Abstract
The worldwide prevalence of stroke continues to rise despite recent successes in treating acute ischemic stroke. With limited patient eligibility and associated risk of tPA and mechanical thrombectomy, new preventive and therapeutic modalities are needed to stave the rising wave of stroke. Inflammation plays a key role in brain damage after cerebral ischemia, and novel therapies that target pro-inflammatory cells have demonstrated promise for treatment for stroke. Partial MHC class II constructs have been shown to prevent and/or reverse clinical signs of various inflammatory diseases such as experimental autoimmune encephalomyelitis, collagen-induced arthritis and experimental autoimmune uveitis, by reducing the number and frequency of activated cells in the damaged CNS. Herein, we review the use of partial MHC class II constructs as a novel treatment for ischemic stroke. These constructs have been shown to reduce infarct volume and neurological deficit in various cerebral ischemia models in young adult and aging male and female mice. In addition, partial MHC class II constructs were shown to reverse stroke-associated splenic atrophy and promote a protective M2 macrophage/microglia phenotype in the CNS which contributes to tissue repair and recovery after stroke. By addressing remaining STAIR criteria, such as efficacy in large animal models of stroke, these constructs will be prime candidates for clinical trials of acute ischemic stroke.
Keywords: Stroke, Inflammation, Immunotherapy, Recombinant T-cell receptor Ligand, partial MHC class II construct
Introduction
Ischemic stroke is a leading cause of death and disability worldwide (Donnan et al., 2008; Lindsay et al., 2014). With the growing aging population, it is anticipated that nearly 4% of the US population will be affected by stroke by 2030 (Ovbiagele et al., 2013); thus the urgent need for novel stroke therapies. It has been well established that inflammation plays a major role in the onset and progression of stroke (Campanella et al., 2002; Chamorro et al., 2012; Elkind et al., 2004; Offner et al., 2006a; Pennypacker and Offner, 2015; Urra et al., 2009). Ischemic central nervous system (CNS) injury is characterized by rapid activation of microglia and time dependent infiltration of activated peripheral immune cells into the affected brain tissue. Neutrophils that were shown to be among the first cells to infiltrate into the CNS are followed by blood-derived macrophages, as well as T and B cells, all of which contribute to the ischemic damage through localized inflammation (Akopov et al., 1996; Gelderblom et al., 2009; Jin et al., 2010; Offner et al., 2006a; Petrovic-Djergovic et al., 2016; Urra et al., 2009; Zhou et al., 2013). T cells which are found in the CNS within hours after experimental stroke perpetuate inflammatory reaction and contribute to increased neuronal damage (Jander et al., 1995; Seifert et al., 2012b). Both T and B cell deficient mice sustain smaller infarcts size after experimental cerebral ischemia (Hurn et al., 2007). Interestingly, in the experimental stroke model of middle cerebral artery occlusion (MCAO) this vast activation of inflammatory cells is followed by immune-suppression that is marked by atrophy of the spleen and thymus (Offner et al., 2006b; Offner et al., 2009; Pennypacker and Offner, 2015; Seifert et al., 2012a). While pro-inflammatory immune cells were shown to be harmful, alternatively activated macrophages and microglia (M2) are instrumental in regulation of inflammation and tissue repair (Gordon, 2003; Miron and Franklin, 2014). Thus, for any immune interventional therapy to be effective, a critical balance needs to achieved between the inhibition of infiltration of pro-inflammatory cells into the CNS and the enhancement of protective M2 cells in the brain.
Currently, the only FDA approved drug for ischemic stroke is tissue plasminogen activator (tPA). However, tPA must be administered within 4.5 hours after the first signs of stroke appear in order to be beneficial (Albers et al., 2000; Lees et al., 2010). Thus, there is an unmet need for developing additional immune-regulating drugs for acute ischemic stroke that meet the Stroke Academic Industry Roundtable (STAIR) criteria. According to these criteria, an optimal therapy should exhibit the following characteristics: 1. Efficacy in aged animals and animals with co-morbidity. 2. Efficacy in both male and female animals. 3. Compatible interaction with tPA. 4. Biomarker endpoints (MRI, serum markers) and 5. Reproducibility in at least one independent laboratory (Albers et al., 2000).
One such potential therapy for ischemic stroke is partial major histocompatibility complex (MHC) class II molecules (pMHC, also known as Recombinant T-cell receptor ligand (RTL). These molecular constructs consist of the α1 and β1 domains of MHC class II molecules expressed as a single polypeptide with or without antigenic amino or carboxy terminal extensions (Burrows et al., 1999; Vandenbark et al., 2003). These molecules were initially designed and developed to regulate T-cell responses and inhibit clinical signs of experimental autoimmune encephalomyelitis (EAE) (Offner et al., 2011; Sinha et al., 2010; Sinha et al., 2007; Wang et al., 2006). However, unlike MS, the involvement of brain antigen specific T cells in stroke was not so widely studied.
Becker et al. demonstrated that adoptive transfer of myelin basic protein (MBP) tolerized splenocytes reduced the infarct size when administered 3 hours after MCAO (Becker et al., 2003). This approach is not inconsistent with our demonstration that transfer of non-tolerized myelin specific cells can infiltrate MCAO lesions and exacerbate stroke severity. In our studies, myelin oligodendrocyte glycoprotein (MOG)-specific splenocytes that were transferred into severe combined immunodeficient (SCID) mice migrated into the lesioned hemisphere of the SCID recipient mice which were subjected to 60 min MCAO and 96 hrs of reperfusion (Ren et al., 2012). Recently, several studies suggested that the pathogenic role of T cells in ischemic stroke is not limited to a rapid response and could be also mediated by neuroantigen specific T cells (Klehmet et al., 2016; Miro-Mur et al., 2016; Urra et al., 2014). Moreover, breakdown of the blood brain barrier (BBB) is likely to promote leakage of neuroantigens. Planas et al. reported that increased immunoreactivity to myelin-derived antigen in the periphery was associated with larger infarctions on brain imaging, and worse outcome at clinical follow-up (Planas et al., 2012).
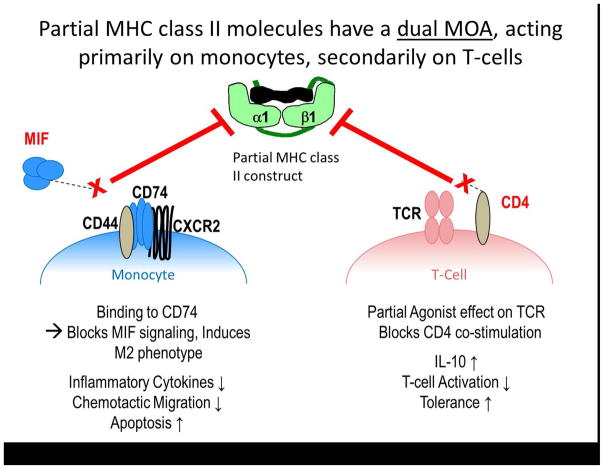
These data suggested that regulating myelin specific T cells by pMHC constructs might improve the outcome of stroke. One key inhibitory activity of the pMHC requires binding to the invariant chain of MHC class II (CD74) and downregulation of its expression on the monocyte cell surface (Vandenbark et al., 2013). We further demonstrated that this binding to CD74 not only modulates cell surface expression, but also blocks the binding of macrophage migration inhibitory factor (MIF), for which CD74 serves as the major receptor (Benedek et al., 2013). Taken together, these finding suggest a dual mechanism of action of the pMHC constructs that appears to primarily affect antigen presenting cells and secondarily T cells, as illustrated in Figure 1. Herein, we will review the involvement of CD74 and MIF in ischemic stroke and the development of pMHC constructs as a novel therapeutic approach for ischemic stroke that meets the STAIR criteria.
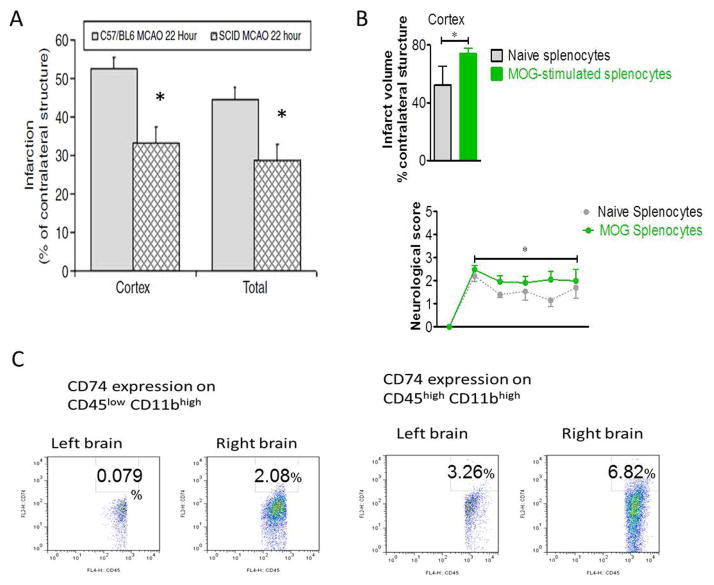
Figure 1. Rationale for the use of partial MHC class II constructs for treating stroke.
A) The reduction of infarct volumes in SCID mice compared to WT C57BL/6 mice implicates the involvement of the immune system in the outcome of stroke. Reprinted from J Cereb Blood Flow Metab. DOI: 10.1038/sj.jcbfm.9600482 http://jcb.sagepub.com/content/27/11/1798.full. With permission from SAGE Journals. B) Myelin specific cells exacerbate stroke severity in SCID mice after 60min MCAO, 96 hrs reperfusion. Reprinted from Metabolic Brain Disease. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. 27(1):7–15, 2011, Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS, Offner H. © Springer Science+Business Media, LLC 2011. With permission of Springer. C) The expression of the partial MHC class II receptor, CD74, is up regulated on microglia and infiltrating macrophages in the ischemic hemisphere 96 hrs after reperfusion (* p<0.05).
CD74 and MIF in ischemic stroke
CD74 is a type II transmembrane protein with a dormant transcription factor in the intracytoplasmic domain, a transmembrane region and an extracellular domain thought to be the receptor for MIF binding (Leng et al., 2003). Intracellular CD74 has an essential role in the transport of newly synthesized MHC class II proteins from the endoplasmic reticulum through the Golgi to the cell surface of APCs (Cresswell, 1996). It was reported that 2–5% of the cellular CD74 is expressed on the cell surface independently of MHC class II (Wraight et al., 1990). CD74 was shown to have an accessory role in immune cell stimulation (Shi et al., 2006). CD74, in combination with CD44 and CXCR2/4/7, has been reported to transduce signaling by MIF, one of the first cytokine mediators that was described (Leng et al., 2003; Naujokas et al., 1993; Shi et al., 2006). The role of cell surface CD74 that could be expressed in the ischemic brain by activated microglia or infiltrating macrophages was not well studied. CD74 was found to be up-regulated on both CD11b+CD45int and CD11b+CD45hi cells in the ischemic hemisphere compared with the non-ischemic hemisphere after MCAO (Unpublished data from our laboratory). We and others demonstrated that CD74 is upregulated on antigen presenting cells (APC) in inflammatory sites, including spinal cord during the acute phase of EAE (Benedek et al., 2013; Herrero et al., 2013; Tan et al., 2014; Wang et al., 2014).
Macrophage migration inhibitory factor (MIF) is a 115 amino acid residue protein that is widely expressed in leukocytes, corticotrophic pituitary cells, epithelial and endothelial cells, and neurons (Bernhagen et al., 1993; Bernhagen et al., 2007; Bernhagen et al., 1994; Bucala, 2013; Calandra et al., 1995; Calandra et al., 1994; Calandra et al., 2003; Schwartz et al., 2009). MIF heads a distinct structural superfamily and exhibits a unique biology that includes cytokine, hormone, chemokine and enzymatic activity that contributes centrally to numerous autoimmune diseases and cancer (Bucala, 2013; O’Reilly et al., 2016). The MIF activation pathway has been well described, with an initial high affinity binding to the CD74/CD44 receptor complex that results in sustained activation of ERK1/2 MAPK (Leng et al., 2003; Shi et al., 2006) and ultimately gene transcription and synthesis of pro-inflammatory factors, increased cell survival and proliferation and trafficking to the sites of acute and chronic inflammation (Gore et al., 2008; Mitchell et al., 2002).
Although MIF was one of the first pro-inflammatory factors to be described, its role in ischemic stroke remains controversial. MIF expression was reported to be elevated in the periphery and around the infarct core in the brain after induction of experimental stroke in rodents (Inacio et al., 2011c; Wang et al., 2009). In addition, MIF plasma levels were found to be elevated in patients within few days after stroke and these levels were correlated with neurological deficits (Wang et al., 2009). Inacio et al. reported that male MIF knockout (KO) mice had reduced infarct volumes compared to C57BL/6 WT mice after 45 min of MCAO. Moreover, male MIF KO mice performed better in sensory-motor tests after MCAO (Inacio et al., 2011b). It was also suggested that increased expression of MIF by reactive astrocytes is associated with sensory-motor deficits (Inacio et al., 2011c). However, MIF deficiency did not affect the Th1/Th2 balance in mouse brain after 45 min of MCAO compared with WT mice (Inacio et al., 2011a). On the other hand, Turtzo et al. reported that female MIF KO mice exhibited larger infarct volumes compared to female WT mice, whereas there was no such difference in male mice. In addition to cell surface receptors, MIF was shown to interact with intracellular Jun activation domain-binding protein (JAB1). It was postulated that after stroke, the absence of MIF leads to activation of JAB1, which in turn enters the mitochondria resulting in increased release of cytochrome c and cell apoptosis (Turtzo et al., 2013).
It has been proposed that different occlusion times might explain why similar effects were not observed in male vs. female mice. MIF has a deleterious role after 45 min of occlusion that results in milder infarcts in male MIF KO mice, but a protective role in females after 90 min of occlusion which results in more severe damage in MIF KO mice (Turtzo et al., 2013). This contrasting role of MIF was similarly observed in several EAE studies in which disease induction with reduced adjuvant concentration or reactivity resulted in milder disease severity and partial recovery compared to WT mice. In contrast, in severe EAE no difference was observed between MIF KO mice and WT mice (Cox et al., 2013; Ji et al., 2015). It can be speculated that as with many other inflammatory factors, MIF is involved both in the inflammatory damage and the subsequent tissue repair phases of stroke.
Treating ischemic stroke with partial MHC class II constructs
Partial MHC class II constructs were shown previously to prevent and/or reverse clinical signs of EAE, collagen-induced arthritis and experimental autoimmune uveitis, through reducing the number and frequency of activated cells in the affected tissues, downregulation of endothelial cell adhesion molecules and reduction of CNS chemokines/receptors (Adamus et al., 2012; Adamus et al., 2006; Huan et al., 2008; Sinha et al., 2010; Sinha et al., 2007). Based on these findings, a pMHC construct (RTL551, mouse I-Ab α1β1 domains linked to mouse MOG-35-55 peptide) was used to treat experimental stroke in C57BL/6 mice. Treatment of these mice starting 3 hrs after 60 minutes occlusion, followed by 3 additional treatments at 24, 48 and 72 hrs post-reperfusion resulted in a significant reduction of infarct size volume compared with vehicle treated mice. This treatment reduced the recovery of total brain mononuclear cells in the ischemic hemisphere by 64%, with the largest effect on CD11b+CD45hi cells. In addition, RTL551 significantly reversed the stroke-induced splenic atrophy without changing the distribution of cell subtypes in the periphery (Akiyoshi et al., 2011; Dziennis et al., 2011; Subramanian et al., 2009).
In a recent series of studies, it was demonstrated that pMHC constructs bind to human and mouse monocytes through cell surface CD74 and that this interaction inhibits MIF binding and signaling (Benedek et al., 2013; Vandenbark et al., 2013). In order to test a humanized partial MHC class II construct, the RTL1000 (comprised of covalently linked HLA-DR2 β1 and α1domains tethered to the human (h)MOG-35-55 peptide) (Yadav et al., 2012), MCAO was induced in DR*1502-transgenic (Tg) mice. Similar results of reduction in infarct volume, frequency of activated mononuclear cells in the ischemic hemisphere and splenic atrophy were observed both in male and female mice (Figure 2)(Akiyoshi et al., 2011; Subramanian et al., 2009; Zhu et al., 2015a). Evaluation of long-term neurobehavioral functional outcomes demonstrated that RTL1000 treatment improved body weight recovery and cognitive performance compared to vehicle treated mice 4 weeks after stroke. However, only a modest trend of improvement effect was observed in sensorimotor deficit recovery (Zhu et al., 2015a). Interestingly, it was demonstrated that in C57BL/6J mice that were exposed to two different methamphetamine treatment regimens (using repeated doses of 4 mg/kg or 10 mg/kg, s.c.) and immunotherapy with RTL551 improved the memory impairments assessed by using the Morris water maze, demonstrating an improved cognitive performance in a different model that involves behavioral deficits (Loftis et al., 2013).
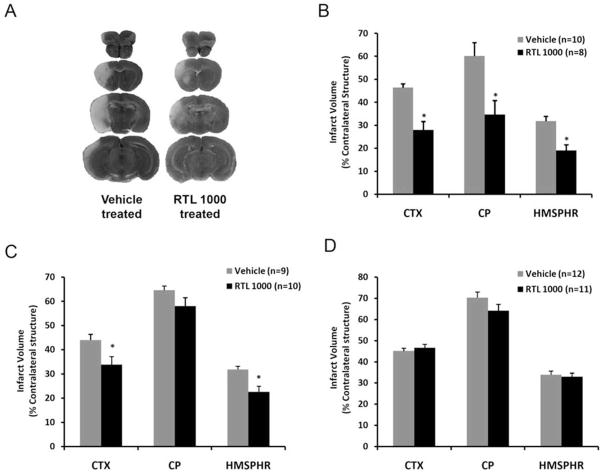
Figure 2. Therapeutic time window of RTL1000 for protection from stroke in DR2-Tg mice.
Male DR2-Tg mice were subjected to 60-min transient MCAO and treated with 100 μg RTL1000 or 100 μl vehicle given subcutaneously (S.C.) starting at 4hr (A, B), 6hr (C) or 8hr (D) after MCAO, followed by additional injections at 24, 48 and 72 hr after MCAO. Brains were harvested 96 hrs after MCAO, brain slices were stained with TTC and infarct volumes measured and expressed as a percentage of the contralateral region. Panel A shows the four 2-mm TTC-stained coronal brain sections that cover the entire MCAO lesion in RTL1000- and vehicle-treated animals. * p<0.05 compared to vehicle group. Data are represented as mean ± SEM. Differences in infarct size were determined by 2-way ANOVA, followed by Holm-Sidak post hoc test, with the two factors being brain region and treatment group. CTX: cortex; CP: striatum; HMSPHR: hemisphere. Reprinted in part from Translational Stroke Research. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. 6(1):60–8. 2015. Zhu W, Casper A, Libal NL, Murphy SJ, Bodhankar S, Offner H, Alkayed NJ. © Springer Science+Business Media New York 2014. With permission of Springer.
Partial MHC class II molecules and t-PA
t-PA is serine protease that induces intravascular thrombolysis by converting plasminogen to plasmin. t-PA is the only FDA approved therapy for ischemic stroke. However, it must be administrated within 4.5 hrs after the appearance of the first clinical signs of stroke and the rate of t-PA use in the clinic remains at less than 4% (Albers et al., 2000; Lees et al., 2010). The use of t-PA in combination with new drugs was recommended by the STAIR committee to induce a wide neuroprotective effect with thrombolysis. Thus, RTL1000 was tested in combination with t-PA in treating ischemic stroke. Infarct volume evaluated 24 or 96 hrs after reperfusion indicated that RTL1000 protected against ischemic stroke in DR2-Tg mice as was observed previously (Zhu et al., 2014). The benefit from t-PA was observed at 24 but not 96 hrs after stroke. Moreover, there was no added benefit of t-PA plus RTL1000 over RTL1000 alone (Zhu et al., 2014). Evaluation of the RTL1000 therapeutic window in DR*1502-Tg mice showed that there was still significant infarct size reduction in the cortex 4 or 6 hours after MCAO treatment, but not after 8 hours of occlusion (Fig. 2). Although this time window still needs to be translated into clinical trials, it is possible that the time window for treating stroke subjects can be extended beyond 4.5 hr by treatment with partial MHC class II constructs. In addition to CD74+ APCs, RTL1000 was shown to bind to platelets, thus providing a level of redundancy since platelets were also reported to partner with leukocytes to amplify the immune response (Itakura et al., 2010). In the context of ischemic stroke, it was demonstrated by Itakura et al. that RTL1000 downregulates platelet aggregation to collagen and to inhibit occlusive thrombus formation (Itakura et al., 2010). Hence, in a combined therapy approach, partial MHC class II might also prevent platelet re-aggregation after thrombolysis by t-PA.
Treating a thromboembolic stroke with partial MHC class II constructs
Several stroke models have been developed in rodents to mimic ischemic stroke in humans. The mouse embolic stroke model best mimics the most common cause of human stroke and yet remains unreliable. Embolic strokes induced by macrospheres, microspheres or autologous clots injected into the brain continue to be infrequently employed due to the high mortality rate, variability of infarct and unsuccessful induction of stroke (Canazza et al., 2014; Howells et al., 2010). One example is the mouse model of in situ thromboembolic stroke, in which an autologous thrombus is directly induced inside the MCA by craniotomy, penetration of dura mater and local microinjection of purified thrombin. Although this procedure results in a stable and reproducible infarct volume with low mortality rate, it may alter intracranial pressure (ICP) (Ansar et al., 2014; Orset et al., 2007). We have recently described a thromboembolic mouse model in C57BL/6 and DR2 male mice that successfully occludes the middle cerebral artery by introducing thrombin through an intraluminal catheter, without the need for craniotomy and without affecting ICP. The new model yields reproducible ischemic lesions, reduces surgery time and better mimics clinical thromboembolic stroke (Chen et al., 2015).
We further demonstrated that thromboembolic mouse model involves activated monocytes/microglia and neutrophils in the ischemic brain, likely caused by an increased frequency of these cells in the spleen. Additionally, treatment of thromboembolic mice with RTL1000 significantly reduced total infarct volume similar to its treatment of MCAO, thus providing additional proof-of-principal therapeutic benefit in a model that better mimics human stroke pathogenesis (Dotson et al., 2016).
Treating stroke in middle-aged and old mice with partial MHC class II constructs
The steady rise in stroke rates worldwide is alarming, and curbing the stroke epidemic requires high efficacy therapy with wider therapeutic window. Unfortunately, over 250 clinical trials and over 1000 neuroprotectant molecules, which were successful in preclinical studies, eventually failed to translate into successful therapies for humans (O’Collins et al., 2006). One of the potential factors that led to this poor outcome was the tendency to use young animals in preclinical studies. Despite the increase in risk of stroke with age, there is underrepresentation of older animals in such research. It is well documented that the immune response changes with age (Deleidi et al., 2015; Goronzy et al., 2015). As an example of such changes that could affect stroke outcome is the balance between M1 and M2 macrophages (Lee et al., 2013). Dependent on the type of stimulation, activated macrophages secrete specific patterns of cytokines and express different surface markers. There are at least two opposing activation states: The classically activated macrophages (M1) express high levels of CD86, CD80 and MHC class II on their cell surface and are very potent in priming T cells and recruiting them to the CNS. On the other side of the activation spectrum, alternatively activated macrophages (M2) express high levels of CD206, CD163, arginase1 and low levels of CD40, CD86 and MHC class II. M2 cells are involved in inhibiting inflammation and tissue repair. It was shown that the switch from M1 to M2 is delayed with ageing (Gordon, 2003; Lee et al., 2013; Miron and Franklin, 2014). Various studies demonstrated the beneficial role of M2 cells in protection against stoke and in the recovery stage (Brifault et al., 2015; Li et al., 2016; Liu et al., 2016). It was reported that M2 microglia/macrophages infiltrated the ischemic core at 24 hrs, peaking at 5 days, and declining in the striatum by 14 days. The expression of CD206+ cells in the cortex at the border zone of ischemia peaked at day 5 after ischemia before being outnumbered by M1 cells (Hu et al., 2012). Furthermore, additional differences were reported in peripheral immune cell frequencies after ischemic stroke in young vs. aged mice. T cells and monocytes are significantly reduced in older mice compared to young mice after MCAO, whereas, CD19+ cell frequency is elevated in older mice (Dotson et al., 2014).
To this end, RTL1000 was evaluated in treating MCAO in middle aged (12 months) and older (16 months) mice. These mice exhibited a significant reduction in infarct volume and inflammatory cells in the brain compared with vehicle-treated mice. RTL1000 treatment also reduced the high mortality that is often observed in aged mice after ischemic stroke. Although the outcome of RTL1000 treatment was the same as in young mice, since the immune response after ischemic stroke differs between young and old mice, it is possible that RTL1000 affects different immunological mechanisms. We hypothesize that in old mice RTL1000 treatment might induce the migration of regulatory CD8+ cells (CD8+CD122+IL-10+) into the CNS, whereas in RTL1000-treated young mice these cells are retained in the periphery. Our studies suggest that age-dependent differences in the immune response after ischemic stroke results in pMHC protection from experimental stoke through peripheral-based immune regulation in young mice and inflammatory tissue-specific protection in older mice (Dotson et al., 2014; Zhu et al., 2015b). These studies address the interaction of stroke therapy with age, one of the important factors that contribute to the likelihood of a success in clinical trials.
The next generation of partial MHC class II constructs
The RTL1000 pMHC class II construct containing the DR2 α1β1 domains was initially developed as a therapy for multiple sclerosis (MS), in which HLA-DR2 is the most prevalent allele. However, treatment with RTL1000 would currently require HLA-DR2 screening. Recently, we found that the HLA-DRα1 domain directly binds to and downregulates cell surface expression of the MHC class II invariant chain (CD74) on CD11b+ monocytes, inhibits binding of MIF to CD74 and blocks downstream inflammatory effects in the CNS (Meza-Romero et al., 2014). We have further demonstrated that the potency of the DRα1 domain could be enhanced by addition of a peptide extension (MOG-35-55 peptide). Moreover, because the DRα1 domain is present in all humans and thus would not be recognized as foreign, treatment using DRα1 constructs does not require HLA screening of potential recipients and can be used for treatment of CNS diseases, including ischemic stroke.
We demonstrated that DRα1-MOG-35-55 reduces infarct size and reverses splenic atrophy after stroke when administered at the clinically relevant time-point of 4 hrs after stroke. The neuroprotective effects of DRα1-MOG-35-55 are mediated in part by reduced expression of the CD74, MIF cell surface receptor and inhibition of migration of CD11b+ monocytes into the ischemic brain (Fig. 3). Four daily treatments with DRα1-MOG-35-55 reduced cortical, striatal and total hemispheric infarct by 40% (Benedek et al., 2014). Interestingly, DRα1-MOG-35-55 treatment of female mice required a higher dose (10x) than males (Pan et al., 2014). The reason for this difference is unclear, but it could be related to gender differences in the immunological response to ischemic stroke. It was demonstrated that the severity of ischemic damage is age- and sex-dependent: Women have lower incidence of stroke and lower mortality compared to males. However with advancing age the incidence of stroke and mortality become equal to or even greater than men (Reeves et al., 2008). On the other hand, females often suffer more from long-term stroke effects (Bushnell et al., 2014; Di Carlo et al., 2003; Dotson et al., 2015; Persky et al., 2010; Turtzo and McCullough, 2010). Various studies using rodent models of stroke have shown that young females have smaller infarcts than males (Alkayed et al., 1998; Banerjee et al., 2013; Cheng and Hurn, 2010; Dotson et al., 2015; Manwani et al., 2013; Murphy et al., 2004). It was also reported that while young male mice exhibit increased infarct lesions compared to females, this ratio is reversed in middle-aged mice, whereas old male and female mice exhibit similar infarct volumes (Manwani et al., 2013). When evaluating the different immune responses to ischemic stroke it appears that males exhibit an early and more robust recruitment of macrophages into the ischemic hemisphere relative to females (Banerjee et al., 2013). This difference is further supported by the removal of the spleen which reduces brain lesion size only in male mice, and the reduced infarct size becomes similar to that in splenectomized and spleen-intact female mice (Dotson et al., 2015). In addition, we have previously reported that microglia from female mice had higher expression of IL-4 and IL-10 receptors, and produced more IL-4 compared to males (Bodhankar et al., 2015). Thus, it is possible that in young females, which appear to have relatively lower inflammation-dependent ischemic brain damage, higher concentrations of DRα1-MOG-35-55 are needed to affect the non-immune mediated damage (as demonstrated in Figure 4).
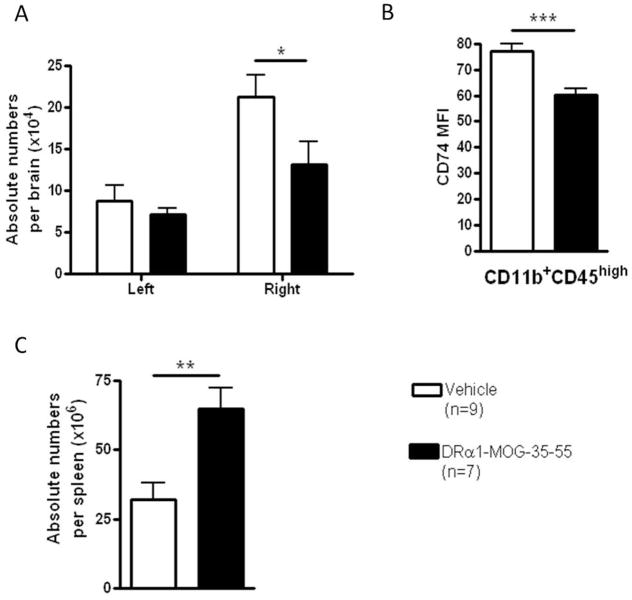
Figure 3. DRα 1-MOG-35-55 treatment reduces number of activated cells and their CD74 surface expression in the ischemic brain 96 hr after MCAO and reverses MCAO-induced splenic atrophy.
A) Absolute lymphocytes numbers in the non-ischemic left and ischemic right brain from DRα1-MOG-35-55-treated versus Vehicle-treated mice. B) CD74 cell surface expression on CD11b+CD45high cells in the right brain. C). Absolute cell numbers in the spleen from DRα1-MOG-35-55-treated versus Vehicle-treated mice; * p<0.05, **p<0.01, ***p<0.001. Reprinted from Metabolic Brain Disease. A novel HLA-DRα1-MOG-35-55 construct treats experimental stroke. 29(1):37-45. 2014. Benedek G, Zhu W, Libal N, Casper A, Yu X, Meza-Romero R, Vandenbark AA, Alkayed NJ, Offner H. © Springer Science+Business Media New York 2013. With permission of Springer.
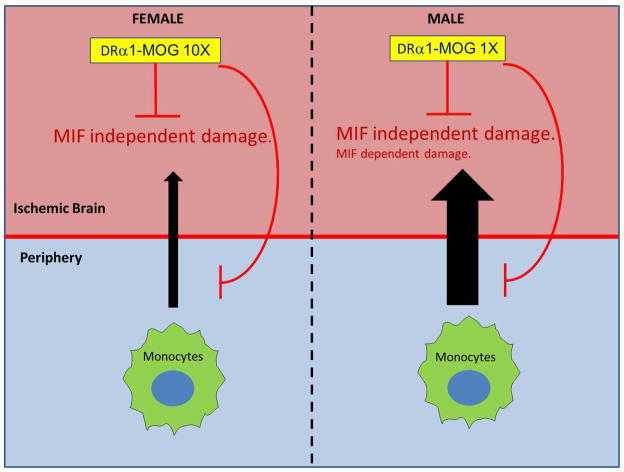
Figure 4. DRα1-MOG-35-55 treatment of MCAO.
DRα1-MOG-35-55 (a partial MHC class II construct that lacks the polymorphic β1 domain) can reduce infarct size in female and male mice after MCAO. In males, where peripheral immune cells cause greater MCAO damage than in females and possibly some MIF dependent inflammation, low concentrations of DRα1-MOG-35-55 are sufficient to reduce the infarct size. In females, which appear to have little if any MIF-dependent MCAO damage, a tenfold higher concentration of DRα1-MOG-35-55 is needed to achieve a similar effect. This differential treatment effect between males and females could be based on gender-associated effects of MIF on infarct size, recruitment of peripheral immune cells into the ischemic brain and axonal death. Reprinted from Translational Stroke Research, Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke, 5(5), 2014, 577–585, Pan et al. Copyright ©The Author(s) 2014.
Nonetheless, DRα1-MOG-35-55 is a novel potential therapy for ischemic stroke that was demonstrated to protect against transient MCAO, similar to its effect in EAE, by downregulating CD74 on APC and enhancing M2 macrophage/microglia and promoting neuroprotection. Recently it was shown that DRα1-MOG-35-55 treatment can reduce infarct volume in a permanent distal MCAO model. This protection was mediated by reduction of infiltrating T cells and increased frequency of CD206+ microglia (manuscript in preparation). It is important to note that partial MHC class II constructs could be detected in the parenchyma of EAE mice, suggesting that they could enter the CNS and bind to CD11b+ cells, and that the mechanism of their therapeutic effect might be linked not only to inhibition of inflammation in the periphery but also to induction of M2 microglial phenotype in the CNS.
In conclusion, both innate and adaptive immunity significantly contribute to CNS inflammation and damage after stroke. This highlights the importance of much needed novel therapies that target the immune system, especially in light of the rising rates of ischemic stroke. Partial MHC class II molecules are capable of treating various models of ischemic stroke in mice and address most of the STAIR criteria. These constructs induce their protective effect by targeting and inhibiting the migration of pro-inflammatory cells into the CNS and by enhancing the M2 regulatory phenotype in microglia (Figure 5).
Figure 5.
DRα1 Partial MHC class II molecules have a dual MOA, acting primarily on monocytes, secondarily on T-cells.
Highlights.
Inflammation plays a key role in brain damage after cerebral ischemia.
Partial MHC constructs are novel therapy for stroke that meets the STAIR criteria.
The constructs inhibit immune cell entry into the CNS and enhance the M2 phenotype.
Acknowledgments
The authors wish to thank Gail Kent for assistance with manuscript submission.
Funding
This work was supported by the National Institutes of Health [grant numbers R01NS075887, R01NS076013 (to HO); R42NS065515 (to HO and NJA)] and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development [grant number BX000226 (AAV)]. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of interest
Drs. Vandenbark, Offner, Benedek, Alkayed and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAPHCS Conflict of Interest in Research Committees.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamus G, Brown L, Andrew S, Meza-Romero R, Burrows GG, Vandenbark AA. Neuroprotective effects of recombinant T-cell receptor ligand in autoimmune optic neuritis in HLA-DR2 mice. Invest Ophthalmol Vis Sci. 2012;53:406–412. doi: 10.1167/iovs.11-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci. 2006;47:2555–2561. doi: 10.1167/iovs.05-1242. [DOI] [PubMed] [Google Scholar]
- Akiyoshi K, Dziennis S, Palmateer J, Ren X, Vandenbark AA, Offner H, Herson PS, Hurn PD. Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res. 2011;2:404–410. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Ansar S, Chatzikonstantinou E, Wistuba-Schier A, Mirau-Weber S, Fatar M, Hennerici MG, Meairs S. Characterization of a new model of thromboembolic stroke in C57 black/6J mice. Transl Stroke Res. 2014;5:526–533. doi: 10.1007/s12975-013-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Benedek G, Meza-Romero R, Andrew S, Leng L, Burrows GG, Bourdette D, Offner H, Bucala R, Vandenbark AA. Partial MHC class II constructs inhibit MIF/CD74 binding and downstream effects. European journal of immunology. 2013;43:1309–1321. doi: 10.1002/eji.201243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Zhu W, Libal N, Casper A, Yu X, Meza-Romero R, Vandenbark AA, Alkayed NJ, Offner H. A novel HLA-DRalpha1-MOG-35-55 construct treats experimental stroke. Metab Brain Dis. 2014;29:37–45. doi: 10.1007/s11011-013-9440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature medicine. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis. 2015;30:1515–1529. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke. 2015;46:520–528. doi: 10.1161/STROKEAHA.114.006864. [DOI] [PubMed] [Google Scholar]
- Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol. 2013;33(Suppl 1):S72–78. doi: 10.1007/s10875-012-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein engineering. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, Olson D, Peterson E. Sex differences in quality of life after ischemic stroke. Neurology. 2014;82:922–931. doi: 10.1212/WNL.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. The Journal of experimental medicine. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T, Froidevaux C, Martin C, Roger T. Macrophage migration inhibitory factor and host innate immune defenses against bacterial sepsis. The Journal of infectious diseases. 2003;187(Suppl 2):S385–390. doi: 10.1086/374752. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Canazza A, Minati L, Boffano C, Parati E, Binks S. Experimental Models of Brain Ischemia: A Review of Techniques, Magnetic Resonance Imaging, and Investigational Cell-Based Therapies. Front Neurol. 2014;5:19. doi: 10.3389/fneur.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu W, Zhang W, Libal N, Murphy SJ, Offner H, Alkayed NJ. A novel mouse model of thromboembolic stroke. J Neurosci Methods. 2015;256:203–211. doi: 10.1016/j.jneumeth.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hurn PD. Sex shapes experimental ischemic brain injury. Steroids. 2010;75:754–759. doi: 10.1016/j.steroids.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Kithcart AP, Pitt D, Guan Z, Alexander J, Williams JL, Shawler T, Dagia NM, Popovich PG, Satoskar AR, Whitacre CC. Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J Immunol. 2013;191:1043–1054. doi: 10.4049/jimmunol.1200485. [DOI] [PubMed] [Google Scholar]
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Jaggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:172. doi: 10.3389/fnins.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D, European BSoSCG. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Dotson AL, Chen Y, Zhu W, Libal N, Alkayed NJ, Offner H. Partial MHC Constructs Treat Thromboembolic Ischemic Stroke Characterized by Early Immune Expansion. Transl Stroke Res. 2016;7:70–78. doi: 10.1007/s12975-015-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Zhu W, Libal N, Alkayed NJ, Offner H. Different immunological mechanisms govern protection from experimental stroke in young and older mice with recombinant TCR ligand therapy. Front Cell Neurosci. 2014;8:284. doi: 10.3389/fncel.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziennis S, Mader S, Akiyoshi K, Ren X, Ayala P, Burrows GG, Vandenbark AA, Herson PS, Hurn PD, Offner HA. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–133. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero LJ, Sheng KC, Jian P, Taylor A, Her Z, Herring BL, Chow A, Leo YS, Hickey MJ, Morand EF, Ng LF, Bucala R, Mahalingam S. Macrophage migration inhibitory factor receptor CD74 mediates alphavirus-induced arthritis and myositis in murine models of alphavirus infection. Arthritis Rheum. 2013;65:2724–2736. doi: 10.1002/art.38090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, Rosloniec EF, Burrows GG, Offner H. MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol. 2008;180:1249–1257. doi: 10.4049/jimmunol.180.2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, Bucala R, Deierborg T. Lack of macrophage migration inhibitory factor in mice does not affect hallmarks of the inflammatory/immune response during the first week after stroke. J Neuroinflammation. 2011a;8:75. doi: 10.1186/1742-2094-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, Ruscher K, Leng L, Bucala R, Deierborg T. Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J Cereb Blood Flow Metab. 2011b;31:1093–1106. doi: 10.1038/jcbfm.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, Ruscher K, Wieloch T. Enriched environment downregulates macrophage migration inhibitory factor and increases parvalbumin in the brain following experimental stroke. Neurobiol Dis. 2011c;41:270–278. doi: 10.1016/j.nbd.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Itakura A, Aslan JE, Sinha S, White-Adams TC, Patel IA, Meza-Romero R, Vandenbark AA, Burrows GG, Offner H, McCarty OJ. Characterization of human platelet binding of recombinant T cell receptor ligand. J Neuroinflammation. 2010;7:75. doi: 10.1186/1742-2094-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Ji N, Kovalovsky A, Fingerle-Rowson G, Guentzel MN, Forsthuber TG. Macrophage migration inhibitory factor promotes resistance to glucocorticoid treatment in EAE. Neurol Neuroimmunol Neuroinflamm. 2015;2:e139. doi: 10.1212/NXI.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehmet J, Hoffmann S, Walter G, Meisel C, Meisel A. Stroke induces specific alteration of T memory compartment controlling auto-reactive CNS antigen-specific T cell responses. J Neurol Sci. 2016;368:77–83. doi: 10.1016/j.jns.2016.06.039. [DOI] [PubMed] [Google Scholar]
- Lee DC, Ruiz CR, Lebson L, Selenica ML, Rizer J, Hunt JB, Jr, Rojiani R, Reid P, Kammath S, Nash K, Dickey CA, Gordon M, Morgan D. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol Aging. 2013;34:1610–1620. doi: 10.1016/j.neurobiolaging.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Ecass AN, Group Er-PS, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. The Journal of experimental medicine. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang C, Yao Y, Chen L, Liu G, Zhang R, Liu Q, Shi FD, Hao J. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. 2016 doi: 10.1096/fj.201600495R. [DOI] [PubMed] [Google Scholar]
- Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke. 2014;9(Suppl A100):4–13. doi: 10.1111/ijs.12371. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Wilhelm CJ, Vandenbark AA, Huckans M. Partial MHC/neuroantigen peptide constructs: a potential neuroimmune-based treatment for methamphetamine addiction. PLoS One. 2013;8:e56306. doi: 10.1371/journal.pone.0056306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Romero R, Benedek G, Yu X, Mooney JL, Dahan R, Duvshani N, Bucala R, Offner H, Reiter Y, Burrows GG, Vandenbark AA. HLA-DRalpha1 constructs block CD74 expression and MIF effects in experimental autoimmune encephalomyelitis. J Immunol. 2014;192:4164–4173. doi: 10.4049/jimmunol.1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro-Mur F, Urra X, Gallizioli M, Chamorro A, Planas AM. Antigen Presentation After Stroke. Neurotherapeutics. 2016 doi: 10.1007/s13311-016-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993;74:257–268. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, Doroudian M, Mawhinney L, Donnelly SC. Targeting MIF in Cancer: Therapeutic Strategies, Current Developments, and Future Opportunities. Med Res Rev. 2016;36:440–460. doi: 10.1002/med.21385. [DOI] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a Phase I clinical study. J Neuroimmunol. 2011;231:7–14. doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG American Heart Association Advocacy Coordinating C, Stroke C. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- Pan J, Palmateer J, Schallert T, Hart M, Pandya A, Vandenbark AA, Offner H, Hurn PD. Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke. Transl Stroke Res. 2014;5:577–585. doi: 10.1007/s12975-014-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Offner H. The role of the spleen in ischemic stroke. J Cereb Blood Flow Metab. 2015;35:186–187. doi: 10.1038/jcbfm.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010;12:6–13. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory Disequilibrium in Stroke. Circ Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188:2156–2163. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS, Offner H. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis. 2012;27:7–15. doi: 10.1007/s11011-011-9267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, Bucala R, Weber C, Bernhagen J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS letters. 2009;583:2749–2757. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012a;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012b;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Miller L, Subramanian S, McCarty OJ, Proctor T, Meza-Romero R, Huan J, Burrows GG, Vandenbark AA, Offner H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;225:52–61. doi: 10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27:12531–12539. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Wu Q, Cai Y, Zhao X, Wang S, Gao Z, Yang Y, Li X, Qian J, Wang J, Su B, Chen H, Han B, Jiang G, Lu D. Novel association between CD74 polymorphisms and hematologic toxicity in patients with NSCLC after platinum-based chemotherapy. Clin Lung Cancer. 2014;15:67–78. e12. doi: 10.1016/j.cllc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Li J, Persky R, Benashski S, Weston G, Bucala R, Venna VR, McCullough LD. Deletion of macrophage migration inhibitory factor worsens stroke outcome in female mice. Neurobiol Dis. 2013;54:421–431. doi: 10.1016/j.nbd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future Neurol. 2010;5:47–59. doi: 10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- Urra X, Miro F, Chamorro A, Planas AM. Antigen-specific immune reactions to ischemic stroke. Front Cell Neurosci. 2014;8:278. doi: 10.3389/fncel.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Meza-Romero R, Benedek G, Andrew S, Huan J, Chou YK, Buenafe AC, Dahan R, Reiter Y, Mooney JL, Offner H, Burrows GG. A novel regulatory pathway for autoimmune disease: binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. Journal of autoimmunity. 2013;40:96–110. doi: 10.1016/j.jaut.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- Wang C, Gold BG, Kaler LJ, Yu X, Afentoulis ME, Burrows GG, Vandenbark AA, Bourdette DN, Offner H. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98:1817–1827. doi: 10.1111/j.1471-4159.2006.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin J, Schlotterer A, Wu L, Fleming T, Busch S, Dietrich N, Hammes HP. CD74 indicates microglial activation in experimental diabetic retinopathy and exogenous methylglyoxal mimics the response in normoglycemic retina. Acta Diabetol. 2014;51:813–821. doi: 10.1007/s00592-014-0616-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Zis O, Ma G, Shan Z, Zhang X, Wang S, Dai C, Zhao J, Lin Q, Lin S, Song W. Upregulation of macrophage migration inhibitory factor gene expression in stroke. Stroke. 2009;40:973–976. doi: 10.1161/STROKEAHA.108.530535. [DOI] [PubMed] [Google Scholar]
- Wraight CJ, van Endert P, Moller P, Lipp J, Ling NR, MacLennan IC, Koch N, Moldenhauer G. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. The Journal of biological chemistry. 1990;265:5787–5792. [PubMed] [Google Scholar]
- Yadav V, Bourdette DN, Bowen JD, Lynch SG, Mattson D, Preiningerova J, Bever CT, Jr, Simon J, Goldstein A, Burrows GG, Offner H, Ferro AJ, Vandenbark AA. Recombinant T-Cell Receptor Ligand (RTL) for Treatment of Multiple Sclerosis: A Double-Blind, Placebo-Controlled, Phase 1, Dose-Escalation Study. Autoimmune diseases. 2012;2012:954739. doi: 10.1155/2012/954739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, Grabe N, Veltkamp R. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23:34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Casper A, Libal NL, Murphy SJ, Bodhankar S, Offner H, Alkayed NJ. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. Transl Stroke Res. 2015a;6:60–68. doi: 10.1007/s12975-014-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Dotson AL, Libal NL, Lapato AS, Bodhankar S, Offner H, Alkayed NJ. Recombinant T-cell receptor ligand RTL1000 limits inflammation and decreases infarct size after experimental ischemic stroke in middle-aged mice. Neuroscience. 2015b;288:112–119. doi: 10.1016/j.neuroscience.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Libal NL, Casper A, Bodhankar S, Offner H, Alkayed NJ. Recombinant T cell receptor ligand treatment improves neurological outcome in the presence of tissue plasminogen activator in experimental ischemic stroke. Transl Stroke Res. 2014;5:612–617. doi: 10.1007/s12975-014-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]