Abstract
The aims of this study were to (1) determine if older people at their fast walking speed (FWS) are able to reach the speed required at pedestrian crossings (>1.2 m/s) and (2) determine the role of cognitive impairment on the ability to alter speed and walk quickly. Participants were recruited from the Angers Memory Clinic, France. Gait speed was assessed at preferred and FWS using a GAITRite walkway. Walking speed reserve (WSR) was calculated as the difference between FWS and preferred speeds. Participants were classified into cognitive stages (cognitively healthy, mild cognitive impairment, mild and moderate dementia) based on neuropsychological evaluations. The proportion of participants with a FWS of <1.2 m/s was reported. The association between cognitive stage and preferred, fast and walking speed reserve was assessed using multivariable regression, adjusting for covariates. The mean age of the sample (n = 681) was 73.3 (SD 5.8) years. At preferred speed 73.7%, and at FWS 12.8%, of participants had speeds less than 1.2 m/s. Poorer cognitive stage was associated with slower preferred speed (β −0.08, 95% CI −0.10, −0.06), FWS (β −0.13, 95% CI −0.16, −0.10) and also with smaller WSR (m/s) (β −0.05, 95% CI −0.07, −0.03), but not WSR (%) (β −1.73, 95% CI −4.38, 0.93). In older people, worse stages of cognitive impairment were associated with poorer ability to increase speed and walk quickly. Such limitations may result in reduced ability to access the community.
Keywords: Cognition, Dementia, Gait, Fast walking speed
Introduction
In order to successfully ambulate in the community, being able to alter speed and walk quickly is important. For example, a decline in preferred walking speed (PWS) with age (Callisaya et al. 2008) may require an older person to increase their walking speed in order to cross the road where pedestrian signals are based on minimum speeds of 1.2 m/s (Donoghue et al. 2016). Walking quickly may be necessary for keeping up with peers or increasing the intensity of physical activity for better health (Office of Disease Prevention and Health Promotion 2008). Slower fast walking speeds (FWS) have also been found to be clinical markers of adverse health outcomes in older age such as falls (Middleton et al. 2016), disability (Artaud et al. 2015) and mortality (Studenski et al. 2011; Sabia et al. 2014). Furthermore, the extent of increase from PWS to FWS (termed walking speed reserve [WSR]) may provide additional information compared with PWS or FWS alone (Middleton et al. 2016). However, there is limited understanding of the factors that contribute to either WSR or FWS.
Altering speed and walking quickly is a challenging task that may require greater cognitive resources than PWS in order to maintain balance and rapidly take in changing surroundings (Fitzpatrick et al. 2007). Two prior studies reported that slower FWS is associated with poorer global cognition (Fitzpatrick et al. 2007; Deshpande et al. 2009), and one further study showed associations with poorer memory, visuospatial ability, executive function and processing speed (Callisaya et al. 2012). No studies to our knowledge have examined whether cognitively impaired older people can reach walking speeds required at pedestrian crossings or examined the associations between different stages of cognitive impairment and FWS or WSR. The aims of this study were to (i) determine the proportion of people in the overall sample, and at each cognitive stage (cognitively healthy, mild cognitive impairment (MCI), mild and moderate dementia), unable to reach walking speeds of 1.2 m/s and (ii) examine the associations between cognitive stage and the ability to increase walking speed (WSR) and walk quickly (FWS). The results of this study may lead to a better understanding of the mechanisms underlying FWS, and assist clinicians develop programs to help older people maintain access to the community.
Methods
Participants were from the Gait and Alzheimer Interactions Tracking (GAIT) study. GAIT is a study of older people from the Angers Memory Clinic in France with the aim to compare gait characteristics during different challenging tasks among older people at different stages of cognitive impairment. The study design and assessments have been previously described in detail (Beauchet et al. 2013). Assessment was from November 2009 to 2015. Participants were excluded if they had an acute medical illness in the past month, extrapyramidal rigidity of the upper limbs, neurological or psychiatric diseases (other than cognitive impairment), severe medical conditions affecting walking like major orthopaedic diagnoses (e.g. severe osteoarthriti) involving the lumbar vertebra, pelvis or lower extremities or were unable to walk 15 m unassisted.
Medical assessment
A standardized questionnaire and interview was used to record age, sex, education level (level was divided into a binary term at secondary education or above), number of drugs taken (used as a measure of morbidity burden (de Decker et al. 2013)) and use of psychoactive (i.e. benzodiazepines, antidepressants or neuroleptics) and diabetes drugs were recorded. High blood pressure was defined as a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or the use of antihypertensive drugs defined by the use of at least one of the following drug therapies: renin-angiotensin inhibitor agents, beta-blocking agents, diuretics, calcium channel blockers and central antihypertensive agents. Functional ability was assessed with the Instrumental Activities of Daily Living scale and depression with the 4-item geriatric depression scale (scores ≥2 were classified as depressed). Height and weight were recorded to calculate body mass index.
Cognition
A comprehensive cognitive battery including the Mini-Mental State Examination (MMSE) (Folstein et al. 1975), Frontal Assessment Battery (Dubois et al. 2000), Alzheimer’s Disease Assessment Scale-Cognitive (Rosen et al. 1984), Trail Making Test parts A and B (Lezak 1995) and the French version of the Free and Cued Selective Reminding Test (Grober et al. 1988) were administered by a qualified neuropsychologist.
Participants were classified as having mild cognitive impairment (MCI) following the Winblad consensus criteria (Winblad et al. 2004) during multidisciplinary meetings of the Angers University Memory Clinic. Further classification was made into amnesic, non-amnesic domains or multi-domain MCI (having both amnesic and non-amnesic impairments). A diagnosis of dementia was also made during multidisciplinary meetings based on the NINCDS/ADRDA criteria (McKhann et al. 1984). The level of severity of dementia was classified as mild if MMSE scores were above 19 with any impairment in activities of daily living. Moderate dementia was classified as scores of 10–19 on the MMSE and any impairment in activities of daily living.
Gait assessment
Gait speed was assessed using the GAITRite walkway (Gold walkway, 972 cm long, active electronic surface area 792 × 610 cm, total 29,952 pressure sensors, scanning frequency 60 Hz; CIR System, Havertown, PA, USA). Participants completed one trial at their PWS and one trial at their FWS (asked to walk as quickly, but as safely as possible) in a quiet, well-lit environment wearing their own footwear. All participants completed the tests without a gait aid. WSR was calculated as the raw difference between FWS-PWS and WSR (%) was calculated as *100.
Data analysis
STATA 12 (StataCorp LP Texas, USA) was used for all statistical analyses. Baseline characteristics of the sample and the gait measures were described stratified by sex as frequencies (%) or means (SD) as appropriate. For the first aim, the proportion of participants not able to reach 1.2 m/s for both preferred and fast speeds were determined for the overall sample and for each cognitive stage. For aim two, linear regressions were used to examine the association between stages of cognitive impairment (independent variable) and each gait outcome measure (dependent variable). Multivariable linear regression was performed adjusting for age, sex and education. Additional adjustment was made for psychoactive or diabetic drug use, hypertension, number of medications, depression and body mass index if their addition changed the beta-coefficient of the gait variable by >10% (Maldonado and Greenland 1993). The MMSE was not included as a covariate as it was used in the assessment of cognitive stage and would result in collinearity if both were included in the model. Interactions between cognitive stage and sex were assessed with their product term in the model. Results are presented as an overall test of trend across cognitive stages. The strength of associations between cognitive stage and each gait variable are summarized as partial r 2 values from the final models. We also performed a secondary analysis to compare the differences in gait measures between subtypes of MCI (amnestic, non-amnestic or multi-domain type) and cognitively healthy individuals with a one-way ANOVA adjusting for multiple comparisons using the Tukey–Kramer test. Ethics approval was obtained from the ethics committee of Angers University Hospital. Informed consent was obtained from all participants.
Results
The average age of the sample (n = 681; range 59–91) was 73.3 years (SD 5.8), and 56.8% (n = 387) were male. Forty-seven percent (n = 321) were classified as cognitively healthy, 37% (n = 252) as having MCI, 10.3% (n = 70) with mild dementia and 5.6% (n = 38) with moderate dementia. The number of people with different types of dementia were as follows: Alzheimer’s (n = 89), neurodegenerative non-Alzheimer (n = 4), vascular (n = 3) and mixed (n = 12). Table 1 provides the characteristics of the sample by cognitive stage. For the full sample, the average speed was 1.04 m/s (SD 0.23) for PWS and 1.56 m/s (SD 0.34) for FWS. The mean WSR was 0.52 m/s (SD 0.20) or 51.82% (SD 23.95). Women were slightly older than men (74.4 SD 6.5 vs 72.4 SD 5.0; p < 0.001), more likely to report depression (10.2 vs 5.4%; p = 0.02) and have less education (secondary above 50 vs 63.3%; p < 0.001).
Table 1.
Participant characteristics by cognitive stage
| Characteristic, n % unless indicated | Total n = 681 | Healthy n = 321 | MCI n = 252 | Mild n = 70 | Moderate n = 38 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years mean SD | 73.3 | 5.8 | 70.8 | 3.5 | 73.1 | 5.1 | 80.1 | 5.5 | 82.9 | 5.9 |
| Sex (male) | 387 | 56.8 | 193 | 60.1 | 158 | 62.7 | 24 | 34.3 | 12 | 31.6 |
| Education (≥secondary) | 392 | 57.6 | 221 | 68.9 | 134 | 53.2 | 28 | 40.0 | 9 | 23.7 |
| Height cm, mean SD | 164.5 | 9.2 | 166.0 | 8.7 | 165.2 | 9.0 | 159.5 | 8.9 | 157.1 | 9.2 |
| Weight kg, mean SD | 70.9 | 13.0 | 71.1 | 11.9 | 73.2 | 13.5 | 66.3 | 13.3 | 62.3 | 13.7 |
| BMI, mean SD | 26.1 | 4.02 | 25.7 | 3.4 | 26.8 | 4.4 | 26.0 | 4.6 | 25.1 | 4.4 |
| Hypertension | 231 | 33.9 | 89 | 27.7 | 100 | 39.7 | 29 | 41.4 | 13 | 34.2 |
| Taking diabetes medications | 28 | 4.1 | 7 | 2.1 | 13 | 5.2 | 5 | 7.1 | 3 | 7.9 |
| Number of drugs | 3.3 | 3.1 | 2.6 | 2.4 | 3.5 | 3.3 | 4.9 | 3.5 | 4.9 | 3.7 |
| Psychoactive medications | 129 | 18.94 | 43 | 13.4 | 45 | 17.9 | 25 | 35.7 | 16 | 42.1 |
| Depression | 126 | 18.5 | 43 | 13.4 | 45 | 17.9 | 24 | 34.3 | 14 | 36.8 |
| MMSE, mean SD | 26.7 | 3.5 | 28.5 | 1.4 | 27.1 | 1.8 | 22.7 | 2.4 | 16.7 | 3.0 |
MCI mild cognitive impairment, Mild mild dementia, Moderate moderate dementia, BMI body mass index, MMSE mini mental status examination, Depression Geriatric Depression Scale-4 ≥2
Aim 1: the proportion of people unable to reach speeds of 1.2 m/s
Table 2 provides the mean of each gait measure at each cognitive stage (cognitively healthy, MCI, mild and moderate dementia) for men and women separately. Men had better walking performance than women (all p < 0.001), except in the mild and moderate dementia stages where there were no significant sex differences in WSR (%) (p > 0.05). Overall, 73.7% of older people had a preferred walking speed of less than 1.2 m/s, and 12.8% of older people could not reach this speed at their FWS. Of those able to reach 1.2 m/s at FWS, on average, they had to increase their PWS by 0.45 m/s or 39.8%. Nearly all participants with mild or moderate dementia had a PWS slower than 1.2 m/s. Fifty percent of women with mild dementia, and over 65% of men and women with moderate dementia could not reach 1.2 m/s at their FWS. In unadjusted analysis, there was a significant trend across cognitive stage for both men and women for slower preferred speed (both p < 0.001), slower fast speed (both p < 0.001) and smaller WSR m/s (both p < 0.001). For WSR %, the trend was significant for men (p = 0.02), but not women (p = 0.76). There was a significant sex × cognitive stage interaction for preferred speed (interaction p = 0.01), but not for fast speed (interaction p = 0.19), WSR m/s (interaction p = 0.39) or WSR % (interaction p = 0.06).
Table 2.
Univariable associations between cognitive stage and gait measures (n = 681)
| Preferred (m/s) | % under 1.2 m/s | Fast (m/s) | % under 1.2 m/s | WSR (m/s) | WSR (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | Mean | SD | Mean | SD | |
| Men | ||||||||||
| Cognitively healthy (n = 193) | 1.13 | 0.19 | 61.7 | 1.75 | 0.25 | 0.5 | 0.62 | 0.20 | 56.66 | 22.19 |
| MCI (n = 158) | 1.08 | 0.19 | 72.2 | 1.64 | 0.28 | 5.1 | 0.56 | 0.19 | 53.41 | 21.16 |
| Mild dementia (n = 24) | 0.93 | 0.19 | 91.7 | 1.41 | 0.21 | 16.7 | 0.48 | 0.13 | 54.51 | 22.22 |
| Moderate dementia (n = 12) | 0.73 | 0.26 | 100.0 | 1.03 | 0.42 | 66.7 | 0.30 | 0.18 | 39.37 | 18.70 |
| Trend (β, 95% CI) | −0.10 | −0.12, −0.07 | −0.18 | −0.21, −0.14 | −0.08 | −0.10, −0.05 | −0.03 | −0.06, −0.01 | ||
| Women | ||||||||||
| Cognitively healthy (n = 128) | 1.11 | 0.20 | 65.6 | 1.62 | 0.22 | 5.5 | 0.51 | 0.16 | 48.26 | 21.10 |
| MCI (n = 94) | 0.98 | 0.22 | 84.0 | 1.43 | 0.29 | 17.0 | 0.45 | 0.18 | 47.17 | 22.18 |
| Mild dementia (n = 46) | 0.78 | 0.19 | 100.0 | 1.14 | 0.30 | 50.0 | 0.36 | 0.19 | 47.24 | 30.06 |
| Moderate dementia (n = 26) | 0.70 | 0.21 | 100.0 | 1.02 | 0.26 | 76.9 | 0.32 | 0.14 | 51.78 | 47.09 |
| Trend (β, 95% CI) | −0.14 | −0.17, −0.12 | −0.21 | −0.24, −0.18 | −0.06 | −0.08, −0.04 | 0.05 | −0.03, 0.04 | ||
One woman was missing a MCI classification
% percentage, WSR walking speed reserve, MCI mild cognitive impairment, β beta coefficient, CI confidence interval
Aim 2: the association between cognitive stage and PWS, FWS and WSR
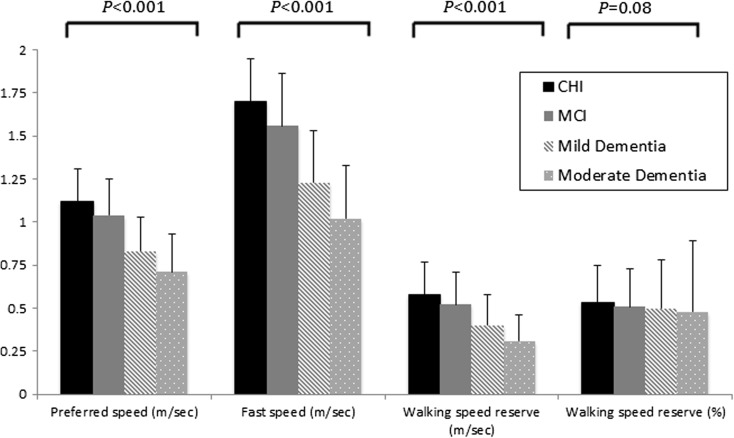
The results are presented for men and women combined as there were no sex interactions in the association between cognitive stage and each gait variable after adjustment for confounders (p > 0.05). There was a significant association between worse cognitive stage and slower PWS, FWS and a smaller WSR m/s (p < 0.05), but not for WSR% (p = 0.08) (Fig. 1). After adjusting for age, sex and education (Table 3), a significant association persisted across poorer stages of cognition and slower PWS (β −0.08 95% CI −0.10, −0.06; trend p < 0.001), slower FWS (β −0.13 95% CI −0.16, −0.01; trend p < 0.001) and a smaller WSR in m/s (β −0.05 95% CI −0.07, −0.03; trend p < 0.001), but not for WSR (%) (β −1.73 95% CI −4.38, 0.93; p = 0.20). The non-significant association for WSR (%) was partially due to an influential female outlier with moderate dementia and a WSR of 270%. Removal of this observation resulted in a slightly stronger association with WSR (%) (p = 0.07). There were also some significant differences between groups as shown in Table 3. The partial r 2 values for cognitive stage were 0.05, 0.07, 0.03 and 0.001 for the regression with PWS, FWS, WSR (m/s) and WSR (%) respectively. The addition of body mass index, number of medications, psychoactive or diabetes medications, hypertension or depression did not change any of the associations by >10% and were therefore not included.
Fig 1.
Preferred, fast and change in walking speed by cognitive stage. CHI cognitively healthy individuals, MCI mild cognitive impairment, Mild mild dementia, Moderate moderate dementia
Table 3.
Multivariable associations between cognitive stage and each gait measure
| Preferred speed (m/s) | Fast speed (m/s) | WSR (m/s) | WSR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p value | β | 95% CI | p value | β | 95% CI | p value | β | 95% CI | p value | |
| Healthy (n = 321) | ref | ref | ref | ref | ||||||||
| MCI (n = 252) | −0.05 | −0.08, −0.17 | 0.003 | −0.09 | −0.13, −0.05 | <0.001 | −0.04 | −0.07, −0.01 | 0.008 | −2.29 | −6.36, 1.77 | 0.27 |
| Mild (n = 70) | −0.17 | −0.23, −0.12a | <0.001 | −0.27 | −0.35, −0.20a | <0.001 | −0.10 | −0.16, −0.05 | <0.001 | −3.06 | −10.32, 4.21 | 0.41 |
| Moderate (n = 38) | −0.26 | −0.33, −0.18a | <0.001 | −0.42 | −0.52, −0.32a,b | <0.001 | −0.16 | −0.24, −0.09a | <0.001 | −4.76 | −14.24, 4.71 | 0.32 |
| P < 0.001 | P < 0.001 | P < 0.001 | P = 0.20 | |||||||||
Adjusted for age, sex and education
WSR walking speed reserve, MCI mild cognitive impairment, Mild mild dementia, Moderate moderate dementia
Other group diffferences are indicated
aDifferent to MCI (p < 0.05)
bDifferent to Mild dementia (p < 0.05)
Secondary analyses of the associations between MCI subtypes and PWS, FWS and WSR
Table 4 provides the mean speeds for each gait measure by subtype of MCI divided into amnestic (n = 59, 12.5%), non-amnestic (n = 169, 67.3%) and multi-domain (n = 23, 9.16%). After adjusting for covariates (age, sex and education), there was a statistically significant difference between groups for PWS (F [3565] = 5.63, p ≤ 0.001), FWS (F [3565] = 7.29, p ≤ 0.001), WSR m/s (F (3565) = 3.14, p = 0.02) and WSR % (F [3565] = 3.06, p = 0.03). Post-hoc testing revealed that at PWS the multi-domain MCI group had slower speeds compared with the amnestic (p = 0.03) and cognitively healthy groups (p = 0.007), and the non-amnestic group had slower speeds than the cognitively healthy group (p = 0.02). At FWS (p < 0.001) and for WSR (m/s; p = 0.02), the non-amnestic group had poorer performance compared to cognitively healthy individuals. Finally, the multi-domain MCI group had greater WSR (%) compared with both the non-amnestic (p = 0.03) and amnestic MCI (p = 0.05) groups.
Table 4.
Differences in gait measures between cognitively healthy individuals and MCI subtypes
| Preferred speed (m/s) | Fast speed (m/s) | WSR (m/s) | WSR (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cognitively healthy (n = 321) | 1.12 | 0.19a,b | 1.70 | 0.25a | 0.58 | 0.19a | 0.53 | 0.22 |
| MCI amnestic (n = 59) | 1.10 | 0.14b | 1.63 | 0.22 | 0.54 | 0.18 | 0.50 | 0.18c |
| MCI non-amnestic (n = 169) | 1.04 | 0.21 | 1.55 | 0.32 | 0.51 | 0.20 | 0.50 | 0.22b |
| MCI multi-domain (n = 23) | 0.94 | 0.24 | 1.51 | 0.31 | 0.56 | 0.19 | 0.63 | 0.25 |
| Unadjusted model | p < 0.001 | p < 0.001 | p = 0.002 | p = 0.03 | ||||
| Adjusted model | F (3565) = 5.63; p ≤ 0.001 | F (3565) = 7.29; p ≤ 0.001 | F (3565) = 3.14; p = 0.02 | F (3565) = 3.06 p = 0.03 | ||||
Adjusted for age, sex, education
WSR walking speed reserve, MCI mild cognitive impairment
aDifferent to the non-amnestic group (p < 0.05)
bDifferent to multi-domain (p < 0.05)
cDifferent to mult-domain (p = 0.05)
Discussion
This is the first study to our knowledge to examine whether older people can increase walking speed to a level of 1.2 m/s required to cross the road. In older people attending a memory clinic, we found that 73.7% of older people would need to increase their PWS to safely cross the road, and that 12.8% were unable to reach this speed at all. Importantly, worse stages of cognitive impairment were associated with slower PWS, FWS, a smaller WSR (m/s) but not WSR (%). These findings increase the understanding of the contribution of cognition to challenging walking tasks and highlight the need to consider longer crossing times for older people in order to improve safety and access.
A minimum walking speed of 1.2 m/s is required to cross the road in many countries (Asher et al. 2012; Donoghue et al. 2016). Although the proportion of people unable to reach this level at FWS was low (12.8%), many individuals were likely extending themselves to close to maximum levels (for example in our study, a further 15% of people had FWS of between 1.2 and 1.4 m/s). Slower FWS and the inability to achieve 1.2 m/s were disproportionately found in women, as well as in those with more severe cognitive impairment, potentially increasing the time of exposure for pedestrian accidents that more commonly occur in older people (Observatoire National Interministériel de Sécurité Routière (ONISR) 2006).
FWS is not only important for community ambulation, but is also a sensitive predictor of adverse health outcomes such as falls, disability and mortality (Deshpande et al. 2009; Studenski et al. 2011; Sabia et al. 2014; Artaud et al. 2015; Middleton et al. 2016). Understanding the underlying mechanisms is important in order to implement appropriate preventative or compensatory interventions. Prior studies have found that FWS is associated with lower scores on continuous measures of either global cognitive function (such as the MMSE or 3MSE) (Fitzpatrick et al. 2007; Deshpande et al. 2009) or with different cognitive domains in those without dementia (Callisaya et al. 2012). Our study extends these findings, by demonstrating associations across stages of cognitive impairment including MCI, mild and moderate dementia. Cognitive stage explained a slightly greater proportion of the variance for FWS than for PWS suggesting that FWS may require slightly greater cognitive resources. We also examined sub-types of MCI. Our findings suggest that a diagnosis of non-amnestic MCI is particularly important in the ability to walk quickly. The non-amnestic subtype broadly represented deficits in executive function. Executive function includes processes such as planning, initiation, attention, cognitive flexibility, judgement and decision making (Lezak 1995), skills that are essential in negotiating a community environment.
The ability to alter walking speed from preferred to fast speed has been termed WSR (Middleton et al. 2016). For example, two people may both have a PWS of 1.0 m/s, but one person can increase to a FWS of 1.2 cm/s (WSR = 0.2 m/s) and another 1.7 m/s (WSR = 0.7 m/s). A greater reserve may indicate better cognitive and physiological ability but few studies have examined whether this is the case (Callisaya et al. 2012). We found a smaller WSR (m/s), but not WSR (%), in those with worse cognitive impairment. Interestingly, there was an influential woman with moderate dementia who started with a PWS less than 0.50 m/s, and increased speed by 270%. It may be that some individuals with greater cognitive impairment increase speed to a level that is too fast for their abilities, but this requires further study. Future research should investigate whether such individuals have different health outcomes, such as falls, to those with a lower WSR (%).
The underlying mechanisms for our findings may include neurodegeneration (Rosano et al. 2007; Callisaya et al. 2013; Del Campo et al. 2016) and vascular pathology (Callisaya et al. 2013). Both cognition and gait impairments are associated with lower grey (Rosano et al. 2007; Jokinen et al. 2012; Callisaya et al. 2014) and white matter volume (Callisaya et al. 2013), as well as cerebrovascular disease such as white matter hyperintensities (Jokinen et al. 2012) and cortical and subcortical infarcts (Rosano et al. 2006; Choi. 2012). Cerebrovascular disease and brain atrophy, particularly in prefrontal and subcortical areas, may underlie the associations between non-amnestic MCI and slower FWS (Rosano et al. 2007; Choi et al. 2012; Callisaya et al. 2014). Associations between brain imaging markers and FWS have not been undertaken, but it could be hypothesised that walking at a fast speed might require greater areas of cerebral brain activation to perform the challenging task.
This study has a number of strengths. Walking speed was measured quantitatively with a sensitive electronic mat under standard protocols. Although we did not use the Clinical Dementia Rating Scale, the diagnosis of cognitive stage was made using both comprehensive clinical and objective tests using standardized criteria. The sample was large allowing us to examine associations and differences across multiple categories. There are, however, some limitations. This was a cross-sectional study and the direction of associations is uncertain. There were few participants in the MCI subtype and moderate dementia categories and therefore caution is required in interpreting results. Although the study population was from a memory clinic making the results less generalizable to the wider population, our results are generalizable to clinical situations where diagnosis of cognitive disorders generally occurs. Those who we classified as cognitively healthy had subjective cognitive complaints or a concerned carer, and therefore may be different to those without such a complaint. However, although from only one trial of each speed, our mean values for gait speed for cognitively healthy people were similar for PWS (Shumway-Cook et al. 2007; Callisaya et al. 2008) and slightly faster for FWS (Shumway-Cook et al. 2007; Callisaya et al. 2012), than those from population-based studies. Participants were excluded if they had major orthopaedic diagnoses (e.g. severe osteoarthritis) that effected walking. However, it is possible that some participants had musculoskeletal conditions which could have influenced gait performance. Finally, it would be interesting in future research to examine the associations between cognitive stage and the patterns or determinants of gait speed (e.g. step length and cadence).
In conclusion, we found that many older people would need to increase their PWS in order to safely cross at pedestrian crossings. Poorer cognitive stage was associated with slower PWS, FWS and a smaller WSR (m/s), which may limit access to the community in these individuals. These results may provide important information for governments to improve the community environment for older people and for clinicians when considering programs to improve community ambulation.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by the French Ministry of Health (Projet Hospitalier de Recherche Clinique national n° 2009-A00533-54).
References
- Artaud F, Singh-Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in fast gait speed as a predictor of disability in older adults. J Am Geriatr Soc. 2015;63(6):1129–1136. doi: 10.1111/jgs.13442. [DOI] [PubMed] [Google Scholar]
- Asher L, Aresu M, Falaschetti E, Mindell J. Most older pedestrians are unable to cross the road in time: a cross-sectional study. Age Ageing. 2012;41(5):690–694. doi: 10.1093/ageing/afs076. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Launay C, Herrmann FR, Annweiler C. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? J Nutr Health Aging. 2013;17(3):235–239. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Sex modifies the relationship between age and gait: a population-based study of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(2):165–170. doi: 10.1093/gerona/63.2.165. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, McGinley JL, Srikanth VK. Risk of falls in older people during fast-walking—the TASCOG study. Gait Posture. 2012;36(3):510–515. doi: 10.1016/j.gaitpost.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, Srikanth VK. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc. 2013;61(7):1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, Chong W, Srikanth V. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke. 2012;43(6):1505–1510. doi: 10.1161/STROKEAHA.111.647271. [DOI] [PubMed] [Google Scholar]
- de Decker L, Launay C, Annweiler C, Kabeshova A, Beauchet O. Number of drug classes taken per day may be used to assess morbidity burden in older inpatients: a pilot cross-sectional study. J Am Geriatr Soc. 2013;61(7):1224–1225. doi: 10.1111/jgs.12345. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, Cesari M, Weiner MW, Andrieu S, Vellas B, M. D. S. Group Relationship of regional brain beta-amyloid to gait speed. Neurology. 2016;86(1):36–43. doi: 10.1212/WNL.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue OA, Dooley C, Kenny RA. Usual and dual-task walking speed: implications for pedestrians crossing the road. J Aging Health. 2016;28(5):850–862. doi: 10.1177/0898264315614004. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Buchanan CK, Nahin RL, Dekosky ST, Atkinson HH, Carlson MC, Williamson JD. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–903. doi: 10.1212/WNL.38.6.900. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Lipsanen J, Schmidt R, Fazekas F, Gouw AA, van der Flier WM, Barkhof F, Madureira S, Verdelho A, Ferro JM, Wallin A, Pantoni L, Inzitari D, Erkinjuntti T, L. S. Group Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology. 2012;78(22):1785–1792. doi: 10.1212/WNL.0b013e3182583070. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Middleton A, Fulk GD, Herter TM, Beets MW, Donley J, Fritz SL. Self-selected and maximal walking speeds provide greater insight into fall status than walking speed reserve among community-dwelling older adults. Am J Phys Med Rehabil. 2016;95(7):475–482. doi: 10.1097/PHM.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Observatoire National Interministériel de Sécurité Routière (ONISR) Grand thèmes de la sécurité routière en France: Piétons. Paris: La Documentation Francaise; 2006. [Google Scholar]
- Office of Disease Prevention and Health Promotion. (2008) Physical Activity Guidelines for Americans. Retrieved 16 January 2016, 2016, from https://health.gov/paguidelines/guidelines/.
- Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26(1):52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Sabia S, Dumurgier J, Tavernier B, Head J, Tzourio C, Elbaz A. Change in fast walking speed preceding death: results from a prospective longitudinal cohort study. J Gerontol A Biol Sci Med Sci. 2014;69(3):354–362. doi: 10.1093/gerona/glt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Guralnik JM, Phillips CL, Coppin AK, Ciol MA, Bandinelli S, Ferrucci L. Age-associated declines in complex walking task performance: the walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55(1):58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment—beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]