Abstract
Background and Aims: Platelet-to-lymphocyte ratio (PLR) has been shown to predict prognosis of cancers. We aimed to evaluate the prognostic value of stratification of PLR in patients after curative liver resection (CLR) for hepatocellular carcinoma (HCC).
Methods: A total of 1804 patients who underwent CLR for suspected HCC between January 2007 and January 2014 were screened for the study. All of the patients were categorized into equal tertiles according to the number of patients and the distribution of PLR. Prognostic significance was determined for overall survival (OS) and was assessed using Kaplan–Meier analysis. Univariate and multivariate Cox proportional hazard regression analyses were evaluated for association of all independent parameters with disease prognosis.
Results: The optimal cut-off points of preoperative PLR were: (T1) 11.98–75.00, (T2) 75.00–113.33 and (T3) 113.33–567.50. There were obvious differences in each PLR tertile with mortality within 36 months of CLR (plog-rank < 0.001). Multivariable analysis suggested that the level of PLR (HR = 1.004, 95%CI: 1.001–1.008, p = 0.006), portal vein thrombosis (HR = 3.406, 95%CI: 1.185–9.794, p = 0.023), number of nodules (HR = 1.810, 95%CI: 1.345–2.437, p < 0.001), Child-Turcotte-Pugh score (HR = 1.741, 95%CI: 1.129–2.684, p = 0.012) and microvascular invasion (HR = 2.730, 95%CI: 1.777–4.196, p < 0.001) were significant predictors of mortality. Kaplan–Meier analysis of overall survival (OS) demonstrated that each PLR tertile showed a progressively worse OS and apparent separation (plog-rank = 0.016). The highest 5-year OS rate following CLR (58%) was revealed in tertile 1. In contrast, the lowest 5-year OS rate (30%) was revealed in tertile 3.
Conclusion: Stratified preoperative PLR could strengthen the predictive power for OS in HCC patients with CLR.
Keywords: Platelet-to-lymphocyte ratio, Hepatocellular carcinoma, Curative liver resection, Overall survival
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide. Recently, there have been approximately 750,000 new cases of liver cancer reported per year.1,2 For men, it is the second leading cause of cancer death worldwide in less developed countries. In more developed countries, it is the sixth leading cause of cancer death among men.3 At present, based on limitations for a more widespread application of liver transplantation for HCC patients (shortage of donor organs, higher perioperative risk, high cost and long-term immunosuppression), hepatectomy is widely accepted as the first treatment option and provides a radical therapy in patients with early stages of HCC.4,5
With appropriate surgical techniques and perioperative management to preserve function of the liver remnant, HCC can be resected safely and with very low operative morbidity and mortality rates.6,7 However, some studies have indicated that linked to the high recurrence rate, patients’ long-term prognosis after radical resection remains poor.4,8 Therefore, it is necessary to monitor patients for progression of HCC by controlling tumor recurrence, ultimately prolonging the survival period in HCC patients after curative liver resection (CLR).
Currently, some studies have shown that genetic, biological aggressiveness and environmental factors are contributory risk factors for the progression and development of HCC.9,10 In addition, numerous pathological features have been identified as prognostic indicators for HCC patients, such as tumor burden, the presence of hepatic vascular invasion, portal vein thrombosis, serum bilirubin, C-reactive protein and the elevated serum levels of alpha-fetoprotein (AFP).11–16 Previous studies have demonstrated that systemic inflammation is related to poor prognosis and increased tumor progression through up-regulation of cytokines in a variety of cancers.17,18 As biomarkers of systemic inflammation, elevated neutrophil-lymphocyte ratio (NLR) and absolute monocyte counts have demonstrated a potential influence for guiding the clinical management of cancer patients.19
Recently, the platelet-to-lymphocyte ratio (PLR), a marker of systemic inflammation, (ratio of the absolute platelet and lymphocyte counts), has been reported to be associated with the progression of various tumor types, including pancreatic ductal adenocarcinoma, epithelial ovarian cancer, and metastatic renal cell cancer.20–22 However, there is conflicting data regarding the ability of predicting prognosis of HCC patients with PLR.23 Li et al.24 demonstrated that elevated PLR was associated with aggressive tumor behavior, and can be identified as a poor independent prognostic factor in advanced HCC patients. However, other studies fail to find correlation between clinical outcome and the level of PLR in HCC patients.25,26 As such, the current opinion on the prognostic role of PLR for HCC is still controversial, and to date there have been no reports regarding PLR in HCC patients undergoing CLR with stratification in order to predict overall survival (OS).
Therefore, the purpose of this study was to use stratification with preoperative PLR to assess the prognostic impact on OS for patients who underwent CLR for suspected HCC.
Methods
Study design
Data was collected from the First Affiliated Hospital of Wenzhou Medical University clinical database, all patients were sampled consecutively from PLR records for suspected HCC between January 2007 and January 2014.
PLR was defined as the absolute platelet count divided by the absolute lymphocyte count prior and closest to the date of resection as part of the routine preoperative assessment of the patients. Furthermore, in view of the number of patients of this study population and the distribution of the level of PLR with the highest differences before surgery, PLR was further categorized into tertiles to observe whether any reinforced predictive performance could be quantified while maintaining sufficient statistical power in each category.
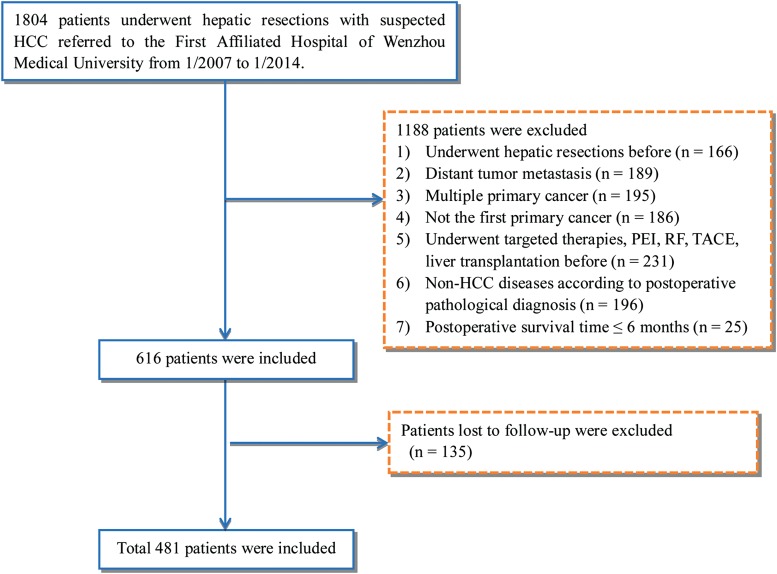
The written informed consent was obtained from each patient included in the study. The study was approved by the Committee on Ethics at the First Affiliated Hospital of Wenzhou Medical University and was performed according to Standards for the Reporting of Diagnostic Accuracy Studies. A study flow diagram is provided in Fig. 1.
Fig. 1. Study flow diagram.
Abbreviations: HCC, hepatocellular carcinoma; PEI, percutaneous ethanol injection; RF, radiofrequency; TACE, transarterial chemoembolization.
Exclusion criteria
All cases of suspected HCC were confirmed by post-operative pathology assessment, and the following exclusion criteria were used: (1) previous hepatic resections; (2) distant tumor metastasis; (3) multiple primary tumors; (4) previous primary cancer; (5) previous transcatheter arterial chemoembolization or radiofrequency treatment, percutaneous ethanol injection, liver transplantation, or targeted therapies; (6) non-HCC disease on the basis of post-operative pathological diagnosis; (7) postoperative survival time of ≤ 6 months; (8) loss during follow-up. In total, 481 HCC patients were identified for this study.
Data collection and follow-up
Standard patient demographic and clinic pathological data were collected from the patients’ medical records, including age, BMI, sex, calculated Child-Turcotte-Pugh (CTP) score and the Cancer of the Liver Italian Program (CLIP) score at initial presentation. Laboratory values, including platelet, total bilirubin, direct bilirubin, albumin, alanine aminotransferase, aspartate aminotransferase (AST), alkaline phosphatase, blood glucose, creatinine, thrombin time, and international normalized ratio, were recorded for all patients before curative liver resection. Clinical values, including liver cirrhosis (LC) and ascites, were recorded for all patients after assessment by physical examination and confirmation by imaging studies such as abdominal ultrasonography, computerized tomography (CT) or magnetic resonance imaging (MRI). The presence of microvascular invasion was defined by evidence of tumor emboli in either the large capsular vessels, or the portal or central hepatic vein based on imaging studies or surgical resection.27 Tumor characteristics, including portal vein thrombosis, were observed during the surgery, and the number of tumor nodules were ascertained based on CT or MRI scan.
Patients were followed-up every 3 months after surgery and OS was based on the time interval between the date of surgery and death, or the date of surgery and the last follow-up. Information on death was collected from the medical records and the social security death index, as well as from families.
Statistical analysis
Data for continuous variables were expressed in mean ± standard deviation or medians and interquartile range, depending on their distribution in the study population tested by Kolmogorov-Smirnov test. Categorical values were presented as relative frequencies and proportions. Comparisons between stratification were performed using the nonparametric Kruskal-Wallis test or one-way analysis of variance (ANOVA) for continuous variables, and the Pearson’s chi-square test or Fisher’s exact test for categorical variables as appropriate. A Cox proportional hazard regression was used to calculate hazard ratios (HRs) and 95 % confidence intervals (CIs) associated with OS. Prognostic factors with significant values of p < 0.05 in a univariate analysis were entered into a multivariate analysis, enabling determination of significant effects while adjusting for multiple factors simultaneously. Then, the Kaplan–Meier curves were used for OS rates to compare patients with each stratification, and statistical difference in the survival curves were evaluated using the log-rank test.
In this study, a two-tailed p value of < 0.05 was recognized as statistically significant. All these statistical calculations were performed using SPSS version 18.0 (SPSS, Chicago, IL, USA) and MedCalc version 12.7 (MedCalc Software, Ostend, Belgium).
Results
The 481 patients who underwent CLR for suspected HCC at the First Affiliated Hospital of Wenzhou Medical University between January 2007 and January 2014 consisted of 411 males (85.4%) and 70 females (14.6%). Their mean age was 56.4 years (range, 23–85 years; Table 1).
Table 1. Characteristics of patients with hepatocellular carcinoma treated by surgical resection according to PLR tertiles.
| Variables | All patients | PLR tertiles | |||
| Tertile 1, n = 160 [11.98–75.00] | Tertile 2, n = 160 [75.00–113.33] | Tertile 3, n = 161 [113.33–567.50] | p-value | ||
| PLR | 91.2 (69.0, 129.2) | 60.0 (48.4, 69.0) | 91.2 (82.5, 102.2) | 155.7 (128.9, 192.5) | <0.001 |
| Demographic parameters | |||||
| Age in years | 56.4 ± 10.9 | 55.6 ± 9.7 | 56.8 ± 10.8 | 56.8 ± 12.2 | 0.507 |
| Sex | 0.335 | ||||
| Male | 411 (85.4%) | 135 (84.4%) | 142 (88.8%) | 134 (83.2%) | |
| Female | 70 (14.6%) | 25 (15.6%) | 18 (11.3%) | 27 (16.8%) | |
| BMI in kg/m2 | 23.0 ± 3.3 | 23.1 ± 3.2 | 23.6 ± 3.9 | 22.3 ± 2.7 | 0.004 |
| Clinical parameters, n (%) | |||||
| Ascites | 0.267 | ||||
| Absence | 366 (90.1%) | 123 (90.4%) | 129 (92.8%) | 114 (87.0%) | |
| Presence | 40 (9.9%) | 13 (9.6%) | 10 (7.2%) | 17 (13.0%) | |
| Liver cirrhosis | 176 (42.6%) | 81 (58.7%) | 59 (41.8%) | 36 (26.9%) | <0.001 |
| Etiology, n (%) | 0.002 | ||||
| Hepatitis B | 325 (68.4%) | 113 (72.9%) | 109 (68.1%) | 103 (64.4%) | |
| Alcohol | 36 (7.6%) | 8 (5.2%) | 9 (5.6%) | 19 (11.9%) | |
| Hepatitis B + hepatitis C | 77 (16.2%) | 31 (20.0%) | 28 (17.5%) | 18 (11.3%) | |
| Other | 34 (7.2%) | 3 (1.9%) | 13 (8.1%) | 18 (11.3%) | |
| Hepatitis C | 3 (0.6%) | 0 | 1 (0.6%) | 2 (1.3%) | |
| Laboratory parameters | |||||
| Total bilirubin in μmol/L | 10.0 (8.0, 15.0) | 12.0 (9.0, 18.0) | 10.0 (8.0, 15.0) | 10.0 (8.0, 14.0) | 0.003 |
| Direct bilirubin in μmol/L | 3.5 (2.0, 6.0) | 4.0 (3.0, 6.0) | 3.0 (2.0, 5.0) | 4.0 (3.0, 6.0) | 0.223 |
| Albumin in g/L | 40.7 (37.3, 43.7) | 39.8 (36.3, 43.3) | 41.3 (38.3, 43.9) | 40.7 (37.6, 43.9) | 0.063 |
| ALT in IU/L | 36.0 (25.0, 55.0) | 38.0 (27.0, 53.0) | 36.0 (24.3, 54.0) | 34.0 (21.0, 55.0) | 0.168 |
| AST in IU/L | 37.0 (27.0, 53.0) | 39.0 (31.0, 54.0) | 34.0 (26.3, 48.0) | 37.0 (25.0, 59.0) | 0.111 |
| Alkaline phosphatase in IU/L | 94.0 (75.0, 115.0) | 96.0 (78.3, 113) | 89.0 (74.0, 112.0) | 97.0 (73.0, 121.0) | 0.283 |
| γ-GT in IU/L | 54.0 (33.0, 106.0) | 53.5 (31.3, 117.0) | 53.0 (35.0, 90.0) | 62.0 (33.5, 127.5) | 0.413 |
| Blood glucose in mmol/L | 5.9 (5.0, 7.3) | 5.7 (4.8, 7.2) | 6.0 (5.1, 7.1) | 6.1 (5.1, 7.5) | 0.371 |
| Creatinine in μmol/L | 67.0 (56.3, 76.0) | 67.0 (58.0, 78.0) | 68.0 (57.0, 76.8) | 66.0 (55.0, 75.0) | 0.434 |
| Serum sodium in mmol/L | 141.0 (139.0, 142.0) | 140.0 (139.0, 142.0) | 141.0 (139.0, 143.0) | 141.0 (138.0, 142.5) | 0.355 |
| PT in s | 13.9 (13.3, 14.7) | 14.4 (13.6, 15.2) | 13.7 (13.2, 14.4) | 13.7 (13.1, 14.3) | <0.001 |
| PTA in % | 88.2 ± 13.7 | 83.4 ± 14.2 | 89.7 ± 11.9 | 91.3 ± 13.6 | <0.001 |
| INR | 1.1 (1.0, 1.2) | 1.1 (1.1, 1.2) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.1) | <0.001 |
| White blood cell in 109/L | 5.3 (4.2, 6.7) | 4.9 (3.6, 6.1) | 5.7 (4.4, 6.8) | 5.2 (4.3, 7.0) | 0.002 |
| AFP in ng/mL | 30.7 (5.4, 447.9) | 34.4 (6.2, 343.1) | 36.9 (5.3, 430.7) | 23.2 (5.0, 596.3) | 0.984 |
| Uric acid in μmol/L | 299.4 ± 88.6 | 313.0 ± 91.2 | 296.4 ± 80.0 | 289.0 ± 92.8 | 0.047 |
| Platelet in 109/L | 138.6 ± 63.5 | 96 ± 42.8 | 138.2 ± 47.1 | 181.3 ± 66.4 | <0.001 |
| Tumor characteristics | |||||
| Number of nodules, n (%) | 0.682 | ||||
| 1 | 403 (87.6%) | 134 (83.8%) | 133 (85.3%) | 136 (88.3%) | |
| 2 | 39 (8.5%) | 12 (7.5%) | 16 (10.3%) | 11 (7.1%) | |
| 3 | 8 (1.7%) | 3 (1.9%) | 3 (1.9%) | 2 (1.3%) | |
| ≥4 | 10 (2.2%) | 1 (0.6%) | 4 (2.6%) | 5 (3.2%) | |
| Greatest tumor diameter in mm | 40.0 (30.0, 60.0) | 30.0 (20.0, 50.0) | 40.0 (30.0, 57.5) | 50.0 (30.0, 80.0) | <0.001 |
| Portal vein thrombosis, n (%) | 12 (3.0%) | 2 (1.5%) | 2 (1.4%) | 8 (6.1%) | 0.054 |
| Microvascular invasion, n (%) | 121 (25.4%) | 42 (26.4%) | 41 (25.9%) | 38 (23.8%) | 0.843 |
| CLIP score, n (%) | <0.001 | ||||
| 0 | 177 (45.3%) | 67 (51.1%) | 60 (44.1%) | 50 (40.3%) | |
| 1 | 85 (21.7%) | 34 (26%) | 37 (27.2%) | 14 (11.3%) | |
| 2 | 72 (18.4%) | 21 (16%) | 24 (17.6%) | 27 (21.8%) | |
| 3 | 42 (10.7%) | 7 (5.3%) | 11 (8.1%) | 24 (19.4%) | |
| 4 | 14 (3.6%) | 2 (1.5%) | 4 (2.9%) | 8 (6.5%) | |
| 5 | 1 (0.3%) | 0 | 0 | 1 (0.8%) | |
| CTP score, n (%) | 0.455 | ||||
| A | 338 (83.9%) | 107 (79.9%) | 120 (87%) | 111 (84.7%) | |
| B | 57 (14.1%) | 23 (17.2%) | 17 (12.3%) | 17 (13.0%) | |
| C | 8 (2.0%) | 4 (3.0%) | 1 (0.7%) | 3 (2.3%) | |
| Follow-up data | |||||
| Death within 36 months of resection | 0.003 | ||||
| Alive | 145 (61.2%) | 60 (71.4%) | 51 (64.6%) | 34 (45.9%) | |
| Deceased | 92 (38.8%) | 24 (28.6%) | 28 (35.4%) | 40 (54.1%) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transferase; PLR, platelet-lymphocyte ratio; PT, prothrombin time; PTA, prothrombin time activity; AFP, alpha-fetoprotein; INR, international normalized ratio; CLIP, Cancer of The Liver Italian Program; CTP, Child-Turcotte-Pugh.
Based on distribution of the level of PLR, all of the patients were categorized into equal tertiles, which ensured the most categories with adequate number of patients per category from the range of 11.98 to 567.50 (T1, 160 patients; T2, 160 patients; T3, 160 patients). The cut-off values for this stratification of the PLR into tertiles were: (T1) 11.98–75.00, (T2) 75.00–113.33, (T3) 113.33–567.50.
The demographic and tumor, laboratory and clinical characteristics of the HCC patients involved in this study with PLR tertiles are summarized in Table 1. Patients with low and high PLR seemed to be similar in regard to laboratory characteristics, except for white blood cells, uric acid platelets, total bilirubin, prothrombin time (PT), prothrombin time activity and INR. The etiology for most of the cases was hepatitis B virus (HBV) (68.4%), followed by superinfection with HBV and hepatitis C virus (16.2%). The majority of patients had a single tumor (87.6%), and higher PLR tertiles were significantly associated with larger tumor diameter when compared with the lower two tertiles (p < 0.001).
Univariate and multivariate analyses by a Cox proportional hazard model were performed to identify independent prognostic factors for OS, as illustrated in Table 2. The univariate Cox proportional hazards analysis demonstrated that PLR, ascites, PT, albumin, AST, alkaline phosphatase, white blood cells, largest tumor diameter, number of nodules, microvascular invasion, portal vein thrombosis, CLIP score and CTP score (all p < 0.05) were statistically significant prognostic factors for OS.
Table 2. Univariate and multivariate cox proportional hazards regression analyses of factors associated with mortality.
| Variables | Univariate analysis | Multivariate analysis | ||||||
| B | HR | 95%CI | p-value | B | HR | 95%CI | p-value | |
| PLR | 0.003 | 1.003 | 1.001–1.006 | 0.015 | 0.004 | 1.004 | 1.001–1.008 | 0.006 |
| Demographic parameters | ||||||||
| Age in years | 0.007 | 1.007 | 0.991–1.024 | 0.399 | ||||
| Sex | −0.206 | 0.814 | 0.466–1.423 | 0.470 | ||||
| BMI | 0.044 | 1.045 | 0.986–1.108 | 0.134 | ||||
| Clinical parameters | ||||||||
| Ascites | 0.464 | 1.591 | 1.189–2.127 | 0.002 | ||||
| Liver cirrhosis | 0.216 | 1.241 | 0.834–1.845 | 0.287 | ||||
| Laboratory parameters | ||||||||
| Total bilirubin in μmol/L | 0.004 | 1.004 | 0.998–1.010 | 0.152 | ||||
| Direct bilirubin in μmol/L | 0.006 | 1.006 | 0.998–1.013 | 0.135 | ||||
| Albumin in g/L | −0.061 | 0.941 | 0.913–0.969 | <0.001 | ||||
| ALT in IU/L | 0.002 | 1.002 | 1.000–1.004 | 0.056 | ||||
| AST in IU/L | 0.001 | 1.001 | 1.000–1.002 | 0.019 | ||||
| Alkaline phosphatase in IU/L | 0.002 | 1.002 | 1.000–1.003 | 0.030 | ||||
| γ-GT in IU/L | 0.001 | 1.001 | 1.000–1.002 | 0.067 | ||||
| Blood glucose in mmol/L | 0.033 | 1.033 | 0.983–1.086 | 0.198 | ||||
| Creatinine in μmol/L | −0.004 | 0.996 | 0.987–1.006 | 0.470 | ||||
| Uric acid in μmol/L | −0.001 | 0.999 | 0.997–1.002 | 0.635 | ||||
| Serum sodium in mmol/L | 0.004 | 1.004 | 0.999–1.009 | 0.160 | ||||
| PT in s | 0.149 | 1.160 | 1.021–1.319 | 0.022 | ||||
| PTA in % | −0.012 | 0.988 | 0.975–1.001 | 0.071 | ||||
| INR | 0.009 | 1.009 | 0.989–1.029 | 0.375 | ||||
| White blood cell in 109/L | 0.027 | 1.027 | 1.01–1.044 | 0.002 | ||||
| Platelet in 109/L | 0.002 | 1.002 | 0.999–1.004 | 0.309 | ||||
| AFP in ng/mL | 0.000 | 1.000 | 1.000–1.000 | 0.244 | ||||
| Tumor characteristics | ||||||||
| Number of nodules | 0.435 | 1.545 | 1.202–1.987 | 0.001 | 0.594 | 1.810 | 1.345–2.437 | <0.001 |
| Greatest tumor diameter in mm | 0.007 | 1.007 | 1.001–1.012 | 0.015 | ||||
| Portal vein thrombosis | 1.880 | 6.554 | 2.619–16.401 | <0.001 | 1.226 | 3.406 | 1.185–9.794 | 0.023 |
| Microvascular invasion | 0.921 | 2.512 | 1.749–3.606 | <0.001 | 1.004 | 2.730 | 1.777–4.196 | <0.001 |
| CLIP score | 0.423 | 1.527 | 1.290–1.809 | <0.001 | ||||
| CTP score | 0.686 | 1.986 | 1.346–2.932 | 0.001 | 0.554 | 1.741 | 1.129–2.684 | 0.012 |
Abbreviations: B, coefficient; HR, hazard ratio; CI, confidence interval; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transferase; PLR, platelet-lymphocyte ratio; PT, prothrombin time; PTA, prothrombin time activity; AFP, alpha-fetoprotein; INR, international normalized ratio; CLIP, Cancer of The Liver Italian Program; CTP, Child-Turcotte-Pugh.
After extensive univariate analysis, these significant factors were included in the multivariable Cox proportional hazards models, and multivariable analysis identified that the level of PLR (HR = 1.004, 95%CI: 1.001–1.008, p = 0.006), number of nodules (HR = 1.810, 95%CI: 1.345–2.437, p < 0.001), presence of microvascular invasion (HR = 2.730, 95%CI: 1.777–4.196, p < 0.001), presence of portal vein thrombosis (HR = 3.406, 95%CI: 1.185–9.794, p = 0.023) and CTP score (HR = 1.741, 95%CI: 1.129–2.684, p = 0.012) were independent prognostic factors for OS.
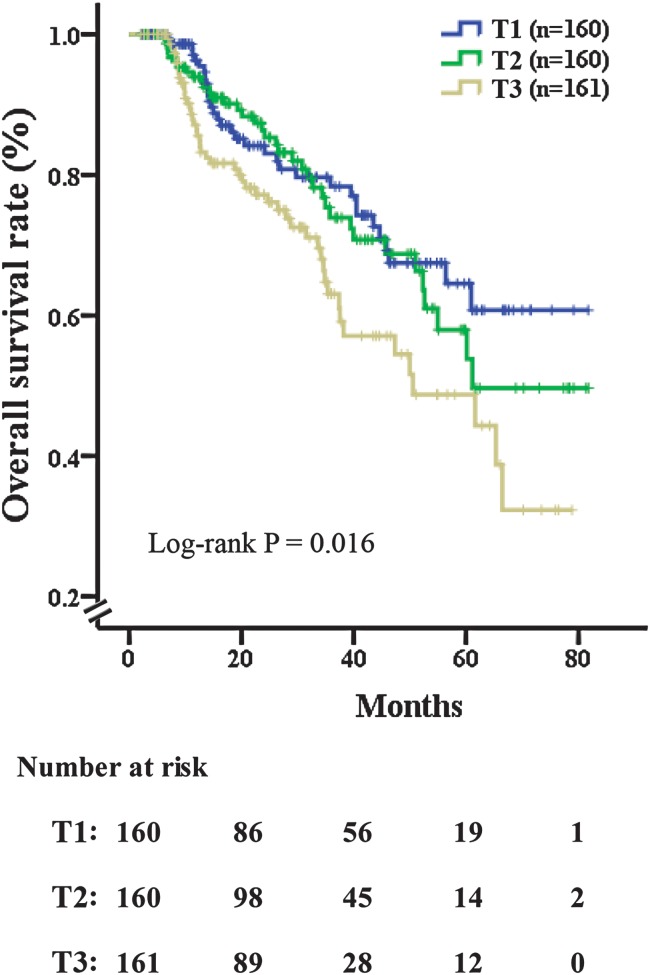
Furthermore, the Kaplan-Meier survival curves of the HCC patients stratified by PLR tertiles demonstrated a higher 5-year OS following CLR (58%) of the lowest PLR tertiles (T1) in comparison to poor outcomes (30%) in the highest tertiles (T3), and each of the tertiles demonstrated a similar difference of OS (log-rank p = 0.016) (Fig. 2).
Fig. 2. Overall survival rate of patients who had received curative liver resection, stratified by tertile of PLR.
The log-rank p-value among all three tertiles was 0.016. (T1) 11.98–75.00, (T2) 75.00–113.33 and (T3) 113.33–567.50. Patients with the lowest tertile of PLR (T1) had favorable 5-year survival following surgery (58%); however, those in the tertile of PLR (T3) had poor outcomes (30%).
Discussion
Postoperative recurrence of HCC is a major barrier for long-term survival for HCC patients after liver resection.28 Hence, in this study, we established as first the stratification of preoperative PLR levels for the prediction of 36-month survival in patients with HCC after CLR. Based on Kaplan-Meier analysis of OS, the elevated level of PLR was demonstrated to be associated with the poor survival of HCC and high tertiles of PLR were related to poor prognosis.
More than a century ago, the association of cancer and inflammation was demonstrated.29 However, the mechanism by which the immune response may be triggered via a tumor is complex,30 and numerous research projects focused on underlying mechanism that associates disease prognosis and tumor inflammation have been undertaken.17,31 Recently, accumulative evidence have demonstrated that increased systemic inflammation is associated with poor prognosis in various kinds of cancers, including pancreatic cancer and ovarian cancer.32 Biomarkers of systemic inflammation such as PLR, elevated NLR, and absolute monocyte counts have a potential role in guiding the clinical management of cancer patients, across a range of malignancies.19 PLR is a basic marker of systemic inflammation and can be easily obtained from routine blood cell testing.23 Previous studies have confirmed that a high preoperative PLR was associated with poor prognosis in patients with non-metastatic non-small cell lung cancer,33 resectable small cell carcinoma of the esophagus and HCC.24,34 Additionally, the level of PLR is a widely accepted independent predictor for OS in patients with advanced HCC.24 We stratified PLR as first to predict prognosis in HCC patients after CLR. We also analyzed whether this could be useful to predict a better performance. We found that the presence of elevated pre-operative PLR was associated with poor survival, which is consistent with the systematic review and clinical trial which reported that a high PLR is associated with worse OS in various solid tumors including HCC.35,36
The elevated peripheral blood platelet counts might reflect the tumor-induced systemic inflammatory response.37 Platelet aggregation and degranulation, along with the consequent release of platelet-derived growth factor, platelet-derived proangiogenic mediators, vascular endothelial growth factor and angiopoetin-1, have been verified as important determinants of tumor growth and probably angiogenesis.38–40 Previous studies have confirmed that activated platelets impel tumor cell escape from immune elimination by promoting their arrest in the endothelium, thereby causing the secondary lesions.24,41,42 Platelets may also promote the growth and spread of malignancies through non-inflammatory mechanisms, including stimulation of metalloproteinase-9 synthesis, and production of adhesion molecules and growth factors (such as EGF, VEGF, TGFb and PDGF).43–45 Carr et al.’s46 study suggested that platelets could also stimulate the growth and invasion of several HCC cell lines in vitro. These studies indicate that platelets may lead to accelerated tumor metastasis and progression in cancers. Therefore, the underlying mechanisms of the interactions of platelet-tumor cells need to be studied more extensively, for the purpose of providing more appropriate treatment plans for individual patients in high-risk situations for HCC.
In recent years, some studies in oncology have explored whether a better effect on disease prognosis can be achieved by stratifying the independent predictor. For instance, Blank et al.47 categorized AFP into quintiles and created the opportunity to observe differences in outcomes among HBV-HCC patients after surgical resection. And, another study categorized patients into equal tertiles according to their baseline of NLR and PLR, demonstrating that an elevated pretreatment NLR is an independent predictor of both worse overall and disease-free survival in colorectal cancer.48 In this study, based on the fact that PLR is a widely accepted HCC risk factor, we categorized PLR into equal tertiles to investigate whether any enhanced predictive effect was detected. Consequently, we gained greater confidence in being able to predict clinical outcome. The Kaplan–Meier curve analyses revealed that patients with the highest tertile of PLR (a 5-year survival of 30%) had significantly shorter OS compared to those with the lowest tertile of PLR (a 5-year survival of 58%). These new categories have shown significant and distinct survival outcomes in HCC patients after CLR. We believe it may be helpful in guiding the clinician to predict the prognosis of cancer and in selecting the most appropriate treatment or palliative care to improve survival rate. Hence, our study suggests that the stratification of PLR could independently and reliably predict the disease prognosis for suspected HCC patients after CLR.
This study has several limitations. First of all, the findings include a relatively homogeneous patient cohort and may not be applicable to HCC patients who received other therapies or surgeries. Moreover, additional large-scale clinical research studies are needed to confirm these findings and to evaluate the effect of categorizing PLR on patients who underwent CLR for suspected HCC. Finally, in view of recurrence after resection being an important prognostic factor, we intend to further record more data in the future.
In summary, this study highlights the potential of PLR as an additional prognostic tool and performs for the first time a categorization of HCC patients with preoperative PLR into tertiles, with significantly improved outcomes among HCC patients following CLR. We suggest that clinicians should consider the level of preoperative PLR as a helpful tool to select the most appropriate therapy scheme for their HCC patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81500665), Scientific Research Foundation of Wenzhou (No. Y20160223), High Level Creative Talents from Department of Public Health in Zhejiang Province, and the Project of New Century 551 Talent Nurturing in Wenzhou to MH Zheng. No writing assistance was obtained for this manuscript.
Abbreviations
- AFP
alpha-fetoprotein
- AST
aspartate aminotransferase
- CI
confidence interval
- CLIP
Cancer of the Liver Italian Program
- CLR
curative liver resection
- CT
computerized tomography
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- LC
liver cirrhosis
- MRI
magnetic resonance imaging
- NLR
neutrophil-to-lymphocyte ratio
- OS
overall survival
- PLR
platelet-to-lymphocyte ratio
- γ-GT
γ-glutamyl transferase
References
- 1.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–245. doi: 10.1097/MCG.0b013e3181d46ef2. doi: 10.1097/MCG.0b013e3181d46ef2. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi NJ, Suh KS, Kim T, Kim J, Shin WY, Lee KU. Current role of surgery in treatment of early stage hepatocellular carcinoma: resection versus liver transplantation. Oncology. 2008;75(Suppl 1):124–128. doi: 10.1159/000173434. doi: 10.1159/000173434. [DOI] [PubMed] [Google Scholar]
- 6.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution’s experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int. 2014;13:153–161. doi: 10.1016/s1499-3872(14)60025-4. doi: 10.1016/S1499-3872(14)60025-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhong JH, Li H, Li LQ, You XM, Zhang Y, Zhao YN, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38:286–295. doi: 10.1016/j.ejso.2012.01.006. doi: 10.1016/j.ejso.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2–14. doi: 10.1159/000339016. doi: 10.1159/000339016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan CK, Law NM, Ng HS, Machin D. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol. 2003;21:2294–2298. doi: 10.1200/JCO.2003.03.151. doi: 10.1200/JCO.2003.03.151. [DOI] [PubMed] [Google Scholar]
- 12.Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103–109. doi: 10.1136/gut.48.1.103. doi: 10.1136/gut.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prajapati HJ, Xing M, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203:W706–W714. doi: 10.2214/AJR.13.12308. doi: 10.2214/AJR.13.12308. [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. doi: 10.1002/1097-0142(20000801)89:3<500::AID-CNCR4>3.0.CO;2-O. [PubMed] [Google Scholar]
- 16.Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. Eur J Surg Oncol. 2008;34:727–729. doi: 10.1016/j.ejso.2008.01.016. doi: 10.1016/j.ejso.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Clarke SJ, Chua W, Moore M, Kao S, Phan V, Tan C, et al. Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther. 2011;90:475–478. doi: 10.1038/clpt.2011.122. doi: 10.1038/clpt.2011.122. [DOI] [PubMed] [Google Scholar]
- 20.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 21.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–273. doi: 10.3802/jgo.2012.23.4.265. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunduz S, Mutlu H, Tural D, Yıldız Ö, Uysal M, Coskun HS, et al. Platelet to lymphocyte ratio as a new prognostic for patients with metastatic renal cell cancer. Asia Pac J Clin Oncol. 2015;11:288–292. doi: 10.1111/ajco.12358. doi: 10.1111/ajco.12358. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Ke Q, Wang Y, Wang W, Zhang M, Shen Y, et al. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol. 2015;13:60. doi: 10.1186/s12957-015-0472-2. doi: 10.1186/s12957-015-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Chen ZH, Xing YF, Wang TT, Wu DH, Wen JY, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2015;36:2263–2269. doi: 10.1007/s13277-014-2833-9. doi: 10.1007/s13277-014-2833-9. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988–993. doi: 10.1038/bjc.2012.354. doi: 10.1038/bjc.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI) J Hepatol. 2012;57:1013–1020. doi: 10.1016/j.jhep.2012.06.022. doi: 10.1016/j.jhep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198:73–79. doi: 10.1016/j.jss.2015.05.003. doi: 10.1016/j.jss.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 30.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 32.Domínguez I, Fernández-del Castillo C. Preoperative platelet-lymphocyte ratio in resected pancreatic ductal carcinoma: is it meaningful? Am J Surg. 2012;203:412. doi: 10.1016/j.amjsurg.2009.05.022. doi: 10.1016/j.amjsurg.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. doi: 10.7314/apjcp.2013.14.9.5237. doi: 10.7314/APJCP.2013.14.9.5237. [DOI] [PubMed] [Google Scholar]
- 34.Feng JF, Huang Y, Zhao Q, Chen QX. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. Scientific World Journal. 2013;2013:504365. doi: 10.1155/2013/504365. doi: 10.1155/2013/504365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 36.Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32–41. doi: 10.1111/tri.12191. doi: 10.1111/tri.12191. [DOI] [PubMed] [Google Scholar]
- 37.Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110:2524–2530. doi: 10.1038/bjc.2014.163. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kepner N, Lipton A. A mitogenic factor for transformed fibroblasts from human platelets. Cancer Res. 1981;41:430–432. [PubMed] [Google Scholar]
- 40.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–362. doi: 10.1016/j.ccr.2006.10.002. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–2760. doi: 10.1002/ijc.27441. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 42.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004;29:132–140. doi: 10.1097/00006676-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Egan K, Crowley D, Smyth P, O’Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6:e26125. doi: 10.1371/journal.pone.0026125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. 2014;31:239. doi: 10.1007/s12032-014-0239-6. doi: 10.1007/s12032-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 46.Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43. doi: 10.1186/1471-2407-14-43. doi: 10.1186/1471-2407-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blank S, Wang Q, Fiel MI, Luan W, Kim KW, Kadri H, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21:986–994. doi: 10.1245/s10434-013-3357-z. doi: 10.1245/s10434-013-3357-z. [DOI] [PubMed] [Google Scholar]
- 48.Azab B, Mohammad F, Shah N, Vonfrolio S, Lu W, Kedia S, et al. The value of the pretreatment neutrophil lymphocyte ratio vs. platelet lymphocyte ratio in predicting the long-term survival in colorectal cancer. Cancer Biomark. 2014;14:303–312. doi: 10.3233/CBM-140416. doi: 10.3233/CBM-140416. [DOI] [PubMed] [Google Scholar]