposition. J Am Acad Dermatol. 2016;75:855–870. doi: 10.1016/j.jaad.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Hara M, Yaar M, Gilchrest BA. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105:744–8. doi: 10.1111/1523-1747.ep12325522. [DOI] [PubMed] [Google Scholar]
- Hauser JE, Kadekaro AL, Kavanagh RJ, Wakamatsu K, Terzieva S, Schwemberger S, Babcock G, Rao MB, Ito S, Abdel-Malek ZA. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19:303–14. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Hill HZ, Hill GJ, Cieszka K, Plonka PM, Mitchell DL, Meyenhofer MF, Xin P, Boissy RE. Comparative action spectrum for ultraviolet light killing of mouse melanocytes from different genetic coat color backgrounds. Photochem Photobiol. 1997;65:983–9. doi: 10.1111/j.1751-1097.1997.tb07958.x. [DOI] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa T, Norris DA, Yohn JJ, Zekman T, Travers JB, Morelli JG. Melanocyte mitogens induce both melanocyte chemokinesis and chemotaxis. J Invest Dermatol. 1995;104:256–9. doi: 10.1111/1523-1747.ep12612795. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–28. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–80. [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–7. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671–5. doi: 10.1016/j.jhevol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Jamal S, Schneider RJ. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest. 2002;110:443–52. doi: 10.1172/JCI13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, D'orazio JA. Hormonal Regulation of the Repair of UV Photoproducts in Melanocytes by the Melanocortin Signaling Axis. Photochem Photobiol. 2016 doi: 10.1111/php.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Horrell EM, Christian PA, Vanover JC, Boulanger MC, Zou Y, D'orazio JA. PKA-mediated phosphorylation of ATR promotes recruitment of XPA to UV-induced DNA damage. Mol Cell. 2014;54:999–1011. doi: 10.1016/j.molcel.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jarrett SG, Wolf Horrell EM, Boulanger MC, D'orazio JA. Defining the Contribution of MC1R Physiological Ligands to ATR Phosphorylation at Ser435, A Predictor of DNA Repair in Melanocytes. J Invest Dermatol. 2015 doi: 10.1038/jid.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Wolf Horrell EM, D'orazio JA. AKAP12 mediates PKA-induced phosphorylation of ATR to enhance nucleotide excision repair. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw871. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jimbow K, Salopek TG, Dixon WT, Searles GE, Yamada K. The epidermal melanin unit in the pathophysiology of malignant melanoma. Am J Dermatopathol. 1991;13:179–88. doi: 10.1097/00000372-199104000-00013. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, Abdel-Malek Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012;10:778–86. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kanto H, Kavanagh R, Abdel-Malek Z. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann N Y Acad Sci. 2003;994:359–65. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Leachman S, Kavanagh RJ, Swope V, Cassidy P, Supp D, Sartor M, Schwemberger S, Babcock G, Wakamatsu K, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. Faseb J. 2010 doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HY, Kang WH, Lee C. Endothelin-B receptor-mediated Ca2+ signaling in human melanocytes. Pflugers Arch. 1998;435:350–6. doi: 10.1007/pl00008082. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Mori N, Nakayama A. Kit(+) melanocytes seem to contribute to melanocyte proliferation after UV exposure as precursor cells. J Invest Dermatol. 2001;116:920–5. doi: 10.1046/j.0022-202x.2001.01370.x. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Khaled M, Levy C, Fisher DE. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24:2276–81. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Scotto J. Melanoma: linked temporal and latitude changes in the United States. Cancer Causes Control. 1993;4:413–8. doi: 10.1007/BF00050859. [DOI] [PubMed] [Google Scholar]
- Li C, Yin M, Wang LE, Amos CI, Zhu D, Lee JE, Gershenwald JE, Grimm EA, Wei Q. Polymorphisms of nucleotide excision repair genes predict melanoma survival. J Invest Dermatol. 2013;133:1813–21. doi: 10.1038/jid.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Elledge SJ, Peterson CA, Bales ES, Legerski RJ. Specific association between the human DNA repair proteins XPA and ERCC1. Proc Natl Acad Sci U S A. 1994;91:5012–6. doi: 10.1073/pnas.91.11.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangahas CR, Dela Cruz GV, Friedman-Jimenez G, Jamal S. Endothelin-1 induces CXCL1 and CXCL8 secretion in human melanoma cells. J Invest Dermatol. 2005;125:307–11. doi: 10.1111/j.0022-202X.2005.23820.x. [DOI] [PubMed] [Google Scholar]
- Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15:465–81. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- Mcgill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–53. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini S, Coin F, Saijo M, Tanaka K, Egly JM. DNA damage recognition by XPA protein promotes efficient recruitment of transcription factor II H. J Biol Chem. 1997;272:22991–4. doi: 10.1074/jbc.272.37.22991. [DOI] [PubMed] [Google Scholar]
- Nordlund JJ. The melanocyte and the epidermal melanin unit: an expanded concept. Dermatol Clin. 2007;25:271–81. vii. doi: 10.1016/j.det.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Park CH, Mu D, Reardon JT, Sancar A. The general transcription-repair factor TFIIH is recruited to the excision repair complex by the XPA protein independent of the TFIIE transcription factor. J Biol Chem. 1995;270:4896–902. doi: 10.1074/jbc.270.9.4896. [DOI] [PubMed] [Google Scholar]
- Pasquali E, Garcia-Borron JC, Fargnoli MC, Gandini S, Maisonneuve P, Bagnardi V, Specchia C, Liu F, Kayser M, Nijsten T, et al. MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: a pooled-analysis from the M-SKIP project. Int J Cancer. 2015;136:618–31. doi: 10.1002/ijc.29018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann N Y Acad Sci. 1999;885:350–63. doi: 10.1111/j.1749-6632.1999.tb08690.x. [DOI] [PubMed] [Google Scholar]
- Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, Bechara EJ, Halaban R, Douki T, Brash DE. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–7. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Zhao B, Shah P, Sample A, Yang S, He YY. Autophagy positively regulates DNA damage recognition by nucleotide excision repair. Autophagy. 2016;12:357–68. doi: 10.1080/15548627.2015.1110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Espinoza CD, Roberts ND, Chen S, Leacy FP, Alexandrov LB, Pornputtapong N, Halaban R, Krauthammer M, Cui R, Timothy Bishop D, et al. Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun. 2016;7:12064. doi: 10.1038/ncomms12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. Faseb J. 2007;21:1844–56. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 2005;571:133–52. doi: 10.1016/j.mrfmmm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Ruwe AR, Koikov L, Abdel-Malek Z, Haskell-Luevano C, Dirain ML, Portillo F, Xiang Z, Wortman M, Knittel JJ. Semi-rigid tripeptide agonists of melanocortin receptors. Bioorg Med Chem Lett. 2009;19:5176–81. doi: 10.1016/j.bmcl.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Scharer OD. Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol. 2013;5:a012609. doi: 10.1101/cshperspect.a012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Brzoska T, Bohm M, Metze D, Scholzen TE, Rougier A, Luger TA. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122:468–76. doi: 10.1046/j.0022-202X.2004.22239.x. [DOI] [PubMed] [Google Scholar]
- Scholzen TE, Kalden DH, Brzoska T, Fisbeck T, Fastrich M, Schiller M, Bohm M, Schwarz T, Armstrong CA, Ansel JC, et al. Expression of proopiomelanocortin peptides in human dermal microvascular endothelial cells: evidence for a regulation by ultraviolet light and interleukin-1. J Invest Dermatol. 2000;115:1021–8. doi: 10.1046/j.1523-1747.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- Schulman JM, Fisher DE. Indoor ultraviolet tanning and skin cancer: health risks and opportunities. Curr Opin Oncol. 2009;21:144–9. doi: 10.1097/CCO.0b013e3283252fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VJ, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci. 2002;115:2349–55. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, et al. The Genetic Evolution of Melanoma from Precursor Lesions. N Engl J Med. 2015;373:1926–36. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- Shenenberger DW. Cutaneous malignant melanoma: a primary care perspective. Am Fam Physician. 2012;85:161–8. [PubMed] [Google Scholar]
- Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J Biol Chem. 2008;283:12564–70. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
- Sober AJ. Solar exposure in the etiology of cutaneous melanoma. Photodermatol. 1987;4:23–31. [PubMed] [Google Scholar]
- Song X, Mosby N, Yang J, Xu A, Abdel-Malek Z, Kadekaro AL. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22:809–18. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- Spry ML, Vanover JC, Scott T, Abona-Ama O, Wakamatsu K, Ito S, D'orazio JA. Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigment Cell Melanoma Res. 2009;22:219–29. doi: 10.1111/j.1755-148X.2008.00536.x. [DOI] [PubMed] [Google Scholar]
- Strub T, Giuliano S, Ye T, Bonet C, Keime C, Kobi D, Le Gras S, Cormont M, Ballotti R, Bertolotto C, et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319–32. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Im S, Tada A, Scott C, Akcali C, Davis MB, Barsh G, Hearing V, Abdel-Malek Z. Participation of the melanocortin-1 receptor in the UV control of pigmentation. J Investig Dermatol Symp Proc. 1999;4:29–34. doi: 10.1038/sj.jidsp.5640177. [DOI] [PubMed] [Google Scholar]
- Swope V, Alexander C, Starner R, Schwemberger S, Babcock G, Abdel-Malek ZA. Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell Melanoma Res. 2014;27:601–10. doi: 10.1111/pcmr.12252. [DOI] [PubMed] [Google Scholar]
- Swope VB, Abdel-Malek ZA. Significance of the Melanocortin 1 and Endothelin B Receptors in Melanocyte Homeostasis and Prevention of Sun-Induced Genotoxicity. Frontiers in genetics. 2016;7:146. doi: 10.3389/fgene.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiukeviciene S, Gollnick H, Stang A. Body-site distribution of common acquired melanocytic nevi associated with severe sunburns among children in Lithuania. Int J Dermatol. 2007;46:1242–9. doi: 10.1111/j.1365-4632.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996;5:1663–6. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- Virador VM, Muller J, Wu X, Abdel-Malek ZA, Yu ZX, Ferrans VJ, Kobayashi N, Wakamatsu K, Ito S, Hammer JA, et al. Influence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. Faseb J. 2002;16:105–7. doi: 10.1096/fj.01-0518fje. [DOI] [PubMed] [Google Scholar]
- Von Koschembahr AM, Swope VB, Starner RJ, Abdel-Malek ZA. Endothelin-1 protects human melanocytes from UV-induced DNA damage by activating JNK and p38 signalling pathways. Exp Dermatol. 2015;24:269–74. doi: 10.1111/exd.12638. [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Kasashima H, Fukase Y, Imura M, Imai R, Yamada S, Cleaver JE, Matsunaga T. Physical and functional interaction between DDB and XPA in nucleotide excision repair. Nucleic Acids Res. 2009;37:516–25. doi: 10.1093/nar/gkn964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock MA, Fisher DE. Indoor ultraviolet tanning: what the data do and do not show regarding risk of melanoma and keratinocyte malignancies. J Natl Compr Canc Netw. 2010;8:867–72. doi: 10.6004/jnccn.2010.0063. quiz 873. [DOI] [PubMed] [Google Scholar]
- Wikonkal NM, Brash DE. Ultraviolet radiation induced signature mutations in photocarcinogenesis. J Investig Dermatol Symp Proc. 1999;4:6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- Wolf Horrell EM, Boulanger MC, D'orazio JA. Melanocortin 1 Receptor: Structure, Function, and Regulation. Frontiers in genetics. 2016;7:95. doi: 10.3389/fgene.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD. Mammalian nucleotide excision repair proteins and interstrand crosslink repair. Environmental and molecular mutagenesis. 2010;51:520–6. doi: 10.1002/em.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jiao G, Wei L, Wang N, Xue Y, Lan J, Wang Y, Liu C, Lou M. Current evidences on the XPG Asp1104His polymorphism and melanoma susceptibility: a meta-analysis based on case-control studies. Mol Genet Genomics. 2015;290:273–9. doi: 10.1007/s00438-014-0917-2. [DOI] [PubMed] [Google Scholar]
- Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–7. [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. BioFactors (Oxford, England) 2009;35:193–9. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988;85:6964–7. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hruby VJ, Chen M, Crasto C, Cai M, Harmon CM. Novel binding motif of ACTH analogues at the melanocortin receptors. Biochemistry. 2009;48:9775–84. doi: 10.1021/bi900634e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100:23–6. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- You JS, Wang M, Lee SH. Biochemical analysis of the damage recognition process in nucleotide excision repair. J Biol Chem. 2003;278:7476–85. doi: 10.1074/jbc.M210603200. [DOI] [PubMed] [Google Scholar]