Abstract
Purpose
To evaluate ocular hypertension (OHT) after Ozurdex injection to determine the incidence of OHT, therapy of OHT, and any associative factors such as diagnosis, underlying glaucoma and therapy, or sequential Ozurdex injection(s).
Methods
Retrospective consecutive case series with patients receiving one or more intravitreal Ozurdex implantations at a tertiary care academic center. OHT was defined as a single measurement of ≥30 mmHg, or an increase of ≥10 mmHg from baseline.
Results
Ninety-four injections in 52 patients (59 eyes) were reviewed. Forty eyes received a single injection, and 19 eyes received multiple injections. OHT developed in 14 patients (26.9%). Thirteen patients (25%) had pre-existing glaucoma or glaucoma suspicion, and six of these developed OHT. Glaucoma eye drops were initiated following 13 injections (13.8%). Invasive glaucoma surgery was required in three patients (3.2%): all had glaucoma or glaucoma suspicion (one case was related to neovascular glaucoma and unlikely related to steroid response after Ozurdex). There was no difference in relative IOP increase (i.e. difference between final follow-up or subsequent intravitreal injection versus baseline) between single versus multiple Ozurdex injections (P = 0.883).
Conclusions
26.9% of patients who received Ozurdex developed OHT. Glaucoma or glaucoma suspicion factors were present in all patients who required invasive glaucoma surgery. A greater proportion of patients who received multiple injections had an IOP elevation, but the relative IOP increase was not significant.
Keywords: Dexamethasone, diabetic macular edema, glaucoma, intraocular pressure, intravitreal, ocular hypertension, Ozurdex, retinal vein occlusion, posterior uveitis
INTRODUCTION
The use of intravitreal corticosteroids for treatment of posterior segment diseases has increased significantly over the last decade.1,2,3,4,5 A commonly recognized complication of intravitreal steroids is ocular hypertension (OHT), which can occur immediately following the direct intraocular volume increase, or weeks to months later due to the steroid effects on aqueous drainage.6 Risk factors included pre-existing glaucoma, higher baseline intraocular pressure (IOP), younger age, OHT following previous injection, uveitis, and higher steroid dosage. Identification of secondary OHT following intravitreal steroid use is important, since OHT in most cases are initially asymptomatic, and can result in permanent vision loss if left untreated.
Dexamethasone implant (Ozurdex®, Allergan Inc, Irvine, CA, USA) is a free-floating device inserted into the vitreous cavity through a 23-gauge needle and releases corticosteroid for up to 6 months.7 The implant is bioerodible resulting in a gradual sustained-release of medication after the polymer undergoes hydrolysis to carbon dioxide and water, which results in complete dissolution of the device within the vitreous cavity.7,8 Animal studies show that a peak concentration of dexamethasone in the vitreous at 2 months is followed by a relatively rapid decline between 2 and 3 months.7 The IVT concentration then reaches a steady state through 6 months.9 The pharmacokinetics of 0.7 mg Ozurdex is similar in non-vitrectomized and vitrectomized eyes.10,11,12 The US Food and Drug Administration approved 0.7 mg Ozurdex for the treatment of macular edema following retinal vein occlusions (RVOs) in June 2009, for non-infectious posterior uveitis (NIPU) in September 2010, and for diabetic macular edema (DME) in September 2014.13,14
OHT was defined as an IOP ≥ 25 mm Hg or ≥10 mm Hg from baseline in previous large multicentered studies involving Ozurdex.1,2,3 Many clinical trials have exclusion criteria which may not accurately reflect the complexity of patients seen routinely in a rea-world clinical setting. For example, some of the exclusion criteria included: visual acuity worse than 20/ 200, history of vitrectomy surgery; use of corticosteroids (systemic, periocular, or intraocular) within 30 days of enrollment, moderate or severe glaucoma, poorly controlled hypertension, and poorly controlled diabetes.2
In this report, we evaluated the incidence of OHT following single and multiple Ozurdex injections. We investigated the occurrence in those with and without a glaucoma history, as well as the mean time of follow-up at which an elevated IOP may occur across various vitreoretinal diseases.
MATERIALS AND METHODS
This was a retrospective consecutive single-center study at a tertiary care academic center. After University of Iowa institutional review board approval was obtained, we evaluated all patients who underwent Ozurdex injection in one or both eyes between October 2009 and April 2014. Only those patients who had at least one month of follow-up were included. The final follow-up point was either a maximum of fourteen months, or the nearest time to subsequent Ozurdex injection.
Baseline clinical factors included clinical indication for injection, history of known primary or secondary glaucoma (e.g. primary open angle glaucoma, uveitic glaucoma, neovascular glaucoma, or angle closure glaucoma) or glaucoma suspicion (e.g. past history of OHT defined as a single pre-injection IOP measurement ≥23 mmHg, steroid-responsive IOP elevation, optic nerve asymmetry defined as a cup-to-disc ratio difference ≥0.3 and/or optic nerve cupping defined as a cup-to-disc ratio ≥0.6 in either eye; collectively defined as “glaucoma risk” for the remainder of the manuscript).
Outcome measures included the greatest difference of IOP between the baseline (time of Ozurdex injection) and at subsequent follow-up visit after Ozurdex injection; the proportion of patients with OHT at any point after an Ozurdex injection; and, the proportion of patients requiring medical or surgical intervention for OHT. Laser destructive intervention such as trabeculoplasty or diode laser cyclophotocoagulation were considered surgical intervention. The number of eyes with OHT was defined as either a single measurement of ≥30 mmHg, or an increase of ≥10 mmHg from baseline. IOP was obtained by Tonopen tonometry (Reichert Tono-Pen XL, USA) at each visit. The type of surgical glaucoma intervention was determined by the glaucoma specialist.
Descriptive statistics (frequencies and proportions for discrete variables) were used to determine patient characteristics and the occurrence of events, such as the need for IOP-lowering medical therapy or surgical procedures. Continuous descriptive statistics (mean, median, standard deviation [SD], range) were determined for measures of IOP (mmHg). The mean change in IOP from preoperative to postoperative visits for implant placement was analyzed using the two-tailed t test and Tukey’s multiple comparison test, where P < 0.05 was considered statistically significant. The distribution of eyes that experienced an IOP spike between different groups was analyzed using the two-tailed Fisher’s exact test, where P < 0.05 was considered statistically significant.
RESULTS
Demographics and Clinical Indications
A total of 105 Ozurdex implants were administered to eyes of 58 patients between October 2009 and April 2014. Demographic characteristics, including their clinical indication for Ozurdex injection, are listed in Table 1. Eight patients had bilateral disease, of which one patient had bilateral branch retinal vein occlusion (BRVO), three patients had bilateral DME, and four patients had bilateral NIPU. Thirteen patients (22%; 14 eyes) had a past history of glaucoma risk as defined previously.
TABLE 1.
Patient Demographic Characteristics
| Total Number of Ozurdex Injections | 105 injections | |
| Total Number Patients | 58 patients | |
| Number of Included Patients | 94 injections (52 patients, 60 eyes) | |
| Number of Excluded Injections | 11 injections (6 patients) | |
| Age (Years) | 63.9 | |
| Gender | 30 male / 26 female | |
| Laterality | 46 right eye / 59 left eye | |
| Clinical Indication for Ozurdex Injection | ||
| Branch Retinal Vein Occlusion | 27 injections, 14 patients, 15 eyes | |
| Central Retinal Vein Occlusion | 12 injections, 8 patients, 8 eyes | |
| Non-infectious Posterior Uveitis | 10 injections, 5 patients, 6 eyes | |
| Diabetic Macular Edema | 20 injections, 9 patients, 12 eyes | |
| Other* | 25 injections, 16 patients, 19 eyes | |
Ozurdex for cystoid macular edema following retinal detachment repair, epiretinal membrane peeling, or cataract surgery.
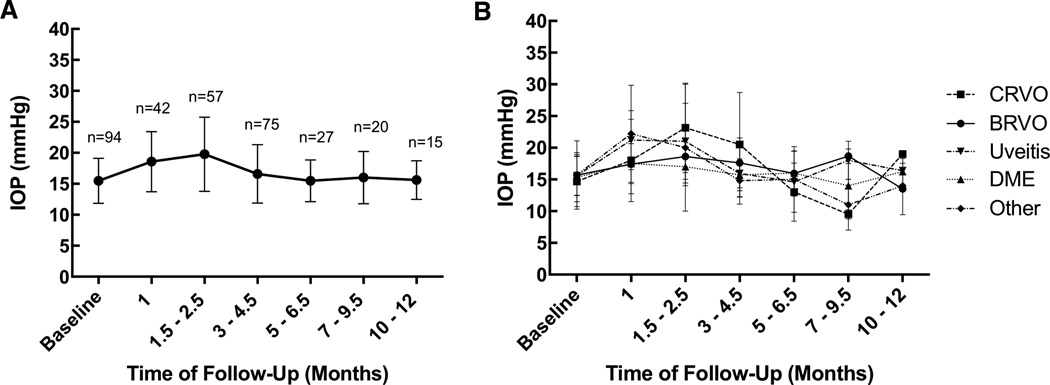
The average IOP across all patients who received Ozurdex injection were plotted against the various follow-up intervals (Figure 1A). The average change in IOP (between final follow-up and baseline) across all patients was an increase of 4.8 mmHg. The greatest IOP elevation was seen at the 1.5 to 2.5 month follow-up interval. The average IOP separated by various clinical indications show a general upward trend in the early post-injection follow-up time period, followed by a return to baseline (Figure 1B). There was no statistically significant difference between relative IOP increase from baseline among the various clinical indications for Ozurdex (Table 2).
FIGURE 1.
Average intraocular pressure (IOP) at baseline and subsequent follow-up relative to the time of initial injection among all patients (1A), and separated by clinical indication (1B).
TABLE 2.
Mean difference in IOP compared between various clinical indications (above), as well as statistical significant (below).
| Mean Difference in IOP | |||||
|---|---|---|---|---|---|
| BRVO | CRVO | DME | Uveitis | Other | |
| BRVO | −4.530 | 1.610 | −2.390 | −1.390 | |
| CRVO | −4.530 | 6.140 | 2.140 | 3.140 | |
| DME | 1.610 | 6.140 | −4.000 | −3.000 | |
| Uveitis | −2.390 | 2.140 | −4.000 | 1.000 | |
| Other | −1.390 | 3.140 | −3.000 | 1.000 | |
| Significance (Tukey’s Multiple Comparison Test; P<0.05 level) | |||||
| BRVO | CRVO | DME | Uveitis | Other | |
| BRVO | ns | ns | ns | ns | |
| CRVO | ns | ns | ns | ns | |
| DME | ns | * | ns | ns | |
| Uveitis | ns | ns | ns | ns | |
| Other | ns | ns | ns | ns | |
ns = not statistically significant (P > 0.05);
statistically significant (P < 0.05)
Fourteen patients (27 injections) received Ozurdex for BRVO, and two patients had glaucoma risk. There were five instances (four patients) of an IOP elevation ≥10 mmHg from baseline, of which one patient had glaucoma risk. There was one instance from one patient (with no history of glaucoma) who had a single IOP measurement of ≥30 mmHg at follow-up. Three patients were started on IOP-lowering medication, none of whom had glaucoma risk. No patients with BRVO required glaucoma surgery.
Nine patients (13 injections) received Ozurdex for CRVO, and three patients had glaucoma risk. There were two instances (two patients) who had an IOP elevation ≥10 mmHg from baseline; both had a history of glaucoma risk. There were three instances (two patients) who had a single IOP measurement of ≥30 mmHg; both had a history of glaucoma risk. Two patients, both with a history of glaucoma risk, were started on IOP-lowering medication. Two patients, both with a history of glaucoma risk, required glaucoma surgery: one patient had a history of steroid-induced OHT following intravitreal triamcinolone injections, who was treated with trabectome surgery; the second patient had a history of ischemic CRVO with neovascular glaucoma (less likely related to steroid response after Ozurdex) which was managed with a glaucoma drainage device.
Nine patients (20 injections) received Ozurdex for DME, and two patients had glaucoma risk. There was one patient who had a history of glaucoma risk who experienced an IOP elevation ≥10 mmHg from baseline. There were no instances of an IOP measurement of ≥30 mmHg. No patients were initiated on IOP-lowering medications, and no patients required glaucoma surgery.
Fourteen patients (24 injections) received Ozurdex for NIPU, three patients had glaucoma risk. There were five instances (four patients) who had an IOP elevation ≥10 mmHg from baseline, none had a history of glaucoma risk. There were four instances (two patients) who had a single IOP measurement of ≥30 mmHg at follow-up, and neither had a history of glaucoma risk. Four patients, none with a history of glaucoma risk, were initiated on IOP-lowering medication. No patients with NIPU required glaucoma surgery.
Six patients (10 injections) received Ozurdex for cystoid macular edema following retinal detachment repair, epiretinal membrane peeling, or cataract surgery, three patients had glaucoma risk. There were two instances (two patients) who had an IOP elevation ≥10 mmHg from baseline IOP, of which one patient had a history of glaucoma risk. There were two instances (two patients) who had a single IOP measurement of ≥30 mmHg at follow-up, of which one had a history of glaucoma risk. Two patients were started on IOP lowering medication, of which one had a history of glaucoma risk. One patient, who had a known history of primary open angle glaucoma and prior glaucoma drainage device, required invasive glaucoma surgery by way of trans-scleral cyclophotocoagulation.
History of Glaucoma or Glaucoma Suspicion
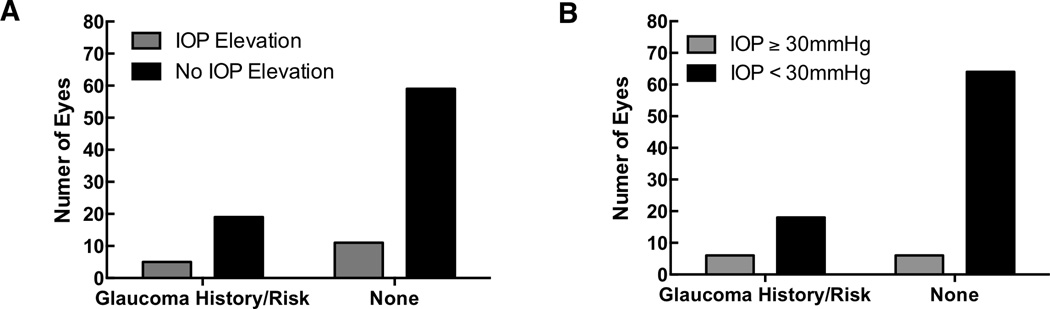
A total of 13 patients (22.4%, 14 eyes) had prior glaucoma or glaucoma suspicion (collectively “glaucoma risk”): one patient had primary open angle glaucoma, one patient had neovascular glaucoma from an ischemic CRVO, one patient had uveitic glaucoma (with a past history of trabeculectomy surgery three years prior), five patient had a history of steroid induced glaucoma, two patient was a glaucoma suspect based on optic nerve cupping, and three patients had a history of ocular hypertension. There were 14 instances (14.9%; 13 patients) with an IOP elevation of ≥10 mmHg from baseline, of which five patients (38.5%) had glaucoma risk (P = 0.5443, Figure 2A). There were 10 instances (10.6%; 7 patients) of an IOP elevation of 30 mmHg or greater, of which three patients (42.9%) had glaucoma risk (P = 0.0696, Figure 2B). Glaucoma eye drops were initiated following 13 injections (13.8%; 11 patients), and only two patients (18.2%) had glaucoma risk. Invasive glaucoma surgery was required following 3 injections (3.2%; 3 patients), of which all three patients had a history of glaucoma risk.
FIGURE 2.
Proportion of patients with and without glaucoma or glaucoma suspicion who experienced a relative increase in intraocular pressure of ≥10 mmHg (1A), and the proportion of patients with and without glaucoma or glaucoma suspicion who experienced a single IOP measurement of ≥30 mmHg after Ozurdex injection (1B).
Single versus Multiple Ozurdex Injections
A subset of 18 patients (34.6%; 54 injections in 19 eyes) received two or more (multiple) consecutive Ozurdex injections. Of these patients, 9 patients received two injections, 3 patients received 3 injections, 3 patients received four injections, and three patients received five injections. Among those who received multiple injections, the average time between injections of all patients was 8.4 months (or 252.6 days). When any outliers of 9 months or beyond between injections were excluded (i.e. six patients who had 11, 11, 12, 13, 20, and 48 months between injections), the average time was 5.4 months (or 162 days).
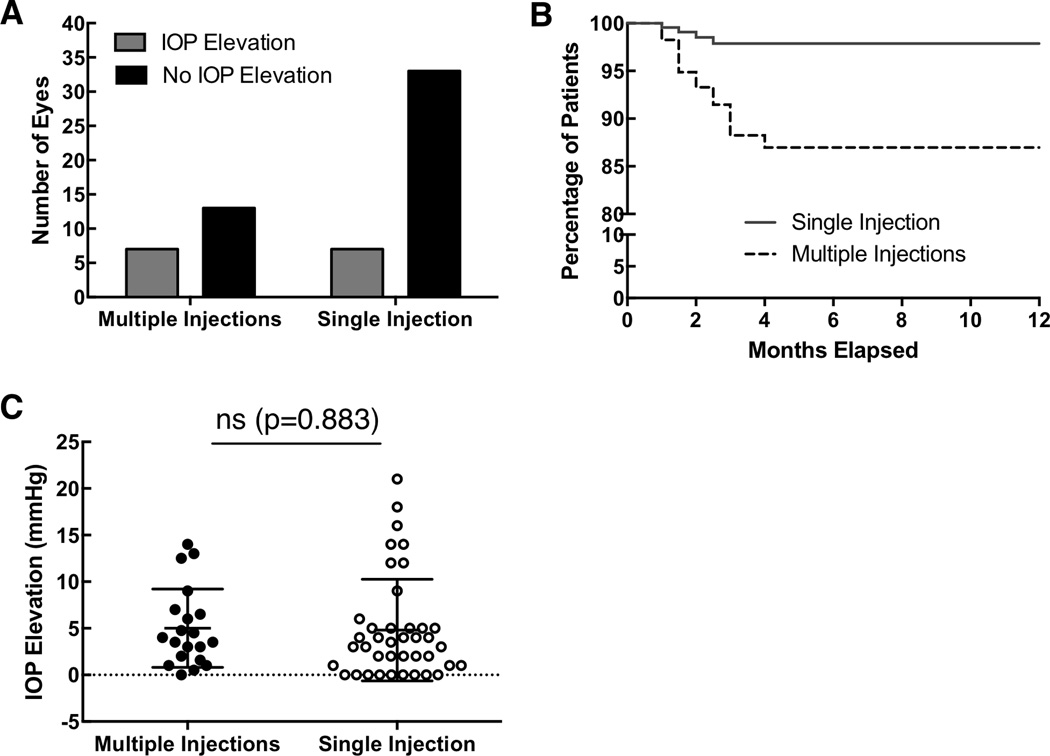
Seven out of 40 eyes (17.5%) who received a single Ozurdex injection experienced a relative IOP elevation of ≥10 mmHg from the time of the first Ozurdex injection. In contrast, seven out of 19 eyes (36.8%) who received multiple consecutive Ozurdex injections experienced a follow-up IOP elevation of ≥10 mmHg relative to the time immediately prior to the Ozurdex injection (Figure 3A). Three out of the seven of these eyes experienced OHT at the time of their initial injection, whereas the remainder were at the time of subsequent injection. The Kaplan-Meier survival curve between the two groups demonstrates that the greatest risk for IOP elevation is in the 1.5 to 2.5 month interval (Figure 3B). No long-term risk beyond four-month follow-up was appreciated. When mean IOP elevation (difference between maximum follow-up and the time of the first Ozurdex injection) was compared between single versus multiple Ozurdex injections (Figure 3C), no statistically significant difference was observed (P = 0.883).
FIGURE 3.
Comparison between patients who had single versus multiple Ozurdex injections and the number of eyes that experienced an intraocular pressure increase of ≥10 mmHg (3A). Kaplan-Meier survival curve comparing single versus multiple Ozurdex injections (3B). The mean IOP elevation (difference between maximum follow-up and baseline) was compared between single versus multiple Ozurdex injections (3C).
DISCUSSION
Common side effects of intraocular corticosteroids may include cataract and OHT. The latter is usually mild to moderate, but transient. The peak of IOP is between the 1.5 to 2.5 month interval, and we observed normalization in IOP at the 5 to 6.5 month follow-up. These findings were consistent with the pharmacokinetics in animal studies.10 Among those who had an increase in IOP, topical treatment was sufficient in a majority of cases. Only three patients required invasive glaucoma surgery, all of whom had glaucoma or glaucoma suspicion factors for which Ozurdex may have partly contributed to the need for more advanced glaucoma management. The patient who required a glaucoma drainage device had a history of ischemic CRVO and neovascularization of the iris. This patient’s elevated IOP is likely multifactorial and may have been partly or wholly-related to the underlying diagnosis which was also treated by panretinal photocoagulation. A second patient who required trabectome surgery received one prior Ozurdex injection for non-ischemic CRVO, but had a known history of steroid-induced OHT. And lastly, a third patient who received transcleral cyclophotocoagulation laser one month following their first Ozurdex injection had a known history of primary open angle glaucoma and prior glaucoma surgery one year prior to Ozurdex injection.
Some studies define “IOP elevation” as an increase of at least 5 mmHg from baseline at follow-up, or an isolated measurement of more than 25 mmHg. We used four commonly used parameters to more precisely capture such adverse events: IOP elevation ≥10 mmHg from baseline IOP, ≥30 mmHg at any visit, the initiation of IOP lowering drops, and the number of patients requiring incisional glaucoma surgery. The percentages for these in our study were 14.9%, 10.6%, 13.8%, and 3.2%, respectively. These subset groups are not mutually exclusive, and the OHT adverse event occurred in a total of 14 patients or 26.9% when combined. Caution should be made in comparing percentages across studies, as we have observed varying definitions of IOP-related adverse events. Additionally, many larger randomized clinical trials had exclusion criteria that did not allow inclusion of known glaucoma or OHT subjects.
We initially hypothesized that patients with NIPU may be more prone to IOP elevations, since uveitis alone can cause elevated IOP. Interestingly, there was no statistically significant difference in change in IOP across all clinical indications (Figure 1B, Table 2). We looked more specifically at the 1.5 to 2.5 month interval where patients may be most prone to IOP elevations, but again did not find a significant difference. We suspect that this may relate to the slow controlled-release of dexamethasone. One retrospective study evaluating Ozurdex for uveitis found 7 instances of increased IOP (defined as >21 mmHg), out of 38 eyes and 61 injections, all of which responded to pharmacologic treatment, and none required incisional or laser surgery even if they were previously diagnosed as steroid responders.15 In a large three-year randomized study evaluating Ozurdex for DME, 41.5% (among 347 patients) who received the 0.7 mg Ozurdex injection required IOP lowering medication and 5 patients (1.5%) required glaucoma surgery.13 Our results, albeit smaller in numbers and retrospective, had no instances of elevated IOP following Ozurdex injections for twenty cases of DME.
In our report, there was no difference in IOP adverse events between those who received one compared to multiple Ozurdex injections. The Kaplan-Meier curve suggests that the greatest likelihood of having an IOP elevation is consistent with the greatest IOP measurements in the 1.5 to 2.5 month interval. We had several patients who received two or more injections (maximum five), and we did not observe a trend or bias towards IOP elevations with greater number of Ozurdex injections. To support this, three of the seven patients who had both OHT and multiple Ozurdex injections, had the OHT event following their first Ozurdex injection. The remainder four patients experienced OHT at the time of subsequent Ozurdex injections. This is consistent with the recent three-year results of Ozurdex for DME, where the incidence of IOP adverse events did not increase after subsequent treatments or in year 2 or 3, and the proportion of patients using IOP-lowering medications in the study eye remained similar from year to year.13 The results indicated that were was no cumulative effect of Ozurdex on IOP which our data supports. For comparison, one case series of twelve patients (15 eyes) found three instances of elevated IOP that were all responsive to topical therapy alone (one after the first, one after the second, and one after the third intravitreal injection).16
Differences in pharmacologic and pharmacokinetic profiles of the available intravitreal steroid treatments may account for their differing safety profiles with regard to OHT. Dexamethasone, fluocinolone acetonide, and triamcinolone have been shown to activate different patterns of gene expression in human trabecular meshwork cell lines.17 Dexamethasone differs from triamcinolone in pharmacologic activity, lipid solubility, and delivery requirements. Dexamethasone is less lipophilic and does not accumulate to the same extent in the trabecular meshwork, and therefore may have lower risk of IOP increases.18,19 Our findings suggest that Ozurdex may provide a higher benefit-risk ratio than the fluocinolone implant with regard to lower incidence of IOP elevation requiring surgery20,21,22; however, head-to-head prospective clinical studies would be necessary to directly compare the safety and efficacy of the Ozurdex implant with other intravitreal steroid modalities.
This study has limitations inherent in its uncontrolled and retrospective nature. Selection bias may affect our results because of a treatment bias against Ozurdex implantation in patients with established glaucoma or glaucoma suspicion. The long-term prognosis of those patients who developed isolated IOP increases is not known and they should be followed closely for the possibility glaucoma development.23
Summary statement.
26.9% patients who received a single or multiple Ozurdex injections developed ocular hypertension. Glaucoma or glaucoma suspicion factors were present in all patients who required invasive glaucoma surgery. A greater proportion of patients who received multiple injections had an IOP elevation, but the relative IOP increase was not significant.
Acknowledgments
Funding/Support: Unrestricted grant from the Research to Prevent Blindness; GV is supported by T32GM007337. The funding organization had no role in the study.
Footnotes
Statement: None of the authors have any proprietary interests.
REFERENCES
- 1.Haller JA, Dugel P, Weinberg DV, et al. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29:46–51. doi: 10.1097/IAE.0b013e318188c814. [DOI] [PubMed] [Google Scholar]
- 2.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 3.Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Zarranz-Ventura J, Carreno E, Johnston RL, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014;158:1136–1145. doi: 10.1016/j.ajo.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema. Ophthalmology. 2014;121:2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SR, Isa H, Joshi L, et al. New developments in corticosteroid therapy for uveitis. Ophthalmologica. 2010;224(Suppl 1):46–53. doi: 10.1159/000318021. [DOI] [PubMed] [Google Scholar]
- 7.Kiddee W, Trope GE, Sheng L, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013;58:291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Ciulla TA, Walker JD, Fong DS, et al. Corticosteroids in posterior segment disease: An update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004;15:211–220. doi: 10.1097/01.icu.0000120711.35941.76. [DOI] [PubMed] [Google Scholar]
- 9.Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 10.Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52:4605–4609. doi: 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros MD, Alkabes M, Navarro R, et al. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2013;30:709–716. doi: 10.1089/jop.2014.0010. [DOI] [PubMed] [Google Scholar]
- 12.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 13.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28:351–366. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 14.Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Tomkins-Netzer O, Taylor SR, Bar A, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121:1649–1654. doi: 10.1016/j.ophtha.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Scaramuzzi M, Querques G, Spina C, Lattanzio R, Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex) for diabetic macular edema. Retina. 2015 Jun;35(6):1216–1222. doi: 10.1097/IAE.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 17.Nehmé A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structuers but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58. doi: 10.1186/1755-8794-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman JL. Differentiated intraocular glucocorticoids. Ophthalmologica. 2010;224(suppl):25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 19.Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129:914–920. doi: 10.1001/archophthalmol.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125:1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]
- 23.Kitazawa Y, Horie T. The prognosis of corticosteroid responsive individuals. Arch Ophthalmol. 1981;99:819–823. doi: 10.1001/archopht.1981.03930010819005. [DOI] [PubMed] [Google Scholar]