Abstract
Humans in modern societies typically consume food at least three times daily, while laboratory animals are fed ad libitum. Overconsumption of food with such eating patterns often leads to metabolic morbidities (insulin resistance, excessive accumulation of visceral fat, etc.), particularly when associated with a sedentary lifestyle. Because animals, including humans, evolved in environments where food was relatively scarce, they developed numerous adaptations that enabled them to function at a high level, both physically and cognitively, when in a food-deprived/fasted state. Intermittent fasting (IF) encompasses eating patterns in which individuals go extended time periods (e.g., 16–48h) with little or no energy intake, with intervening periods of normal food intake, on a recurring basis. We use the term periodic fasting (PF) to refer to IF with periods of fasting or fasting mimicking diets lasting from 2 to as many as 21 or more days. In laboratory rats and mice IF and PF have profound beneficial effects on many different indices of health and, importantly, can counteract disease processes and improve functional outcome in experimental models of a wide range of age-related disorders including diabetes, cardiovascular disease, cancers and neurological disorders such as Alzheimer’s disease Parkinson’s disease and stroke. Studies of IF (e.g., 60% energy restriction on 2 days per week or every other day), PF (e.g., a 5 day diet providing 750–1100 kcal) and time-restricted feeding (TRF; limiting the daily period of food intake to 8 h or less) in normal and overweight human subjects have demonstrated efficacy for weight loss and improvements in multiple health indicators including insulin resistance and reductions in risk factors for cardiovascular disease. The cellular and molecular mechanisms by which IF improves health and counteracts disease processes involve activation of adaptive cellular stress response signaling pathways that enhance mitochondrial health, DNA repair and autophagy. PF also promotes stem cell-based regeneration as well as long-lasting metabolic effects. Randomized controlled clinical trials of IF versus PF and isoenergetic continuous energy restriction in human subjects will be required to establish the efficacy of IF in improving general health, and preventing and managing major diseases of aging.
Keywords: Alzheimer’s disease, Blood pressure, Cardiovascular disease, Diabetes, Insulin resistance, Intermittent fasting, Ketone bodies, Obesity
1. Introduction
The survival and reproductive success of all organisms depends upon their ability to obtain food. Accordingly, animals have evolved behavioral and physiological adaptations that enable them to survive periods of food scarcity or absence. When food is not available for extended time periods some organisms become dormant; for example, yeast enter a stationary phase, nematodes enter the dauer state, and ground squirrels and some bears hibernate (Calixto, 2015). Mammals have organs such as the liver and adipose tissue which function as energy depots that enable fasting/starvation for varying lengths of time depending upon the species. Importantly, metabolic, endocrine and nervous systems evolved in ways that enabled high levels of physical and mental performance when in the fasted state. In this article we review studies of the effects of regimens of intermittent fasting (IF) diets, which include eating patterns in which individuals go extended time periods (e.g., 16–48h) with little or no energy intake, with intervening periods of normal food intake, on a recurring basis. To distinguish studies of short-term frequent fasting periods from studies of less frequent but longer fasting periods we use the term periodic fasting (PF) to refer to IF with periods of fasting or “fasting mimicking diets” (FMDs) lasting from 2 to as many as 21 or more days. The term time-restricted feeding (TRF) is used to describe an eating pattern in which food intake is restricted to a time window of 8 h or less every day. Studies of laboratory animals have elucidated the cellular and molecular mechanisms by which individuals respond to fasting in ways that can increase their overall fitness and their resistance to injury and a wide array of diseases (Longo and Mattson, 2014). Recent randomized controlled trials in human subjects have demonstrated that IF, including diets that mimic some aspects of FMDs, are achievable in humans and improve many health indicators in healthy individuals and in those with some chronic diseases.
In this article we focus on studies of the effects of IF, including PF and FMD, on animals and humans. Examples of specific IF diets include: complete fasting every other day (Bruce-Keller et al., 1999; Anson et al., 2003); 70% energy restriction every other day (Johnson et al., 2007; Varady et al., 2015); consuming only 500–700 cal two consecutive days/week (Harvie et al., 2011); and restricting food intake to a 6–8 h time period daily, which has also been termed ‘time restricted feeding’ (TRF) (Chaix et al., 2014). Examples of PF include a 4–5 day FMD (Brandhorst et al., 2015), 2–5 days of water only fasting (Raffaghello et al., 2008; Safdie et al., 2009), and 7 days of a FMD (Choi et al., 2016). The vast majority of IF animal studies have involved either alternate day fasting or TRF, and most randomized controlled human trials have involved either 60–75% energy restriction (500–800 kcal) on alternate days or 2 consecutive days/week. Rarely has more that one IF regimen been compared within the same study, and so it is not yet possible to make any clear conclusions as to whether one regimen is superior to another with regards to improving health and disease resistance.
Although results may differ quantitatively depending on the type of IF pattern and the species studied, all of the IF regimens described in the preceding paragraph result in several fundamental metabolic changes that define a fasting period including: maintenance of blood glucose levels in the low normal range, depletion or reduction of glycogen stores, mobilization of fatty acids and generation of ketones, a reduction of circulating leptin and often elevation of adiponectin levels (Johnson et al., 2007; Wan et al., 2010) (Fig. 1). Behavioral changes that occur during the fasting period of IF diets include increased alertness/arousal and increased mental acuity (Fond et al., 2013). As we describe in subsequent sections of this article, both the metabolic shift to ketone utilization, and adaptive responses of the brain and autonomic nervous system to food deprivation, play major roles in the fitness-promoting and disease-allaying effects of IF. Because overall calorie intake is often reduced during IF (e.g., weekly calorie intake in an individual on the ‘5:2′ diet is reduced by 25%; Harvie et al., 2011, 2013a) it is important to know if and to what extent physiological responses to IF are mediated by the overall caloric restriction (CR). In some studies of animals or human subjects, groups maintained on IF or isocaloric CR diets have been compared directly and, in those cases we will describe the similarities and differences. Otherwise, we will not review studies of CR which are much more numerous than studies of IF, and have been reviewed elsewhere recently (Speakman and Mitchell, 2011; Mercken et al., 2012; Longo et al., 2015). It should be noted, however, that the most commonly used method for daily CR in rodent laboratory studies (limited daily feeding) is in fact a form of IF/TRF. Thus, the animals are housed singly and the average amount of food consumed each day when the animals are fed ad libitum is designated the ad libitum food intake. Animals are then randomly assigned to ad libitum control and CR groups, with the animals in the CR group being fed a designated percentage of their normal ad libitum intake (usually 60–80%; i.e., 20–40% CR). Animals on CR are typically provided their daily or, in some cases thrice weekly, food in one portion (Pugh et al., 1999). However, under these circumstances, the animals on CR often consume their entire allotment within a period of several hours of receiving the food and, accordingly, they are fasting intermittently for extended time periods of (for example, 16–20h when fed daily, or 36 h or more when fed thrice weekly). The relative contributions of IF and CR to the lifespan extension and health-enhancing effects reported in studies of standard caloric restriction in these laboratory studies have not been investigated, and therefore represents a major knowledge gap in this field of research. Here we will focus on IF as the role of PF/FMDs on longevity and diseases in laboratory animals and humans. For more comprehensive recent reviews of the physiological and disease-modifying effects of fasting at the cellular and molecular levels, the reader is referred to Longo and Mattson (2014) and Longo and Panda (2016).
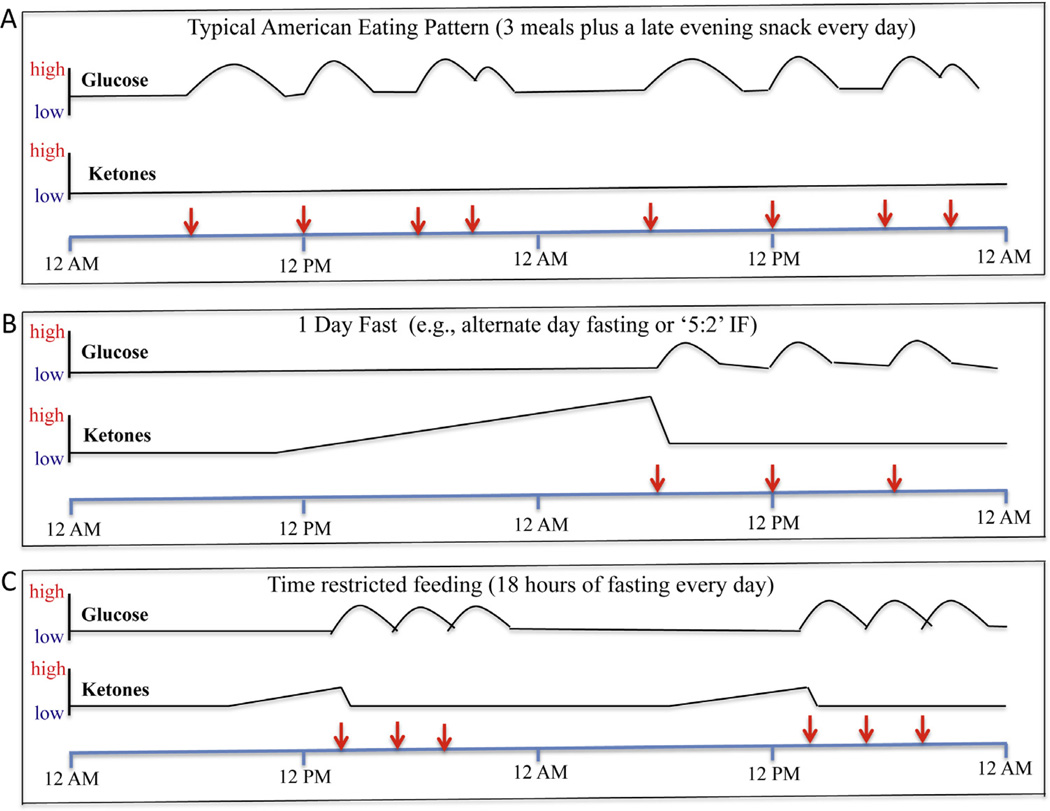
Fig. 1.
Examples of the influence of eating patterns on levels of glucose at ketones in the blood. The red arrows indicate the time of food consumption/meals during a 2 day period of time. A. This is an example of the typical eating pattern in most industrialized countries. Every day the person eats breakfast, lunch and dinner and a late evening snack. With each meal, glucose levels are elevated and then return towards baseline over a period of several hours. Ketone levels remain low, because liver glycogen stores are never depleted. B. This is an example of fasting one day, followed by a three-meal feeding day. During the fasting day, glucose levels remain in the low normal range, and ketone levels (β-hydroxybutyrate and acetoacetate) rise progressively, and then fall when the first meal is consumed on the 2nd day. C. This is an example of an eating pattern in which all food is consumed within a 6h time window each day. Glucose levels are elevated during and for several hours after the 6h period of food consumption and then remain low for the subsequent 16 h until food is consumed the next day. Ketones are elevated during the last 6–8 h of the 18 h fasting period.
2. IF and health indicators in laboratory animals
Studies of IF in animals usually compare a control group fed ad libitum with an IF group; in some cases, a daily CR group(s) is also included. Control laboratory rats and mice are also typically sedentary which, together with ad libitum feeding and an unstimulating environment, renders them rather like the stereotypical human “couch potato” (Martin et al., 2010). This is important to keep in mind when attempting to extrapolate data from IF studies in animals to humans, especially when considering the potential effects of IF amongst normal weight human subjects. The two major IF regimens that have been applied to laboratory rodents are alternate day fasting (ADF) and TRF. When maintained on an ADF diet, rats and mice exhibit lower body weights than do ad libitum fed controls, with the magnitude of the reduction in body weight ranging from 5 to 10% up to 25–30% depending upon the strain of animal (Goodrick et al., 1983; Anson et al., 2003; Wan et al., 2003). Perhaps the first evidence that IF may confer widespread health benefits came from studies in which rats maintained on ADF beginning when they were young lived nearly twice as long as rats on an ad libitum diet (Goodrick et al., 1982). When ADF was initiated in middle age, the rats lived 30–40% longer than rats fed ad libitum, and this life extension could be further increased by regular exercise (Goodrick et al., 1983). ADF also results in preservation of cognitive function and sensory - motor function during aging in rodents (Singh et al., 2015). An anti-aging effect of IF appears to be evolutionarily conserved because IF increases lifespan in lower species such as nematodes (Uno et al., 2013).
Multiple effects of IF on body composition and energy metabolism have been described. A reduction in levels of fat, particularly visceral fat, and retention of lean mass occurs in rats or mice maintained on ADF (Varady et al., 2007; Gotthardt et al., 2016). The lean mass/fat mass ratio is generally greater in animals on an ADF diet compared to those on 30–40% CR diets (Gotthardt et al., 2016). In one study, mice on an ADF diet maintained body weights similar to those of mice fed ad libitum but, nevertheless, exhibit highly significant improvements in glucose metabolism (reduced glucose and insulin levels) and mobilization of fatty acids (increased β-hydroxybutyrate levels) that were as great or even greater than mice on a 40% CR diet (Anson et al., 2003). Body temperature is significantly lower on fasting days compared to feeding days (Wan et al., 2003). In numerous animal and humans studies IF increases insulin sensitivity and improves glucose tolerance (Gotthardt et al., 2016). In mice hyperphagic and obese as a consequence of brain-derived neurotrophic factor (BDNF) hap-loinsufficiency, ADF reverses insulin resistance, and reduces levels of circulating levels of insulin and leptin (Duan et al., 2003a). IF also improves glucose regulation/insulin sensitivity in long-lived Ames Dwarf mice and growth hormone receptor mutant mice, demonstrating beneficial effects of IF in animals that maintain a low body weight during aging (Arum et al., 2014). Interestingly, and in contrast to CR, SIRT1 may not play major roles in physiological adaptations to ADF (Boutant et al., 2016). However, while the vast majority of studies of IF in rodents have demonstrated numerous improvements in health indicators and protection against various diseases (see below), there have been reports of adverse effects of IF in some rodent models. For example, one study found that rats maintained on an ADF diet for one month had improved glucose tolerance, whereas rats maintained on ADF for 8 months had impaired glucose tolerance (Cerqueira et al., 2011). The rats on IF in the latter study maintained a lower body weight than rats fed ad libitum, and the mechanism responsible for the apparent glucose intolerance despite a reduction body weight was not established. It was also reported that IF has adverse effects on glucose metabolism in hypercholesterolemic (low density lipoprotein receptor-deficient) mice (Dorighello et al., 2014), in contrast to the clear beneficial effects of ADF on lipid and glucose metabolism in wild type rodents and human subjects (Varady et al., 2009a, 2009b).
Several changes in circulating hormones have been documented in animal studies of IF. Levels of circulating leptin and insulin are reduced, and levels of adiponectin are increased in animals on an ADF diet (Duan et al., 2003a; Varady et al., 2010; Wan et al., 2010). Levels of corticosterone are significantly elevated in rats in response to ADF (Wan et al., 2003) but, in contrast to chronic uncontrollable stress (e.g., psychosocial stress), corticosterone does not adversely affect neurons in the brains of animals on ADF. It has been shown that chronic uncontrollable stress reduces expression of the mineralocorticoid receptor (MR), while glucocorticoid receptor (GR) levels are sustained, in hippocampal neurons which increases the vulnerability of the neurons to excitotoxic and metabolic stress (McEwen, 2007). On the other hand, IF reduces the expression of GR while sustaining MR, which would be expected to promote synaptic plasticity and neuronal stress resistance (Lee et al., 2000; Stranahan et al., 2010). ADF has been reported to affect levels of sex hormones and gonadal function in rats, with testosterone levels increasing in males but not in females, and marked changes in gene expression in the gonads of males compared to females (Martin et al., 2009).
The cardiovascular system of rats and mice responds to IF in a manner very similar to its responses to aerobic exercise training (Scheuer and Tipton, 1977). Within 1 week of initiation of ADF in rats, resting heart rate and blood pressure are significantly reduced, continue to decrease through 2 weeks, and remain reduced on both fasting and feeding days (Wan et al., 2003). However, within 1–2 weeks following return to an ad libitum diet, the heart rate of rats previously on an ADF diet returns towards pre-ADF diet levels indicating that the benefit for cardiovascular health is not sustained much beyond the IF period (Mager et al., 2006). The reduction of heart rate in response to IF is mediated by increased parasympathetic tone, namely, enhanced activity of brainstem car-diovagal cholinergic neurons (Mager et al., 2006; Wan et al., 2014). These effects of IF on heart rate are not simply the result of caloric restriction, because rats maintained on ADF are only moderately calorie-restricted (10–20%) and yet exhibit greater reductions in resting heart rate than do mice on 40% daily caloric restriction (Mager et al., 2006). Increased BDNF signaling is known to occur in response to both exercise and IF, and BDNF can reduce heart rate by increasing activity of brainstem cardiovagal neurons (Wan et al., 2014). The available data therefore suggest a scenario in which IF and exercise stimulate BDNF signaling which, in turn, results in the enhancement of activity in brainstem cholinergic neurons and a consequent reduction in resting heart rate and blood pressure, and increased heart rate variability. IF has also been shown to enhance cardiovascular stress adaptation in rat models of uncontrollable stress (Wan et al., 2003). The remarkably similar effects of exercise and IF on heart rate and blood pressure suggest that both types of intermittent bioenergetic challenge can promote optimal cardiovascular health and fitness.
IF has effects on animal behavior and circadian rhythms that may have an impact on overall health and longevity. Energy metabolism is regulated in a circadian manner as indicated by circadian oscillations in all of the major energy-regulating hormones including insulin, leptin, corticosterone and adiponectin (Ramsey and Bass, 2011). Meal timing can have a major influence on circadian rhythms and IF can shift circadian aspects of behavior (e.g., activity levels). For example, when food is provided for only a few hours at a designated time each day, animals exhibit increased activity during a 1–2h time period preceding the time they are fed (Stephan, 2002). Effects of IF on circadian regulation of energy metabolism and behavior may occur as a result of changes in the core cell ‘clock’ molecular apparatus in peripheral tissues and/or central (hypothalamic suprachiasmatic nucleus) circadian control centers (Froy and Miskin, 2010). With regards to ADF, Froy and Miskin (2010) proposed that when food is provided or withdrawn during the daytime, the suprachiasmatic nucleus controls metabolic and behavioral rhythms, whereas when food is applied or withdrawn during the nighttime, the peripheral clock is in command. In addition to effects on the hypothalamus and periphery, there is emerging evidence that exercise and IF can affect mitochondrial physiology in neurons in the hippocampus and other brain regions by mechanisms involving increased mitochondrial biogenesis and mitochondrial stress resistance mediated by BDNF, PGC-1α (a master regulator of genes involved in mitochondrial biogenesis) and sirtuin 3 (SIRT3; a mitochondrial protein deacetylase that suppresses oxidative stress and apoptosis) (Cheng et al., 2012, 2016). The latter metabolic adaptations of neurons may contribute to the improvement in cognitive function of rodents maintained on ADF compared to those fed ad libitum (Li et al., 2013).
A general conclusion that can be drawn from the available data from animal studies is that involuntary IF has been a fundamental challenge during the evolution of animals, and the brain and other organ systems therefore respond adaptively to IF in ways that improve the abilities of the individual to optimize their performance and resistance to injury and disease.
2.1. IF and age-related diseases in animal models
2.1.1. Diabetes
Put simply, IF can prevent and cure the disease in rodent models of type 2 diabetes. When sand rats are fed a high fat diet they develop insulin resistance and diabetes, which can be ameliorated by maintaining them on an 8h/day TRF diet (i.e., 16 h of fasting every day) (Belkacemi et al., 2010). Similarly, when C57BL/6 mice are maintained on a high fat diet fed ad libitum they develop hyperinsulinemia, obesity and systemic inflammation, all of which are prevented by restricting food availability to 8 h/day (Hatori et al., 2012). The latter anti-diabetic effect of TRF is not due to caloric restriction because mice provided food for only 8 h/day consume the same amount of food as control mice fed ad libitum. Similar to leptin-deficient mice and leptin receptor mutant mice, mice with reduced BDNF levels are hyperphagic and develop insulin resistance and diabetes (Kernie et al., 2000). Daily intraperitoneal administration of BDNF to leptin receptor mutant mice, reversed obesity and diabetes (Nakagawa et al., 2003). When diabetic BDNF+/− mice are maintained on an ADF diet, their circulating levels of glucose, insulin and leptin are reduced, and glucose tolerance is normalized (Duan et al., 2003a). Interestingly, IF can ameliorate the insulin deficit and glucose intolerance in a rat model of type I diabetes by a mechanism involving preservation of pancreatic β-cells (Belkacemi et al., 2012). Although not yet established, it is likely that enhancement of cellular stress resistance by IF protects β-cells, as has been reported in studies of the effects of IF on other cell types (e.g., myocardial cells and neurons) (Mattson and Wan, 2005; Mattson, 2015).
The cellular and molecular mechanism by which IF prevents and reverses diabetes involves increased sensitivity of insulin receptor signaling such that insulin more readily stimulates glucose uptake by muscle and liver cells, and likely other cell types including neurons (Sequea et al., 2012). Changes in other signaling pathways affected by IF in one or many cell types may include: reductions of mTOR signaling; improved mitochondrial function; stimulation of mitochondrial biogenesis; and up-regulation of CREB, BDNF and autophagy pathways (Descamps et al., 2005; Cheng et al., 2012, 2016; Hatori et al., 2012; Longo and Mattson, 2014; Yuen and Sander, 2014). Inflammation of multiple organ systems occurs in diabetes (Guo, 2014), and IF can suppress inflammation (Arumugam et al., 2010) which may contribute to the anti-diabetic effects of IF.
2.1.2. Cardiovascular disease
Profound cardioprotective effects of IF have been documented in studies of rats and mice. In a model of myocardial infarction (MI; coronary artery ligation), rats that had been maintained on ADF for 3 months prior to MI, exhibited a reduced myocardial infarct size and the number of apoptotic cells in the area at risk (penumbra) was reduced by approximately 75% compared to the ad libitum control rats (Ahmet et al., 2005). Post-MI longitudinal echocardiographic analyses showed that left ventricular remodeling and infarct expansion occurred in rats on the ad libitum diet, but not in those on the ADF diet. Similar to rats, ADF protected the mouse heart against Mi-induced damage (Godar et al., 2015). In contrast to wild type mice, ADF did not protect the hearts of mice with impaired autophagy (Lamp2 heterozygous mutant mice). Instead, ADF worsened myocardial damage in Lamp2-deficient mice, indicating that stimulation of autophagy mediates the cardioprotective actions of ADF (Godar et al., 2015). IF was also reported to greatly improve survival and recovery of heart function in rats when initiated beginning 2 weeks after MI induced by occlusion of the left coronary artery (Katare et al., 2009). Whereas more than 75% of the rats on the ADF diet survived during an 8-week post MI period, less than 25% of the rats on the normal ad libitum diet survived. Data regarding the mechanism of action of IF in the latter study is consistent with the involvement of hormesis/adaptive cellular stress responses in that levels of HIF-1α, BDNF and VEGF were significantly elevated in myocardial tissue of rats on IF compared to those on the control diet.
When initiated in 2 month-old rats, ADF protected the heart against age-related increases in inflammation, oxidative stress and fibrosis (Castello et al., 2010). Age-related increases in myocardial cell ERK1/2 and PBK7 kinases, and altered STAT3 transcription factor activity, were prevented by ADF (Castello et al., 2011). Benefits of IF on cardiac function during aging appear to be highly conserved as it was reported that TRF can attenuate decline of cardiac function during aging in fruit flies (Gill et al., 2015). On the other hand, it was reported that when maintained on ADF for 6 months, rats exhibit a reduction of left ventricular diastolic compliance and evidence of diminished cardiac reserve (Ahmet et al., 2010). However, the interpretation of the latter findings is unclear because the rats on ADF weighed much less than did the rats fed ad libitum and may therefore require less cardiac output to support their requirements when living a sedentary life in laboratory cages.
In humans, hypertension, low heart rate variability, insulin resistance and hyperlipidemia are associated with increased risk for cardiovascular disease and stroke (DeFronzo and AbdulGhani, 2011). IF reduces blood pressure (Wan et al., 2003), increases heart rate variability (Mager et al., 2006) and reduces insulin resistance (Wan et al., 2003; Belkacemi et al., 2010) in laboratory rodents. The reduction in blood pressure may result, in part, from enhanced vascular endothelial cell-dependent vasodilation (Razzak et al., 2011). The increased heart rate variability in rats maintained on ADF may result from enhanced activity of brainstem cholinergic cardiovagal neurons (Mager et al., 2006; Wan et al., 2014). Levels of circulating cholesterol and triglycerides are reduced in animals on ADF and TRF diets (Varady et al., 2007; Belkacemi et al., 2012; Chaix et al., 2014). TRF protects mice against obesity and metabolic syndrome caused by consumption of atherogenic diets including a high fat + glucose diet and a high fructose diet (Chaix et al., 2014). The latter effects of TRF are associated with reductions in hepatic triglyceride content, circulating leptin and triglyceride levels, and reduced levels of proinflammatory cytokines in adipose tissue. Moreover, the physical performance of TRF mice on a rotarod test and treadmill endurance test are superior to mice fed ad libitum (Chaix et al., 2014), suggesting that IF can enhance physical fitness. The latter tests were performed during the feeding phase of the compressed (9 h) daily feeding period suggesting that the improved motor and endurance performance was not due to the feeding state of the mice.
2.1.3. Neurological disorders
Advancing age is the major risk factor for Alzheimer’s disease (AD), Parkinson’s disease (PD) and stroke (Yankner et al., 2008). The degeneration and death of neurons that occurs in each of these disorders is believed to involve impaired mitochondrial function, oxidative damage, impaired lysosome function and dys-regulation of cellular calcium homeostasis. Experimental evidence further suggests that hyperexcitability of neurons contributes to their demise in a process called excitotoxicity (Mattson, 2003). Prior to the current era of transgenic animals, experimental models of neurodegenerative disorders were based on the administration of neurotoxins that cause relatively selective degeneration of one or more populations of neurons that degenerate in the human disease. PD models include administration of the toxins MPTP, 6-hydroxydopamine and rotenone which inhibit mitochondrial complex I and cause degeneration of dopaminergic neurons. Models relevant to AD include hippocampal lesions induced by the excitotoxins (glutamate receptor agonists) kainic acid and domoic acid. The toxins 3-nitropropionic acid (3NPA) and malonate are inhibitors of succinate dehydrogenase (Complex II in the mitochondrial electron transport chain) that selectively kill striatal medium spiny neurons, the neurons most affected in Hunting-ton’s disease (HD). In the 1990s, studies were initiated to test the general hypothesis that, because aging is the major risk factor for neurodegenerative disorders, and because IF can counteract aging processes, IF may protect neurons in animal models of the disorders (see Mattson, 2012 for review).
When rats are maintained on ADF for several months prior to administration of kainic acid, their hippocampal neurons are more resistant to degeneration and learning and memory deficits were ameliorated (Bruce-Keller et al., 1999). Rats on ADF are also more resistant to 3NPA and malonate, exhibit less motor dysfunction and less degeneration of striatal neurons, suggesting a potential therapeutic application to patients with HD (Bruce-Keller et al., 1999). Mice maintained on ADF for several months are more resistant to MPTP as indicated by reduced loss of dopaminergic neurons and improved functional outcome in a PD model (Duan and Mattson, 1999). Moreover, rhesus monkeys maintained on CR for 6 months suffered less motor impairment and less striatal dopamine depletion (Maswood et al., 2004). In the latter study, levels of two neurotrophic factors known to protect dopaminergic neurons against MPTP (BDNF and glial cell line-derived neurotrophic factor) were elevated in the lesioned striatum of CR monkeys compared to those on the control diet.
Several transgenic mouse models of AD have been generated that exhibit age-related accumulation of Aβ without or with Tau pathology, and associated learning and memory deficits. Such ‘AD mice’ express familial AD mutations in the β-amyloid precursor protein (APP) alone or in combination with a familial AD presenilin 1 mutation. Aβ is generated from APP by sequential enzymatic cleavages by β- and 7-secretases, and presenilin 1 is the enzymatic subunit of the 7-secretase enzyme complex (Mattson, 1997). When 3xTgAD mice (which express APP, presenilin 1 and Tau mutations) were maintained for 1 year on either 40% CR or ADF diets beginning when they were 5 months old, they did not develop the cognitive impairment exhibited by 3xTgAD mice fed ad libitum (Halagappa et al., 2007). Interestingly, whereas levels of Aβ and Tau accumulation were reduced in the brains of 3xTgAD mice on the CR diet, they were not reduced in 3xTgAD mice on the ADF diet, suggesting that IF can protect neurons against dysfunction even in the presence of Aβ and Tau pathologies. Other studies have also shown that CR can attenuate Aβ pathology in the brains of APP mutant mice (Patel et al., 2005; Wang et al., 2005). The mechanism(s) by which IF protects against synaptic dysfunction and cognitive deficits in mouse models of AD is unknown, but may include reductions in oxidative stress, preservation of mitochondrial function and increased neurotrophic factor signaling and autophagy because: IF induces the expression of antioxidant enzymes and neurotrophic factors including BDNF and FGF2 (Arumugam et al., 2010); BDNF stimulates mitochondrial biogenesis (Cheng et al., 2012); IF up-regulates autophagy (Godar et al., 2015); neurotrophic factors and interventions that bolster mitochondrial bioenergetics (Mark et al., 1997; Caccamo et al., 2010; Liu et al., 2013) and autophagy (Majumder et al., 2011; Lin et al., 2013) can protect neurons in experimental models of AD.
Mutations in α-synuclein cause some cases of familial PD. Several lines of transgenic mice that overexpress wild type or mutant human α-synuclein exhibit progressive accumulation of α-synuclein in neurons, motor dysfunction and death (Crabtree and Zhang, 2012). Mice expressing mutant (A53T) α-synuclein exhibit impaired autonomic regulation of heart rate characterized by elevated resting heart rate associated with accumulation of α-synuclein aggregates in the brainstem and reduced parasympa-thetic (cardiovagal) tone (Griffioen et al., 2013). Maintenance of the α-synuclein mutant mice on ADF reversed the autonomic deficit, whereas a high fat diet exacerbated the autonomic deficit (Griffioen et al., 2013). Consistent with the latter findings, a high fat diet hastened the onset of motor dysfunction and brainstem pathology in another line of α-synuclein mutant mice, which was associated with reduced activity of kinases known to be involved in neurotrophic factor signaling (Rotermund et al., 2014). In addition to enhancement of neurotrophic factor/BDNF signaling, IF may counteract PD-related pathogenic processes by stimulating autophagy. Indeed, inhibition of mTOR with rapamycin, which stimulates autophagy, reduced oxidative stress and synaptic damage, and improved motor function in a α-synuclein accumulation-based mouse model of PD (Bai et al., 2015).
Huntingtin mutant mice exhibit progressive degeneration of striatal and cortical neurons, and also have reduced expression of BDNF in these brain regions and peripheral insulin resistance. When initiated prior to the onset of motor dysfunction in huntingtin mutant mice, ADF increases brain BDNF levels, normalizes glucose metabolism and significantly delays the onset of neurode-generation and motor dysfunction (Duan et al., 2003b). While ADF is beneficial in animal models of AD, PD and HD, it has been reported not to be beneficial and, instead exacerbates motor dysfunction in a transgenic mouse model of amyotrophic lateral sclerosis (ALS) in which the mice overexpress a mutant form of Cu/Zn superoxide dismutase that causes familial ALS in humans (Pedersen and Mattson, 1999). One potential reason for the lack of benefit in the ALS model is that the neurons affected in ALS (lower and upper motor neurons) are unable to respond adaptively to the bioenergetics challenge of fasting.
Numerous studies have shown that, when initiated prior to the ischemic insult, ADF can reduce brain damage and improve functional outcome in animal models of stroke (Yu and Mattson, 1999; Arumugam et al., 2010). The cellular and molecular mechanisms by which IF protects brain cells against a stroke have not been fully established but involve up-regulation of expression of neurotrophic factors (BDNF and FGF2), antioxidant enzymes (heme oxygenase 1) and protein chaperones (HSP70 and GRP78) (Arumugam et al., 2010). Reduced inflammation may also mediate the beneficial effects of IF in stroke models as indicated by reduced levels of proinflammatory cytokines (TNFα, IL1-β and IL6) and suppression of the ‘inflammasome’ (Arumugam et al., 2010; Fann et al., 2014). Indeed, IF can attenuate cerebral oxidative stress and cognitive impairment induced by lipopolysaccharide in an animal model of systemic inflammation (Vasconcelos et al., 2014, 2015). Reductions in levels of leptin and increased levels of ketones may also contribute to neuroprotection by IF in stroke models (Manzanero et al., 2014). It remains to be determined whether post-stroke IF will modify functional outcome/recovery in animal models, which will be critical to know when considering whether or not IF is likely to benefit human stroke patients.
IF has been reported to improve outcome in animal models of traumatic injury to the nervous system, as well as in models of peripheral neuropathy. In rat models of incomplete cervical spinal cord injury and thoracic contusion injury, ADF initiated prior to the injury and continued thereafter significantly improved functional outcome and reduced spinal cord lesion size (Plunet et al., 2010; Jeong et al., 2011). ADF was also beneficial when initiated after thoracic contusion spinal cord injury (Jeong et al., 2011). However, in a mouse model of spinal cord injury ADF initiated after the injury did not significantly affect functional outcome or spinal cord lesion size (Streijger et al., 2011). The reason why ADF was effective in the rat models, but not in the mouse model, is unclear and merits further investigation. As with spinal cord injuries, traumatic brain injury is a major cause of disability and death, particularly in young active individuals. While IF (per se) has not been evaluated in animals models of traumatic brain injury, it was reported that CR (limited daily feeding with a 30% reduction in calorie intake) initiated 4 months prior to the injury, reduced the extent of brain damage, ameliorated cognitive deficits, and elevated BDNF levels in the affected cerebral cortex and hippocampus (Rich et al., 2010). Finally, recent studies have elucidated the potential impact of IF on peripheral nerve health and disease resistance. In a mouse model of the peripheral demyelinating neuropathic disease Charcot-Marie-tooth type 1A (Trembler mice), 5 months of ADF resulted in improved motor performance, increased myeli-nation and decreased accumulation of PMP22 protein aggregates (Madorsky et al., 2009). Additional findings suggest that the beneficial effects of IF on peripheral nerve health and disease resistance are mediated, in part, by up-regulation of autophagy and related protein quality control mechanisms (Lee and Notterpek, 2013).
2.1.4. Cancer
Recently a series of studies in animal models have shown that periodic fasting (PF) lasting 2 or more days can be as effective as chemotherapy in delaying the progression of a wide range of cancers but, more importantly, can protect normal cells from the toxic effects of chemotherapy drugs while sensitizing cancer cells to the treatment (Raffaghello et al., 2008; Lee et al., 2012; Safdie et al., 2009; Dorff et al., 2016; Lee and Longo, 2011). A severely restricted diet that mimics PF started at middle age was effective in causing a major reduction in tumor incidence, in addition to delaying tumor onset and reducing the number of sites with tumor-like lesions, suggesting a reduction in metastatic cancers (Brandhorst et al., 2015). The role of PF and FMDs in cancer prevention and treatment has been discussed in more detail elsewhere (Longo and Mattson, 2014; Longo and Panda, 2016). Here we will focus on IF and cancer.
IF has been studied in murine cancer models, although mostly in cancer prevention. Siegel et al. studied the effects of ADF on the survival of 3–4 month-old tumor-free and tumor-bearing Fisher rats. 50% of the ADF rats survived to day 10 compared to 12.5% survival in the control diet group (Siegel et al., 1988). In addition, this study included both a tumor prevention and a tumor treatment component since the ADF was initiated one week before rats were inoculated intraperitoneally with ascites tumor cells, making it difficult to understand the mechanisms responsible for its effects.
In another study, p53+/− mice with an accelerated cancer death phenotype, undergoing one day per week fasting survived significantly, albeit modestly, longer than mice on an ad libitum diet (Berrigan et al., 2002). The one day per week IF diet resulted in only an 8% reduction in IGF-1 levels, which may explain in part its limited efficacy.
Considering the recent development of relatively high calorie FMDs tested in both mice and humans (Brandhorst et al., 2015), and the ability of the combination of PF or FMDs with chemotherapy to have very strong effects including cancer-free survival in multiple murine cancer models, it will be important to directly compare PF or FMDs, with IF diets with shorter periods of fasting such as ADF, the 5:2 diet and TRF. Future studies on IF and cancer treatment should consider the potential toxicity of its combination with chemotherapy, particularly during the feeding days, which may cause an increase in the proliferation of various cell types and promote the generation of secondary tumors. In addition, it will be important to determine whether IF diets affect the metabolism of chemotherapeutic agents, as this could influence the impact of the drug treatment on the cancer cells.
3. IF in humans
3.1. Weight loss and maintenance amongst overweight and obese subjects
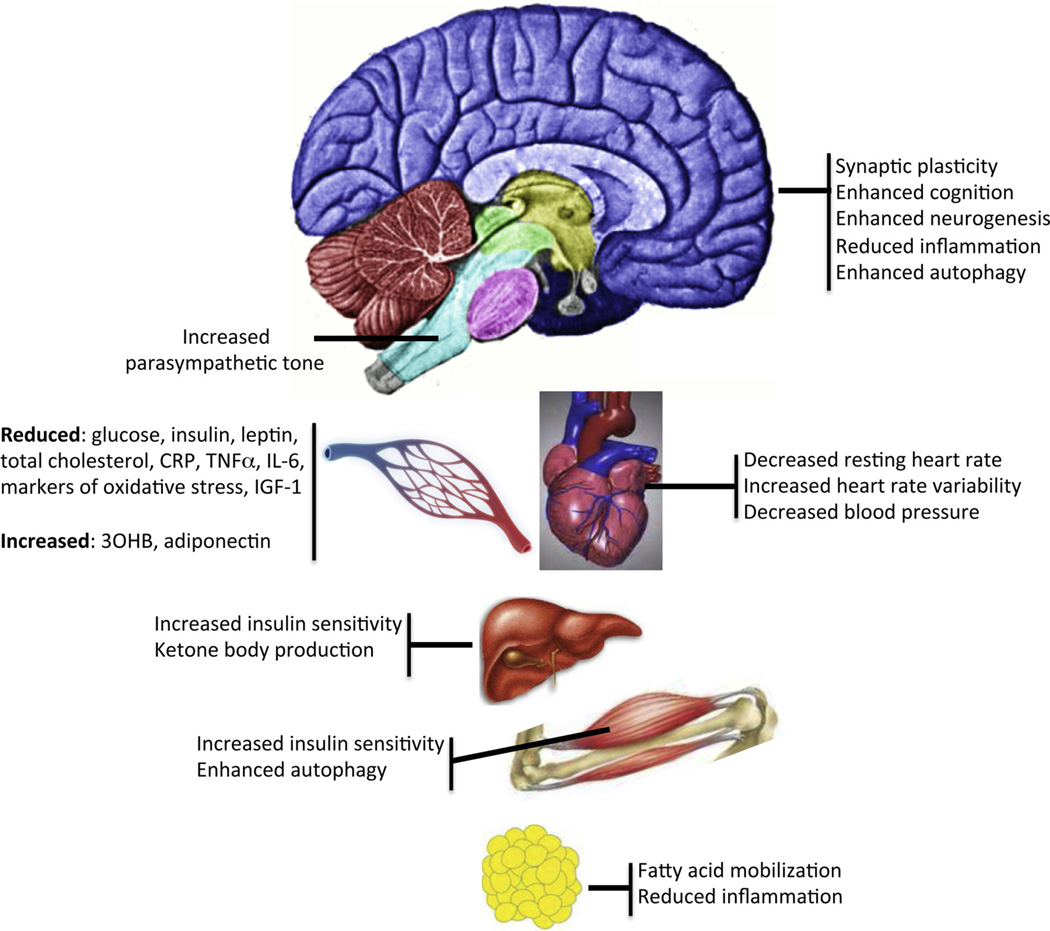
The majority of studies of IF in humans have considered whether IF can be a potential strategy to reduce weight and correct adverse metabolic parameters amongst obese and overweight subjects (Fig. 3). This is important since the problems of long term adherence to continuous energy restriction (CER) for weight management are well known (Anastasiou et al., 2015). Johnson et al. undertook the first trial of IF for weight loss amongst 10 obese subjects with asthma which tested alternate days of an 85% energy restricted low carbohydrate diet regimen. This study reported beneficial reductions in serum cholesterol and triglycerides, markers of oxidative stress (8-isoprostane, nitrotyrosine, protein carbonyls, and 4-hydroxynonenal adducts) and inflammation (serum tumor necrosis factor-α) (Johnson et al., 2007). Circulating ketone levels were also elevated on the fasting days (Johnson et al., 2007) (Fig. 1). This study was the first to show the feasibility of IF amongst obese subjects, however, the lack of a CER comparison group means that we cannot distinguish if the benefits were as a result of the overall energy restriction and weight loss or a specific effect of the IF regimen. The most studied IF regimen has been alternate days of 70% CR, a modified form of ADF. Most studies of ADF summarized in recent reviews show benefits in terms of reductions in weight (−3 to −7%), body fat (3 −5.5 kg), total serum cholesterol(−10 to −21%) and triglycerides (−14 to −42) (Tinsley and La Bounty, 2015), as well as improvements in glucose homeostasis (Seimon et al., 2015). However the lack of a CER comparator in most of these studies means again, we cannot determine if these effects are a function of the overall energy restriction/weight loss or a specific effect of the IF regimen.
Fig. 3.
Examples of effects of intermittent fasting on different organ systems.
Abbreviations;: 30HB, 3-hydroxybutyrate; CRP, C-reactive protein; IGF-1, insulin-like growth factor 1; IL-6, interleukin 6; TNFα, tumor necrosis factor α
To date only a few published randomized controlled trials (RCTs) have assessed whether IF may be equivalent or superior to an isocaloric CER for managing weight and metabolic risk amongst overweight or obese subjects. These trials have tested various IF regimens; 2 consecutive days of a 55–70% CR every week (Harvie et al., 2011, 2013a; Fig. 2), 4 days of 50% CR each week (Ash et al., 2003), an alternating pattern of 3–7 days of 70%, 60%, 45% and 10% calorie restriction per week (Hill et al., 1989a), and alternating days of a 70% CR and ad libitum eating (Varady et al., 2011). These studies have been relatively small and all reported equivalent weight loss between IF and CER. The only study to show a difference was by Harvie et al. (2013a,b). In this study there was no significant difference in weight loss between the groups, but there was a greater loss of body fat with two different low carbohydrate IF regimens compared with CER over 4 months (Harvie et al., 2013a). The two IF regimens allowed two consecutive days/week of either a low carbohydrate, low energy IF diet (70% CR, 600 kcal, 40 g carbohydrate/d) or a less restrictive low carbohydrate IF which allowed ad libitum protein and monounsaturated fatty acids (55% CR, 1000 kcal, 40 g carbohydrate/d), both with 5 days of a healthy Mediterranean type diet (45% energy from low glycemic load carbohydrates 30% fat; 15% monounstaturated fatty acids, 8% polyunsaturated fatty acids and 7% saturate fatty acids). They were compared to an isocaloric 25% CER Mediterranean type diet. The differences in overall carbohydrate intake between the diet groups were modest (41% and 37% of energy for the two IF diets compared to 47% of energy for CER, which is unlikely to account for any differences in adherence and reductions in adiposity between the diets (Sacks et al., 2009).
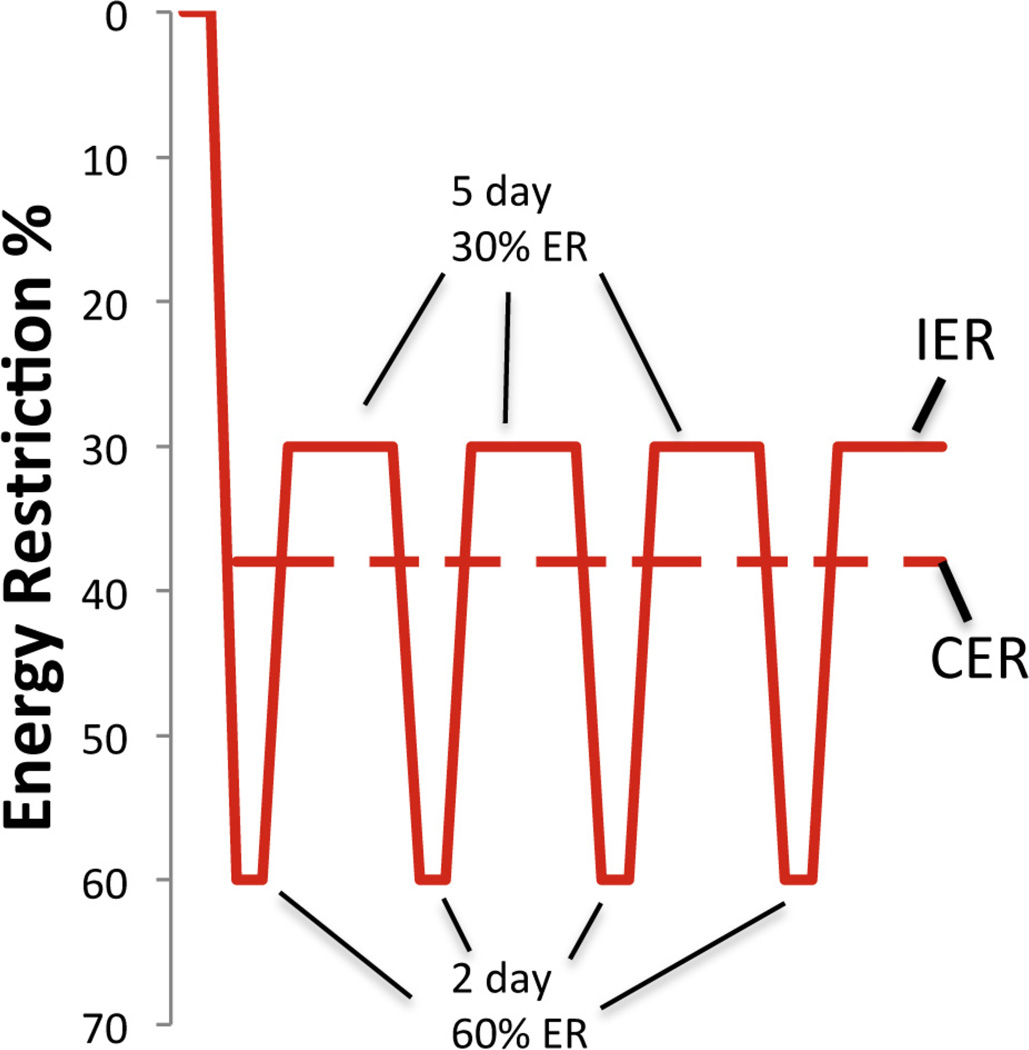
Fig. 2.
Patterns of energy intake in subject on either the ‘5:2′ intermittent energy restriction (IER) diet or continuous (daily) energy restriction (CER). Based on Harvie et al., 2011.
Drop out from the selected studies was between 0 and 40%, and similar between the IF and CER groups. Studies which reported adherence to the IF days show a good level of compliance, with 65–75% of potential IF days achieved during the trial period. (Harvie et al., 2011,2013a). Importantly, IF does not appear to lead to compensatory over-consumption on the non-dieting days in any of the trials. The two Manchester trials reported a ‘carry over effect’ of reduced energy intake by 23–32% on non-restricted days. Thus on these days the energy restriction was similar to the planned 25% restriction in women taking CER (Harvie et al., 2011, 2013a). The greater loss of fat with the two-day low carbohydrate IF diet compared to CER in our 2013 study appears to be linked to good adherence to the restricted days and the spontaneous restriction of energy intake on non-restricted days.
Weight loss diets aim to maximize loss of body fat and minimize loss of fat-free mass (FFM) to maintain physical function and attenuate declines in resting energy expenditure and to help prevent weight gain. Proponents of IF diets claim they may preserve FFM, and allowed our Palaeolithic hunter gatherer ancestors to survive spells of food shortage. There are, however, few data to support this assertion as the modest sized IF trials which have been undertaken are unlikely to be powered sufficiently to demonstrate changes in FFM (Heymsfield et al., 2014). Weight loss trials amongst overweight and obese subjects suggest losses of FFM with IF and CER are equivalent and are dependent on the overall protein content of the IF and CER diet, rather than the pattern of energy restriction, which is well established for CER diets (Soenen et al., 2013). Our first IF trial reported an equivalent loss of weight as FFM with IF and CER (both 20%) when both diets provided 0.9 g protein/kg body weight (Harvie et al., 2011). Our 2013 trial reported equal losses of FFM (both 30% of weight lost) with a standard protein (1.0 g protein/kg body weight) IF compared to a standard protein CER (1.0 g protein/kg body weight), but a greater preservation of FFM (20% of weight loss) with a higher protein IF (1.2 g protein/kg weight) (p<0.05) (Harvie et al., 2013b). Studies of ADF have reported the proportion of weight lost as FFM to be as low as 10% in obese women (Varady et al., 2009a, 2009b) and as high as 30% amongst non-obese subjects (Varady et al., 2013) (Heilbronn et al., 2005b). Subsequent studies have shown that exercise helps to retain FFM amongst subjects undergoing IF (Hill et al., 1989b; Bhutani et al., 2013) which is well documented with CER (Weinheimer et al., 2010). Indeed, a study of young adult men who performed resistance training demonstrated that eight weeks of TRF (8h feeding period every day) resulted in loss of fat mass, with retention of lean mass and improved muscle endurance (Moro et al., 2016; Tinsley et al., 2016). The latter findings are consistent with results of TRF in mice (Chaix et al., 2014) and show that at least some IF diets do not adversely affect, and can even enhance, physical performance.
While the equivalent or sometimes superior effects of IF versus CER in the aforementioned studies is of interest, these studies have been short term (≤6 months). The true test of a successful weight loss diet is its ability to maintain weight loss long term. Estimates of successful weight loss maintenance with CER (defined as >10% weight loss maintained at ≥12 months), vary between 20% and 50% (Anastasiou et al., 2015; Wing and Phelan, 2005), which depends on the level of ongoing support. In a recent study, obese subjects were randomized to either zero calorie ADF or daily CER (400 cal/day below baseline intake) for 2 months, followed by 6 months of unsupervised follow-up (Catenacci et al., 2016). During the 2-month diet intervention period, both groups exhibited weight and fat mass loss; however, at the 6 month post-diet follow-up, changes from baseline in lean mass and fat mass were more favorable in the subjects in the ADF group. However, the latter study is the only published data on weight loss maintenance with IF which is a major gap in the evidence base. There are no data on the potential for IF regimes to prevent weight gain amongst normal weight subjects. Strategies to prevent weight gain in normal weight subjects are important for public health, since adult weight gain is a major public health problem linked to risk of many non-communicable diseases including cancer, diabetes, CVD and dementia (Sun et al., 2009). Reports of sustained hunger with IF (Heilbronn et al., 2005b; Wegman et al., 2014), and difficulties maintaining daily living activities during restricted days of IF (Wegman et al., 2014) in non-obese subjects suggests a more limited compliance and potential efficacy with these specific regimens in the non-obese. However, other patterns of IF, e.g. 1 day/week of CR, may be better tolerated and need to be studied in non-obese subjects.
Finally, in addition to controlled studies of IF in human subjects, there have been several studies of health indicators in subjects who fast from dawn until dusk during the month of Ramadan (Mazidi et al., 2015). Overall, it appears that many subjects lose weight during Ramadan and exhibit improvements in some health indicators. However, such studies are generally not well controlled because the time period during which the individuals fast each day varies considerably (9–20 h) depending upon the day length in the region of the world where the subjects reside.
3.2. IF and age-related diseases in humans
3.2.1. Type 2 diabetes
There are minimal data on the effects of IF versus CER on glucose homeostasis amongst overweight/obese individuals with type 2 diabetes. Ash et al. (2003) reported that a four day IF led to comparable reductions in percentage body fat and reductions in HbA1c to an isocaloric CER. Mean (SD) reduction for the overall group was 1.0 (8.4)%, although this small study may have been underpowered to show significant differences (Ash et al., 2003). Williams et al. (1998) assessed the effect of enhancing a standard 25% CER diet with periods of IF, (75% ER either 5 days/week every 5 weeks or 1 day/week for 15 weeks). Predictably, additional periods of ER increased weight loss. The 5 days/week every 5 weeks intervention resulted in the greatest normalization of HbA1c, independent of weight loss suggesting a potential specific insulin-sensitizing effect of this pattern of IF added to CER (Williams et al., 1998).
The two studies of a 2 days/week IF mentioned previously have reported greater reductions in insulin resistance versus CER amongst overweight and obese non-diabetic subjects (Harvie et al., 2011, 2013a). In the first study the subjects on the IF diet exhibited a 25% greater reduction in insulin resistance compared to the CER group when measured on the morning after five normal feeding days, with a further 25% reduction in insulin resistance compared with CER on the morning after the two energy restricted days. These differences in insulin sensitivity occurred despite comparable reductions in body fat between the groups (Harvie et al., 2011, 2013a). Our follow up study reported greater reductions of insulin resistance with the 2 days/week low carbohydrate IF compared to CER, which this time was associated with a greater loss of fat with the IF regimen (Harvie et al., 2011, 2013a).
Three studies have assessed the effects of 2–3 weeks of IF with alternating 20–24 h periods of a total fast interspersed with 24–28 h periods of hyperphagia (175–200% of normal intake) and were designed to ensure there was no overall energy deficit or weight loss. Results have been variable between the studies. Halberg et al. reported improvements in insulin-mediated whole body glucose uptake and insulin-induced inhibition of adipose tissue lipolysis when measured after two normal feeding days, (Halberg et al., 2005), whereas Soeters et al. failed to replicate these findings (Soeters et al., 2009). Heilbronn assessed 3 weeks of ADF (24 h total fast and 24 h ad lib feeding) amongst 16 normal and overweight men and women (Heilbronn et al., 2005a). Glucose uptake during a test meal (peripheral insulin sensitivity) was assessed on the morning after a fasting day, i.e. after a 36 h fast; interestingly, insulin sensitivity was increased in men but decreased in women. The latter observation may be a benign observation linked to greater fluxes of free fatty acids amongst fasting women (Hedrington and Davis, 2015), and likely to be a normal physiological adaptation to fasting rather than a cause for concern (Gormsen et al., 2008). Thus, IF has been reported to have variable effects on peripheral and hepatic insulin sensitivity which may be different in obese and normal weight subjects and may be gender-specific. Further studies are required using more robust measures of insulin sensitivity e.g. insulin clamp or other techniques.
3.2.2. Cardiovascular disease
Varady et al. performed several different studies to evaluate the effects of modified ADF on cardiovascular risk factors in overweight and obese subjects. In one study, ADF for 2 months resulted in decreases in resting heart rate, and circulating levels of glucose, insulin and homocysteine, all of which are favorable with regards to the risk of cardiovascular disease (Klempel et al., 2012). In another study, 2 months of ADF reduced fat mass, total cholesterol, LDL cholesterol and triglyceride concentrations (Varady et al., 2015). However, there have been few studies that have evaluated the relative effects of IF and CER on cardiovascular risk markers. The randomized comparisons of IF and CER have reported equivalent reductions in blood pressure (Hill et al., 1989a; Harvie et al., 2011, 2013a) and triglycerides (Hill et al., 1989a), (Ash et al., 2003). (Harvie et al., 2011, 2013a), and increased LDL particle size (Varady et al., 2011). Whilst Hill et al. reported a greater reduction in serum cholesterol with IF (14%) versus CER (6%) (Hill et al., 1989a).
3.2.3. IF and cancer
There are no data on the effects of IF on cancer rates in humans. Weight control is likely to reduce the incident risk of thirteen cancers which have been linked to obesity (Renehan et al., 2008), although the role of weight management after diagnosis on the outcome of obesity related cancers is not known (WCRF, 2014; Goodwin et al., 2015). Surrogate evidence that IF may reduce cancer risk can be derived from its effects on a number of cancer risk biomarkers such as insulin, cytokines, and the inflammation-related molecules leptin and adiponectin, which are thought to mediate the effects of adiposity and excessive energy intake on the development and growth of cancers in humans (Hursting et al., 2012; Wei et al., 2016).
The effect of IF on total and bioavailable insulin-like growth factor 1 (IGF-1) in human studies has been variable. This reflects the fact that, in contradistinction to animal studies, circulating levels of total IGF-1 and bioactive IGF-1 (determined by measuring IGF-binding proteins 1,2 and 3) are poor markers of the effects of energy restriction and weight loss in humans (Byers and Sedjo, 2011), and do not relate well to IGF-1 bioactivity at the tissue level (Ramadhin et al., 2014). We reported no change in total circulating IGF-1 alongside weight loss with IF or CER in either of our studies (Harvie et al., 2011, 2013a). IF and CER both increased IGF binding protein 1 (26% and 28%) and IGFBP-2 (22% and 36%), but did not change serum bioavailable IGF-1 (ultrafiltered) when measured after feeding days. There is a further acute 17% increase in IGF binding protein 2 on the morning after the two restricted days of a 70% ER, but no measurable change in total or serum bioavailable IGF-1 (ultrafiltered) (Harvie et al., 2011). Reductions in IGF-1 (−15%) have been reported in normal and overweight subjects who had followed an IF diet which involved 5 days per month of a low protein, low energy diet (~0.25g protein/kg weight, 34–54% of normal energy intake) interspersed with normal intake for the remaining 25 days of the month. These reductions were observed after 5 days of normal eating after three months alongside modest reductions in body weight (−2%) (Brandhorst et al., 2015). The aforementioned effects of IF in relation to insulin resistance and diabetes risk (see references in the section on diabetes above) may therefore have an important role in protecting against obesity-related cancers (Goodwin et al., 2015).
Adipose tissue exhibits increased leptin and decreased adiponectin production with increasing adiposity, which is thought to have a role in cancer development and progression via effects on insulin sensitivity, inflammation, and direct effects on cell proliferation and apoptosis (Hursting et al., 2012). Adiponectin levels only increase in overweight humans following CER when there are large reductions in body and visceral fat (>10%) (Klempel and Varady, 2011). Some studies of IF have reported increases in adiponectin with more modest weight loss. i.e. a 30% increase in plasma adiponectin on both restricted and feeding days, alongside modest reductions in weight (−4%) and body fat (−11%) (Bhutani et al., 2010). We reported a tendency for a greater increase in adiponectin with IF than CER despite a comparable reductions in weight and adiposity (p = 0.08) (Harvie et al., 2011). These observations are interesting; however our follow up IF study reported no change in adiponectin levels with either IF or CER. (Harvie et al., 2013a). IF brings about large and comparable reductions in leptin as CER (both 40%) and the leptin: adiponectin ratio (Harvie et al., 2011, 2013a). Weight loss with CER reduces circulating levels of C reactive protein (CRP) by 2–3% for every 1% weight loss, whereas TNF-α and IL-6 are reduced by around 1–2% per 1% weight loss (Byers and Sedjo, 2011). Reductions of inflammatory markers with IF are comparable to CER for a given weight loss (Harvie et al., 2011). Thus, although limited, the available biomarker data suggest that IF leads to comparable changes in most cancer risk biomarkers to CER, with the possible exceptions of insulin resistance and adiponectin which require further study using robust methodologies.
4. Conclusions and future directions
Numerous physiological indicators of health are improved in laboratory rats and mice maintained on IF diets including alternate day fasting and time-restricted feeding. Among such responses to IF are: reduced levels of insulin and leptin which parallel increases in insulin and leptin sensitivity; reduced body fat; elevated ketone levels; reduced resting heart rate and blood pressure, and increased heart rate variability (resulting from increased parasympathetic tone); reduced inflammation; increased resistance of the brain and heart to stress (e.g., reduced tissue damage and improved functional outcome in models of stroke and myocardial infarction); and resistance to diabetes. IF can delay onset and slow the progression of neuronal dysfunction and degeneration in animal models of Alzheimer’s, Parkinson’s and Huntington’s diseases. Emerging findings are revealing cellular and molecular mechanisms by which IF increases the resistance of cells, tissues and organs to stress and common diseases associated with aging and sedentary, overindulgent lifestyles. The results of human studies in which various health indicators are measured at baseline and after periods of IF of 2–6 months or more, suggest that IF can protect against the metabolic syndrome and associated disorders including diabetes and cardiovascular disease. Recent small trials of IF in patients with cancer (Safdie et al., 2009) or multiple sclerosis (Choi et al., 2016) have generated promising results that provide a strong rationale for moving forward with larger clinical trials in patients with a range of chronic age- and obesity-related disorders.
Acknowledgments
This work was supported, in part, by the Intramural Research Program of the National Institute on Aging.
Abbreviations
- AD
Alzheimer’s disease
- ADF
alternate day fasting
- ALS
amyotrophic lateral sclerosis
- APP
β-amyloid precursor protein
- BDNF
brain-derived neurotrophic factor
- CER
continuous energy restriction
- CR
caloric restriction
- CREB
cyclic AMP response element-binding protein
- CVD
cardiovasculardisease
- ERK
extracellular signal regulated kinase
- FFM
fat-free mass
- FGF2
fibroblast growth factor 2
- FMD
fasting mimicking diet
- GR
glucocorticoid receptor
- GRP-78
glucose regulated protein 78
- HD
Huntington’s disease
- HSP-70
heat-shock protein 70
- IF
intermittent fasting
- IGF-1
insulin-like growth factor 1
- IL-6
interleukin 6
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MI
myocardial infarction
- MR
mineralocorticoid receptor
- mTOR
mammalian target of rapamycin
- PGC-1α
peroxisome proliferator-activator receptor 7 coactivator 1α
- PD
Parkinson’s disease
- PF
periodic fasting
- PMP22
peripheral myelin protein 22
- SIRT1
sirtuin 1
- SIRT3
sirtuin 3
- TNF-α
tumor necrosis factor α
- TRF
time-restricted feeding
References
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan MI. Chronic alternate-day fasting results in reduced diastolic compliance and diminished systolic reserve in rats. J. Card. Fail. 2010;16:843–853. doi: 10.1016/j.cardfail.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou CA, Karfopoulou E, Yannakoulia M. Weight regaining: from statistics and behaviors to physiology and metabolism. Metabolism. 2015;64:1395–1407. doi: 10.1016/j.metabol.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Saleh JK, Boparai RK, Kopchick JJ, Khardori RK, Bartke A. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age (Dordr) 2014;36:9651. doi: 10.1007/s11357-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: a randomised trial. Int. J. Obes. Relat. Metab. Disord. 2003;27:797–802. doi: 10.1038/sj.ijo.0802295. [DOI] [PubMed] [Google Scholar]
- Bai X, Wey MC, Fernandez E, Hart MJ, Gelfond J, Bokov AF, Rani S, Strong R. Rapamycin improves motor function, reduces 4-hydroxynonenal adducted protein in brain, and attenuates synaptic injury in a mouse model of synucleinopathy. Pathobiol. Aging Age Relat. Dis. 2015;5:28743. doi: 10.3402/pba.v5.28743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi L, Seiselet-Attou G, Louchami It, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in sand rats: II. In vivo investigations. Int. J. Mol. Med. 2010;26:759–765. doi: 10.3892/ijmm_00000523. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Seiselet-Attou G, Hupkens E, Nguidjoe E, Louchami It, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int. J. Endocrinol. 2012;2012:962012. doi: 10.1155/2012/962012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver. Spring) 2010;18:2152–2159. doi: 10.1038/oby.2010.54. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver. Spring) 2013;21:1370–1379. doi: 10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- Boutant M, Kulkarni SS, Joffraud M, Raymond F, Metairon S, Descombes P, Canto C. SIRT1 Gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep. 2016;14:2068–2075. doi: 10.1016/j.celrep.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes. Metab. 2011;13:1063–1072. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto A. Life without food and the implications for neurodegeneration. Adv. Genet. 2015;92:53–74. doi: 10.1016/bs.adgen.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Radic. Biol. Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Castello L, Maina M, Testa G, Cavallini G, Biasi F, Donati A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting reverses the age-associated hypertrophy phenotype in rat heart by influencing the ERK and PI3 K signaling pathways. Mech. Ageing Dev. 2011;132:305–314. doi: 10.1016/j.mad.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Donahoo WT. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity. 2016;24:1874–1883. doi: 10.1002/oby.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, da Cunha FM, Caldeira da Silva CC, Chausse B, Romano RL, Garcia CC, Colepicolo P, Medeiros MH, Kowaltowski AJ. Long-term intermittent feeding, but not caloric restriction, leads to redox imbalance, insulin receptor nitration, and glucose intolerance. Free Radic. Biol. Med. 2011;51:1454–1460. doi: 10.1016/j.freeradbiomed.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-l(in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi It, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;2016(23):128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TE, Wei M, Paul F, Bock M, Longo VD. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15:2136–2146. doi: 10.1016/j.celrep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree DM, Zhang J. Genetically engineered mouse models of Parkinson’s disease. Brain Res. Bull. 2012;88:13–32. doi: 10.1016/j.brainresbull.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am. J. Cardiol. 2011;108(Suppl. (3)):3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Descamps O, Riondel J, Ducros V, Roussel AM. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate day fasting. Mech. Ageing Dev. 2005;126:1185–1191. doi: 10.1016/j.mad.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, Cheng CW, Brandhorst S, Cohen P, Wei M, Longo V, Quinn DI. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360. doi: 10.1186/s12885-016-2370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorighello GG, Rovani JC, Luhman CJ, Paim BA, Raposo HF, Vercesi AE, Oliveira HC. Food restriction by intermittent fasting induces diabetes and obesity and aggravates spontaneous atherosclerosis development in hypercholesterolaemic mice. Br. J. Nutr. 2014;111:979–986. doi: 10.1017/S0007114513003383. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003a;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2003b;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM, Mattson MP, Arumugam TV. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp. Neurol. 2014;257:114–119. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Fond G, Macgregor A, Leboyer M, Michalsen A. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209:253–258. doi: 10.1016/j.psychres.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy. 2015;11:1537–1560. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J. Gerontol. 1983;38:36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ambrosone CB, Hong CC. Modifiable lifestyle factors and breast cancer outcomes: current controversies and research recommendations. Adv. Exp. Med. Biol. 2015;862:177–192. doi: 10.1007/978-3-319-16366-6_12. [DOI] [PubMed] [Google Scholar]
- Gormsen LC, Gjedsted J, Gjedde S, Norrelund H, Christiansen JS, Schmitz O, et al. Dose-response effects of free fatty acids on amino acid metabolism and ureagenesis. Acta Physiol. (Oxf) 2008;192:369–379. doi: 10.1111/j.1748-1716.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Gotthardt JD, Verpeut JL, Yeomans BL, Yang JA, Yasrebi A, Roepke TA, Bello NT. Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Ygene expression in diet-induced obese male mice. Endocrinology. 2016;157:679–691. doi: 10.1210/en.2015-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen KJ, Rothman SM, Ladenheim B, Wan R, Vranis N, Hutchison E, Okun E, Cadet JL, Mattson MP. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol. Aging. 2013;34:928–935. doi: 10.1016/j.neurobiolaging.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005;99:2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v: daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013a;110:1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013b:1–14. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrington MS, Davis SN. Sexual dimorphism in glucose and lipid metabolism during fasting, hypoglycemia, and exercise. Front. Endocrinol. (Lausanne) 2015;6:61. doi: 10.3389/fendo.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes. Res. 2005a;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition and energy metabolism. Am. J. Clin. Nutr. 2005b;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes. Rev. 2014;15:310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Schlundt DG, Sbrocco T, Sharp T, Pope-Cordle J, Stetson B, et al. Evaluation of an alternating-calorie diet with and without exercise in the treatment of obesity. Am. J. Clin. Nutr. 1989a;50:248–254. doi: 10.1093/ajcn/50.2.248. [DOI] [PubMed] [Google Scholar]
- Hill JO, Schlundt DG, Sbrocco T, Sharp T, Pope-Cordle J, Stetson B, et al. Evaluation of an alternating-calorie diet with and without exercise in the treatment of obesity. Am. J. Clin. Nutr. 1989b;50:248–254. doi: 10.1093/ajcn/50.2.248. [DOI] [PubMed] [Google Scholar]
- Hursting SD, DiGiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, energy balance: and cancer: new opportunities for prevention. Cancer Prev. Res. (Phila) 2012;5:1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong MA, Plunet W, Streijger F, Lee JH, Plemel JR, Park S, Lam CK, Liu J, Tetzlaff W. Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J. Neurotrauma. 2011;28:479–492. doi: 10.1089/neu.2010.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3 K signaling pathway. J. Mol. Cell. Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBOJ. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Varady KA. Reliability of leptin, but not adiponectin: as a biomarker for diet-induced weight loss in humans. Nutr. Rev. 2011;69:145–154. doi: 10.1111/j.1753-4887.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr. J. 2012;11:98. doi: 10.1186/1475-2891-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- Lee S, Notterpek L. Dietary restriction supports peripheral nerve health by enhancing endogenous protein quality control mechanisms. Exp. Gerontol. 2013;48:1085–1090. doi: 10.1016/j.exger.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Herman JP, Mattson MP. Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp. Neurol. 2000;166:435–441. doi: 10.1006/exnr.2000.7512. [DOI] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003293. 124ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013;33:1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging. 2013;34:1564–1580. doi: 10.1016/j.neurobiolaging.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;4:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madorsky I, Opalach K, Waber A, Verrier JD, Solmo C, Foster T, Dunn WA, Jr, Notterpek L. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol. Dis. 2009;34:146–154. doi: 10.1016/j.nbd.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEBJ. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Erion JR, Santro T, Steyn FJ, Chen C, Arumugam TV, Stranahan AM. Intermittent fasting attenuates increases in neurogenesis after ischemia and reperfusion and improves recovery. J. Cereb. Blood Flow Metab. 2014;34:897–905. doi: 10.1038/jcbfm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Keller JN, Kruman I, Mattson MP. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997;756:205–214. doi: 10.1016/s0006-8993(97)00196-0. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Brenneman R, Golden E, Wood W, Prabhu V, Becker KG, Mattson MP, Maudsley S. Gonadal transcriptome alterations in response to dietary energy intake: sensing the reproductive environment. PLoS One. 2009;4(1):e4146. doi: 10.1371/journal.pone.0004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. Control laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res. Rev. 2015;20:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi M, Rezaie P, Chaudhri O, Karimi E, Nematy M. The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: a systematic review. Pak. J. Med. Sci. 2015;31:1250–1255. doi: 10.12669/pjms.315.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016;14:290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Ogawa Y, Ebihara K, Yamanaka M, Tsuchida A, Taiji M, Noguchi H, Nakao K. Anti-obesity and anti-diabetic effects of brain-derived neurotrophic factor in rodent models ofleptin resistance. Int. J. Obes. Relat. Metab. Disord. 2003;27:557–565. doi: 10.1038/sj.ijo.0802265. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Mattson MP. No benefit of dietary restriction on disease onset or progression in amyotrophic lateral sclerosis Cu/Zn-superoxide dismutase mutant mice. Brain Res. 1999;833:117–120. doi: 10.1016/s0006-8993(99)01471-7. [DOI] [PubMed] [Google Scholar]
- Plunet WT, Lam CK, Lee JH, Liu J, Tetzlaff W. Prophylactic dietary restriction may promote functional recovery and increase lifespan after spinal cord injury. Ann. N. Y. Acad. Sci. 2010;1198(Suppl. (1)):E1–E11. doi: 10.1111/j.1749-6632.2010.05564.x. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol. Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadhin C, Pillay B, Olaniran AO. Cell-based assays for IGF-I bioactivity measurement: overview: limitations and current trends. Growth Factors. 2014;32:130–138. doi: 10.3109/08977194.2014.939806. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Bass J. Circadian clocks in fuel harvesting and energy homeostasis. Cold Spring Harb. Symp. Quant. Biol. 2011;76:63–72. doi: 10.1101/sqb.2011.76.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzak RL, Abu-Hozaifa BM, Bamosa AO, Ali NM. Assessment of enhanced endothelium-dependent vasodilation by intermittent fasting in Wistar albino rats. Indian J. Physiol. Pharmacol. 2011;55:336–342. [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Rich NJ, Van Landingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J. Neurosci. Res. 2010;88:2933–2939. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- Rotermund C, Truckenmüller FM, Schell H, Kahle PJ. Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice. J. Neurochem. 2014;131:848–858. doi: 10.1111/jnc.12813. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein and carbohydrates. New Engl. J. Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu. Rev. Physiol. 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? a systematic review of clinical trials. Mol. Cell Endocrinol. 2015;1:153–172. doi: 10.1016/j.mce.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Sequea DA, Sharma N, Arias EB, Cartee GD. Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:1279–1285. doi: 10.1093/gerona/gls085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel I, Liu TL, Nepomuceno N, Gleicher N. Effects of short-term dietary restriction on survival of mammary ascites tumor-bearing rats. Cancer Invest. 1988;6:677–680. doi: 10.3109/07357908809078034. [DOI] [PubMed] [Google Scholar]
- Singh R, Manchanda S, Kaur T, Kumar S, Lakhanpal D, Lakhman SS, Kaur G. Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology. 2015;16:775–788. doi: 10.1007/s10522-015-9603-y. [DOI] [PubMed] [Google Scholar]
- Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance: and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J. Nutr. 2013;143:591–596. doi: 10.3945/jn.112.167593. [DOI] [PubMed] [Google Scholar]
- Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans M, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid or protein metabolism. Am. J. Clin. Nutr. 2009;90:1244–1251. doi: 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol. Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The other circadian system: food as aZeitgeber. J. Biol. Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Lee K, Mattson MP. Mineralocorticoid receptor activation restores medial perforant path LTP in diabetic rats. Synapse. 2010;64:528–532. doi: 10.1002/syn.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streijger F, Plunet WT, Plemel JR, Lam CK, Liu J, Tetzlaff W. Intermittent fasting in mice does not improve hindlimb motor performance after spinal cord injury. J. Neurotrauma. 2011;28:1051–1061. doi: 10.1089/neu.2010.1715. [DOI] [PubMed] [Google Scholar]
- Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women: prospective cohort study. BMJ. 2009;339:b3796. doi: 10.1136/bmj.b3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015;73:661–674. doi: 10.1093/nutrit/nuv041. [DOI] [PubMed] [Google Scholar]
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 2016;22:1–8. doi: 10.1080/17461391.2016.1223173. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Uno M, Honjoh S, Matsuda M, Hoshikawa H, Kishimoto S, Yamamoto T, Ebisuya M, Yamamoto T, Matsumoto K, Nishida E. A fasting-responsive signaling pathway that extends life span in C elegans. Cell Rep. 2013;3:79–91. doi: 10.1016/j.celrep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Varady KA, Roohk DJ, Loe YC, McEvoy-Hein BK, Hellerstein MK. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J. Lipid Res. 2007;48:2212–2219. doi: 10.1194/jlr.M700223-JLR200. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009a;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- Varady KA, Hudak CS, Hellerstein MK. Modified alternate-day fasting and cardioprotection: relation to adipose tissue dynamics and dietary fat intake. Metabolism. 2009b;58:803–811. doi: 10.1016/j.metabol.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J. Nutr. Biochem. 2010;21:188–195. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011;10:119. doi: 10.1186/1476-511X-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Dam VT, Klempel MC, Horne M, Cruz R, Kroeger CM, Santosa S. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci. Rep. 2015;5:7561. doi: 10.1038/srep07561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J. Neuroinflammation. 2014;11:85. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Kinoshita PF, Yshii LM, Marques Orellana AM, Böhmer AE, de Sá Lima L, Alves R, Andreotti DZ, Marcourakis T, Scavone C, Kawamoto EM. Effects of intermittent fasting on age-related changes on Na,K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol. Aging. 2015;36:1914–1923. doi: 10.1016/j.neurobiolaging.2015.02.020. [DOI] [PubMed] [Google Scholar]
- WCRF. (16-2014). Systematic review on diet, nutrition, physical activity and survival and second cancers in breast cancer survivors. 7-1-2016. Ref Type: Online Source. [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J. Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J. Neurochem. 2014;129:573–580. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’sdisease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wegman MP, Guo M, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation. Res. 2014 doi: 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Ye P, Peng X, Wu LL, Yu GY. Circulating adiponectin levels in various malignancies: an updated meta-analysis of 107 studies. Oncotarget. 2016 doi: 10.18632/oncotarget.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr. Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998;21:2–8. doi: 10.2337/diacare.21.1.2. [DOI] [PubMed] [Google Scholar]
- Wing RR, Phelan S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu. Rev. Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Yuen AW, Sander JW. Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav. 2014;33:110–114. doi: 10.1016/j.yebeh.2014.02.026. [DOI] [PubMed] [Google Scholar]