Abstract
Age is the single greatest risk factor for most causes of morbidity and mortality in humans and their companion animals. As opposed to other model organisms used to study aging, dogs share the human environment, are subject to similar risk factors, receive comparable medical care, and develop many of the same age-related diseases humans do. In this study, 24 middle-aged healthy dogs received either placebo or a non-immunosuppressive dose of rapamycin for 10 weeks. All dogs received clinical and hematological exams before, during, and after the trial and echocardiography before and after the trial. Our results showed no clinical side effects in the rapamycin-treated group compared to dogs receiving the placebo. Echocardiography suggested improvement in both diastolic and systolic age-related measures of heart function (E/A ratio, fractional shortening, and ejection fraction) in the rapamycin-treated dogs. Hematological values remained within the normal range for all parameters studied; however, the mean corpuscular volume (MCV) was decreased in rapamycin-treated dogs. Based on these results, we will test rapamycin on a larger dog cohort for a longer period of time in order to validate its effects on cardiac function and to determine whether it can significantly improve healthspan and reduce mortality in companion dogs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9972-z) contains supplementary material, which is available to authorized users.
Keywords: Dogs, Cardiac aging, Rapamycin, Echocardiogram, Blood chemistry, Healthspan, Sirolimus
Introduction
Age is the single greatest risk factor for nearly every major cause of morbidity and mortality in humans and their companion animals (Kaeberlein et al. 2016; Pitt and Kaeberlein 2015). Recent advances in the field of geroscience have identified several hallmarks of aging that begin to explain the molecular mechanisms linking aging with disease (Lopez-Otin et al. 2013; Kennedy et al. 2014; Sierra and Kohanski 2017). Interventions that target these hallmarks of aging in laboratory animals are emerging as potential therapeutic strategies for delaying age-related disability and disease and increasing healthy lifespan (Kaeberlein et al. 2015). Among these, rapamycin is the drug that has been shown to most robustly and reproducibly increase lifespan and healthspan in laboratory mice (Kaeberlein 2014).
Rapamycin (also referred to as sirolimus) is a specific inhibitor of the mechanistic target of rapamycin complex I (mTORC1), a central regulator of cell growth and nutrient response (Laplante and Sabatini 2012). It is an FDA-approved drug that has been used clinically in human medicine for many years to prevent organ transplant rejection (Kaeberlein 2013). Rapamycin and rapamycin derivatives are commonly labeled as immunosuppressants, although more recent data indicates that the effect of mTOR inhibitors on immune function is complex and, depending on dose and delivery regimen, may enhance immune function in some contexts (Mannick et al. 2014). Rapamycin is also used clinically for some rare forms of cancer (Xie et al. 2016), to treat tuberous sclerosis complex, and in cardiac stents, to prevent restenosis (Kaeberlein 2013). Several side effects have been associated with rapamycin and rapamycin derivatives at the doses used in human medicine, including stomatitis (Boers-Doets et al. 2013), impaired wound healing (Weinreich et al. 2011), hyperlipidemia (Brattstrom et al. 1998), hyperglycemia (Khan et al. 2016), and thrombocytopenia (Alexandre et al. 1999).
Evidence that rapamycin can delay aging and increase healthspan comes primarily from studies in laboratory organisms. Treatment with rapamycin increases lifespan in evolutionarily divergent organisms including yeast, nematodes, fruit flies, and mice (Johnson et al. 2013; Johnson et al. 2015). In addition to increasing lifespan, treatment with rapamycin has also been shown to improve a variety of age-associated conditions in mice, including reducing cancer incidence (Anisimov et al. 2011), improving cognitive function (Halloran et al. 2012; Majumder et al. 2012), reversing cardiac (Dai et al. 2014; Flynn et al. 2013; Neff et al. 2013) and immune (Chen et al. 2009) declines, restoring stem cell function (Chen et al. 2009; Yilmaz et al. 2012), and improving muscle function (Bitto et al. 2016; Fischer et al. 2015) in aged animals. Of particular note, initiating treatment with rapamycin late in mid-life appears to increase longevity to an extent comparable to treatment beginning early in life (Harrison et al. 2009), and one recent study reported increases in life expectancy from 20 to 60% following single transient treatments with rapamycin from 20 to 23 months of life in mice (Bitto et al. 2016).
The beneficial effects associated with low-dose rapamycin treatment in mice occur generally in the absence of significant side effects, although long-term treatment with rapamycin in mice is associated with defects in spermatogenesis, poorer performance following a glucose tolerance test, and may increase the risk of cataracts (Wilkinson et al. 2012; Lamming et al. 2012). One report found no significant clinical side effects in marmoset monkeys (Callithrix jacchus) treated with up to1 mg/kg/day rapamycin for up to 14 months (Tardif et al. 2015). Limited studies in companion dogs indicate that low doses of rapamycin (0.08 mg/kg/day) are well-tolerated (Paoloni et al. 2010), and one study of a canine glycogen storage disease treated laboratory dogs with 1 mg/kg/day rapamycin for several months without side effects (Yi et al. 2014). Taken together, these observations raise the possibility that non-immunosuppressive doses of rapamycin or rapamycin derivatives could reduce morbidity and mortality in larger animals, including companion dogs and people, at an acceptably low risk of side effects. Consistent with this possibility, one recent study found that 6 weeks of treatment with a rapamycin derivative was sufficient to rejuvenate immune function in healthy elderly people as measured by response to an influenza vaccine (Mannick et al. 2014).
Compared to the commonly used model organisms, companion dogs (Canis lupus familiaris) have great translational potential for geroscience because they uniquely recapitulate many aspects of human aging, including sharing our environment, receiving comparable medical care, and often having detailed medical records available (Pitt and Kaeberlein 2015; Neff and Rine 2006; Gilmore and Greer 2015; Kaeberlein 2015). In this context, the Dog Aging Project seeks to carry out both longitudinal and interventional studies of aging in the privately owned domestic dog, both to gain new insights into mechanisms of human aging and to enhance healthspan and lifespan in dogs (Kaeberlein et al. 2016; Kaeberlein 2015).
Recently, we carried out a randomized double-blind veterinary clinical trial to assess dosing, safety, and effects of rapamycin treatment for 10 weeks in healthy middle-aged companion dogs. Here, we report the results of this study, finding that 10 weeks of rapamycin treatment at either 0.05 or 0.1 mg/kg delivered orally three times per week did not cause significant clinical side effects or abnormal hematological changes, but did result in favorable changes in cardiac left ventricular function during both diastole and systole.
Methods
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC) under protocol number 4359-02. All of the owners completed a written informed consent prior to their first study-related veterinary visit.
Enrollment and selection for this study has been described previously (Urfer et al. 2017). Briefly, 40 companion dogs from the greater Seattle area were recruited through owner submission of an internet-based form on the Dog Aging Project website (www.dogagingproject.com) and were selected for further consideration if the owners reported that they met the following criteria:
the owners agreed to bring the dog to the study veterinary clinic three times during an 11-week period,
the dog weighed at least 18 kg (40 lbs),
the dog was at least 6 years old (no upper age limit), and
the dog did not have any prior or current significant health problems or current medication that might be contraindicated with rapamycin.
Eighty-three dogs were identified that lived within approximately 50 miles of the study clinic and met the age and weight criteria. Seventeen of these dogs were excluded at this stage based on owner-reported pre-existing health conditions or current medications that might be contraindicated with rapamycin, and six dogs were excluded at the recommendation of the clinical trial monitor (SM) based on owner-reported information regarding personality and/or aggression. Sixty owners were invited to participate in the study, 48 were scheduled for an initial exam, ten were non-responsive to email follow-up, and two were unable to schedule an appointment at a mutually agreeable time. Of the 48 owners scheduled for an initial exam, seven canceled their appointments prior to enrollment, and one failed to appear and was non-responsive to follow-up communication.
Forty dogs attended an initial study-related veterinary exam at the VCA Specialty Clinic in Lynnwood, WA, between August 2015 and December 2015 (Fig. 1). At the first examination, medical history was taken and a physical examination performed for each dog. A standard minimum database including a complete blood count, chemistry profile, serum total T4 and free T4ED concentration, and urinalysis was performed on each dog using a commercial reference laboratory (Antech Diagnostics, Kent, WA). Standard echocardiography with continuous ECG monitoring was performed by a board-certified veterinary cardiologist using a GE Vivid i, GEMS ultrasound machine with a 3.0 MHz phased-array transducer (GE Healthcare, Chicago, IL). Standard right parasternal short axis, right parasternal long axis, and left apical views were obtained while the dogs were gently restrained in lateral recumbency without sedation or anesthesia. Color flow Doppler was used to determine the presence or absence of valvular regurgitation. If valvular regurgitation was detected, continuous wave (CW) spectral Doppler imaging was used in the left apical view to measure the peak regurgitant velocity and velocity-derived pressure gradients. CW Doppler recordings were made at a sweep speed of 100 mm/s. The average of three consecutive regurgitant profiles was then calculated, and the severity of the valvular regurgitation was subjectively classified as trace, mild, moderate, or severe for each dog.
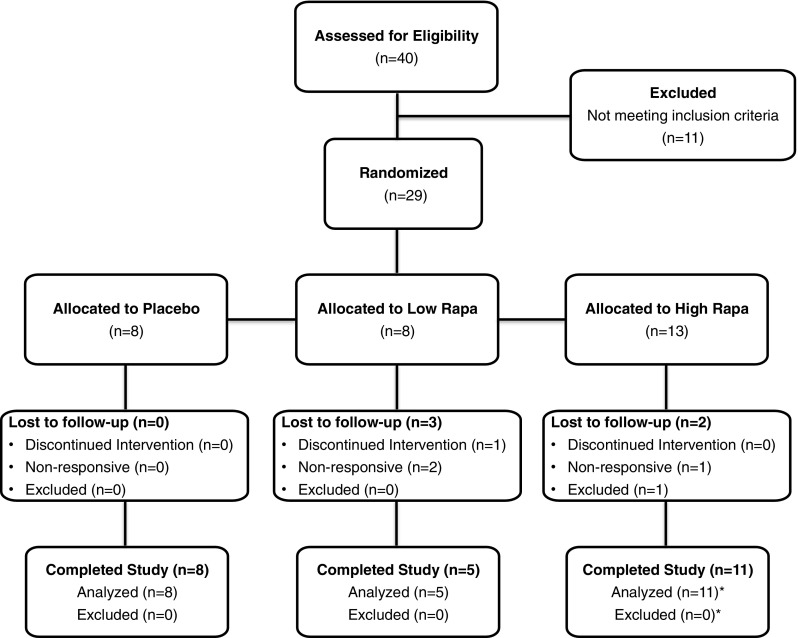
Fig. 1.
Dog Aging Project Rapamycin Intervention Phase I Trial Flow Diagram. Forty dogs were assessed for eligibility at the first veterinary visit, and 11 dogs were excluded due to pre-existing conditions that were inconsistent with the study protocol. Twenty-nine dogs were allocated into treatment groups according to the pre-determined randomization schedule based on their chronological order of entry into the study. Two dogs were lost to follow-up in the low rapamycin group because the owners failed to complete the required veterinary visits. One dog was lost to follow-up in the low rapamycin group because the owner discontinued the medication. Two dogs were lost to follow-up in the high rapamycin group because one owner failed to complete the required veterinary visits and it was discovered during the study period that one owner failed to disclose a pre-existing skin condition requiring medication, resulting in removal of this dog from the study. Asterisk signs indicate a dog in the high-dose rapamycin group completed the entire 10-week study, but it was discovered upon reviewing the dosing log that the owners had mistakenly provided the dog with 0.025 mg/kg three times each week, one quarter the intended dose of the study drug. This dog was removed from analysis of the high rapamycin group, but was included in analysis of the pooled (low and high dose) rapamycin-treated dogs
After the initial visit, each dog was evaluated to determine whether it met the study criterion of being free from a significant pre-existing health condition. Eleven dogs were excluded at this stage (Fig. 1); seven dogs were excluded because of valvular regurgitation (Urfer et al. 2017), and four dogs were excluded because of abnormal blood work (two hypothyroid, one elevated creatinine, one elevated liver enzymes).
Twenty-nine dogs were randomized into a placebo group, a low rapamycin group, or a high rapamycin group according to a pre-established randomization schedule (Table 1). Randomization was performed by the clinic director. Other study personnel (including the cardiologist) and the owners were blind to the randomization. The owners were instructed to administer the study drug to their dogs orally three times per week on Monday, Wednesday, and Friday in the morning at breakfast time and were provided with a dosing log to record study drug administration. The placebo group received a sugar pill (Katterman’s pharmacy, Seattle, WA). The low rapamycin group received 0.05 mg/kg rapamycin (sirolimus, Dr. Reddy’s Laboratories, Shreveport, LA) at each dosing. The high rapamycin group received 0.1 mg/kg rapamycin at each dosing. The dogs in the rapamycin treatment groups were dosed to the nearest 0.5 mg based on their body weight at the initial exam.
Table 1.
Randomization schedule for the Dog Aging Project Rapamycin Intervention Phase I Trial
| Dog no. | Randomized treatment group | Dog no. | Randomized treatment group | Dog no. | Randomized treatment group |
|---|---|---|---|---|---|
| 1 | placebo | 11 | placebo | 21 | 0.1 mg/kg |
| 2 | 0.05 mg/kg | 12 | 0.1 mg/kg | 22 | 0.1 mg/kg |
| 3 | 0.05 mg/kg | 13 | 0.1 mg/kg | 23 | 0.1 mg/kg |
| 4 | 0.05 mg/kg | 14 | placebo | 24 | 0.1 mg/kg |
| 5 | placebo | 15 | 0.1 mg/kg | 25 | placebo |
| 6 | 0.05 mg/kg | 16 | 0.1 mg/kg | 26 | 0.1 mg/kg |
| 7 | 0.05 mg/kg | 17 | placebo | 27 | 0.1 mg/kg |
| 8 | 0.05 mg/kg | 18 | 0.1 mg/kg | 28 | 0.1 mg/kg |
| 9 | 0.05 mg/kg | 19 | 0.1 mg/kg | 29 | placebo |
| 10 | 0.05 mg/kg | 20 | placebo |
The dogs were randomized into the study in chronological order following the first exam upon determination that they met the study criteria, including no significant pre-existing conditions. The study medication was allocated at the corresponding dose according to the dog weight at the first visit, and the owners were given instructions to provide the study medication three times each week on Monday, Wednesday, and Friday in the morning. The owners and study staff, with the exception of the clinic director, were blinded to the randomization groups
Eight dogs were randomized into the placebo group, and all eight dogs completed the entire 10-week treatment period and were included in subsequent analyses.
Eight dogs were randomized into the low rapamycin group, and five dogs completed the entire 10-week treatment period and were included in subsequent analyses. Two dogs were lost to follow-up because the owners failed to complete the required veterinary visits. One dog was lost to follow-up because the owner discontinued the medication. One dog was diagnosed with putative geriatric onset laryngeal paralysis polyneuropathy during the study but completed the study without complications.
Thirteen dogs were randomized into the high rapamycin group, and 11 dogs completed the entire 10-week treatment period and were included in subsequent analyses. Two dogs were lost to follow-up, one because the owner failed to complete the required study visits and one because it was discovered during the study period that the dog did not meet the enrollment criteria, as the owner had failed to disclose a pre-existing skin condition requiring medication. One dog in the high-dose group completed the entire 10-week study, but it was discovered upon reviewing the dosing log that the owners had provided the dog with 0.025 mg/kg rapamycin three times a week instead of 0.1 mg/kg. The data corresponding to this dog were removed from analysis of the high rapamycin group but were included in analysis of the pooled data for all rapamycin-treated dogs.
Following randomization, the owners were asked to complete weekly email surveys describing any perceived changes in the mood, activity, or health of their dog. An exam was scheduled for each dog during their third week in the study. This third week exam included a physical and a blood draw as during the initial exam; however, no echocardiographic exam was carried out during this visit.
A final exam was scheduled for each dog during their eleventh week in the study (within 1 week after cessation of the study medication). The procedures carried out at this time were identical to those used during the initial exam, including echocardiography.
All age and weight values are given as mean ± standard deviation. All statistical analyses were carried out using R (Team RC 2016).
Echocardiographic data were analyzed in a linear regression model that used dogs at baseline as their own controls to compare to end-of-study measurements. This allowed us to correct for the assumption that baseline levels and end-of-study levels were correlated within individual dogs. Based on the literature, we focused on indicators of both diastolic and systolic functions. We also analyzed the differences between end-of-study and baseline scores between rapamycin and placebo using two-sided Wilcoxon rank sum tests.
Echocardiographic parameters are defined as follows:
Ejection Fraction (EF): Percentage of the end-diastolic left ventricular blood volume that is ejected from the left ventricle during systole.
Fractional Shortening (FS): Percentage by which the left ventricular interior diameter is reduced at peak systole as compared to that of end-diastole.
E/A Ratio: Ratio of early- to late-diastolic velocity of blood flowing from the left atrium into the left ventricle.
Clinicopathologic data were analyzed analogous to the heart data. In addition, we performed a principal component analysis to differentiate between the placebo and rapamycin-treated dogs in an attempt to identify principal components that were associated with observed changes.
Results
Twenty-three dogs completed the 10-week study with adherence to the study protocol, and one dog completed the study but was given the drug at one quarter the intended dose (Fig. 1). The age at the start of treatment ranged from 6.6 to 12.7 years (9.7 ± 1.84), and weight ranged from 21 to 44 kg (30.6 ± 5.88). The demographic details of the study subjects are provided in Table 2.
Table 2.
Demographic information for all the dogs that completed the Dog Aging Project Rapamycin Intervention Phase I Trial
| Group | Breed | Sex | Age (years) | Weight (kg) |
|---|---|---|---|---|
| Placebo | Standard poodle | FS | 9.7 | 21 |
| Placebo | Mixed breed | MN | 10 | 44 |
| Placebo | Mixed breed | MN | 6.6 | 31 |
| Placebo | Mixed breed | FS | 7.2 | 25 |
| Placebo | Labrador retriever | FS | 6.8 | 26 |
| Placebo | Mixed breed | MN | 8.3 | 27 |
| Placebo | Great Dane | FS | 10.4 | 37 |
| Placebo | Mixed breed | MN | 7.5 | 33 |
| Low | Mixed breed | MN | 8.8 | 34 |
| Low | Mixed breed | FS | 12.7 | 34 |
| Low | Golden retriever | FS | 12 | 31 |
| Low | English bulldog | MN | 7.2 | 34 |
| Low | Mixed breed | FS | 10.8 | 31 |
| High | Golden retriever | FS | 9.2 | 31 |
| High | Mixed breed | FS | 8 | 36 |
| High | Wheaten terrier | MN | 8.3 | 22 |
| High | Labrador retriever | MI | 7.9 | 34 |
| High | Chow | FS | 9.6 | 23 |
| High | Mixed breed | FS | 11.5 | 22 |
| High | Doberman | FS | 6.8 | 24 |
| High | Labrador retriever | MN | 11.1 | 38 |
| High | Labrador retriever | FS | 8 | 36 |
| High | Mixed breed | MN | 6.8 | 31 |
| Higha | Mixed breed | MN | 11.1 | 29 |
The breed indicates owner-reported information. Age is owner-reported age at the time of the first exam, verified by age indicated on veterinary records. The weight is the measured weight at the first exam
Sex is indicated as follows, verified at initial exam. FS female spayed, FI female intact, MN male neutered, MI male intact
aA dog that was randomized to the high rapamycin group and completed the entire 10-week study, but it was discovered upon reviewing the dosing log that the owners had mistakenly provided the dog with 0.025 mg/kg rapamycin three times each week. This dog was removed from analysis of the high rapamycin group, but was included in analysis of the pooled (low and high dose) rapamycin-treated dogs
For all comparisons throughout the remainder of this manuscript, the dogs that received the placebo and completed the study (n = 8) will be referred to as the “placebo group”, the dogs that received 0.05 mg/kg rapamycin three times each week and completed the study (n = 5) will be referred to as the “low rapamycin group”, and the dogs that received 0.1 mg/kg rapamycin three times each week and completed the study (n = 10) will be referred to as the “high rapamycin group”. A forth comparison group comprised of all dogs that received rapamycin and completed the study group (n = 16) is referred to as the “pooled rapamycin group” and includes the low rapamycin group, high rapamycin group, and the one dog that was erroneously given 0.025 mg/kg rapamycin three times each week.
Side effects and clinical pathology
There were no significant differences in body weight before and after treatment in either the placebo or rapamycin groups. Adverse events, potential side effects, and changes in behavior were tracked through weekly owner surveys. The frequency and percent of dogs for which a particular symptom or behavioral change was reported during the study is shown in Table 3. All reported symptoms and changes in behavior occurred at similar frequencies in the placebo and treatment groups, with the possible exception of increased activity, which was reported in 70% (7/10) of the dogs in the high rapamycin group, 2/5 (40%) of the dogs in the low rapamycin group, and 25% (2/8) of the dogs in the placebo group (P = 0.066, linear model).
Table 3.
Owner-reported symptoms and changes in behavior
| Symptom/behavioral change | Placebo (%) | Low rapa (%) | High rapa (%) |
|---|---|---|---|
| Increased water consumption | 3 (38) | 1 (20) | 4 (40) |
| Fatigue/lethargy | 1 (13) | 1 (20) | 3 (30) |
| Vomiting | 2 (25) | 2 (40) | 2 (20) |
| Diarrhea | 3 (38) | 1 (20) | 4 (40) |
| Cough | 2 (25) | 1 (20) | 3 (30) |
| Skin irritation/rash | 2 (25) | 2 (40) | 2 (20) |
| Lipoma | 1 (13) | 0 (0) | 0 (0) |
| Limping/slipping | 2 (25) | 2 (40) | 0 (0) |
| Constipation | 1 (13) | 0 (0) | 0 (0) |
| Eye irritation | 1 (13) | 0 (0) | 1 (10) |
| Increased appetite | 1 (13) | 2 (40) | 0 (0) |
| Decreased appetite | 1 (13) | 0 (0) | 0 (0) |
| Weight loss | 1 (13) | 0 (0) | 0 (0) |
| Increased energy | 2 (25) | 2 (40) | 7 (70) |
| More affectionate | 0 (0) | 2 (40) | 2 (20) |
| Increased alertness | 0 (0) | 0 (0) | 1 (10) |
Symptoms and changes in behavior reported by owners during the 10 weeks that their dog participated in the study. The number of different dogs within each treatment for which a particular symptom or change in behavior was reported at least once during the study is shown with the percentage of dogs shown in parentheses
Routine clinical pathology remained within normal limits for both hematologic and plasma/chemistry parameters. The rapamycin-treated dogs did show a significant decrease in the volume of the red blood cells over the course of the study (mean corpuscular volume, MCV) when compared to placebo (F 1, 22 = 7.54, P = 0.012, linear model; W = 23.5, P = 0.0086, Wilcoxon two-sided test). A full table of blood parameters studied and changes within treatment groups at weeks 0, 3, and 11 is provided in Supplemental Table 1.
We also performed principal component analysis of the clinical pathology data in order to identify possible principal components that would separate rapamycin-treated from placebo dogs. Analysis of the first 14 principal components suggested no significant differences between profiles of placebo and rapamycin-treated dogs (data not shown).
Echocardiography
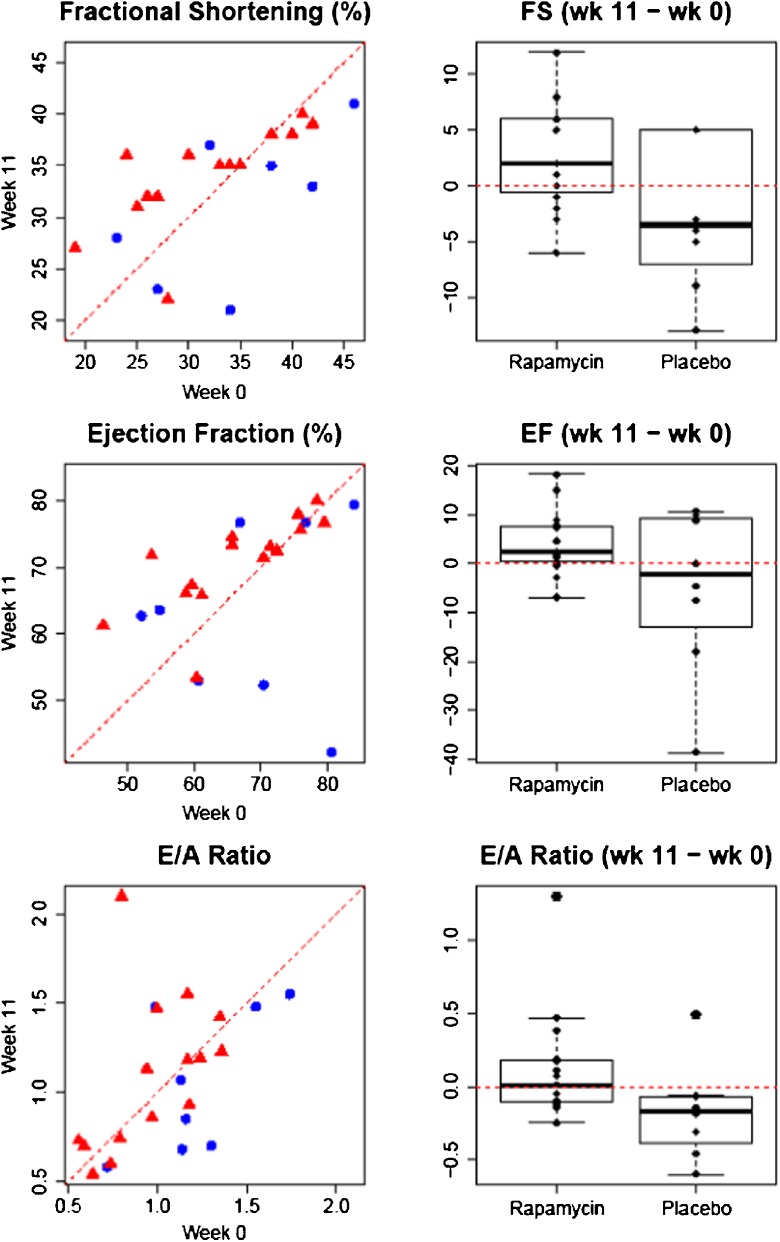
Over the 11-week course of the study, we observed significant changes in both diastolic and systolic function in rapamycin-treated dogs, but not in those treated with placebo. Both the low- and high-dose rapamycin treatment groups showed a trend toward improved fractional shortening (FS), ejection fraction (EF), and E/A ratio. When considered as a single group, the dogs receiving rapamycin had improved FS that reached statistical significance (p < 0.05) relative to the placebo by both the linear model and the non-parametric Wilcoxon rank sum test (linear model: F 2, 20 = 5.05, P = 0.036; Wilcoxon two-sided test: W = 91.0, P = 0.045). Neither E/A ratio (linear model: F 2, 20 = 1.78, P = 0.197; Wilcoxon two-sided test: W = 93.5, P = 0.029) nor EF (linear model: F 2, 20 = 4.06, P = 0.058; Wilcoxon two-sided test: W = 77.5, P = 0.27) reached statistical significance by linear model; however, E/A ratio did by Wilcoxon two-sided test (Fig. 2). Low- and high-dose rapamycin results are presented separately in Supplemental Fig. 1.
Fig. 2.
Differences in fractional shortening (FS), ejection fraction (EF), and E/A ratio in rapamycin (red triangles) vs. placebo (blue circles). x-axis value at baseline; y-axis value at week 11; dotted red lines no change. Rapamycin significantly improved both FS as a measure of systolic function and the E/A ratio as a measure of diastolic function in rapamycin-treated dogs when compared to placebo. Note that FS improvement was more marked in dogs whose baseline scores were low, which is presumably indicative of the existence of a maximum possible FS percentage dictated by cardiac physiology
Discussion
Interventions that delay or reverse molecular mechanisms of aging have the potential to greatly improve health for companion animals and humans alike; however, major challenges must be overcome before such interventions can be brought from the laboratory to the clinic (Kaeberlein et al. 2015). In this study, we have taken a first step toward this goal by testing the effects of rapamycin, the most effective pharmacological agent for increasing lifespan and healthspan in mice and on age-related cardiac function in companion dogs. We find that 10 weeks of low-dose rapamycin treatment in middle-aged dogs is well-tolerated, with no overt side effects relative to placebo, and with improvements in left ventricular cardiac function that are comparable to what has been previously reported from a similar regimen in middle-aged mice (Dai et al. 2014; Flynn et al. 2013; Neff et al. 2013).
Side effects
Rapamycin has been reported to cause certain clinicopathologic changes when given to human patients at immunosuppressive doses (Brattstrom et al. 1998; Khan et al. 2016; Alexandre et al. 1999), but this study provides the first comparable data for dogs given rapamycin in non-immunosuppressive doses.
While the pharmacokinetics and pharmacodynamics of both orally and parenterally administered rapamycin in dogs are known (Paoloni et al. 2010; Larson et al. 2016), no data on its use for periods exceeding 7 days in companion dogs have been previously published. Thus, the primary goal of this study was to determine whether low, non-immunosuppressive doses of rapamycin would cause significant clinical side effects in privately owned domestic dogs when given orally over a 10-week period. This included screening the dogs for clinical changes, as well as hematological and echocardiographic changes.
In this study, all parameters considered in routine bloodwork remained within normal limits in both the treatment and the placebo groups for the duration of the study period. This mirrors findings in marmosets, where 14 months of oral rapamycin treatment did not result in significant changes to the animals’ hematocrit and red and/or white blood cell counts (Tardif et al. 2015), their blood lipids, or their blood glucose (Ross et al. 2015). Changes in behavior such as increased water consumption and altered appetite occurred with similar frequencies in the placebo and treatment groups in the dogs of our report, as did minor adverse events such as vomiting, fatigue, diarrhea, lethargy, and skin irritation (Table 3).
It is interesting to note that the mean corpuscular volume was significantly diminished in rapamycin-treated dogs after 10 weeks when compared to that of placebo. Hematocrit did not change significantly between treatment and placebo (P = 0.803), which in combination with the observed decrease in the MCV may indicate longer red blood cell survival, as the MCV tends to decrease as the red blood cells age. A decrease in red blood cell size in response to treatment with rapamycin has previously been described in vitro (Edinger et al. 2003). It is also interesting to note that older humans have lowered red blood cell survival times, which are reflected in an MCV increase with age, and increased MCV has also been linked to decreased cognitive performance in older humans (Gamaldo et al. 2013). This may indicate an attenuation by rapamycin of an age-related change in red blood cell volume that is known to predict adverse outcomes in humans, and it provides additional justification for studying cognitive function in future studies of rapamycin in aging dogs.
Effects on cardiac function
Both systolic and diastolic functions are known to deteriorate with age in dogs (Templeton et al. 1976) as well as in humans (Xu and Daimon 2016; Parikh et al. 2016). Among these age-associated changes are reduced stroke volume and cardiac output as signs of systolic dysfunction (though not necessarily accompanied by a reduced EF), and a reduced E/A ratio as a sign of diastolic dysfunction. Additionally, age increases the risk of degenerative valve disease (DVD) in both humans (Singh et al. 1999) and dogs (Urfer et al. 2017; Detweiler and Patterson 1965; Jones and Zook 1965).
While there is evidence that rapamycin improves age-related deterioration of cardiac function in laboratory mice (Dai et al. 2014; Flynn et al. 2013), no such effect has been demonstrated in dogs or other animals existing in a natural environment. Our study provides the first evidence that rapamycin may partially reverse age-related heart dysfunction in dogs by improving measures of both diastolic and systolic functions. Although the effects reported here do not reach statistical significance (p < 0.05) for each measure, likely due to the relatively small sample size and high individual heterogeneity (see Supplemental Fig. 2 for power analysis), all three of the outcomes (EF, FS, and E/A ratio) showed trends toward improved function following rapamycin treatment, and two of them (FS and E/A ratio) reached statistical significance. In this context, caloric restriction (CR) is known to inhibit mTOR and also shows beneficial effects on heart function in aged mice that are similar to those induced by rapamycin (Dai et al. 2014), and one study reports lifespan and healthspan extension from CR in dogs maintained in the laboratory (Kealy et al. 2002), possibly also through the inhibition of mTOR. Our results thus indicate that pharmacological inhibition of mTOR in older dogs likely has effects on heart function that are consistent with a reversal of age-related functional changes.
Comparing the scatter plots in Fig. 2, it appears that FS improvement was most prominent in rapamycin-treated dogs with lower baseline values, while dogs that had higher values at baseline did not improve as much in response to rapamycin. This may point toward the existence of a physiological upper limit to FS during systole, which would make using individual dogs as their own controls (as in the linear model applied here) a more powerful approach to detect improvement. In addition, this may suggest that at least for certain functional parameters, the impact of rapamycin on age-related decline in heart function will depend on the degree of decline already present in each individual. It will be of interest to determine whether differential individual outcomes are observed for other functional measures following rapamycin treatment in future studies.
Possible effects of rapamycin on behavior
The relatively small number of dogs studied here prohibits us from reaching a definite conclusion regarding any behavioral effects of rapamycin. However, it is interesting to note that some changes in behavior that were considered to be positive by owners appeared to occur with greater frequency in the rapamycin-treated dogs. Seventy percent (7/10) of the owners whose dogs received the higher rapamycin dose reported that their dog displayed increased activity and energy, as did 40% (2/5) of those whose dogs received the lower dose, compared to 25% (2/8) of owners whose dogs received the placebo. While we make no claims as to whether this represents a real effect of rapamycin in these dogs, such an effect would reflect previous reports of increased activity in rapamycin-treated mice (Flynn et al. 2013; Miller et al. 2011) and would be consistent with anecdotal reports from owners who have independently begun treating their dogs with rapamycin (Cohen 2016).
Similarly, 20% (2/10) of owners in the high rapamycin group and 40% (2/5) of owners in the low rapamycin group reported that their dogs’ behavior changed in a way they interpreted as more affectionate, while none of the owners in the placebo group reported this behavioral change. If such an effect can be confirmed in a future larger scale trial, one possible explanation could be the anti-inflammatory effects of rapamycin reducing pain associated with arthritis. Rapamycin is also known to improve cognitive function in middle-aged mice (Halloran et al. 2012; Majumder et al. 2012), and it is possible that rapamycin could alter cognitive function in dogs in a way that induces these behavioral changes. It will be of particular interest to determine whether similar effects are observed in future longer term studies and to directly assess cognitive function and inflammatory state in dogs receiving rapamycin.
Conclusion
To summarize, this initial study of rapamycin in healthy, middle-aged dogs showed that low-dose rapamycin treatment is safe over a period of 10 weeks and appears to recapitulate some of the same beneficial effects that have been described for mTOR inhibition in mice. The low number of dogs and multiple comparisons performed limit our statistical power, and the relatively short duration did not allow us to determine whether the rapamycin-treated dogs had increased lifespan and healthspan compared to placebo. The absence of significant side effects here is consistent with findings from studies on marmosets in captivity (Ross et al. 2015) as well as prior studies of rapamycin in laboratory dogs (Yi et al. 2014). Unlike these prior studies, however, the dogs in this study were living in their normal home environment, providing an important demonstration that rapamycin can be safely administered to companion dogs with the potential for significant health benefits. Based on these findings, we are planning additional randomized clinical trials involving larger cohorts of middle-aged dogs that will be studied over a longer period of time. This will allow us to perform more powerful analyses of rapamycin’s effects on heart function and behavior and help us determine whether there are differences in mortality, as well as the onset and prevalence of the various diseases that share aging as their common risk factor.
Electronic supplementary material
(PDF 256 kb)
Acknowledgements
We would like to thank Dr. Karen Kline and Dr. Heidi MacLean for clinical veterinary support. This work was partially supported by the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIH Grant P30AG013280). SU was supported by donations from the Irish Wolfhound Association of New England and the Donner Foundation to the Dog Aging Project. DP and KC received support from NIH Grant R24AG044284.
Compliance with ethical standards
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC) under protocol number 4359-02. All of the owners completed a written informed consent prior to their first study-related veterinary visit.
References
- Alexandre J, Raymond E, Armand JP. Rapamycin and CCI-779. Bull Cancer. 1999;86(10):808–811. [PubMed] [Google Scholar]
- Anisimov VN, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Bitto A, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers-Doets CB, et al. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013;9(12):1883–1892. doi: 10.2217/fon.13.141. [DOI] [PubMed] [Google Scholar]
- Brattstrom C, et al. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin) Transplantation. 1998;65(9):1272–1274. doi: 10.1097/00007890-199805150-00023. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E (2016) This pill could make your dog (and maybe you) live longer. (CNN)
- Dai DF, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13(3):529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci. 1965;127(1):481–516. doi: 10.1111/j.1749-6632.1965.tb49421.x. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63(23):8451–8460. [PubMed] [Google Scholar]
- Fischer KE, et al. Health effects of long-term rapamycin treatment: the impact on mouse health of enteric rapamycin treatment from four months of age throughout life. PLoS One. 2015;10(5):e0126644. doi: 10.1371/journal.pone.0126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12(5):851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo AA, Ferrucci L, Rifkind J, Longo DL, Zonderman AB. Relationship between mean corpuscular volume and cognitive performance in older adults. J Am Geriatr Soc. 2013;61(1):84–89. doi: 10.1111/jgs.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore KM, Greer KA. Why is the dog an ideal model for aging research? Exp Gerontol. 2015;71:14–20. doi: 10.1016/j.exger.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Halloran J, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving youth: does rapamycin deliver? Sci Transl Med. 2013;5(211):211fs240. doi: 10.1126/scitranslmed.3007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–127. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- Jones TC, Zook BC. Aging changes in the vascular system of animals. Ann N Y Acad Sci. 1965;127(1):671–684. doi: 10.1111/j.1749-6632.1965.tb49434.x. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. mTOR inhibition: from aging to autism and beyond. Scientifica (Cairo) 2013;2013:849186. doi: 10.1155/2013/849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. Rapamycin and aging: when, for how long, and how much? J Genet Genomics. 2014;41(9):459–463. doi: 10.1016/j.jgg.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M (2015) The biology of aging: citizen scientists and their pets as a bridge between research on model organisms and human subjects. Vet Pathol [DOI] [PMC free article] [PubMed]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350(6265):1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mammalian genome: official journal of the International Mammalian Genome Society. 2016;27(7–8):279–288. doi: 10.1007/s00335-016-9638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy RD, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. 2002;220(9):1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KH, et al. Hyperglycemia and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) inhibitors in phase I trials: incidence, predictive factors, and management. Oncologist. 2016;21(7):855–860. doi: 10.1634/theoncologist.2015-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JC, et al. Pharmacokinetics of orally administered low-dose rapamycin in healthy dogs. Am J Vet Res. 2016;77(1):65–71. doi: 10.2460/ajvr.77.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11(2):326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MW, Rine J. A fetching model organism. Cell. 2006;124(2):229–231. doi: 10.1016/j.cell.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Neff F, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123(8):3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoloni MC, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5(6):e11013. doi: 10.1371/journal.pone.0011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh JD, Hollingsworth KG, Wallace D, Blamire AM, MacGowan GA. Normal age-related changes in left ventricular function: role of afterload and subendocardial dysfunction. Int J Cardiol. 2016;223:306–312. doi: 10.1016/j.ijcard.2016.07.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JN, Kaeberlein M (2015) Why is aging conserved and what can we do about it? PLoS Biol [DOI] [PMC free article] [PubMed]
- Ross C, et al. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus) Aging (Albany NY) 2015;7(11):964–973. doi: 10.18632/aging.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39(1):1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83(6):897–902. doi: 10.1016/S0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- Tardif S, et al. Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015;70(5):577–587. doi: 10.1093/gerona/glu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC . R: a language and environment for statistical computing. Vienna: R foundation for statistical Computing; 2016. [Google Scholar]
- Templeton GH, Willerson JT, Platt MR, Weisfeldt M. Contraction duration and diastolic stiffness in aged canine left ventricle. Recent Adv Stud Cardiac Struct Metab. 1976;11:169–173. [PubMed] [Google Scholar]
- Urfer SR, et al. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39(1):43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich J, et al. Rapamycin-induced impaired wound healing is associated with compromised tissue lactate accumulation and extracellular matrix remodeling. Eur Surg Res. 2011;47(1):39–44. doi: 10.1159/000327972. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang X, Proud CG (2016) mTOR inhibitors in cancer therapy. F1000Res 5 [DOI] [PMC free article] [PubMed]
- Xu B & Daimon M (2016) Cardiac aging phenomenon and its clinical features by echocardiography. J Echocardiogr [DOI] [PubMed]
- Yi H, et al. Correction of glycogen storage disease type III with rapamycin in a canine model. J Mol Med. 2014;92(6):641–650. doi: 10.1007/s00109-014-1127-4. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486(7404):490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 256 kb)