Abstract
Background
The 12-item MS Walking Scale (12-MSWS), is a validated questionnaire which assessed walking function; it has been widely adopted in multiple sclerosis (MS) clinical research.
Objective
Identify and validate clinically-meaningful 12-MSWS benchmarks in MS.
Methods
Cross-sectional study of 159 MS patients permitted identification of clinically-meaningful 12-MSWS benchmarks based on their relationship to real-life anchors. Identified 12-MSWS benchmarks were then validated in a second population of 96 subjects using measures of ambulation, cognition, and patient-reported outcomes.
Results
12-MSWS score of 0–24.99 was associated with working outside the home and assistance-free mobility; 25–49.99 was associated with gait disability and difficulty doing housework; 50–74.99 was associated with unemployment, government healthcare, cane use, and difficulty performing instrumental activities of daily living (IADLs); and 75–100 was associated with change in occupation due to walking, mobility impairment requiring bilateral assistance, and inability to perform IADLs. During the validation step, strong linear associations identified between 12-MSWS benchmarks and other MS-related disability outcome measures, including ambulatory and non-ambulatory measures.
Conclusion
We have identified clinically-meaningful 12-MSWS benchmarks which define 4 groups differentiated by increasing levels of mobility impairment and associated loss of functional independence. These data provide insight into how 12-MSWS translate to meaningful functional limitations in MS.
Keywords: Multiple Sclerosis, Outcome Research, Clinically Meaningful, MS Walking Scale, Walking Impairment
INTRODUCTION
Mobility impairment is prevalent among patients with multiple sclerosis (MS)1, and is often observed early in disease course even at otherwise low degrees of clinical disease burden2,3. Mobility decline in MS is correlated with decreased health-related quality of life and increased indirect costs such as lost work-place productivity4. Patient-perceived mobility impairment tends to increase with lengthening duration of disease5.
Multiple measures exist for the assessment of walking function in MS6. The majority of these depend on objective measures of walking times. The Timed 25-Foot Walk (T25FW) is one such measure, and clinically meaningful benchmarks in T25FW performance have been identified – demonstrating associations with occupational changes and disability due to MS, need for assistive devices, loss of independence with activities of daily living, and government income and health-care assistance7,8. The 12-item MS Walking Scale (12-MSWS), is a validated and reliable questionnaire assessing the impact of MS on walking, can be easily administered in a clinical setting, and has been widely adopted in MS clinical research9,10,11 [appendix 1]. The 12-MSWS results in a total calculated score range from 12 to 60, which is then transformed into scores ranging from 0 to 100. A higher score is indicative of increased walking impairment. Of the available walking measures in MS, the 12-MSWS is unique as a measure of patient-reported perception of ambulatory function. We hypothesized that, similar to the T25FW, 12-MSWS benchmarks could be identified to indicate significant and meaningful changes in real-world disability.
METHODS
Standard protocol approvals, registrations, and patient consent
This study involved a secondary analysis of existing cross-sectional data7 and identified and validated clinically meaningful 12-MSWS benchmarks as was done for the T25FW. Study protocols were approved by the University of Virginia (Identification Step) and University of Illinois at Peoria (Validation Step) institutional review boards. All participants provided informed written consent.
MS populations
A questionnaire was mailed to all 300 individuals with MS (relapsing or progressive forms) who had available T25FW test available as part of their routine clinical care in the 15 months prior to survey mailing (2/2011). No respondents were having an MS-relapse at time of T25FW measurement. Surveys included general demographic information, as well as validated scales of MS disability and activities of daily living. Respondents provided clinically meaningful information regarding marital status, current employment, instrumental activities of daily living (IADL)7,12, and use of walking assistive devices. Surveys also included the 12-MSWS, Patient Determined Disease Steps (PDDS) and Performance Scales (PS)13,14, and Beck Depression Inventory-Fast Screen15,16.
The mean and median 12-MSWS scores of our population were calculated within all of the real-life anchor categories collected (e.g. married vs divorced, working full-time, government healthcare assistance, and IADL independence). Using mean and median 12-MSWS scores, we identified and grouped subjects by candidate benchmarks. We then formed potential benchmark groupings in 10, 20, or 25 increments to determine the proportion of subjects in a real-life anchor category within each candidate benchmark group and the discriminate value of the different incremental groupings.
The benchmark validation step recruited 96 subjects from three area neurologists in mid-2011. All had confirmed MS (relapsing or progressive form) and were without acute relapse at time of study visit. Inclusion criteria were ability to ambulate independently or with an assistive device. Participants completed all testing in a single, 2-hour session, which included the following: Expanded Disability Status Scale (EDSS), objective ambulation testing (T25FW, Timed Up and Go [TUG]17, Six Spot Step Test [SSST]18, Six-Minute Walk Test [6MWT]3,19, with associated oxygen cost of walking [O2 cost]20,21, a 7.9-m GAITRite electronic mat [CIR Systems Inc., Havertown, PA] for overall gait based on the functional ambulation performance (FAP) score22,23, and cognitive function (3-second Paced Auditory Serial Addition Task [PASAT] and Symbol Digit Modalities Test [SDMT])24,25. Subjective measures included the 12-MSWS, functional limitations portion of the Late-Life Function and Disability Inventory (LL-FDI)26 and Symptom Inventory (SI)14. Participants then wore a triaxial accelerometer during the waking hours of a 7-day period to capture free-living ambulation as counts per day; this metric is a summary indicator of the volume (intensity and duration) of ambulatory physical activity accumulated over the course of the day. Accelerometers were returned through the US Postal Service.
Statistical analysis
Identification step data were analyzed using SAS 9.4 software (SAS Institute Inc., Cary, NC). Descriptive statistics of demographics and real-life anchor 12-MSWS scores were used to identify candidate 12-MSWS performance benchmarks. Candidate 12-MSWS benchmark-defined group differences on real-life anchors and self-report measures were completed using analysis of variance and χ2 test, as appropriate. During the benchmark validation step using SPSS 19.0 (IBM Corp., Armonk, NY), a second sample of MS participants were stratified by 12-MSWS benchmarks (group 1: 0–24.99, group 2: 25–49.99, group 3: 50–74.99, group 4: 75–100). We performed analysis of variance with a priori linear contrasts on the different outcomes; the univariate F-ratios, t-values assuming equal and unequal variances, and partial η2 values were used to examine the presence and magnitude of linear differences in the dependent outcome per 12-MSWS group. Of note, η2 is analogous with R2 and reflects variance explained in the outcome variable. The linear contrast provides a direct indication of a linear change (either increase or decreases) in the dependent outcomes per level of 12-MSWS benchmark, and the η2 provides an indication of the strength of linear change per level of 12-MSWS benchmark.
RESULTS
During the benchmark identification step, 169 completed surveys were returned of the 300 sent, a 56.3% response rate. This response rate is similar to other mail surveys in the medical literature27. For analyses, we included only those subjects with a T25FW recorded within 6 months of survey completion, which resulted in exclusion of 8 subjects. Two additional subjects were excluded, one for a T25FW of >2 minutes and one who was no longer walking at the time of the survey. Ultimately, 159 MS subjects we utilized for analyses. Survey responders had similar T25FW performance compared to survey non-responders (mean 8.2±7.89 seconds [range 3.25–65 seconds] and mean 8.55±9.79 seconds [range 3.12–104 seconds], respectively).7
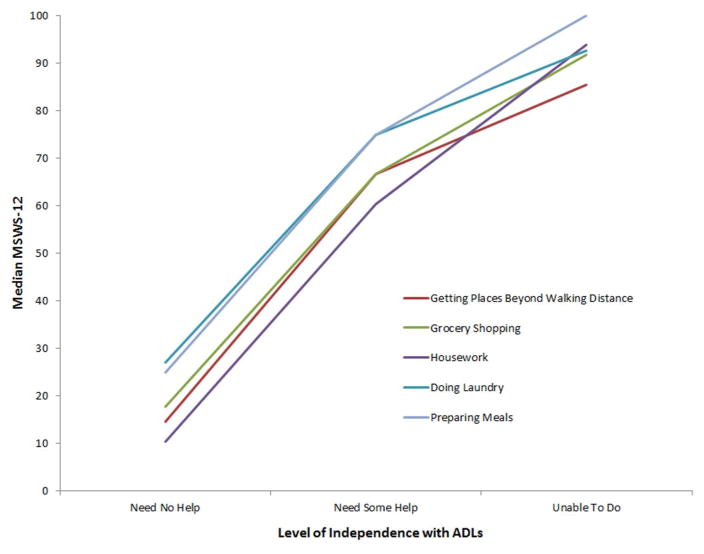
Of the 159 subjects, 68% were women with an average age of 48 ± 12.6 years. Additional study subject population demographics are detailed in table 1. For survey responders, the 12-MSWS median was 43.8 and 45% were employed, either outside or within the home. Thirty-seven percent self-identified as disabled. Only 37.8% of all responders were working full-time (60/159 subjects) and 44% reported a change in occupational status due to MS. Potential 12-MSWS benchmarks were identified using the median 12-MSWS across real-life anchors (table 2; figure 1). Review of the median scores within real-life anchor categories was investigated for information regarding both extreme cut-points (lowest and highest ends of scores), overlapping values across similar anchor groups, or differences between levels of disability within single anchor. For example, 12-MSWS median < 10 was identified as a distinct cut point. The only anchors with a median score in the 0–25 range were: PDDS and all Performance Scores = 0 and full independence with several IADLs. Alternatively, a median score of 60–65 was found across several anchors – e.g. disabled, change in occupation due to MS, government healthcare assistance, and cane use. Next we grouped subjects using different increments, e.g. 10 [0–9.99, 10–19.99,…], 20 [0–19.99, 20–39.99,...] or 25 [0–24.99, 25–49.99…] to evaluate the proportion of subjects found within meaningful anchor categories. Using this approach, we found a 25 point interval was most discriminate across clinically meaningful and distinct real-life anchors (table 3, figure 1). For example, a 12-MSWS of 0–24.99 was associated with working outside the home, no change in occupation, and assistance-free mobility. Whereas, a 12-MSWS score of 25–49.99 was associated with moderate disability reported on the PDDS (median 35.4), and a 57% of subjects reporting a loss of full independence in performing housework. 12-MSWS of 50–74.99 was associated with loss of employment, government healthcare assistance, and the majority of subjects reporting a loss of independence in IADLs. A 12-MSWS of 75–100 was associated with change in occupation due to walking disability, mobility impairment requiring assistive-device with mobility, and 10 – 35 % reporting an inability to preform several IADLs. There was an overall statistically significant difference between 12-MSWS benchmark groups and age, T25FW, employment, government healthcare assistance, IADL scores, and PDDS (table 3). There was no statistically significant relationship between 12-MSWS and marital status, although overall numbers of divorced subjects were low and a relative higher proportion of divorce found in the more impaired 12-MSWS groups.
Table 1.
Study Participant
| Demographics | Identification Study (n=159) | Validation Study (n=96) |
|---|---|---|
|
| ||
| Age, y, mean ± sd | 48.3 ± 12.6 | 52.8 ± 11.1 |
|
| ||
| Female, n (%) | 108 (67.9) | 77 (80) |
|
| ||
| Race, n (%) | ||
| Caucasian | 132 (83) | 77 (80) |
| African American | 25 (15.7) | 19 (20) |
|
| ||
| Marital Status, n (%) | ||
| Single | 26 (16.4) | 14 (14.6) |
| Married | 108 (68) | 70 (72.9) |
| Live with partner | 9 (5.6) | 7 (7.3) |
| Divorced | 9 (5.6) | 5 (5.2) |
|
| ||
| MS Disease Classification, n (%) | ||
| Relapsing MS | 116 (73) | 78 (81.3) |
| Progressive MSa | 43 (27) | 18 (18.7) |
| MS Disease Duration, mean ± sd | 10.1 ± 8.6 | 11.9 ± 10.0 |
|
| ||
| PDDS, n (%) | ||
| 0 = normal | 37 (23.3) | 19 (19.8) |
| 1 = mild disability | 25 (15.7) | 14 (14.6) |
| 2 = moderate disability | 11 (6.9) | 8 (8.3) |
| 3 = gait disability | 26 (16.4) | 19 (19.8) |
| 4 = early cane | 28 (17.6) | 23 (24.0) |
| 5 = late cane | 11 (6.9) | 10 (10.4) |
| 6 = bilateral support | 18 (11.3) | 3 (3.1) |
| 7 = wheelchair/scooter | 3 (1.9) | 0 (0) |
Abbreviation: MS = multiple sclerosis; PDDS = Patient Determined Disease Steps
Inclusive of Primary Progressive and Secondary Progressive subjects
Table 2.
Identification step -- Real-life anchor categories
| Variable | Frequency | Median 12-MSWS |
|---|---|---|
| Marital status | ||
| Married | 108 | 43.6 |
| Divorced | 9 | 77.1 |
| Employment status | ||
| Working outside home | 68 | 17.7 |
| Disabled by self-report | 58 | 63.5 |
| Government health care | ||
| Not receiving | 110 | 27.1 |
| Receiving | 49 | 64.6 |
| Occupation change due to MS | ||
| No | 73 | 14.6 |
| Yes | 86 | 60.4 |
| Occupation change due to walking disability | ||
| Yes | 41 | 75 |
| Mobility assistance | ||
| No assistance | 102 | 20.8 |
| Cane | 24 | 65.6 |
| Walker | 16 | 79.2 |
| Housework, IADL survey | ||
| Need no help | 67 | 10.4 |
| Need some help | 73 | 60.4 |
| Unable to do | 19 | 93.8 |
| Preparing Meals, IADL survey | ||
| Need no help | 104 | 25 |
| Need some help | 51 | 75 |
| Unable to do | 4 | 100 |
Abbreviations: MS = multiple sclerosis; 12-MSWS = Multiple Sclerosis Walking Scale-12; IADL = instrumental activities of daily living.
Figure 1.
Instrumental Activities of Daily Living
Abbreviations: 12-MSWS = Multiple Sclerosis Walking Scale -12; ADLs = Activities of Daily Living.
Table 3.
Identification step -- 12-MSWS benchmarks and survey results
| MSWS12 response range | 0–24.99 | 25–49.99 | 50–74.99 | 75–100 | p Value |
|---|---|---|---|---|---|
|
| |||||
| Subject no. | 54 | 32 | 33 | 40 | |
|
| |||||
| Age, mean y | 42.3 | 48.2 | 52.9 | 52.5 | <0.0001 |
|
| |||||
| T25FW, mean | 5.04 | 6.9 | 9.2 | 12.7 | <0.0001 |
|
| |||||
| Beck Depression Inventory, mean | 2.01 | 3.5 | 3.3 | 4.7 | <0.0009 |
|
| |||||
| Marital status, % | |||||
| Married | 66.7 | 71.9 | 66.7 | 67.5 | 0.212 |
| Divorced | 1.9 | 6.3 | 3 | 12.5 | |
|
| |||||
| Employed outside home, % | 66.7 | 53.1 | 24.2 | 17.5 | <0.0001 |
| If employed, working full-timea, % yes | 78.1 | 94.1 | 50 | 88.9 | 0.067a |
| Change in occupation due to MS, % yes | 18.6 | 22.1 | 24.4 | 34.9 | <0.0001 |
| Disabled, % | 14.8 | 31.3 | 48.5 | 60 | <0.0001 |
| Has government health care assistance, % | 11.1 | 15.6 | 51.5 | 52.5 | <0.0001 |
| Activities of daily living, mean score | 15.3 | 14.2 | 12.7 | 10.1 | <0.0001 |
|
| |||||
| Groceries, % | |||||
| Independent | 92.6 | 78.1 | 42.4 | 12.5 | <0.0001 |
| Needs help | 7.4 | 21.9 | 54.6 | 62.5 | |
| Unable to do | 0 | 0 | 3 | 25 | |
|
| |||||
| Housework, % | |||||
| Independent | 81.5 | 43.8 | 21.2 | 5 | <0.0001 |
| Needs help | 18.5 | 53.1 | 66.7 | 60 | |
| Unable to do | 0 | 3.1 | 12.1 | 35 | |
|
| |||||
| Laundry, % | |||||
| Independent | 94.4 | 78.1 | 72.7 | 30 | <0.0001 |
| Needs help | 5.6 | 21.9 | 24.2 | 57.5 | |
| Unable to do | 0 | 0 | 3 | 12.5 | |
|
| |||||
| Preparing Meal, % | |||||
| Independent | 92.6 | 81.3 | 57.6 | 22.5 | <0.0001 |
| Needs help | 7.4 | 18.8 | 42.4 | 67.5 | |
| Unable to do | 0 | 0 | 0 | 10 | |
|
| |||||
| PDDS, % | |||||
| 0 = normal | 35 | 1 | 1 | 0 | <0.0001 |
| 1 = mild disability | 13 | 9 | 3 | 0 | |
| 2 = moderate disability | 3 | 5 | 1 | 2 | |
| 3 = gait disability | 1 | 13 | 11 | 1 | |
| 4 = early cane 1 | 3 | 12 | 12 | ||
| 5 = late cane | 1 | 0 | 3 | 7 | |
| 6 = bilateral support | 0 | 1 | 1 | 16 | |
| 7 = wheelchair/scooter | 0 | 0 | 1 | 2 | |
Calculated with denominator as total number reporting any employment (n 5 75).
Abbreviations: MS = multiple sclerosis; 12-MSWS = MS Walking Scale-12; T25FW = Timed 25-Foot Walk; PDDS = Patient
Determined Disease Steps.
The identification step results supported 12-MSWS performances of 0–24.99, 25–49.99, 50–74.99, and 75–100 to represent clinically meaningful benchmarks of MS-related gait impairment (table 3). In those with 12-MSWS 0–24.99, we found nearly 67% were working outside the home and > 80% were able to perform instrumental activities of daily living without help. Within the 25 – 49.99 12-MSWS group, there was a notable increase in disability with 31.3% self-identified as disabled and >50% reporting minimal or mild gait disability on the PDDS. Only 43.8% report independence in doing housework and there is an overall 10–15% loss of full independence with grocery shopping, laundry and preparing a meal, demonstrating a meaningful loss of complete independence in these activities in individuals with 12-MSWS scores 25–49.99. In the 12-MSWS 50–74.99 group, we found only 50% were working full time and 24% employed outside the home. Over 50% were receiving government assistance, reported “occasional cane use” on the mobility performance scale, and required “some help” with grocery shopping and housework. In the most limited group, those with 12-MSWS 75–100 only 17.5% report working full-time, 60% were disabled, and > 50% had government assistance. A total of 40% required bilateral support for ambulation and > 50% reported either severe or total gait disability on the mobility performance scale. Less than 30% maintained independence in any IADLs domain and > 25% were unable to grocery shop or perform any housework.
Interestingly, within these 12-MSWS benchmark groups, we also found a clear and recurring escalation in disability across several other domains (Supplemental table 1). For example, in the 12-MSWS 25–49.99 group we see a shift from 61% normal hand function (12-MSWS 0–24.99 group) to only 28% reporting normal hand function. Instead 50% reported minimal disability with hand function and similarly 30–60% reporting minimal – mild disability in vision, fatigue, cognitive, bladder/bowel, sensory, spasticity, pain, depression, and tremor/loss of coordination domains on Performance Scales in the 12-MSWS 25–49.99 group. Moving up in mobility impairment, the 12-MSWS 50–74.99 again see a corresponding increase in other disability domains. For example, > 50% now report mild-moderate hand impairment and 30–57% report mild-moderate disability in vision, fatigue, cognitive, bladder/bowel, sensory, spasticity, pain, depression, and tremor/loss of coordination domains on Performance Scales. In the most impaired mobility group, 12-MSWS 75–100, 20% report either severe or total hand disability and again we see a notable across the board increase in disability across other domains in this group with 20–50 % reporting severe-total disability in vision, fatigue, bladder/bowel, sensory, spasticity, pain, depression, and tremor/loss of coordination domains on Performance Scales. To confirm and validate these 12-MSWS benchmarks, we conducted additional analysis in a second, independent MS sample recruited from a regionally different institution. During the benchmark validation step, 96 subjects with MS completed the assessment. This was a separate cohort from the first 169. Average age was 52.7 and the median EDSS score was 4.5 with a range between 2 and 6.5. The median 12-MSWS was 50.0 with a mean of 44.9 (SD = 27.5). Approximately 25% of the population had a 12-MSWS < 25 (group 1, n = 24), 25% had a 12-MSWS of 25–49.99 (group 2, n = 24), 34% had a 12-MSWS of 50–74.99 (group 3, n = 33, and 16% had a 12-MSWS of 75–100 (n = 16). Median EDSS scores differed significantly across groups (chi-square = 40.9, df = 3, p < .0001) with median values for 12-MSWS groups as follows: group 1: <25 = 2.5 (1.5); group 2: 25–49.99 = 4.0 (1.5); group 3: 50–74.99 = 6.0 (1.5); and group 4: 75–100 = 6.0 (0).
We then tested all measures for expected linear differences/trends across groupings of 12-MSWS scores (table 4). There were statistically significant linear trends for stratified 12-MSWS benchmarks across both objective and subjective measures: 6MWT (p < 0.001), TUG (p < 0.001), SSST (p < 0.001), T25FW (p < 0.001), accelerometer counts per day (p < 0.001) and steps per day (p < 0.001), Functional Ambulation Performance (FAP) score from the GAITRite (p < 0.001), SDMT (p < 0.001), LL-FDI (p < 0.001), and SI (p < 0.001). Mean scores for these benchmarks are reported in table 5. The linear trends indicate a linear, systematic change in the outcomes per level of 12-MSWS benchmark.
Table 4.
Validation Step - Linear contrast analysis comparing outcomes across 12-MSWS benchmarks
| F-value for linear term | p-value | t-value, equal variance | p-value | t-value, unequal variance | p-value | Partial eta-squared | |
|---|---|---|---|---|---|---|---|
| T25FW | 39.7 | <.001 | 6.3 | <.001 | 4.1 | <.001 | 0.3 |
| TUG | 47.5 | <.001 | 6.9 | <.001 | 4.8 | <.001 | 0.34 |
| 6MW | 64.3 | <.001 | 8 | <.001 | 7.7 | <.001 | 0.41 |
| SSST | 53.4 | <.001 | 7.3 | <.001 | 5.3 | <.001 | 0.37 |
| Steps/day | 45.1 | <.001 | 6.7 | <.001 | 7.2 | <.001 | 0.33 |
| Counts/day | 29.5 | <.001 | 5.4 | <.001 | 5.5 | <.001 | 0.24 |
| O2 Cost | 23.7 | <.001 | 4.9 | <.001 | 4.3 | <.001 | 0.21 |
| FAP score | 44.7 | <.001 | 6.8 | <.001 | 5.2 | <.001 | 0.36 |
| PASAT | 1.8 | 0.18 | 1.3 | 0.18 | 1.5 | 0.15 | 0.02 |
| SDMT | 13.3 | <.001 | 3.6 | <.001 | 3.9 | <.001 | 0.13 |
| LL-FDI | 141.2 | <.001 | 11.9 | <.001 | 11 | <.001 | 0.61 |
| SI | 67.2 | <.001 | 8.2 | <.001 | 7.2 | <.001 | 0.42 |
Abbreviations: 12-MSWS = Multiple Sclerosis Walking Scale – 12; T25FW = Timed 25-Foot Walk; TUG = Timed Up and Go; 6MW = 6 Minute Walk; SSST = Six Spot Step Test; FAP = Functional Ambulation Performance; PASAT = Paced Auditory Serial Addition Test; SDMT = Symbol Digit Modalities Test; LL-FDI = Late Life Function and Disability Instrument; SI = Symptom Inventory.
Table 5.
Descriptive statistics for validation outcomes across four benchmarks for 12-MSWS
| 12-MSWS Group | ||||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| 0–24.99 | 25–49.99 | 50–74.99 | 75–100 | |
| T25FW | 4.7 (1.0) | 5.9 (1.7) | 7.8 (2.3) | 9.7 (5.2) |
| TUG | 5.9 (1.8) | 7.5 (2.1) | 10.6 (3.9) | 14.1 (7.2) |
| 6MW | 523 (78) | 444 (93) | 348 (102) | 293 (112) |
| SSST | 7.0 (1.6) | 9.0 (2.9) | 13.2 (4.5) | 16.3 (7.6) |
| Steps/day | 6097 (2387) | 4895 (2522) | 2737 (1371) | 2267 (1342) |
| Counts/day | 200753 (92259) | 163732 (83311) | 102610 (50588) | 87455 (54596) |
| O2 Cost | 0.187 (0.032) | 0.201 (0.041) | 0.243 (0.790) | 0.278 (0.088) |
| FAP score | 96.4 (3.7) | 94.3 (6.5) | 86.3 (11.3) | 76.0 (15.0) |
| PASAT | 45.5 (11.0) | 42.4 (10.2) | 36.8 (15.8) | 41.5 (11.6) |
| SDMT | 51.5 (10.6) | 44.8 (9.1) | 41.6 (11.3) | 39.8 (8.8) |
| LL-FDI | 67.0 (5.5) | 54.3 (7.9) | 45.4 (7.2) | 40.1 (9.2) |
| SI | 17.0 (11.1) | 42.0 (14.1) | 47.6 (13.7) | 54.7 (18.7) |
Abbreviations: Values represent mean (standard deviation). T25FW = Timed 25-Foot Walk; TUG = Timed Up and Go; 6MW = 6 Minute Walk; SSST = Six Spot Step Test; FAP = Functional Ambulation Performance; PASAT = Paced Auditory Serial Addition Test; SDMT = Symbol Digit Modalities Test; LL-FDI = Late Life Function and Disability Instrument; SI = Symptom Inventory.
DISCUSSION
The impact of mobility impairment on employment and functional status in MS has been well characterized28. The 12-MSWS is a validated patient-reported outcome measure (PRO) of walking impairment in MS, and translating these PROs typically used in research settings to clinically meaningful benchmarks can be a useful way to classify an individual’s disability and monitor its accrual over time. Our study approach and resultant data adds to the available literature regarding clinically meaningful benchmarks in MS outcome measures, which was previously limited to only objective measures7,8.
12-MSWS scores of 50–74.99 and >75 appear to represent a substantial shift in functional independence. Patients with 12-MSWS of 50–74.99 are more likely to have employment changes related to MS, receive government healthcare assistance, experience cognitive difficulty and fatigue, and require a cane for mobility. Across all groups, increases in 12-MSWS were associated with more difficulty completing IADLs and increased likelihood of receiving disability benefits. Ambulatory and functional limitations further increase beyond an 12-MSWS of 75, as these patients are more likely to require bilateral assistance, a walker, or wheelchair for ambulation and are more likely to be unable to complete their own IADLs. Interestingly, our data also demonstrate that increasing mobility difficulty is associated with increasingly severe disability across multiple other domains; demonstrating our clinical experience that disability is not domain specific and comprehensive measurement/assessment is needed to capture the spectrum of MS-related disability. These results also demonstrate that increasing disability in one domain is associated with increasing disability across other domains.
The validation step in our study further confirmed the strong linear association between levels of 12-MSWS benchmarks and other MS-related disability measures, including objective ambulatory measures (T25FW, TUG, 6MW, SSST, FAP, and accelerometer data) and non-ambulatory measures (SDMT, LL-FDI, and SI). Regarding cognitive measures, significant differences were seen with increased 12-MSWS benchmarks and SDMT, but not PASAT, scores, yet these differences were small in magnitude. Such results suggests some degree of specificity of the 12-MSWS benchmarks for MS-related mobility disability that is relatively unaffected by general cognitive impairment based on SDMT and PASAT scores. It is important to note that we have identified and validated cross-sectional benchmarks, and we have not demonstrated that a 25 point “change” in the 12-MSWS represents the minimally clinically important (MCID) and/or detectable change; the MCID for the 12-MSWS has been reported to be 4–22 depending on the statistical approach and population studied.11,29,30 While this cross-sectional approach is one limitation to our study, we believe that these 12-MSWS benchmarks are a meaningful way to describe and understand what a sample score means for real-world consequences. This should not be confused with the MCID value, which reflects a meaningful change brought about by an intervention or change over time.
It is important to note, that both of our study populations where from a U.S. sample, and therefore, some of the anchors used (e.g. government healthcare) may not translate to other non-US populations. In addition, choices of when to leave the work force and/or work from home are likely also shaped by work flexibility and opportunities that may have U.S. biased elements. Finally, the 12-MSWS was designed from and for an MS population, and the tool has been applied to other neurologic populations (e.g. stroke).31 Caution should be taken when applying these benchmarks to non-MS patients, due to important differences in tempo and pattern of disability progression in neurologic disease (e.g. MS vs. stroke).
There has been increasing emphasis on patient-reported outcomes (PROs) recently, so PROs have been included as secondary or tertiary endpoints in recent clinical trials of disease modifying therapies for MS. PROs are obviously important, but at times there may be a seeming discordance between clinical data and patient-reported perceptions. One potential explanation for this disconnect may be the presence of comorbidities; indeed, a recent study suggested that the impact of physical disability and that of depression were almost identical on another Health Related Quality of Life PRO (Health Utilities Index Mark 3) in people with MS32. Given this, it is useful to be able to correlate PRO changes with meaningful clinical benchmarks when interpreting the implications of PRO endpoints in clinical trials and clinical practice.
Furthermore, PROs will also have medico-economic implications as healthcare reimbursements, at least in the United States, shift from a “fee for service” paradigm to a Merit-Based Incentive Payment System (MIPS) based on the value and quality of the service provided. The identification of benchmarks is important for helping research and clinicians interpret 12-MSWS scores based on standards with associated real-world relevance and may suggest that this PRO is appropriate to include as a quality measure for MIPS which would directly impact practicing neurologists. While, we do not anticipate that the 12-MSWS would be used in substitution to the neurologic exam, but rather augment the objective data of an exam. The added value of these benchmarks, allows the 12-MSWS data provided by the individual patient to then be contextualized into a larger framework of population-based MS disability. Our data provide this framework, with new insight into how categories of mobility impairment on the 12-MSWS may translate to other, more broad functional limitations in MS.
Supplementary Material
Acknowledgments
STUDY FUNDING:
Funded by an Investigator Initiated Trial Grant from Biogen Idec. Additional funding provided by the ziMS Foundation. M.D. Ward received funding from a Clinical Multiple Sclerosis Fellowship from the National MS Society. M.D. Goldman was funded by in part by the NIH (K23NS062898).
Footnotes
AUTHOR CONTRIBUTIONS:
M.D. Goldman and R.W. Motl: study design, data collection, statistical analysis and interpretation, manuscript drafting and revising. M.D. Ward: data interpretation, manuscript drafting and revising. D.E. Jones: data interpretation, manuscript drafting and revising. JH Pula: Data collection and manuscript revising. D Cadavid: Study design, manuscript revising.
DISCLOSURES
M. Goldman has received personal consultancy funds from Genzyme and Serepta, institutional consultancy and/or research funds from Acorda, Biogen Idec, and Novartis Pharmaceuticals, and grant supported by the NIH (K23NS062898). R. Motl has consulting relationships with and/or research support from Biogen Idec, Acorda, and Sun Health Technologies. M. Ward reports no disclosures. D.E. Jones has received personal consulting fees from Novartis and Biogen Idec, institutional consulting agreements from Biogen Idec, Genzyme, and Novartis, and research support from Biogen Idec. J. Pula reports no disclosures. D. Cadavid is a full-time employee at Biogen, owns Biogen stock and stock options.
References
- 1.Swingler RJ, Compston DA. The morbidity of multiple sclerosis. Q J Med. 1992;83(300):325–27. [PubMed] [Google Scholar]
- 2.Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006;12(5):620–628. doi: 10.1177/1352458506070658. [DOI] [PubMed] [Google Scholar]
- 3.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 4.Coleman C, Sidovar M, Roberts M, et al. Impact of Mobility Impairment on Indirect Costs and Health-Related Quality of Life in Multiple Sclerosis. PLoS One. 2013;8(1):e54756. doi: 10.1371/journal.pone.0054756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox R, Bacon T, Chamot E, et al. Prevalence of multiple sclerosis symptoms across lifespan: data from the NARCOMS Registry. Neurodegener Dis Manag. 2015;5(6s) doi: 10.2217/nmt.15.55. [DOI] [PubMed] [Google Scholar]
- 6.Kieseier B, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. 2012;18(7):914–924. doi: 10.1177/1352458512444498. [DOI] [PubMed] [Google Scholar]
- 7.Goldman M, Motl R, Scagnelli J, et al. Clinically meaningful performance benchmarks in MS: Timed 25-Foot Walk and the real world. Neurology. 2013;81:1856–1863. doi: 10.1212/01.wnl.0000436065.97642.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedict R, Drake A, Irwin L, et al. Benchmarks of meaningful impairment on the MSFC and BICAMS. Mult Scler. 2016 doi: 10.1177/1352458516633517. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (12-MSWS) Neurology. 2003;60(1):31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Motl R, Snook E. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (12-MSWS) J Neurol Sci. 2008;268:69–73. doi: 10.1016/j.jns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Learmonth Y, Dlugonski D, Pilutti L, et al. The reliability, precision, and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013;19:1784–1791. doi: 10.1177/1352458513483890. [DOI] [PubMed] [Google Scholar]
- 12.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 13.Marrie R, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007;13:1176–1182. doi: 10.1177/1352458507078388. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz C, Volmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS. North American Research Consortium on Multiple Sclerosis Study Group. Neurology. 1999;52:63–70. doi: 10.1212/wnl.52.1.63. [DOI] [PubMed] [Google Scholar]
- 15.Benedict R, Fishman I, McClellan M, et al. Validity of the Beck Depression Inventory-Fast Screen in multiple sclerosis. Mult Scler. 2003;9:393–396. doi: 10.1191/1352458503ms902oa. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Steer R, Brown G, editors. BDI-FastScreen for Medical Patients Manual. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 17.Nilsagard Y, Lundholm C, Gunnarsson L, et al. Clinical relevance using timed walk tests and “timed up and go” testing in persons with multiple sclerosis. Physiother Res Int. 2007;12:105–114. doi: 10.1002/pri.358. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwenhuis M, Van Tongeren H, Sorensen P, et al. The Six Spot Step Test: a new measurement for walking ability in multiple sclerosis. Mult Scler. 2006;12:495–500. doi: 10.1191/1352458506ms1293oa. [DOI] [PubMed] [Google Scholar]
- 19.Heffernan K, Ranadive S, Weikert M, et al. Pulse pressure is associated with walking impairment in multiple sclerosis. J Neurol Sci. 2011;309:105–109. doi: 10.1016/j.jns.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Motl R, Suh Y, Dlugonski D, et al. Oxygen cost of treadmill and over-ground walking in mildly disabled persons with multiple sclerosis. Neurol Sci. 2011;32:255–262. doi: 10.1007/s10072-010-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motl R, Dlugonski D, Weikert M, et al. Multiple Sclerosis Walking Scale-12 and oxygen cost of walking. Gait Posture. 2010;31:506–510. doi: 10.1016/j.gaitpost.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Sosnoff J, Sandroff B, Motl R. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture. 2012;36:154–156. doi: 10.1016/j.gaitpost.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Sosnoff J, Weikert M, Dlugonski D, et al. Quantifying gait impairment in multiple sclerosis using GAITRite technology. Gait Posture. 2011;34:145–147. doi: 10.1016/j.gaitpost.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Drake A, Weinstock-Guttman B, Morrow S, et al. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler. 2010;16:228–237. doi: 10.1177/1352458509354552. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JS, Rudick RA, Cutter GR, et al. National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 26.Motl R, McAuley E, Suy Y. Validity, invariance, and responsiveness of a self-report measure of functional limitations and disability in multiple sclerosis. Disabil Rehabil. 2010;32:1260–1271. doi: 10.3109/09638280903464463. [DOI] [PubMed] [Google Scholar]
- 27.Asch D, Jedrziewski M, Christakis N. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 28.Slater A, Cutter G, Tyry T, et al. Impact of loss of mobility on instrumental activities of daily living and socioeconomic status in patients with MS. Curr Med Res Opin. 2010;26:493–500. doi: 10.1185/03007990903500649. [DOI] [PubMed] [Google Scholar]
- 29.Baert I, Freeman J, Smedal T, et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabilitation Neuroal Repair. 2014;28:621–631. doi: 10.1177/1545968314521010. [DOI] [PubMed] [Google Scholar]
- 30.Motl R, Learmonth Y, Pilutti L, et al. Validity of minimal clinically important difference values for the multiple sclerosis walking scale-12? European Neurology. 2014;71:196–202. doi: 10.1159/000356116. [DOI] [PubMed] [Google Scholar]
- 31.Holland A, O’Conner RJ, Thompson AJ, et al. Talking the talk on walking the walk: A 12-item generic walking scale suitable for neurologic conditions? Journal of Neurology. 2006;253:1594–1602. doi: 10.1007/s00415-006-0272-2. [DOI] [PubMed] [Google Scholar]
- 32.Berrigan LI, Fisk JD, Patten SB, et al. Health-related quality of life in multiple sclerosis: Direct and indirect effects of comorbidity. Neurology. 2016 doi: 10.1212/WNL.0000000000002564. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.