Abstract
Background
Effectiveness data on novel oral anticoagulants (NOACs) versus warfarin for stroke prevention in non-valvular atrial fibrillation (NVAF) by prior warfarin use are limited.
Methods
We used data from the US MarketScan databases from 2009–2012. NVAF patients initiating dabigatran or rivaroxaban were matched with up to 5 warfarin users. Propensity score-adjusted Cox regression was used to calculate hazard ratios (HR) and 95% confidence intervals (95%CI) for relevant endpoints in NOACs versus warfarin users. Separate analyses were conducted to compare anticoagulant-naïve users of NOACs and those switching from warfarin.
Results
Among 32,918 dabigatran, 3,301 rivaroxaban, and 109,447 warfarin users with NVAF, 225 intracranial bleeds, 1035 ischemic strokes, 958 myocardial infarctions, and 1842 gastrointestinal bleeds were identified. Compared to warfarin users, patients initiating NOACs had similar ischemic stroke rates and lower intracranial bleeding rates, while the gastrointestinal bleeding rate was higher in dabigatran users than warfarin users. Associations of dabigatran with ischemic stroke risk differed between anticoagulant-naïve initiators and patients switching from warfarin; dabigatran was associated with lower ischemic stroke rates in naïve users (HR 0.65, 95%CI 0.52–0.82) but not in switchers (HR 1.20, 95%CI 0.95–1.51), compared to warfarin. Risk of stroke and bleeding was not different between rivaroxaban and warfarin users.
Conclusions
Real-world effectiveness of NOACs (compared to warfarin) for diverse outcomes was comparable to efficacy reported in published clinical trials. However, harms and benefits of switching from warfarin to dabigatran need to be evaluated.
Keywords: Atrial fibrillation, Oral anticoagulants, Stroke, Intracranial bleeding, Gastrointestinal bleeding
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with increased morbidity, mortality, and healthcare costs; the estimated number of cases in the USA ranged from 2.7 to 6.1 million in 2010, and is expected to rise to between 5.6 and 12 million in 2050 [1]. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society Guideline for the Management of Patients with AF recommends chronic oral anticoagulation in patients with a moderate or greater risk of ischemic stroke [2]. Whereas Vitamin K antagonists (VKA) (i.e. warfarin in the USA) were historically the only available oral anticoagulant, recent US Food and Drug Administration approvals provided four alternative novel oral anticoagulants (NOACs) for the prophylaxis of ischemic stroke and other cardioembolic complications. Randomized controlled trials (RCTs) of dabigatran, a direct thrombin inhibitor, and rivaroxaban, apixaban, and edoxaban, direct factor Xa inhibitors, demonstrated that these agents are at least as efficacious as warfarin for stroke prevention and are associated with lower rates of hemorrhage [3–7].
In addition to the results from the RCTs, several observational studies examining the effectiveness of dabigatran versus warfarin in AF patients, recently summarized in a meta-analysis [8], have been published. These studies suggest that dabigatran is comparable to warfarin in the prevention of ischemic stroke, is associated with lower risk of intracranial bleeding than warfarin, and higher risk of gastrointestinal (GI) bleeding [8]. Despite the results from these RCTs and observational studies, clinically-relevant issues regarding the use of NOACs in real-world clinical practice need to be resolved. Particularly, given the substantial AF population presently being treated with warfarin and the large proportion of newly diagnosed AF patients who receive warfarin as a first-line anticoagulant treatment, it is important to determine if the effectiveness of NOACs, compared to warfarin, is similar among patients who switch from warfarin to a NOAC and among treatment-naïve patients who initiate treatment with a NOAC. In a prespecified substudy of the RE-LY trial comparing the effectiveness of dabigatran versus VKA in patients naïve to and experienced with VKA [9], prior exposure to VKA did not affect the effectiveness of dabigatran. A similar analysis in the ROCKET-AF trial, however, found that bleeding risks were lower in VKA-naïve patients compared to VKA-experienced patients receiving rivaroxaban [10]. A French cohort study did not find differences in the risk of stroke and bleeding in VKA users switching to a NOAC compared to those maintained on the VKA over 10 months of follow-up [11]. To date, however, no systematic assessment of NOACs effectiveness by prior warfarin exposure, including both warfarin-naïve and warfarin-experienced patients, in usual clinical practice has been done.
To provide innovative real-world evidence on whether the effectiveness of dabigatran and rivaroxaban (versus warfarin) in prevention of ischemic stroke differs between switchers from warfarin to NOACs and anticoagulant-naïve patients and to assess the overall safety profile of oral anticoagulants, we used a large healthcare utilization database to study outcomes of AF patients who were receiving dabigatran, rivaroxaban, or warfarin.
METHODS
Data source and study sample
We conducted a retrospective cohort study utilizing health care claims data from the Truven Health MarketScan® Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics Inc., Ann Arbor, MI, USA) from January 1, 2009, through December 31, 2012. The FDA approved dabigatran in October 2010 and rivaroxaban in November 2011 for the prevention of stroke and systemic embolism in patients with non-valvular AF. The MarketScan databases contain enrollment data and health insurance claims for inpatient and outpatient services as well as outpatient pharmacy services. These data are collected from large US employers and health plans that provide private coverage for employees, their spouses, and dependents and for individuals and their dependents with Medicare supplemental coverage. Patient enrollment data are linked with medical and outpatient prescription drug claims and encounter data enabling individual-specific clinical utilization, cost, and outcomes for inpatient and outpatient services and outpatient pharmacy services.
The analysis was restricted to individuals with medical and outpatient pharmaceutical data, with ≥6 months of continuous enrollment prior to first anticoagulant use. Patients were eligible if they had at least one inpatient or two outpatient claims for AF [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.3, 427.31, and 427.32, in any position] and at least one prescription for warfarin or one of the NOACs (dabigatran or rivaroxaban) after their initial AF diagnosis. A recent systematic review of ICD-9-CM codes for AF identification reported a positive predictive value of approximately 90% and sensitivity of approximately 80% [12]. Consistent with previous studies, two outpatient claims for AF are required to minimize the impact of rule-out diagnoses and improve algorithm specificity [13]. Patients with ICD-9-CM diagnostic codes for valvular disease or procedure codes for valvular repair or replacement before or at AF diagnosis were excluded because the NOACs have received FDA approval for non-valvular AF only. The index date was defined as the date of the first anticoagulant prescription following AF diagnosis. Patients with AF enrolled in the MarketScan Medicare Supplemental Database have similar demographic characteristics to patients with AF in the general fee-for-service Medicare population [13, 14].
All patient information was Health Insurance Portability and Accountability Act compliant, deidentified, commercially available secondary data; therefore, the Institutional Review Board at the University of Minnesota deemed this analysis exempt from review.
Anticoagulant use
Each outpatient pharmaceutical claim includes the National Drug Code, the prescription fill date, and the number of days supplied. All prescriptions for oral anticoagulants (dabigatran, rivaroxaban, and warfarin) from January 1, 2009 to December 31, 2012 were identified. Patients using NOACs were initially categorized according to their first anticoagulant prescription as either dabigatran or rivaroxaban users. After classification as a dabigatran or rivaroxaban user, these patients were classified further based on their use of warfarin prior to their NOAC use; those with no history of warfarin were classified as new (“naïve”) anticoagulant users (no previous recorded warfarin exposure) while those with prior warfarin use were classified as switchers (previous warfarin user, switching to a NOAC). Each NOAC user was matched with up to 5 warfarin users by age (±3 years), sex, and time since database enrollment. Individuals who switched to NOACs were matched with AF patients who had ≥3 months of continuous warfarin use before the index date of the matched NOAC user. New NOAC users were matched with AF patients who had initiated warfarin use <3 months before the index date of the matched NOAC user. The validity of warfarin claims in administrative data is excellent with a sensitivity of 94% and a positive predictive value of 99% [15].
Outcome ascertainment
The outcomes of interest were obtained from inpatient claims, using validated algorithms, and included intracranial bleeding, ischemic stroke, myocardial infarction (MI), GI bleeding, and hip/pelvic fracture obtained from inpatient claims. Hip/pelvic fracture was included as a “neutral” endpoint, for which an association with type of anticoagulant would not be expected; similar hip/pelvic fracture risk between groups provides indirect evidence of no confounding. Intracranial bleeding was defined based on the presence of ICD-9-CM codes 430 (subarachnoid hemorrhage), and 431 (intracerebral hemorrhage) as the primary discharge diagnosis in an inpatient claim after the index date. Positive predictive value (PPV) of this definition has been >90% in many different validation studies [16]. Ischemic stroke was defined based on the presence of ICD-9-CM codes 434.xx (occlusion of cerebral arteries) and 436.xx (acute but ill-defined cerebrovascular disease) as the primary discharge diagnosis in any inpatient claim following their index date. Several validation studies have reported PPV of >80% for this definition [16]. MI was defined as the presence of an inpatient claim with an ICD-9-CM discharge diagnosis code of 410.xx (excluding 410.x2, used to indicate follow-up of the initial episode) in the first or second position. This algorithm has had a PPV between 88–94% in validation studies [17, 18]. GI bleeding was defined according to an algorithm developed by Cunningham et al. [19]. This algorithm considers presence of bleeding-related ICD-9-CM codes in inpatient claims as primary and secondary diagnoses, presence of transfusion codes (hospital revenue code indicating transfusion/cross-matching for transfusion), and presence/absence of trauma codes to exclude trauma-related bleeding. This algorithm PPV is 86% [19], which compares favorably to other peer-reviewed algorithms [18]. Hip/pelvic fracture was defined according to the Centers for Medicare and Medicaid Chronic Conditions Data Warehouse algorithm, developed from published literature [20].
Assessment of covariates
Covariates were defined based on inpatient and outpatient claims during the enrollment period prior to the index date (≥6 months) with validated published algorithms [19, 21]. Demographic characteristics, comorbidities, procedures, and pharmacy fills were ascertained. Comorbidities of interest were ascertained with published algorithms from inpatient and outpatient claims and include prior stroke/transient ischemic attack (TIA), hemorrhagic stroke, heart failure, MI, hypertension, diabetes, peripheral arterial disease, liver disease, kidney disease, chronic pulmonary disease, malignancies (except malignant skin neoplasm), metastatic cancer, history of bleeding, hematological disorders (anemia, coagulation defects), dementia, depression, and alcohol abuse [19, 21]. Cardiac, vascular, GI, and neurologic procedures also were identified from inpatient and outpatients claims. Presence of prescription fills for the following medication groups were ascertained: digoxin, clopidogrel, other antiplatelets, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, antiarrhythmics, statins, and antidiabetic medications.
Statistical analysis
The primary analysis included patients categorized according to the anticoagulant used at their index date. Cox proportional hazards models were utilized to assess the association between anticoagulant type (separately for dabigatran and rivaroxaban vs warfarin) and the time to each outcome. The time to event was censored at the earliest of health plan disenrollment or the end of study follow-up. Separate models were estimated to compare new NOAC users to new warfarin users and for switchers to NOACs to existing warfarin users.
High-dimensional propensity scores were calculated for each of the main comparisons. Methodology proposed by Schneeweiss et al. was used and included the following dimensions: age, sex, inpatient diagnostic codes, inpatient procedure codes, outpatient diagnostic does, outpatient procedure codes, and outpatient pharmacy claims [22]. High-dimensional propensity scores were calculated with the SAS macros developed by Rassen et al. and included both empirical variables and covariates described above [23]. For each outcome, Cox proportional hazards models were adjusted for high-dimensional propensity score decile as well as age, sex, and CHADS2 score, to allow stratification of results by these three covariates.
We assessed the proportional hazards assumptions and found that it did not hold for all outcomes. The results are presented overall and stratified by follow-up time, classified as early (<90 days) and late (≥90 days). A sensitivity analysis was performed among high-dimensional propensity score-matched dabigatran and warfarin users. A greedy matching technique, which is an efficient approximation of a nearest neighbor matching approach, where the comparator with the closest propensity score is selected was implemented with a published SAS macro for the matched analysis [24]. Kaplan-Meier survival curves were used to calculate the probability of survival free of each outcome of interest separately for dabigatran and warfarin new users and switchers. Effect measure modification by sex, age (≤75 and >75), and CHADS2 score (0–1 classified as low risk and ≥2 classified as moderate/high risk) was explored via stratified analysis. Due to the small number of rivaroxaban users and correspondingly few events, new users and switchers were pooled for analysis. All statistical analyses were performed with SAS version 9.3 (SPSS, Chicago, IL, USA).
RESULTS
A total of 61,648 anticoagulant initiators (18,981 dabigatran users, 2,100 rivaroxaban users, and 40,567 matched warfarin users) and 84,018 switchers (13,937 dabigatran users, 1,202 rivaroxaban users, and 68,880 matched warfarin users) met all inclusion criteria and formed the primary study population. Most patient characteristics differed between dabigatran initiators and warfarin initiators, with a slightly healthier profile in dabigatran compared to warfarin users. However, age, liver disease, alcohol abuse, antiplatelet medication use, and prior neurological procedures were similar (Table 1). A similar pattern was observed between switchers to dabigatran and persistent warfarin users. Age and sex distributions, as well as peripheral arterial disease, liver disease, alcohol abuse, antiplatelet medication use, angiotensin-converting-enzyme inhibitors, and CHADS2 score were similar between the groups. In the analysis of rivaroxaban users, patient characteristics followed a similar pattern between groups as those for dabigatran and warfarin users. Rivaroxaban users were significantly younger, had a lower CHADS2 score, and had fewer comorbidities compared to warfarin users. The prevalence of diabetes, heart failure, ischemic stroke/TIA, hemorrhagic stroke, renal disease, peripheral arterial disease, dementia, chronic pulmonary disease, malignancy, hematological disorders, and other bleed were higher among warfarin users compared to rivaroxaban users. Propensity score distributions were more similar among dabigatran switchers and persistent warfarin users than among dabigatran new users and warfarin new users. Propensity score distributions were similar for all outcomes; distributions for stroke can be seen in Supplemental Figure 1.
Table 1.
Characteristics of atrial fibrillation patients by anticoagulant use, MarketScan Databases, 2009 to 2012
| New Users | Switchers | Pooled (New Users and Switchers) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dabigatran | Matched | Dabigatran | Matched | Rivaroxaban | Matched | |
| User | Warfarin User | User | Warfarin User | User | Warfarin User | |
| Age, years | 68.5±12.3 | 70.8±12.1 | 70.9±11.3 | 71.5±11.4 | 70.4±12.0 | 72.5±12.2 |
| Female, % | 36.2 | 38.8 | 37.9 | 38.0 | 39.8 | 41.2 |
| Comorbidities, % | ||||||
| Hypertension | 75.2 | 72.9 | 82.0 | 80.2 | 85.6 | 84.7 |
| Diabetes | 28.6 | 32.1 | 32.2 | 33.8 | 30.7 | 35.4 |
| Myocardial infarction | 7.6 | 9.5 | 7.6 | 9.2 | 10.5 | 11.7 |
| Heart failure | 24.3 | 30.4 | 35.2 | 36.6 | 31.5 | 39.3 |
| Ischemic stroke/TIA | 20.6 | 22.3 | 25.4 | 24.0 | 26.3 | 30.9 |
| Hemorrhagic stroke | 0.7 | 1.1 | 1.1 | 1.4 | 1.3 | 2.1 |
| Renal disease | 7.6 | 12.9 | 10.0 | 13.0 | 11.2 | 16.0 |
| PAD | 15.5 | 18.0 | 19.8 | 20.3 | 21.4 | 25.6 |
| Dementia | 1.0 | 2.0 | 1.4 | 2.1 | 1.7 | 3.0 |
| Chronic pulmonary disease | 24.8 | 28.0 | 28.9 | 30.6 | 34.0 | 36.0 |
| Liver disease | 4.8 | 4.9 | 5.8 | 5.7 | 7.5 | 7.7 |
| Malignancy | 13.4 | 14.7 | 15.2 | 16.4 | 15.8 | 19.3 |
| Depression | 7.8 | 9.2 | 8.5 | 9.6 | 12.2 | 12.3 |
| Hematological disorders | 9.2 | 14.1 | 19.0 | 20.0 | 17.0 | 22.5 |
| Metastatic cancer | 1.6 | 2.3 | 1.4 | 2.2 | 1.9 | 2.5 |
| Alcohol abuse | 0.4 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 |
| GI bleed | 7.6 | 8.3 | 10.4 | 11.4 | 13.2 | 14.5 |
| Other bleed | 3.6 | 5.0 | 7.9 | 8.4 | 7.6 | 9.5 |
| CHADS2 score | 2.0±1.4 | 2.2±1.5 | 2.4±1.4 | 2.4±1.5 | 2.4±1.5 | 2.7±1.6 |
| Initial (first) dose of novel oral anticoagulant*, % | ||||||
| Rivaroxaban | ||||||
| 10 mg | – | – | – | – | 15.7 | – |
| 15 mg | – | – | – | – | 15.2 | – |
| 20 mg | – | – | – | – | 69.2 | – |
| Dabigatran | ||||||
| 75 mg | 8.3 | – | 6.6 | – | – | – |
| 150 mg | 91.7 | – | 93.4 | – | – | – |
| Prior procedures | ||||||
| Cardiac | 69.0 | 70.9 | 75.9 | 78.2 | 81.3 | 80.2 |
| Vascular | 4.0 | 7.9 | 5.3 | 6.9 | 6.9 | 9.3 |
| Gastrointestinal | 35.3 | 32.4 | 41.8 | 39.8 | 51.9 | 50.1 |
| Neurological | 15.4 | 15.6 | 19.4 | 17.2 | 29.3 | 22.6 |
| Medications | ||||||
| Digoxin | 14.9 | 16.2 | 28.9 | 27.6 | 21.9 | 25.3 |
| Clopidogrel | 14.0 | 12.0 | 10.8 | 10.1 | 15.7 | 13.0 |
| Antiplatelets | 2.1 | 2.0 | 1.5 | 1.6 | 2.9 | 2.3 |
| Angiotensin-converting-enzyme inhibitors | 36.0 | 37.6 | 42.5 | 43.3 | 40.3 | 43.9 |
| Angiotensin receptor blockers | 23.5 | 20.5 | 28.1 | 23.9 | 29.3 | 25.7 |
| β-blockers | 71.1 | 64.8 | 79.4 | 76.2 | 77.6 | 76.4 |
| Calcium channel blockers | 41.7 | 39.4 | 48.9 | 44.4 | 48.3 | 46.5 |
| Anti-arrhythmias | 29.4 | 20.4 | 39.3 | 29.1 | 41.5 | 29.4 |
| Statins | 54.3 | 51.7 | 64.2 | 61.5 | 61.3 | 62.5 |
| Diabetes medications | 21.5 | 23.7 | 24.0 | 24.8 | 21.2 | 24.8 |
Values correspond to mean ± standard deviation or percentage
A small number of patients (<0.1%) had multiple prescriptions with conflicting medication strengths on the same day; the most common dose was assigned, 150 mg and 20 mg for dabigatran and rivaroxaban, respectively.
TIA, transient ischemic attack; PAD, peripheral arterial disease; GI, gastrointestinal.
Dabigatran vs warfarin
During a median follow-up duration from anticoagulant initiation of 15 months, new users of dabigatran had a significantly lower risk of intracranial bleeding, stroke, and MI, after adjustment for age, sex, CHADS2 score, and propensity score decile compared to new users of warfarin (Table 2). After multivariable and propensity-score adjustment, there was no difference in the rate of GI bleeding or hip/pelvic fracture among new users of dabigatran compared to warfarin initiators.
Table 2.
Adjusted hazard ratios (95% confidence intervals) comparing dabigatran new users to warfarin new users for the treatment of non-valvular atrial fibrillation
| Number of events | Early (<90 days) | Late (≥90 days) | |||
|---|---|---|---|---|---|
| Dabigatran | Warfarin | Adjusted hazard ratio (95% CI)* |
Adjusted hazard ratio (95% CI)* |
Adjusted hazard ratio (95% CI)* |
|
| Intracranial bleed | 15 | 79 | 0.37 (0.20, 0.67) | 0.43 (0.13, 1.42) | 0.34 (0.17, 0.68) |
| Ischemic stroke | 127 | 369 | 0.65 (0.52, 0.82) | 0.32 (0.22, 0.47) | 0.99 (0.75, 1.31) |
| Myocardial infarction | 115 | 335 | 0.72 (0.57, 0.91) | 0.32 (0.21, 0.51) | 0.99 (0.75, 1.31) |
| Gastrointestinal bleed | 265 | 593 | 1.04 (0.88, 1.22) | 1.11 (0.82, 1.51) | 1.01 (0.83, 1.22) |
| Hip/pelvic Fracture | 108 | 275 | 1.02 (0.80, 1.30) | 0.52 (0.28, 0.97) | 1.16 (0.88, 1.51) |
Adjusted for age, sex, CHADS2 score, and propensity score decile
The multivariable and propensity-score adjusted rate of stroke, MI, and hip/pelvic fracture was similar among switchers to dabigatran compared to persistent warfarin users (Table 3). The rate of intracranial bleeding was significantly lower among dabigatran switchers compared to persistent warfarin users, after adjustment for age, sex, CHADS2 score, and propensity score decile (HR 0.42 95% CI 0.23, 0.75), while the rate for GI bleeding was significantly higher (HR 1.83 95% CI 1.58, 2.13). Similar results were observed in sensitive analyses among propensity score matched patients (Supplemental Tables 1 and 2).
Table 3.
Adjusted hazard ratios (95% confidence intervals) comparing switchers to dabigatran to warfarin users for the treatment of non-valvular atrial fibrillation
| Number of events | Early (<90 days) | Late (≥90 days) | |||
|---|---|---|---|---|---|
| Dabigatran | Warfarin | Adjusted hazard ratio (95% CI) |
Adjusted hazard ratio (95% CI) |
Adjusted hazard ratio (95% CI) |
|
| Intracranial bleed | 13 | 110 | 0.42 (0.23, 0.75) | 0.25 (0.06, 1.07) | 0.48 (0.25, 0.91) |
| Ischemic stroke | 99 | 377 | 1.20 (0.95, 1.51) | 0.90 (0.51, 1.59) | 1.26 (0.98, 1.61) |
| Myocardial infarction | 105 | 359 | 1.24 (0.99, 1.56) | 1.33 (0.82, 2.14) | 1.23 (0.96, 1.59) |
| Gastrointestinal bleed | 261 | 649 | 1.83 (1.58, 2.13) | 1.23 (0.96, 1.59) | 1.67 (1.40, 1.98) |
| Hip/pelvic Fracture | 96 | 498 | 0.89 (0.71, 1.11) | 1.02 (0.63, 1.66) | 0.85 (0.66, 1.09) |
Adjusted for age, sex, CHADS2 score, and propensity score decile
The difference in rates between dabigatran and warfarin users for intracranial bleeding occurred early after the index date and increased over time (Fig. 1A). The decreased rate among dabigatran users relative to warfarin users was seen in both new users and switchers. The rate of ischemic stroke was significantly lower among new dabigatran users compared to new warfarin users (Fig. 1B). This was especially true in the early period following treatment initiation (Table 2). Dabigatran switchers and persistent warfarin users had fairly similar rates (Fig. 1B). New warfarin users had an increased rate of MI compared to new dabigatran users (Fig. 1C). The rate of MI was similar between dabigatran switchers and persistent warfarin users (Fig. 1C). The rate of GI bleeding was higher among dabigatran switchers and was similar among the other anticoagulant users (Fig. 1D).
Figure 1.
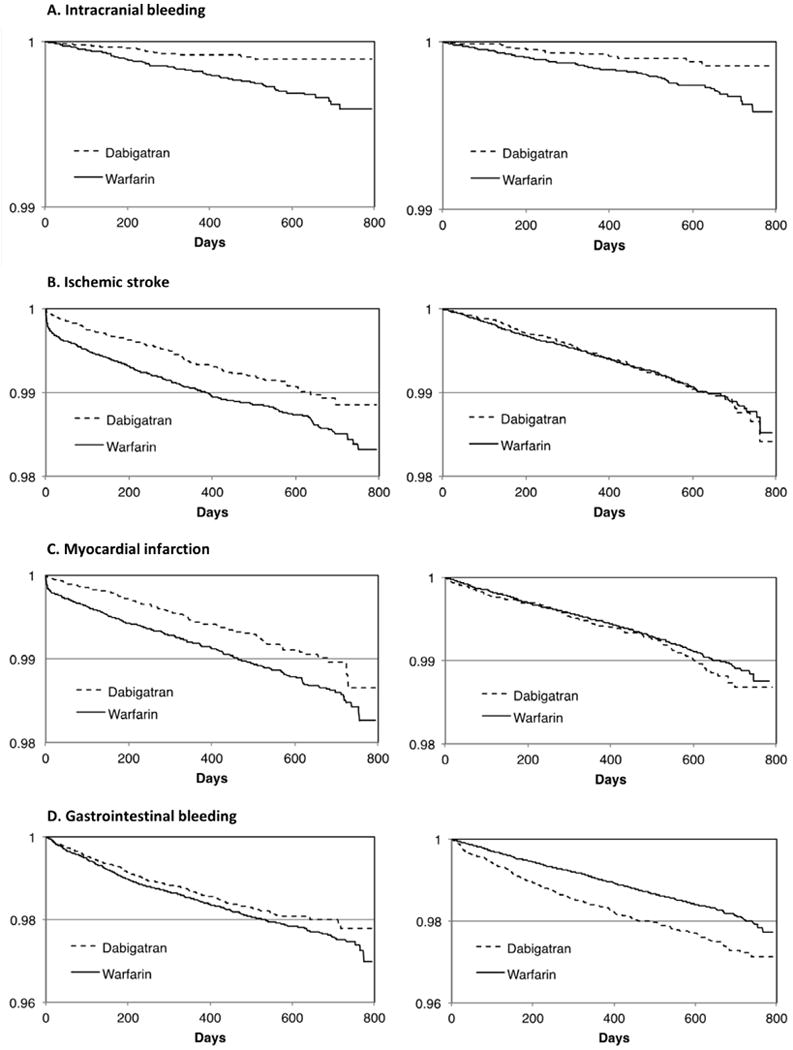
Kaplan-Meier estimates of endpoint-free survival during follow-up for dabigatran and warfarin users. Left panels show new users of dabigatran versus new users of warfarin. Right panels show switchers to dabigatran versus persistent warfarin users. (A) Intracranial bleeding. (B) Ischemic stroke. (C) Myocardial infarction. (D) Gastrointestinal bleeding.
Subgroup analysis
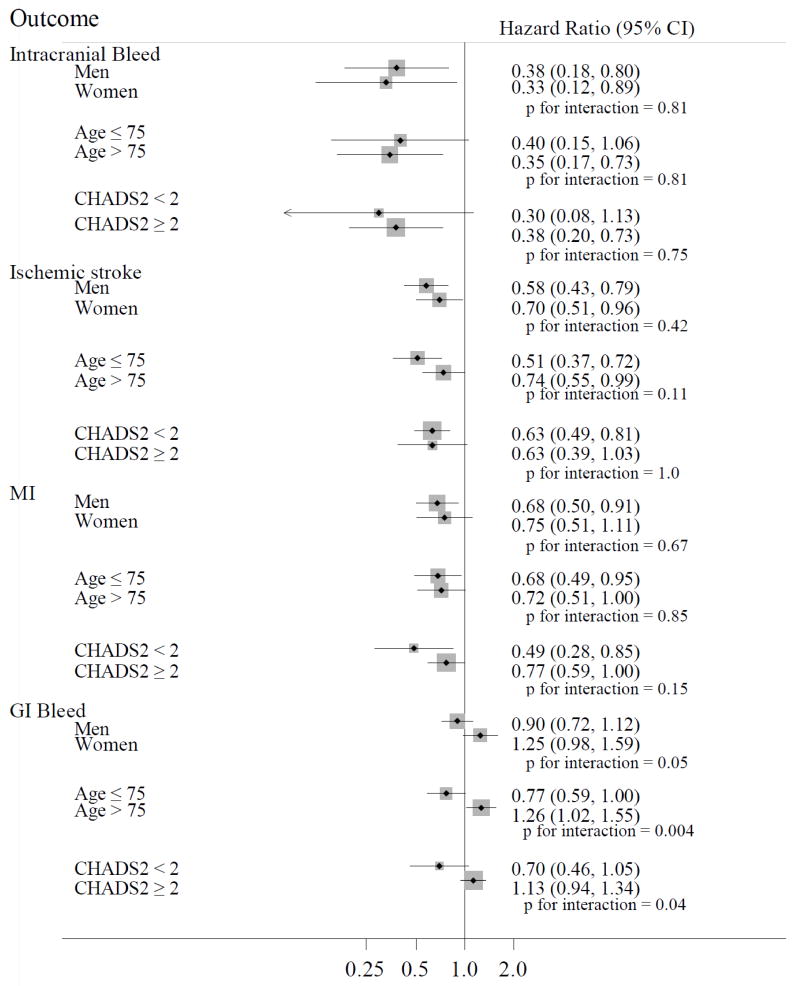
In stratified analyses, among new anticoagulant users, the associations between anticoagulant type and intracranial bleeding, stroke, and MI were similar in men and women, younger and older patients, and those with a low or moderate/high stroke risk (Fig. 2). The association between anticoagulant type and GI bleeding differed by sex (p for interaction=0.05), age (p for interaction=0.004), and stroke risk (p for interaction=0.04). The occurrence of GI bleeding was lower among new dabigatran users compared to new warfarin users for men (HR 0.90, 95% CI 0.72, 1.12), younger patients (HR 0.77, 95% CI 0.59, 1.00), and those with a low stroke risk (HR 0.70 95% CI 0.46, 1.05), while the inverse was observed for women (HR 1.25 95% CI 0.98, 1.59), older patients (HR 1.26, 95% CI 1.02, 1.55), and those with a moderate/high stroke risk (HR 1.13, 95% CI 0.94, 1.34).
Figure 2.
Adjusted hazard ratios (95% confidence intervals) of outcomes in dabigatran new users versus warfarin new users across selected subgroups, MarketScan Databases, 2009 to 2012.
MI, myocardial infarction; GI, gastrointestinal.
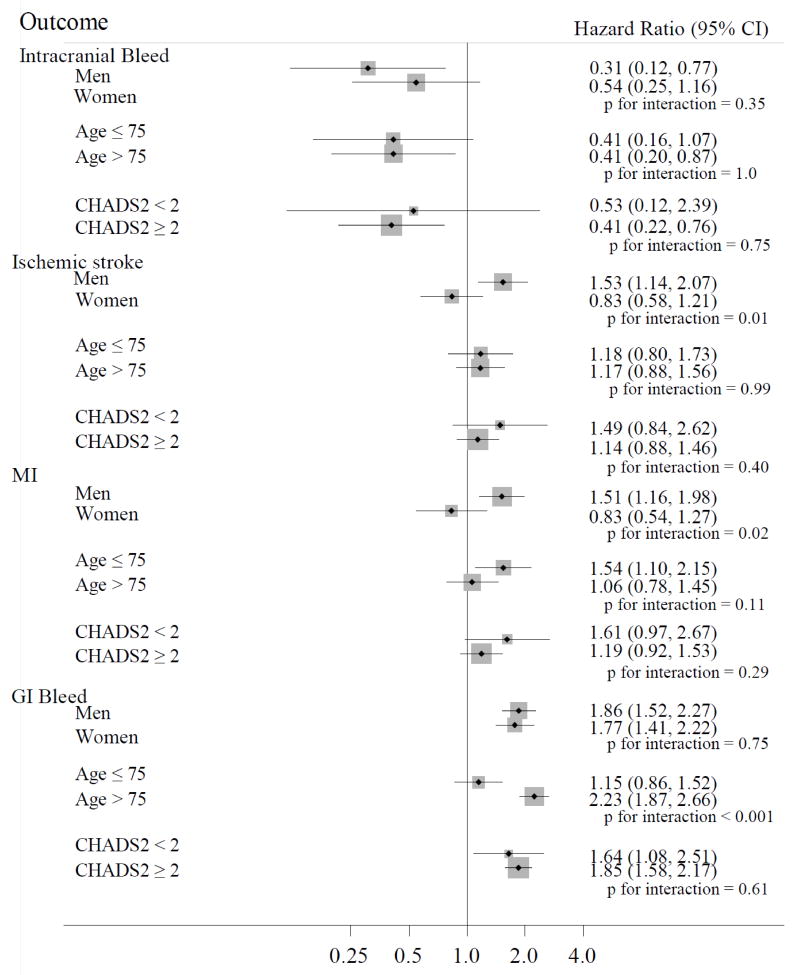
Among switchers (Fig. 3), stratified analyses revealed a significant interaction (on the multiplicative scale) with sex for stroke (p for interaction=0.01) and MI (p for interaction=0.02) and with age for GI bleeding (p for interaction<0.001). Among men, the occurrence of stroke (HR 1.53, 95% CI 1.14, 2.07) and MI (HR 1.51, 95% CI 1.16, 1.98) were higher among dabigatran switchers compared with persistent warfarin users, while the opposite was detected among women (HR 0.83, 95% CI 0.58, 1.21) and (HR 0.83, 95% CI 0.54, 1.27), respectively. The association between anticoagulant use and GI bleeding differed in magnitude between younger and older patients; the risk of GI bleeding was greater among switchers to dabigatran compared to persistent warfarin users but the association was stronger among older patients (HR 1.15 95% CI 0.86, 1.52 in those ≤75 years of age vs. HR 2.23 95% CI 1.87, 2.66, in those older than >75 years).
Figure 3.
Adjusted hazard ratios (95% confidence intervals) of outcomes in switchers to dabigatran to warfarin users across selected subgroups, MarketScan Databases, 2009 to 2012.
MI, myocardial infarction; GI, gastrointestinal.
Rivaroxaban vs warfarin
Due to a short follow-up period (median follow-up of 8 months) and few outcome events (<25 for any outcome among rivaroxaban users), analyses were pooled for new users of and switchers to rivaroxaban. There were no significant differences in outcomes between rivaroxaban and warfarin users (Supplemental Table 3); however, these analyses were underpowered to detect an association and are presented as a first examination of real-world data only.
DISCUSSION
This retrospective administrative claims analysis found that among AF patients, the risks of intracranial bleeding, ischemic stroke, and MI were lower among new users of dabigatran compared to new users of warfarin. Notably, the difference in ischemic stroke rates was present during the 90 days following treatment initiation, after which the rates were similar between new users of dabigatran and new users of warfarin. In contrast, the risk of ischemic stroke was not significantly different among those who switched from warfarin to dabigatran compared to persistent warfarin users. Furthermore, the results identified other differences in the risks for new users and switchers according to key patient characteristics, an area with a dearth of research to date.
Our findings have direct clinical implications. First, in anticoagulant-naïve patients NOACs are at least as effective and safe as warfarin. Second, patients already using warfarin may not experience any clinical benefit in the prevention of stroke if switching to a NOAC. And, third, individual patient characteristics modify the risk-benefit balance of NOACs, pointing to potential benefits of more individualized and precise approaches to the choice of oral anticoagulation.
Effectiveness results from the present analysis are fairly consistent with efficacy results from the RE-LY trial where dabigatran was non-inferior or superior to warfarin for the prevention of stroke or systemic embolism [3, 9]. Additionally, these results corroborate other real-world analyses [8, 25–29]. However, these previous studies focused on comparing dabigatran initiators with warfarin initiators, without consideration of associations among switchers. Three recent publications, two reporting secondary analyses of the RE-LY and ROCKET-AF trials and one using French administrative health data, have explored the risk of stroke and bleeding among switchers from warfarin to NOACs, with inconsistent results [9–11]. Switchers comprise a highly clinically relevant group, considering the large number of AF patients presently using warfarin. Given the various benefits of NOACs (e.g. no need for anticoagulation monitoring and fewer adverse dietary and pharmacologic interactions) current warfarin users may consider a switch to a NOAC; our results, however, indicate that switching from warfarin to dabigatran does not provide any additional benefit for stroke prevention.
During the first 90 days following treatment initiation, the rate of ischemic stroke was substantially lower in new users of dabigatran versus new warfarin users. The stroke rate was, however, similar after 90 days as well as among switchers to dabigatran and persistent warfarin users. A potential explanation for this difference is that it takes time for new warfarin users to stabilize in the therapeutic range, during which these patients may be particularly at risk of cardioembolic complications [30]. A similar pattern was observed for MI, whereby new users of warfarin also had a greater risk, compared to new users of dabigatran while persistent warfarin users had similar risk of MI compared to dabigatran users (new users and switchers), and for GI bleeding, with no differences in the risk among new users of anticoagulants, but higher risk among switchers to dabigatran than continuous users of warfarin. This finding suggests that persistent users of warfarin are a selected group who may be free of complications from warfarin and switching to dabigatran may not offer any added benefit for stroke prevention and may even increase the risk of some complications such as GI bleeding. However, intracranial bleeding risk was lower for dabigatran users (initiators and switchers) compared to warfarin users (initiators and persistent users). Our results suggest that risk-benefit considerations should be different in anticoagulant-naïve users and those already using warfarin.
Among new users of anticoagulants, there was evidence that sex, age, and CHADS2 score modified the risk of GI bleeding associated with dabigatran compared to warfarin. Specifically, dabigatran was associated with an increased risk of GI bleeding particularly among women, patients >75 years of age, and those with a CHADS2 score ≥2. These results were consistent with previously reported subgroup analyses which found increased risk of GI bleeding for those ≥75 years and treated with dabigatran compared to warfarin [26] and that the risk of GI bleeding was increased for women aged 75 years and older using dabigatran compared to warfarin [25]. Subgroup analysis among patients who switched to dabigatran compared to persistent warfarin users found that dabigatran was associated with an increased risk of ischemic stroke and MI in men but not in women. Potential explanations for this difference are not fully elucidated and it is possible that this finding is due to chance. Upholding and expanding on both RCT and observational studies, compared with persistent warfarin use, switching to dabigatran was associated with higher risk of GI bleeding among patients aged >75 years than among patients aged ≤75 years.
Despite the limited number of events among rivaroxaban users, there was no evidence that the risk of GI bleeding was disproportionally higher among rivaroxaban users compared to warfarin users. Even though these real-world effectiveness findings have wide confidence intervals the results are consistent with the ROCKET-AF efficacy results. Future studies should explore determinants of adverse endpoints in users of NOACs [31].
This study has several limitations which should be considered. First, unmeasured confounding is a major drawback of observational studies utilizing administrative claims. Particularly, we lacked specific information on the reasons why patients switched from warfarin to dabigatran. High-dimensional propensity scores were utilized to adjust for both pre-defined and empirically identified confounders. This approach has been studied empirically and found to be reasonably effective [22]. The similar risk of hip fracture among dabigatran and warfarin users provides indirect evidence of no residual uncontrolled confounding. Second, the results of this analysis rely on the ability to ascertain accurately both covariates and outcomes in administrative data. Validated algorithms were utilized to ascertain events of interest and it is likely that any misclassification is non-differential. Third, follow-up data were limited in duration given the recent FDA approval of dabigatran (October 2010) and rivaroxaban (November 2011). Fourth, medication adherence following initiation was not considered in this analysis.
Strengths of this study include, first, the availability of administrative data across the continuum of care for a relatively large number of dabigatran users, which enabled the use of state-of-the-art methodology for confounding adjustment. Second, this is the first real-world analysis, to our knowledge, to stratify the analysis by new user and switcher status. Third, many real-world analyses focus on a single outcome, usually bleeding. The present analysis includes a number of important outcomes for AF patients.
CONCLUSIONS
In conclusion, these real-world effectiveness results for dabigatran users compared to warfarin users were similar to RCT efficacy results. The risks of intracranial bleeding and ischemic stroke were lower among new users of dabigatran compared to new users of warfarin. Our results provide information on the safety profile of dabigatran and may help clinicians and patients make informed decisions when selecting an oral anticoagulant for thromboembolic prevention in AF.
Supplementary Material
Acknowledgments
FUNDING
Grant R01-HL122200 from the National Heart, Lung and Blood Institute, grant 16EIA2640001 from the American Heart Association, the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114, and a Small Grant from the University of Minnesota Academic Health Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr Bengtson is an employee of Optum. The other authors have no conflicts of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open studies. J Cardiol. 2015;66:466–74. doi: 10.1016/j.jjcc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Romanelli RJ, Nolting L, Dolginsky M, Kym E, Orrico KB. Dabigatran versus warfarin for atrial fibrillation in real-world clinical practice: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2016;9:126–34. doi: 10.1161/CIRCOUTCOMES.115.002369. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz MD, Wallentin L, Connolly SJ, Parekh A, Chernick MR, Pogue J, Aikens TH, Yang S, Reilly PA, Lip GY, Yusuf S, RE-LY Steering Committee and Investigators Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. Circulation. 2010;122:2246–53. doi: 10.1161/CIRCULATIONAHA.110.973735. [DOI] [PubMed] [Google Scholar]
- 10.Mahaffey KW, Wojdyla D, Hankey GJ, White HD, Nessel CC, Piccini JP, Patel MR, Berkowitz SD, Becker RC, Halperin JL, Singer DE, Califf RM, Fox KA, Breithardt G, Hacke W. Clinical outcomes with rivaroxaban in patients transitioned from vitamin K antagonist therapy: a subgroup analysis of a randomized trial. Ann Intern Med. 2013;158:861–8. doi: 10.7326/0003-4819-158-12-201306180-00003. [DOI] [PubMed] [Google Scholar]
- 11.Bouillon K, Bertrand M, Maura G, Blotiere PO, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2:e150–9. doi: 10.1016/S2352-3026(15)00027-7. [DOI] [PubMed] [Google Scholar]
- 12.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 1):141–7. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naccarelli GV, Johnston SS, Dalal M, Lin J, Patel PP. Rates and implications for hospitalization of patients >=65 year of age with atrial fibrillation/flutter. Am J Cardiol. 2012;109:543–9. doi: 10.1016/j.amjcard.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Garg RK, Glazer NL, Wiggins KL, Newton KM, Thacker EL, Smith NL, Siscovick DS, Psaty BM, Heckbert SR. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf. 2011;20:313–6. doi: 10.1002/pds.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 1):100–28. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Wahl PM, Rodgers K, Schneeweiss S, Gage BF, Butler J, Wilmer C, Nash M, Esper G, Gitlin N, Osborn N, Short LJ, Bohn RL. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf. 2010;19:596–603. doi: 10.1002/pds.1924. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–6. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter NN, Haberman EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–93. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–22. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox. Boston, MA: http://www.hdpharmacoepi.org. [Google Scholar]
- 24.Parsons LS. SUGI 26: Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Available from: www2.sas.com/proceedings/sugi26/p214-26.pdf.
- 25.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–64. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez I, Baik SH, Piñera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. doi: 10.1001/jamainternmed.2014.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4:e001798. doi: 10.1161/JAHA.115.001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290–8. doi: 10.1160/TH15-06-0453. [DOI] [PubMed] [Google Scholar]
- 29.Seeger JD, Bykov K, Bartels DB, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114:1277–89. doi: 10.1160/TH15-06-0497. [DOI] [PubMed] [Google Scholar]
- 30.Nelson WW, Desai S, Damaraju CV, Lu L, Fields LE, Wildgoose P, Schein JR. International normalized ratio stabilization in newly initiated warfarin patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30:2437–42. doi: 10.1185/03007995.2014.957822. [DOI] [PubMed] [Google Scholar]
- 31.Nishino M, Okamoto N, Tanaka A, Mori N, Hara M, Yano M, Makino N, Egami Y, Shutta R, Tanouchi J. Different risk factors for bleeding and discontinuation between dabigatran and rivaroxaban. J Cardiol. 2016;68:156–60. doi: 10.1016/j.jjcc.2015.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.