Abstract
Frailty and the metabolic syndrome are each associated with poor outcomes, but in very old people (90+ years) only frailty was associated with an increased mortality risk. We investigated the relationship between frailty, metabolic syndrome, and mortality risk, in younger (20–65 years) and older (65+ years) people. This is a secondary analysis of the US National Health and Nutrition Examination Survey (NHANES) datasets for 2003–2004 and 2005–2006, linked with mortality data up to 2011. The metabolic syndrome was defined using the International Diabetes Federation criteria. Frailty was operationalized using a 41-item frailty index (FI). Compared to the younger group (n = 6403), older adults (n = 2152) had both a higher FI (0.10 ± 0.00 vs. 0.22 ± 0.00, p < 0.001) and a greater prevalence of the metabolic syndrome (24.1 vs. 45.5%, p < 0.001). The metabolic syndrome and FI were correlated in younger people (r = 0.25, p < 0.001) but not in older people (r = 0.08, p < 0.1). In bivariate analyses, the FI predicted mortality risk in both age groups whereas the metabolic syndrome did so only in the younger group. In Cox models, adjusted for age, sex, race, education, and each other, the FI was associated with increased mortality risk at both ages (younger HR 1.05 (1.04–1.06); older HR 1.04 (1.03–1.04) whereas the metabolic syndrome did not contribute to mortality risk. The FI better predicted mortality than did the metabolic syndrome, regardless of age.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9967-9) contains supplementary material, which is available to authorized users.
Keywords: Frailty index, Metabolic syndrome, Frailty in older people, Deficit accumulation, NHANES
Introduction
The metabolic syndrome describes a cluster of risk factors for poor cardiovascular outcomes (Alberti et al. 2006; Maggi et al. 2006). It is defined as central obesity, plus two or more of elevated triglyceride (TG) level, low high-density lipoprotein (HDL) cholesterol level, high blood pressure (BP) or treatment for previous diagnosis of hypertension, and abnormal fasting blood glucose (FBG) level or previous diagnosis of type 2 diabetes (Alberti et al. 2006). Frailty is an age-related, multidimensional condition that represents an increased vulnerability to adverse health outcomes compared with others of the same age (Collard et al. 2012; Hubbard and Theou 2012). The key concept is that frailty captures unexplained heterogeneity in risk for people of the same age (Vaupel et al. 1979). Frailty can be assessed by a frailty index (FI) based on health deficit accumulation (Mitnitski et al. 2002; Rockwood and Mitnitski 2007; Mitnitski et al. 2013). The FI counts the number of health deficits a person has and divides this by the total number of possible deficits evaluated to give an FI score between 0 and 1 (Rockwood and Mitnitski 2007).
Both frailty and the metabolic syndrome are common in adults worldwide, and are associated with adverse outcomes. The FI is associated with an increased risk of institutionalization, disability, and all-cause mortality at all ages, especially in older people (Rockwood et al. 2011; Clegg et al. 2013; Mohler et al. 2014). In adults, the metabolic syndrome is associated with significantly increased risk of cardiovascular disease (CVD), type 2 diabetes, and both cardiovascular and all-cause mortality (Lakka et al. 2002; Meigs et al. 2003; Hu et al. 2004; McNeill et al. 2005; Sattar et al. 2008). The association of metabolic syndrome with adverse outcomes, particularly mortality, in older people is less clear. Some studies report an increased risk of diabetes, CVD, and stroke in older people with the metabolic syndrome (Scuteri et al. 2005; Maggi et al. 2006; Wang et al. 2008). Two studies in older populations found an association of metabolic syndrome with cardiovascular mortality, but only in men (Maggi et al. 2006; Wang et al. 2007). On the other hand, several studies have shown no association of the metabolic syndrome with either CVD (Sattar et al. 2008; Singh et al. 2013) or mortality risk (Hao et al. 2016) in older adults. Alexander and colleagues found an increased mortality risk with some of the individual metabolic syndrome components including high BP, elevated HDL cholesterol, and diabetes but not with metabolic syndrome itself (Alexander et al. 2003). Studies have also reported that some components of the metabolic syndrome including high BP (Blom et al. 2013) and increased waist circumference (Visscher et al. 2001; Price et al. 2006) are actually linked to a reduction in mortality risk in old age (Le Couteur and Simpson 2011).
The relationship between the metabolic syndrome and FI in both younger and older people is also not clear. In adults aged 50+ years, people who are frail are more likely to have metabolic syndrome (Viscogliosi 2016), and likewise, the metabolic syndrome is linked to an increased risk of frailty (Tang et al. 2013; Lin et al. 2015). Other studies, however, report no association between metabolic syndrome and frailty in older populations (70+ years) (Barzilay et al. 2007; Hao et al. 2016). A recent study in very old people (90+ years) showed that while a higher FI score was associated with a greater risk of mortality, metabolic syndrome status was not (Hao et al. 2016). The relative contributions of the FI and metabolic syndrome to mortality risk in other age groups have not been investigated. The aims of this study were to describe the relationship between FI and metabolic syndrome in both younger and older people, and to evaluate how the FI and the metabolic syndrome affect mortality risk in these two groups.
Methods
Sample and study design
This was a secondary analysis of the US National Health and Nutrition Examination Survey (NHANES) dataset for 2003–4 and 2005–6. The NHANES protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention; further details about the study design and participants are found elsewhere (Zipf et al. 2013). There were 10,020 participants aged 20 years or older in the cohort. Individuals missing data for central obesity or more than one other factor in the metabolic syndrome definition were excluded (n = 1412). A further 53 were excluded for missing frailty or mortality data, for a final sample size of 8555 (6403 participants <65 years and 2152 participants ≥65 years).
Self-reported demographic, health, and functional data were collected via interview and questionnaires. A standardized clinical exam was undertaken with each participant to obtain laboratory results and clinical measurements (Zipf et al. 2013). NHANES-linked mortality files were used to identify mortality status at the time of follow up (December 31, 2011). Time to death was calculated from the date of the clinical examination.
Metabolic syndrome
Metabolic syndrome was determined with the International Diabetes Federation (IDF) criteria (Alberti et al. 2006) with some adaptations for the dataset used. The definition used was central obesity as defined by Europid cut-points (waist circumference ≥94 cm for men; ≥80 cm for women), plus two or more other criteria: elevated TG level (≥150 mg/dL), low HDL cholesterol level (<40 mg/dL for men and <50 mg/dL for women), high BP (systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg) or previous diagnosis of hypertension, and abnormal FBG level (≥100 mg/dL) or previous diagnosis of type 2 diabetes. The original definition also included “treatment of previously diagnosed hypertension” or “treatment for lipid abnormalities,” but, as treatment data were not available, previous diagnosis of hypertension was used as a surrogate identifier for high BP, and lipid abnormalities were based only on blood test results. For some analyses, the degree of metabolic syndrome was determined from 0 to 5 based on the number of criteria present.
Frailty index
Frailty was determined with a previously published 46-item FI created for the NHANES dataset (Blodgett et al. 2015). From that 46-item FI, we excluded items associated with, or used to define the metabolic syndrome (high BP diagnosis, diabetes diagnosis, systolic BP, homocysteine, glycohemoglobin); therefore, we finally used a 41-item FI. The FI is a continuous score from 0 to 1, although for some analyses frailty was stratified by FI score according to the following groups: <0.1, 0.1–0.21, 0.21–0.45, and >0.45, as in previous studies (Blodgett et al. 2015; Hao et al. 2016).
Statistical analysis
Sample characteristics are presented as mean ± SD, or percentage present, and differences across age groups were compared with t tests or chi-squared tests as appropriate. Spearman correlation coefficients were calculated to examine the association between FI and the level of metabolic syndrome for each age group. Kaplan-Meier curves with log-rank analysis were used to compare survival probability stratified by FI level and metabolic syndrome status for both younger (<65 years) and older people (≥65 years), with censoring for non-responders. Unadjusted Cox proportional hazard ratios for mortality risk were calculated for each of the components of the FI and the metabolic syndrome, FI score, metabolic syndrome status, education, race, age, and sex, for each age group. Adjusted Cox proportional hazard ratios for mortality were determined for FI and metabolic syndrome status with covariates of age, sex, education, and race for the whole sample and after stratifying by age. Data analysis was conducted using SPSS v20 and graphs were created in SigmaPlot v.11. A value of p < 0.05 was considered statistically significant.
Results
Relationship between frailty and metabolic syndrome in younger and older groups
The older group had a lower proportion of women than the younger group, and there were differences between the age groups for education level and proportions of each race (Table 1). The older group also had a higher mean FI score than the younger group (0.22 ± 0.13 vs. 0.10 ± 0.10). The maximum FI scores in the younger and older groups respectively were 0.65 and 0.78. In both age groups, women had higher FI scores than men (younger 0.12 ± 0.10 vs. 0.09 ± 0.09, p < 0.001; older 0.24 ± 0.13 vs. 0.21 ± 0.13, p < 0.001). The FI score was correlated with age in both groups (younger r = 0.36, p < 0.001; older r = 0.26, p < 0.001). There was a greater proportion of people with metabolic syndrome in the older group than the younger group (45.49 vs. 24.14%, p < 0.001), and the mean values for each of the measured metabolic syndrome items, except TG level, were higher in the older group (Table 1). In the older group but not the younger group, there was a greater proportion of women with metabolic syndrome than men (younger 23.3 vs. 25.1%, p = 0.09; older 49.5 vs. 41.9%, p < 0.001). There was a significant correlation between FI and level of metabolic syndrome (0–5) in the whole sample (r = 0.32, p < 0.001), and the younger group (r = 0.25, p <0.001) but this association was weak in the older group (r = 0.08, p < 0.01). In the younger group, there was a weak correlation between FI score and each of the metabolic syndrome items (Supplementary Table S1). In the older group, there was no correlation between FI and most of the metabolic syndrome items, apart from a weak correlation with waist circumference and a weak negative correlation with diastolic BP (Supplementary Table S1). The mean FI of those who had metabolic syndrome in each age group was higher than the mean FI for those who did not have metabolic syndrome (younger 0.14 ± 0.12 vs. 0.09 ± 0.09, p < 0.001; older 0.24 ± 0.13 vs. 0.21 ± 0.12, p < 0.001).
Table 1.
Demographic and clinical characteristics of the sample by age group
| Whole sample (n = 8555a) | Age groups | |||
|---|---|---|---|---|
| Under 65 (n = 6403b) | Over 65 (n = 2152c) | p value | ||
| Women (%) | 51.4 | 52.7 | 47.5 | <0.001 |
| Mean age (years ± SD) | 48.9 ± 18.9 | 40.3 ± 13.0 | 74.7 ± 6.5 | <0.001 |
| Education level (%): Less than Year 12 |
27.9 | 24.4 | 38.2 | <0.001 |
| Year 12 or equivalent | 24.3 | 23.5 | 26.5 | |
| More than Year 12 | 47.7 | 52.0 | 35.0 | |
| Race (%): Mexican-American |
20.3 | 21.7 | 16.1 | <0.001 |
| Non-Hispanic White | 52.0 | 47.8 | 64.5 | |
| Non-Hispanic Black | 20.4 | 22.3 | 15.0 | |
| Other | 7.3 | 8.2 | 4.4 | |
| Mean FI (± SD) | 0.13 ± 0.12 | 0.10 ± 0.10 | 0.22 ± 0.13 | <0.001 |
| Metabolic syndrome (%) | 29.5 | 24.1 | 45.5 | <0.001 |
| Mean waist circumference (cm ± SD) | 98.0 ± 15.3 | 97.3 ± 15.7 | 100.4 ± 13.6 | <0.001 |
| HDL level (mg/dL ± SD) | 54.9 ± 16.5 | 54.7 ± 16.5 | 55.6 ± 16.5 | <0.05 |
| TG level (mg/dL ± SD) | 148.1 ± 122.9 | 147.5 ± 133.6 | 149.8 ± 83.7 | 0.61 |
| SBP (mm Hg ± SD) | 124.8 ± 20.2 | 120.2 ± 16.8 | 138.6 ± 23.1 | <0.001 |
| DBP (mm Hg ± SD) | 69.2 ± 13.7 | 70.6 ± 12.4 | 65.1 ± 16.3 | <0.001 |
| FBG level (mg/dL ± SD) | 104.9 ± 35.1 | 102.0 ± 35.2 | 113.3 ± 33.5 | <0.001 |
FI frailty index, HDL high-density lipoprotein, TG triglyceride, SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, SD standard deviation
a n = 8469 for HDL level, n = 4106 for TG level, n = 8294 for SBP and DBP, n = 4118 for FBG level
b n = 6336 for HDL level, n = 3067 for TG level, n = 6220 for SBP and DBP, n = 3078 for FBG level
c n = 2133 for HDL level, n = 1039 for TG level, n = 2074 for SBP and DBP, n = 1040 for FBG level
The relationship between each of the metabolic syndrome components and education level, and race for the whole sample is shown in Supplementary Tables S2 and S3. There was a higher prevalence of metabolic syndrome in those with less than grade 12 education compared to more than grade 12 (35.1 vs. 24.7%, p < 0.001). Those of Mexican-American race had the highest prevalence of metabolic syndrome (31.0%), and those who identified as “other race” had the lowest prevalence (24.4%).
Mortality risk, frailty, and metabolic syndrome in younger and older groups
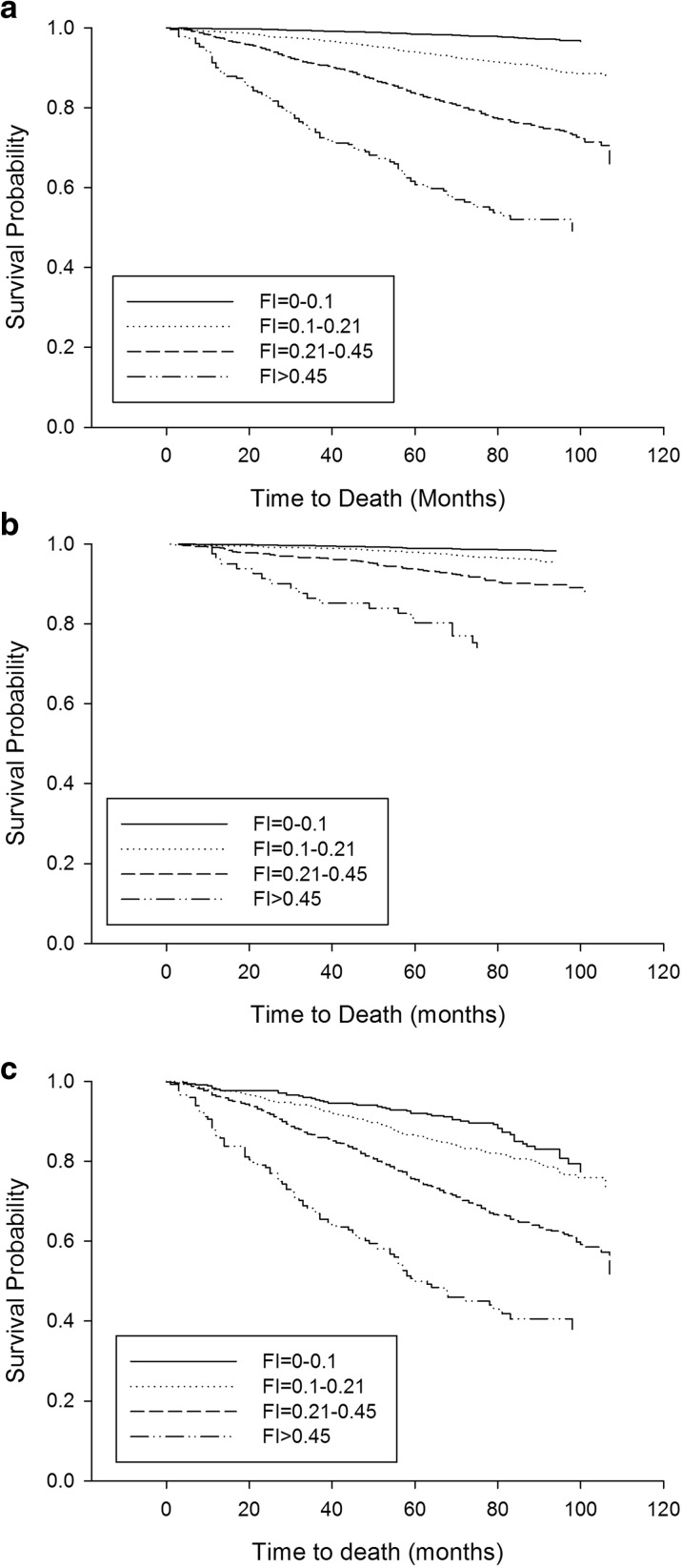
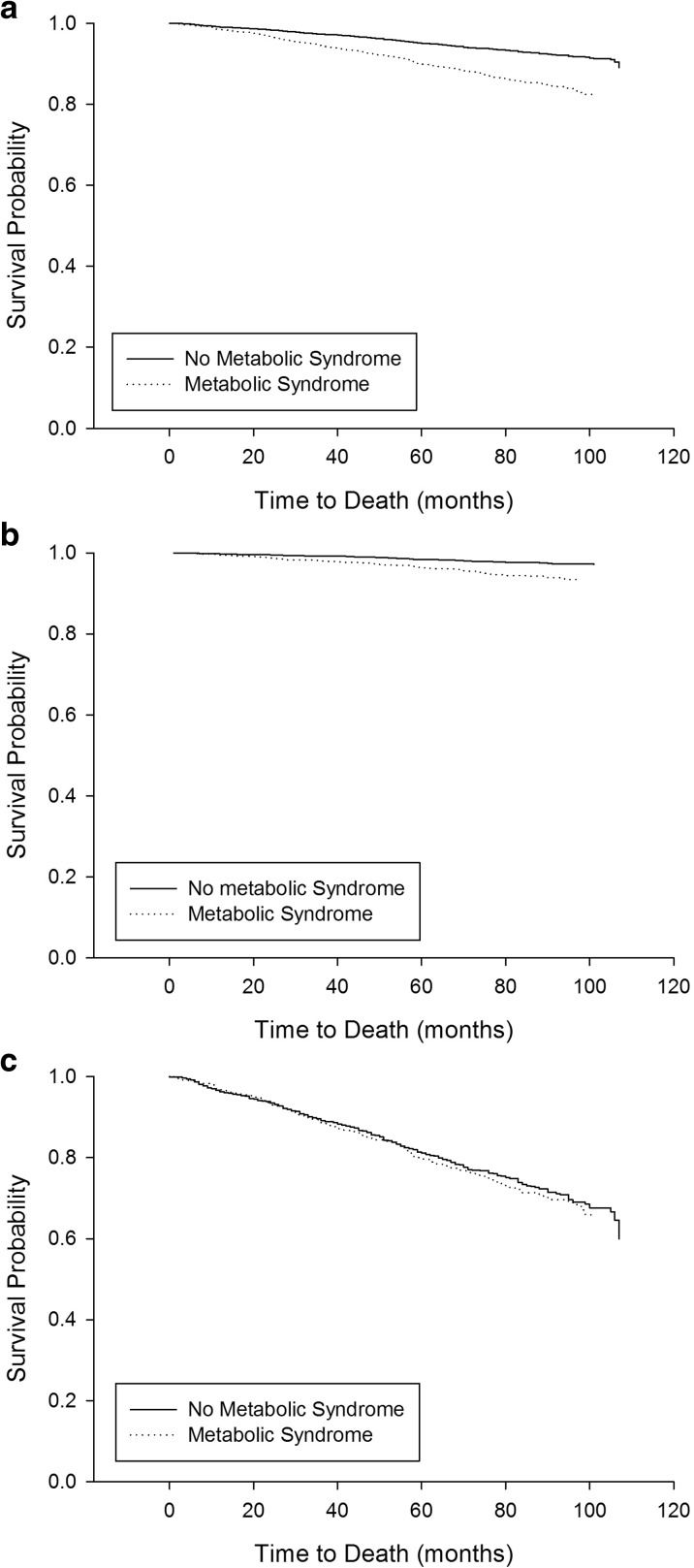
Mortality rate for the whole sample was 9.2% and, as expected, mortality was higher in the older group than the younger group (27.2 vs. 3.1%, p < 0.001). Kaplan-Meier curves, with log-rank analysis, showed a strong association between baseline FI score and 10-year mortality risk for both age groups (p < 0.001) (Fig. 1). There was a relationship between having the metabolic syndrome at baseline and increased mortality risk for the younger group (p < 0.001), but not the older group (p = 0.48) (Fig. 2).
Fig. 1.
Kaplan-Meier curves for 10-year survival probability stratified by frailty index level for the whole sample (a), younger (under 65 years, b), and older (65 years and older, c) groups. Log-rank analysis showed a significant difference between the curves for each age group (p < 0.001)
Fig. 2.
Kaplan-Meier curves for 10-year survival probability stratified by metabolic syndrome status for the whole sample (a), younger (under 65 years, b), and older (65 years and older, c) groups. Log rank analysis showed a significant difference between the curves for the whole sample and the younger group (p < 0.001), but not the older group (p = 0.48)
Whole sample unadjusted Cox proportional hazard ratio analysis showed that most items in the FI, FBG level/score, waist circumference, blood pressure, age, and being of non-Hispanic white race were associated with increased mortality risk. Female sex and higher education level were associated with decreased mortality risk (Supplementary Table S4). In the younger group, most items in the FI, each of the metabolic syndrome components (except TG score/level), and age were associated with increased mortality risk. Female sex and higher education level were associated with decreased risk (Supplementary Table S5). In the older group, most of the FI items, a high BP score, age, and being of non-Hispanic white race were associated with increased risk, whereas female sex and a higher waist circumference score were associated with reduced risk (Supplementary Table S6). FI score was associated with an increased risk of mortality in all age groups, and metabolic syndrome status was associated with increased risk in the whole sample and younger group, but not the older group (Supplementary Table S6). Age-adjusted Cox proportional hazard ratios for each of these items for the whole sample are shown in Supplementary Table S7.
Multivariable Cox proportional hazards regression models, adjusting for age, sex, education, race, FI, and metabolic syndrome, showed that FI was associated with increased mortality risk at both ages (younger HR 1.05 (1.04–1.06); older HR 1.04 (1.03–1.04)) whereas the metabolic syndrome did not contribute to mortality risk in either age group (Table 2). Age, sex, and education level were significant covariates for the younger group, while in the older group age and sex were the only significant covariates (Table 2).
Table 2.
Cox proportional hazard model for 10-year mortality risk for the whole sample, younger and older groups, adjusted for covariates
| Variables | Whole sample | <65 years | ≥65 years | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Agea | 1.07 | 1.07–1.08 | 1.06 | 1.05–1.08 | 1.11 | 1.09–1.12 |
| Sex | 0.53 | 0.46–0.61 | 0.56 | 0.42–0.75 | 0.51 | 0.43–0.60 |
| Metabolic syndrome | 1.08 | 0.94–1.25 | 1.09 | 0.81–1.46 | 1.14 | 0.97–1.35 |
| FIa | 1.04 | 1.03–1.05 | 1.05 | 1.04–1.06 | 1.04 | 1.03–1.04 |
| Raceb: | ||||||
| Non-Hispanic White | 1.09 | 0.88–1.36 | 1.08 | 0.74–1.59 | 1.06 | 0.81–1.39 |
| Non-Hispanic Black | 1.22 | 0.94–1.56 | 1.14 | 0.76–1.73 | 1.31 | 0.95–1.80 |
| Other race | 0.97 | 0.66–1.43 | 1.35 | 0.76–2.41 | 0.76 | 0.44–1.29 |
| Educationc: | ||||||
| Year 12 or equivalent | 0.96 | 0.80–1.16 | 0.64 | 0.44–0.93 | 1.10 | 0.89–1.36 |
| >Year 12 | 0.77 | 0.64–0.92 | 0.44 | 0.31–0.63 | 0.91 | 0.74–1.12 |
aAge, per year; FI per 0.01
bRelative to Mexican-American
cRelative to Less than Year 12
To determine whether these results were consistent across age groups, we repeated the multivariable Cox hazard regression analysis with the older group divided into those aged 65–79 years, and those aged 80 and over. The same result as seen in the original analysis was obtained for both age groups (65–79 years, age HR 1.09 (1.06–1.12), sex HR 0.45 (0.35–0.58), metabolic syndrome not significant (NS), FI 1.04 (1.03–1.05), race NS, education NS; 80 years and over, age HR 1.13 (1.06–1.20), sex HR 0.55 (0.44–0.69), metabolic syndrome NS, FI 1.03 (1.02–1.04), race NS, education NS).
Discussion
This study was the first to look at the relationship between the FI and metabolic syndrome in younger and older people. We found that the FI was a better predictor of mortality risk than metabolic syndrome in those aged both under and over 65 years. We also found that the association between FI and the metabolic syndrome weakened with age.
Previous work has shown that the metabolic syndrome is not associated with increased mortality in a very old (90+ years) Chinese population (Hao et al. 2016). The present study extends this work to show that metabolic syndrome was not associated with increased mortality risk in those aged greater than 65 years. This was seen even when the older group was divided into those aged 65–79 years, and those aged 80 plus. Other studies that found associations of the metabolic syndrome with adverse outcomes in older populations, focused on cardiovascular outcomes, rather than all-cause mortality risk (Scuteri et al. 2005; Maggi et al. 2006; Wang et al. 2007; Wang et al. 2008). In support of the findings of the current paper, there is evidence that some individual components of metabolic syndrome are actually associated with reduced mortality risk in old age, including higher waist circumference (Visscher et al. 2001; Price et al. 2006; Le Couteur and Simpson 2011). Indeed, in the current study, univariate analysis showed that waist circumference was actually protective against mortality risk. High BP has been associated with both increased (Alexander et al. 2003) and decreased (Blom et al. 2013) mortality risk in old age. Interestingly, the current study found that high BP was the only metabolic syndrome component associated with increased mortality risk in the over 65 population. It is likely, however, that the univariate analysis was significantly confounded by age, as seen in the loss of associated risk for BP or waist circumference with age-adjusted Cox regression analysis (Supplementary Table S7).
This study confirms that in studying late life illness, it is important to consider not just a disease or syndrome of interest, such as the metabolic syndrome, but the other health deficits that a patient may have. Other studies have shown that the value of the FI lies in predicting adverse outcomes even when the index is composed of factors not traditionally thought to be related to the outcome (Song et al. 2011). Traditional modeling finds importance in risk when people share the same deficits, but a body of work suggests that at some point, sharing the same number of deficits can be as important as the nature of the deficits that are shared (Rockwood and Mitnitski 2007; Kulminski et al. 2008a). The frailty index approach allows that insight to be formalized.
Interestingly, in the current study, multivariate Cox hazard regression analysis showed that metabolic syndrome was not associated with increased mortality risk in the younger group either. Previous studies have shown an association between metabolic syndrome and all-cause mortality risk in general population studies (Lakka et al. 2002; Hu et al. 2004; McNeill et al. 2005). However, none of these studies included frailty as a covariate. Univariate analysis showed an increased mortality risk with the metabolic syndrome, as well as each of its components, in those aged under 65 years, and it is clear from the literature that the metabolic syndrome is an important risk assessment tool for the adult population (Lakka et al. 2002; Meigs et al. 2003; McNeill et al. 2005). Nevertheless, the fact that the current study found that the FI was a better predictor of all-cause mortality indicates that the FI has great value as a clinical tool for risk assessment in adult populations. This finding also demonstrates that health-related deficits accumulate gradually over the lifespan, rather than only appearing in old age. This supports other literature, especially in relation to late life cognitive impairment (Lafortune et al. 2016), on the importance of midlife health status to late life illness. Further, viewed from a frailty perspective, variability about results in relation to similar age cut-points is likely to be less important than is understanding not just a person’s age, but their overall health status, which is what the FI seeks to quantify.
Common pathophysiological pathways such as inflammation (Cesari et al. 2006; Barzilay et al. 2007; Hubbard and Woodhouse 2010; Collerton et al. 2012) and changes in body composition could help explain the link between frailty and metabolic syndrome in younger adults. The FI quantifies health deficit accumulation across a range of systems, so it seems likely if someone has the metabolic syndrome then they will have more health-related deficits. It is important to note that, for the FI used in the current study, factors associated with the metabolic syndrome were removed from the index. The relationship between metabolic syndrome and the FI was very weak in those aged over 65 years, although those with metabolic syndrome did have higher mean FI scores than those without the syndrome. Other studies have found an association between these syndromes in populations aged 50 plus (Viscogliosi 2016) but not in those aged over 70 (Barzilay et al. 2007) or over 90 (Hao et al. 2016). Overall, the current study indicates that there is a relationship between FI and metabolic syndrome, but this relationship declines in old age.
The metabolic syndrome has previously been associated with both race and education level (Park et al. 2003; Lee et al. 2005). The current study found a negative association of education level with metabolic syndrome in the whole population. However, this association weakened in old age, with education a significant covariate in the Cox hazard regression mortality analysis for the younger group but not the older group. The association between low education level and increased metabolic syndrome risk has been attributed to the association of lower socioeconomic status with other risk factors such as obesity, smoking, and inactivity (Park et al. 2003). Race was not a significant covariate for mortality risk for either age group. There were slight differences across the races, however, in the prevalence of metabolic syndrome with Mexican-Americans having the highest prevalence, as has been seen in previous studies (Meigs et al. 2003; Avila-Funes et al. 2014). There was no clear association of race with any of the metabolic syndrome components.
Female sex was a significantly protective factor for mortality risk in both age groups, despite the fact that women have higher mean FIs, and a higher prevalence of the metabolic syndrome than men in both groups. This paradox has been seen in other studies of the FI (Howlett et al. 2014), and the metabolic syndrome (Maggi et al. 2006; Wang et al. 2007). Studies suggest that there may be health behavior or indeed biological vulnerability explanations for this morbidity-mortality paradox (Case and Paxson 2005; Kulminski et al. 2008b).
There are some limitations to this study. The study population is from the USA, so the generalizability worldwide is unclear. Europid cut-points were also used for the weight circumference component of the metabolic syndrome definition in the current study. However, as recommended by the IDF (Alberti et al. 2006), some analysis was also completed with North American cut-points (waist circumference ≥102 cm for men and ≥88 cm for women). Using these cut-points, the prevalence of the metabolic syndrome was lower but the other outcomes were similar (data not shown). Additionally, the original IDF metabolic syndrome definition included treatment for low HDL or high TG level as an alternative to abnormal serum results for these factors. As treatment data was not available for the current dataset, only abnormal serum results were used in the definition. This may result in a potential for underestimation of the metabolic syndrome in this dataset. Finally, the dataset used in the current study did not provide mortality causes. Future studies looking at the association of FI and metabolic syndrome with specific outcomes such as cardiovascular mortality would be interesting.
Overall, this study found that there is an association between FI and metabolic syndrome but this association weakens with old age. Furthermore, the FI is a better predictor of mortality risk than the metabolic syndrome at all ages. The FI can be incorporated into the examination and risk assessment of patients across the lifespan, and is feasible in clinical epidemiological settings.
Electronic supplementary material
(DOCX 16 kb)
.
(DOCX 17 kb)
.
(DOCX 17 kb)
.
(DOCX 22 kb)
.
(DOCX 22 kb)
.
(DOCX 23 kb)
.
(DOCX 19 kb)
.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (MOP 126018, MOP 97973 and MOP 209888) and the Fountain Innovation Fund of the Queen Elizabeth II Health Sciences Foundation.
References
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- Avila-Funes J, Medina-Campos R, Tamez-Rivera O, et al. Frailty is associated with disability and recent hospitalization in community-dwelling elderly: the Coyoacan Cohort. J Frailty Aging. 2014;3:206–210. doi: 10.14283/jfa.2014.25. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Blodgett J, Theou O, Kirkland S, et al. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Blom JW, de Ruijter W, Witteman JCM, et al. Changing prediction of mortality by systolic blood pressure with increasing age: the Rotterdam study. Age (Dordr) 2013;35:431–438. doi: 10.1007/s11357-011-9349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42:189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Hao Q, Song X, Yang M, et al. Understanding risk in the oldest old: frailty and the metabolic syndrome in a Chinese community sample aged 90+ years. J Nutr Heal Aging. 2016;20:82–88. doi: 10.1007/s12603-016-0680-7. [DOI] [PubMed] [Google Scholar]
- Howlett SE, Rockwood MRH, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi: 10.1186/s12916-014-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Qiao Q, Tuomilehto J, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Theou O. Frailty: enhancing the known knowns. Age Ageing. 2012;41:574–575. doi: 10.1093/ageing/afs093. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Woodhouse KW. Frailty, inflammation and the elderly. Biogerontology. 2010;11:635–641. doi: 10.1007/s10522-010-9292-5. [DOI] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva S, Kulminskaya I, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Culminskaya IV, Ukraintseva SV, et al. Sex-specific health deterioration and mortality: the morbidity-mortality paradox over age and time. Exp Gerontol. 2008;43:1052–1057. doi: 10.1016/j.exger.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune L, Martin S, Kelly S, et al. Behavioural risk factors in mid-life associated with successful ageing, disability, dementia and frailty in later life: a rapid systematic review. PLoS One. 2016;11:1–34. doi: 10.1371/journal.pone.0144405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Simpson SJ. Adaptive senectitude: the prolongevity effects of aging. Journals Gerontol - Ser A Biol Sci Med Sci. 2011;66(A):179–182. doi: 10.1093/gerona/glq171. [DOI] [PubMed] [Google Scholar]
- Lee WY, Jung CH, Park JS, et al. Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res Clin Pract. 2005;67:70–77. doi: 10.1016/j.diabres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lin F, Roiland R, Chen D, Qiu C. Linking cognition and frailty in middle and old age: metabolic syndrome matters. Int J Geriatr Psychiatry. 2015;30:64–71. doi: 10.1002/gps.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi S, Noale M, Gallina P, et al. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: the Italian Longitudinal Study on Aging. Journals Gerontol Ser A Biol Sci Med Sci. 2006;61:505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Wilson PWF, Nathan DM, et al. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460. doi: 10.1016/S0047-6374(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14:709–717. doi: 10.1007/s10522-013-9446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler MJ, Fain MJ, Wertheimer AM, et al. The frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi: 10.1016/j.exger.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Park Y, Zhu S, Palaniappan L, et al. The metabolic syndrome. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GM, Uauy R, Breeze E, et al. Weight, shape, and mortality risk in older persons: elevated waist- hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. Journals Gerontol Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Song X, Mitnitski AB. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;138:487–494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, McConnachie A, Shaper AG, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Najjar SS, Morrell C, Lakatta E. The metabolic syndrome in older individuals: prevalence and prediction. Diabetes Care. 2005;28:882–887. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77:227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Wang C, Song X, et al. Co-occurrence of cardiometabolic diseases and frailty in older Chinese adults in the Beijing longitudinal study of ageing. Age Ageing. 2013;42:346–351. doi: 10.1093/ageing/aft004. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. doi: 10.2307/2061224. [DOI] [PubMed] [Google Scholar]
- Viscogliosi G. The metabolic syndrome: a risk factor for the frailty syndrome? J Am Med Dir Assoc. 2016;17:364–366. doi: 10.1016/j.jamda.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC, Molarius A, et al. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- Wang J, Ruotsalainen S, Moilanen L, et al. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–864. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- Wang J, Ruotsalainen S, Moilanen L, et al. The metabolic syndrome predicts incident stroke: a 14-year follow-up study in elderly people in Finland. Stroke. 2008;39:1078–1083. doi: 10.1161/STROKEAHA.107.499830. [DOI] [PubMed] [Google Scholar]
- Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. 2013;1:1–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)
(DOCX 17 kb)
(DOCX 17 kb)
(DOCX 22 kb)
(DOCX 22 kb)
(DOCX 23 kb)
(DOCX 19 kb)