Abstract
Experimental, clinical, and epidemiological findings support the concept of developmental origins of health and disease (DOHAD), suggesting that early-life hormonal influences during a sensitive period around adolescence have a powerful impact on cancer morbidity later in life. The endocrine changes that occur during puberty are highly conserved across mammalian species and include dramatic increases in circulating GH and IGF-1 levels. Importantly, patients with developmental IGF-1 deficiency due to GH insensitivity (Laron syndrome) do not develop cancer during aging. Rodents with developmental GH/IGF-1 deficiency also exhibit significantly decreased cancer incidence at old age, marked resistance to chemically induced carcinogenesis, and cellular resistance to genotoxic stressors. Early-life treatment of GH/IGF-1-deficient mice and rats with GH reverses the cancer resistance phenotype; however, the underlying molecular mechanisms remain elusive. The present study was designed to test the hypothesis that developmental GH/IGF-1 status impacts cellular DNA repair mechanisms. To achieve that goal, we assessed repair of γ-irradiation-induced DNA damage (single-cell gel electrophoresis/comet assay) and basal and post-irradiation expression of DNA repair-related genes (qPCR) in primary fibroblasts derived from control rats, Lewis dwarf rats (a model of developmental GH/IGF-1 deficiency), and GH-replete dwarf rats (GH administered beginning at 5 weeks of age, for 30 days). We found that developmental GH/IGF-1 deficiency resulted in persisting increases in cellular DNA repair capacity and upregulation of several DNA repair-related genes (e.g., Gadd45a, Bbc3). Peripubertal GH treatment reversed the radiation resistance phenotype. Fibroblasts of GH/IGF-1-deficient Snell dwarf mice also exhibited improved DNA repair capacity, showing that the persisting influence of peripubertal GH/IGF-1 status is not species-dependent. Collectively, GH/IGF-1 levels during a critical period during early life determine cellular DNA repair capacity in rodents, presumably by transcriptional control of genes involved in DNA repair. Because lifestyle factors (e.g., nutrition and childhood obesity) cause huge variation in peripubertal GH/IGF-1 levels in children, further studies are warranted to determine their persisting influence on cellular cancer resistance pathways.
Keywords: Growth hormone; Insulin-like growth factor-1; Lifespan, health span; Longevity; Endocrine; Cellular resilience; Stress resistance
Introduction
Laboratory and epidemiological studies during the past two decades support the developmental origins of health and disease (DOHAD) hypothesis suggesting that early-life events during a sensitive period of development have a fundamental effect on the organism’s susceptibility for malignancies in later life(Johnson et al. 2009; Walker and Ho 2012). Growing clinical and experimental evidence suggests that the endocrine milieu present around puberty, when rapid physical growth occurs, induces persisting cellular resilience programs and stress response molecular networks(Dominick et al. 2016; Leiser et al. 2006; Murakami et al. 2003; Page et al. 2009; Leiser and Miller 2010; Panici et al. 2010) that affect the pathogenesis of cancer in adulthood. Accordingly, there is evidence that the origins of breast cancer occur during puberty, a time of rapid breast development (Zhao et al. 2013; Haslam and Schwartz 2011; Olson et al. 2010). Other studies link early-life endocrine influences to the pathogenesis of prostate cancer, bladder cancer, and colon cancer in later stages of life. The endocrine changes that occur during development and puberty are highly conserved across mammalian species and include dramatic increases in GH and the anabolic hormone insulin-like growth factor-1 (IGF-1) (Sonntag et al. 2012; Carter et al. 2002a; D’Costa et al. 1993; Deak and Sonntag 2012; Sonntag et al. 1999, 2000, 2005a). Recent experimental studies have uncovered evidence suggesting that levels of GH and IGF-1 during early life are important in determining susceptibility to cancer later in life (Panici et al. 2010; Ramsey et al. 2002). Importantly, in humans, the peripubertal GH/IGF-1 surge is highly variable. A breakthrough longitudinal study of individuals who carry mutations in the growth hormone receptor gene demonstrates that severe childhood IGF-1 deficiency results in zero cancer incidence later in life (Guevara-Aguirre et al. 2011). Experimental findings obtained in rodent models of developmental GH/IGF-1 deficiency corroborate the clinical data (Panici et al. 2010). Yet, the mechanisms by which early-life GH/IGF-1 levels impact carcinogenesis later in life remain elusive.
To explain the causal link between IGF-1 and carcinogenesis, the growth-promoting and antiapoptotic functions of the IGF-1 pathway were evoked (Pollak et al. 2004; Yakar et al. 2004). It is well known that IGF-1 is a potent growth factor, and proliferation of tumor cells in vitro is stimulated by administration of IGF-1 (Osborne et al. 1976). While the aforementioned studies primarily focused on the possible role of growth-promoting functions of IGF-1, emerging evidence points to potentially highly important, yet under-studied, links among peripubertal GH and IGF-1 levels, cellular resilience programs, and susceptibility to carcinogenesis. Accordingly, there are reports that rodents with developmental GH/IGF-1 deficiency exhibit marked resistance to chemically induced cancers and their cells are resistant to the damaging effects of known mutagens in vitro (Panici et al. 2010; Ramsey et al. 2002; Yakar et al. 2004; Sonntag et al. 2005b; Moore et al. 2008; Olivo-Marston et al. 2009; Ikeno et al. 2003; Bokov et al. 2009; Brown-Borg et al. 2009; Salmon et al. 2005). Studies in rat and murine models of GH and/or IGF-1 deficiency suggest that a critical time window exists around puberty when circulating levels of GH and/or IGF-1 determine both susceptibility to chemically induced carcinogenesis (Ramsey et al. 2002) and incidence of spontaneously occurring malignancies later in life (Panici et al. 2010; Ikeno et al. 2003). These studies suggest that developmental GH/IGF-1 deficiency enhances cellular resistance to genotoxic stresses (Page et al. 2009; Panici et al. 2010). Both in humans and experimental animals, cellular DNA repair pathways determine risk of cancer.
The present study was designed to test the hypothesis that GH/IGF-1 status in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes. To test these hypotheses, we assessed repair of γ-irradiation-induced DNA damage (by single-cell gel electrophoresis/comet assay) and analyzed basal and post-irradiation expression of DNA repair-related genes (by qPCR) in primary fibroblasts derived from control rats, Lewis dwarf rats, and Lewis dwarf rats with peripubertal GH replacement. The Lewis dwarf rat is a useful model of human isolated developmental GH/IGF-1 deficiency, as these animals have normal pituitary function except for a selective genetic GH deficiency, and they mimic many of the pathophysiological alterations present in human GH/IGF-1-deficient children. DNA repair capacity was also assessed in primary fibroblasts derived from Snell dwarf mice and respective wild-type control mice. Snell dwarf mice lack GH, prolactin (PRL), and thyroid-stimulating hormone (TSH) throughout development and exhibit resistance to spontaneous cancers.
Methods
Experimental animals
Lewis dwarf rats
In the present study, we used male Lewis rats that are heterozygous or homozygous for the spontaneous autosomal recessive dw-4 mutation, which causes a decrease in GH secretion from the pituitary gland beginning around post-natal day 26 (Carter et al. 2002a, b; Charlton et al. 1988). Lewis dwarf (dw-4/dw-4) rats have chronically low levels of GH and IGF-1 and make an excellent animal model of isolated GH/IGF-1 deficiency (Charlton et al. 1988; Bailey-Downs et al. 2012a; Ungvari et al. 2010; Ungvari et al. 2011; Yan et al. 2014). Female heterozygous (dw-4/–) Lewis rats were bred with male homozygous Lewis dwarf rats (dw-4/dw-4) to generate heterozygous (dw-4/–) offspring with a normal phenotype (“control”) or homozygous rats (dw-4/dw-4) with a dwarf phenotype (“dwarf”). Classification as control or dwarf was based on their body weight as well as serum IGF-1 levels at 33 days of age. Total IGF-1 levels in serum were determined as previously reported (Ungvari et al. 2011). Beginning on day 35, dwarf rats were divided into two experimental groups: (1) dwarf rats given saline (n = 6) and (2) GH-replete dwarf rats with GH administered beginning at 5 weeks of age and continued throughout the experimental period of 30 days (termed “GH-replete,” n = 6). Saline or GH (300 μg recombinant porcine GH, Alpharma, Victoria, Australia) was injected s.c. twice daily. The heterozygous rats were used as controls and given saline injections twice daily from 5 weeks of age to the end of the experimental period. Recently, we reported that at the end of the experimental period, in this experimental cohort, control and dwarf GH-replete rats had significantly higher serum IGF-1 levels (control: ∼900–1000 ng/mL; dwarf: ∼400 ng/mL; GH-replete: ∼800–900 ng/mL) compared to the untreated dwarf rats, indicating that twice daily administration of GH to the dwarf rats effectively normalizes serum IGF-1 (Ungvari et al. 2011). Rats had access to food and water ad libitum and were housed in pairs in the specific pathogen-free barrier facility of the University of Oklahoma Health Sciences Center. Animals were fed standard rodent chow (PicoLab Rodent Diet 20 from Purina Mills [Richmond, IN], containing 20% protein by mass, 24% protein by caloric content). Animals were killed by decapitation 30 days after the treatment period to establish primary fibroblast cultures.
Snell dwarf mice
Snell dwarf (n = 5) and littermate control mice (n = 5) were produced by a cross between (DW/J × C3H/HeJ)-dw/+ heterozygous parents and maintained in the specific pathogen-free barrier facility of the University of Michigan using husbandry conditions that have been described previously (Vergara et al. 2004). Mice with the dw/dw genotype were identified at approximately 3 weeks of age by their small body size (dwarfs). The mice were housed in microisolator cages with 1/8 in. Bed-O-Cob bedding (The Andersons, Maumee, OH), free access to tap water and Purina 5001 Rodent Chow (St. Louis, MO); in addition, moist or crushed pellets of chow were placed on the floor of cages housing dw/dw animals. Dwarf mice were caged with normal sized females (“warmer” mice) to prevent premature death of the dwarf mice from hypothermia.
Post-developmental liver-specific knockdown of Igf1 in mice
In additional studies, to separate the effects of early- and late-life IGF-1 deficiency, blood that was collected from the tail vein of a mouse model of adult-onset isolated circulating IGF-1 deficiency (Igf1 f/f + TBG-Cre-AAV8) was used. The generation and husbandry of these mice were recently reported (Tarantini et al. 2016a, b; Toth et al. 2014a, 2015). In brief, male mice homozygous for a floxed exon 4 of the Igf1 gene (Igf1 f/f) in a C57BL/6 background were purchased from Jackson Laboratories. These mice have the entirety of exon 4 of the Igf1 gene flanked by loxP sites, which allows for genomic excision of this exon when exposed to Cre recombinase. Transcripts of the altered Igf1 gene yield a protein upon translation that fails to bind the IGF receptor. To target hepatocytes, adeno-associated viruses (AAVs) were purchased from the University of Pennsylvania Vector Core (Philadelphia, PA). At 4 months of age, approximately 1.3 × 10 (Haslam and Schwartz 2011) viral particles (as assayed by genome content at the University of Pennsylvania) of AAV8.TBG.PI.Cre.rBG or AAV8.TBG.PI.eGFP.WPRE.bGH were administered to Igf1 f/f mice to knockdown IGF-1 or as a control, respectively. Mice were anesthetized with ketamine/xylazine (100 and 15 mg/kg, respectively) and given retro-orbital injections of virus diluted to the appropriate concentration in 100-μL 0.9% saline. While AAV8 is effective at transducing multiple tissues after i.v. delivery, including the liver, the thyroxine binding globulin (TBG) promoter restricts expression solely to hepatocytes (Toth et al. 2014b, 2015). Blood was collected at 6 months of age. All studies were approved by the Institutional Animal Care and Use Committees of the respective institutions.
Isolation of fibroblasts and cell culture techniques
Primary fibroblast cell lines were established from rats as previously described (Ungvari et al. 2011). In brief, skin samples were digested with collagenase (at 37 °C and 5% CO2 for 30 min), then washed twice with MEM, supplemented with 10% heat-inactivated fetal bovine serum (Hyclone). Cells were plated into 100-mm dishes with MEM media supplemented with 10% heat-inactivated fetal bovine serum plus penicillin/streptomycin/Fungizone (at 5% CO2 and 3% O2, at 37 °C). After 18 h, the media was changed to discard unattached cells. The fibroblasts were subsequently cultured, as described previously (Labinskyy et al. 2009).
Primary fibroblast cultures from mice were generated using a previously published protocol (Salmon et al. 2005). In brief, tail skin biopsies ∼3–5 mm in length were obtained from the latter half of the intact tail of isoflurane-anesthetized mice after skin sterilization with 70% ethanol. Biopsies were further washed in 70% ethanol, placed in DMEM (high-glucose variant; GIBCO-Invitrogen, Carlsbad, CA), diced to <0.5 mm, and digested overnight with collagenase type II (400 U/mL, 1000 U total per tail, GIBCO-Invitrogen) dissolved in DMEM supplemented with 20% heat-inactivated fetal bovine serum, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Sigma, St. Louis, MO), and 0.25 μg/mL fungizone (Biowhittaker-Cambrex Life Sciences, Walkersville, MD) at 37 °C in a humidified incubator with 5% CO2 in air. After collagenase treatment, cells were dislodged from digested tissue by repeated pipetting and passed through sterile nylon netting into sterile 14-mL centrifuge tubes (BD Dickenson, Bedford, MA). Samples were centrifuged for 5 min at 200g, and collagenase solution was drawn off the cell pellet. Cells were resuspended in DMEM with 20% heat-inactivated fetal bovine serum, antibiotics, and fungizone. Approximately 2.5 × 105 cells in 3 mL of medium were seeded into tissue culture flasks of 25-cm2 surface area (Corning Costar, Corning, NY). After 3 days, ∼2/3 total volume of medium was removed on day 3 and replaced with fresh DMEM with 20% heat-inactivated fetal bovine serum, antibiotics, and fungizone. Six or seven days after seeding, initial cultures (designated first-passage cells) were either 1) split six- or ninefold by volume to create second-passage cells, with twofold or threefold dilutions at each subsequent passage, or (2) split and seeded at a density of 1 × 105 cells/cm2 flask surface area at each passage into tissue culture flasks of 75 or 175-cm2 surface area (Corning Costar). Cells were split by first washing flasks with 1× phosphate-buffered saline solution (PBS: 8.8 g NaCl, 2.25 g Na2HPO4, and 0.26 g NaH2PO4/L distilled H2O, pH 7.3) followed by incubation with ∼3 mL of trypsin/100 cm2 surface area of flask 1× trypsin-EDTA (GIBCO-Invitrogen) for ∼5 min at 37 °C in a humidified incubator with 5% CO2 in air. Trypsin activity was inhibited with an equal volume of DMEM with 20% heat-inactivated fetal bovine serum, antibiotics, and fungizone. Subsequent passages were split at 6-day intervals with ∼2/3 total volume of medium removed on day 3 and replaced with fresh DMEM with 20% heat-inactivated fetal bovine serum, antibiotics, and fungizone.
Multiple cell lines were established from each experimental group (n = 5–6 for each group). One cell line per animal was used at the end of the third passage for measurement of cellular DNA repair capacity and expression of DNA repair-related genes.
γ-Irradiation protocol
γ-Irradiation to the cells was administered using a 137Cs gamma irradiator (GammaCell 40, Nordion International). The irradiator was activated for a time calculated to deliver from 3 to 9 Gy, depending on the protocol. Dosimetry was performed as previously described (Warrington et al. 2011, 2012) to confirm the dose received.
DNA damage analysis by alkaline single-cell gel electrophoresis
To compare susceptibility of cultured fibroblasts to DNA damage induced by γ-irradiation, single-cell gel electrophoresis was performed following our published protocol (Ungvari et al. 2013a; Csiszar et al. 2007, 2008; Ungvari et al. 2007). In brief, 105 cells in 100 μL PBS were mixed with 100 μL of 1.5% low-melting agarose and 90 μL of this mixture spotted on CometAssay slides (Trevigen, Gaithersburg, MD) between two layers of 1% low-melting agarose. DNA damage was induced by exposure of the slides to γ-irradiation (4 Gy). The extent of DNA fragmentation was examined by single-cell electrophoresis (“comet assay”), as reported (Ungvari et al. 2013a, 2007; Csiszar et al. 2007, 2008). The comet assay is based on the alkaline lysis of labile DNA at sites of damage. The slides were treated with a lysis solution (NaCl 2.5 M, Na2EDTA 100 mM, Triton X-100 1% DMSO 10%, Trizma base 10 mM; pH 10; for 1 h at 4 °C in the dark), rinsed with a neutralization buffer (3 × 5 min, 0.4 M Tris, pH 7.5) to remove detergents and salts, then submerged in a high pH buffer (NaOH 300 mM, Na2EDTA 1 mM, pH > 13) for 20 min to allow for unwinding of the DNA and the expression of alkali-labile damage. Electrophoresis was performed using the same buffer at 25 V (∼0.74 V/cm) and 300 mA for 20 min. At the end of the run, the slides were neutralized in Tris-HCl 0.4 M, pH 7.5, submersed in absolute ethanol for 3 min, air-dried for 5–10 min, and DNA was stained with SYBR green (Invitrogen). Fluorescent images of the nuclei were captured using an EVOS FL Cell Imaging System (Invitrogen) at 20× magnification. DNA damage was quantified by measuring the tail DNA content (as a percentage of total DNA) using the Comet Assay-IV software (Perceptive Instruments, Haverhill, Suffolk, UK). For assessment of DNA repair efficiency, the cells were irradiated with 3 Gy, and the extent of DNA damage was assessed by the comet assay at 10, 20, 30, 40, 60, and/or 90 min post-irradiation (Csiszar et al. 2007). The percentage of residual DNA damage was plotted as a function of time. The time constant calculated from this plot and the residual DNA damage at 20 to 60 min post-irradiation were used as indices of DNA repair capacity.
Targeted qPCR array to analyze mRNA expression of genes involved in regulation of DNA repair processes
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of genes known to be involved in regulation of DNA repair pathways in fibroblasts derived from control rats, Lewis dwarf rats, and GH-replete dwarf rats as reported (Tarantini et al. 2016b; Csiszar et al. 2013; Tucsek et al. 2013, 2014; Toth et al. 2013). The fibroblasts were harvested for analysis before or 2, 4, or 6 h after exposure to γ-irradiaton (3 Gy). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (Bailey-Downs et al. 2012b). mRNA expression of DNA repair genes was analyzed using validated TaqMan probes (Applied Biosystems) and a Strategen MX3000 platform, as previously reported (Tucsek et al. 2014). Quantification was performed using the ΔΔCt method as described (Tarantini et al. 2016b; Csiszar et al. 2013; Tucsek et al. 2013; Toth et al. 2013; Tucsek et al. 2014).
Statistical analysis
Data were normalized to the respective control mean values and are expressed as means ± SEM. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. All statistical comparisons were performed using Prism 5.0 for Windows (Graphpad Software, La Jolla, CA) and were considered significant at p < 0.05.
Results
Early-life GH/IGF-1 status of donor animals does not affect the severity of γ-irradiation-induced initial DNA damage in cultured primary fibroblasts
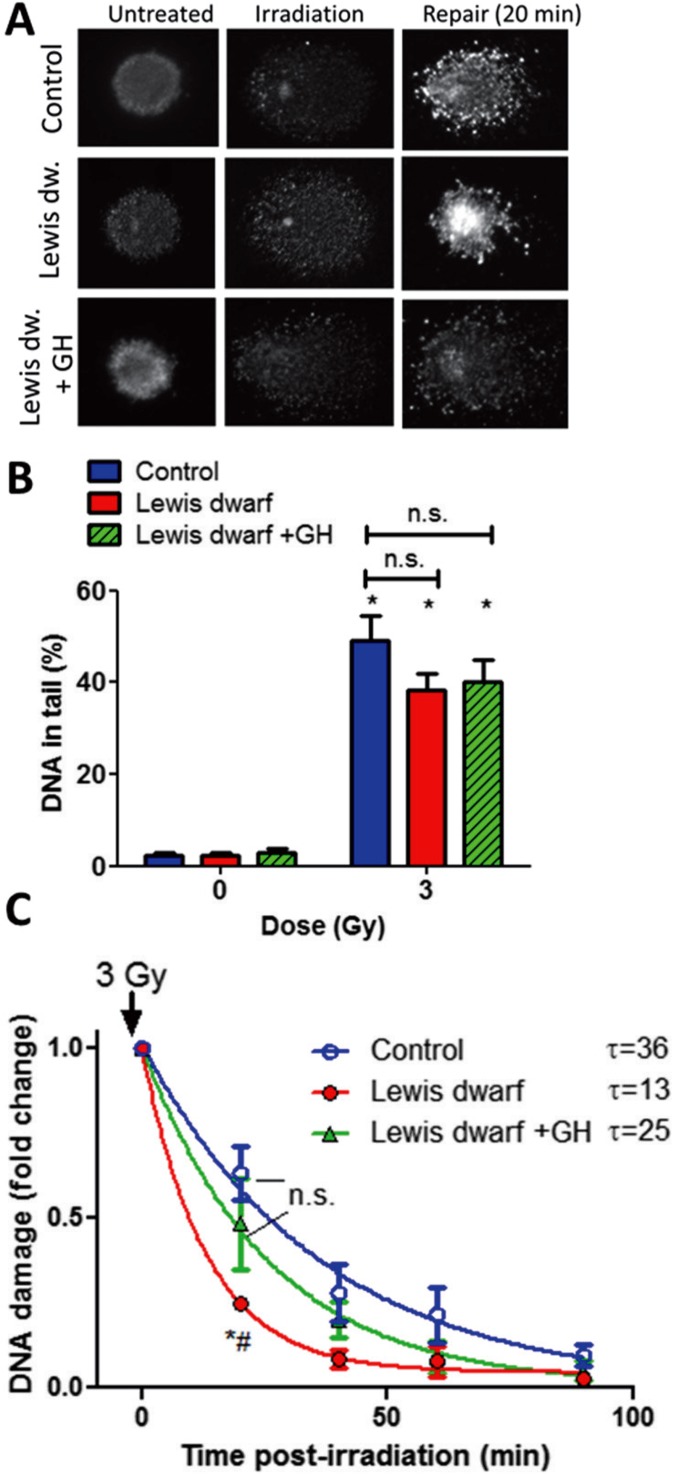
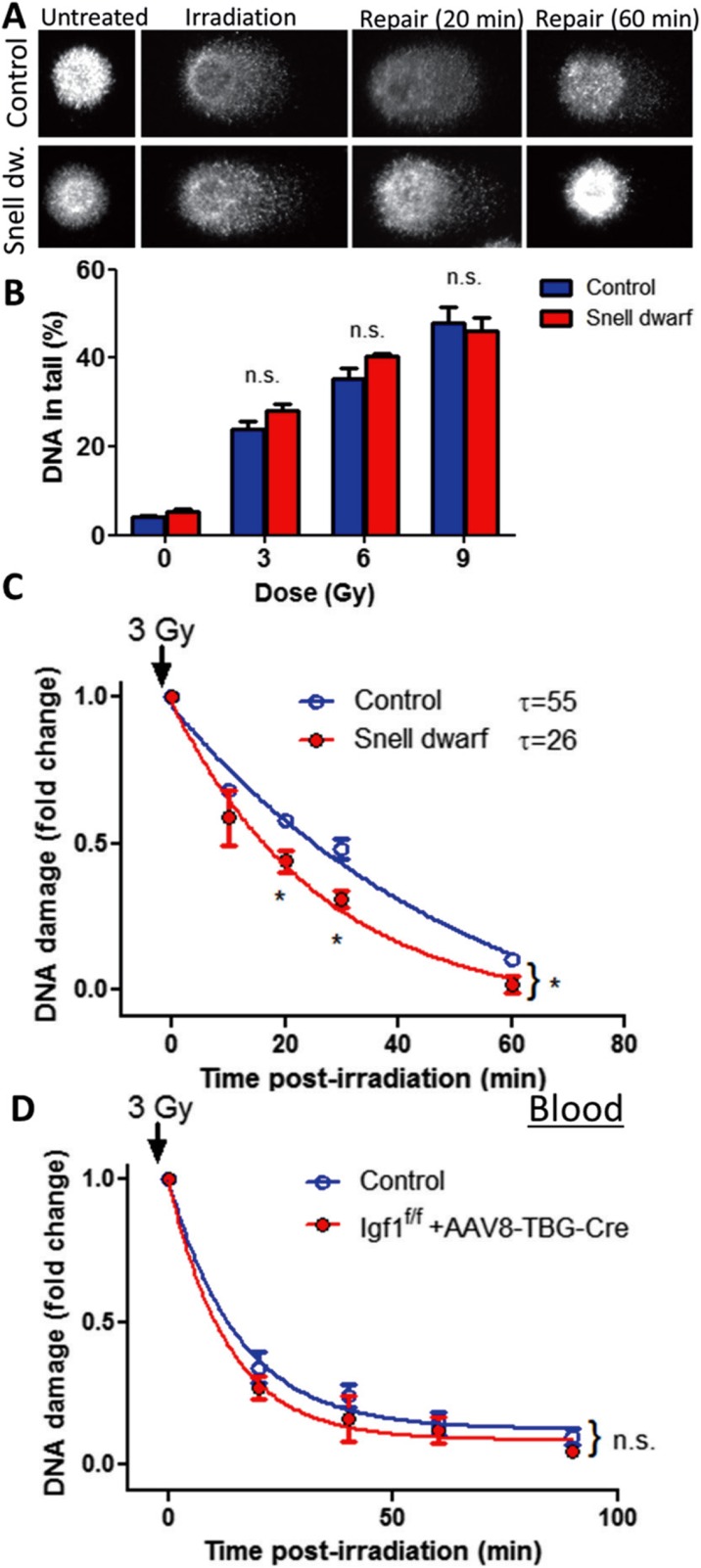
To assess DNA damage induced by γ-irradiation, a random sample of 200 cells was analyzed from each slide. Figures 1a and 2a show examples of images of the comet assay using cells derived from Lewis dwarf rats and Snell dwarf mice, respectively. In each cell line without γ-irradiation, all the DNA was confined to the nuclei, as indicated by the percentage of DNA in the tail less than 5%. In cells with γ-irradiation-induced DNA strand breaks, a bright fluorescent tail along the electric field was observed because small DNA fragments migrated out of the nuclei (Figs. 1a and 2a). Figures 1b and 2b show the DNA content in the tail (indicating the severity of DNA damage) as a function of the dose of γ-irradiation in each experimental group. The severity of γ-irradiation-induced DNA damage in Lewis dwarf fibroblasts (Fig. 1b) and Snell dwarf fibroblasts (Fig. 2b) did not differ significantly from that in their respective controls.
Fig. 1.
Early-life GH/IGF-1 status of donor rats elicits persisting alterations in DNA repair capacity in cultured primary fibroblasts. Fibroblasts were γ-irradiated, and DNA damage was assessed by single-cell electrophoresis (“comet assay”) at different time points post-irradiation. Damaged DNA migrates during electrophoresis from the nucleus toward the anode, forming the shape of a “comet” with a head (cell nucleus with intact DNA) and a tail (relaxed and broken DNA). a Representative images of damaged DNA in irradiated (3 Gy) fibroblasts derived from control rats, Lewis dwarf rats, and Lewis dwarf rats with early-life GH treatment. Median values of tail DNA content in each sample were determined. Note the marked decline in tail DNA content at 20 min post-irradiation due to DNA repair processes. b γ-Irradiated fibroblasts exhibit comparable DNA damage. Shown are γ-irradiation (3 Gy)-induced increases in tail DNA content in fibroblasts derived from control rats, Lewis dwarf rats, and Lewis dwarf rats with peripubertal GH treatment. *p < 0.05 vs respective non-irradiated controls (one-way ANOVA, Tukey’s multiple comparisons). c Fibroblasts derived from control rats Lewis dwarf rats exhibit increased DNA repair capacity, which is prevented by peripubertal GH treatment. For assessment of DNA repair efficiency, the percentage of residual DNA damage was plotted as a function of time post-irradiation. Tail DNA content at each time point is shown. The residual DNA damage at 20 min post-irradiation was the lowest in fibroblasts from untreated Lewis dwarf rats, indicating more efficient DNA repair processes in these cells, which was reversed by peripubertal GH treatment. *p < 0.001 Lewis dwarf vs control; # Lewis dwarf vs Lewis dwarf GH (one-way ANOVA, Tukey’s multiple comparisons); data are mean ± SEM (n = 5–6 for each time-point). The time constant calculated from these plots (τ) is shown next to the legends
Fig. 2.
Developmental GH/IGF-1 deficiency in Snell dwarf mice associates with increased DNA repair capacity in cultured primary fibroblasts. a Representative images of damaged DNA in irradiated (3 Gy) fibroblasts derived from control mice and GH/IGF-1-deficient Snell dwarf mice. Note the greater decline in tail DNA content at 20 and 60 min post-irradiation in cells derived from Snell dwarf mice due to improved DNA repair processes. b γ-Irradiated fibroblasts exhibit comparable DNA damage. Shown are increases in tail DNA content induced by increasing doses of γ-irradiation (3 to 9 Gy) in fibroblasts derived from control mice and Snell dwarf mice (n.s.: not significant vs control; t test). c Fibroblasts derived from Snell dwarf mice exhibit increased DNA repair capacity as compared to controls. For assessment of DNA repair efficiency, the percentage of residual DNA damage was plotted as a function of time post-irradiation. Tail DNA content at each time point is shown. The time constant calculated from this plot was shorter, and the residual DNA damage post-irradiation was lower in fibroblasts from Snell dwarf mice as compared to cells derived from control mice, indicating a more efficient DNA repair capacity in these cells. *p < 0.001 Snell dwarf vs control (t test). Data are mean ± SEM (n = 5 for each time-point). d White blood cells derived from mice with adult-onset IGF-1 deficiency (Igf1 f/f + TBG-Cre-AAV8) and control mice exhibit comparable DNA repair capacity. Data are mean ± SEM (n = 5 for each time-point)
Early-life GH/IGF-1 status of donor animals elicits persisting alterations in DNA repair capacity in cultured primary fibroblasts
For assessment of DNA repair efficiency, the percentage of residual DNA damage in cultured fibroblasts was plotted as a function of time post-irradiation. We found that the percentage of residual DNA damage post-irradiation was lower in fibroblasts derived from Lewis dwarf rats than in control cells (Fig. 1c), indicating that early-life GH/IGF-1 deficiency associates with persisting increases in cellular DNA repair capacity. The percentage of residual DNA damage post-irradiation did not differ significantly in fibroblasts derived from Lewis dwarf rats with early-life GH treatment and in control cells (Fig. 1c), indicating that GH treatment of donor Lewis dwarf rats decreased cellular DNA repair capacity. These results highlight the importance of early-life GH/IGF-1 status in regulation of cellular DNA repair capacity. This concept is also supported by findings obtained in Snell dwarf fibroblasts showing decreased residual DNA damage post-irradiation in this mouse model of developmental GH/IGF-1 deficiency (Fig. 2c). To separate the effects of early- and late-life IGF-1 deficiency on cellular DNA repair in mice, a comet assay was performed using blood derived from a mouse model of adult-onset isolated circulating IGF-1 deficiency (Igf1 f/f + TBG-Cre-AAV8). The IGF-1 levels in this model are decreased by ∼70% as reported (Tarantini et al. 2016a, b; Toth et al. 2015, 2014a). We found that adult-onset IGF-1 deficiency did not have any obvious effect on cellular DNA repair (Fig. 2d).
Early-life GH/IGF-1 status of donor animals elicits persisting alterations in cellular expression of DNA repair-related genes
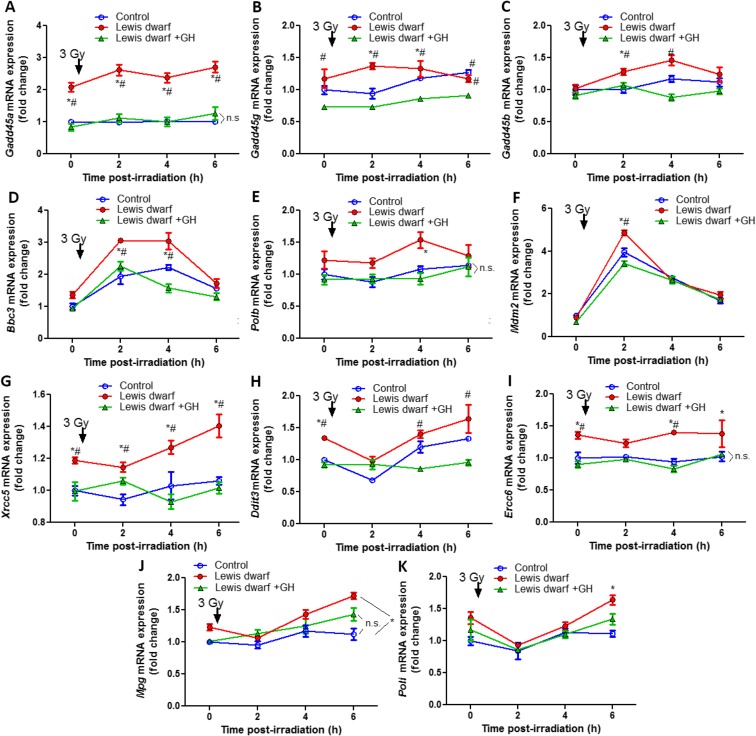
Expression of genes involved in regulation of DNA repair pathways was analyzed in fibroblasts derived from control rats, Lewis dwarf rats, and GH-replete dwarf rats. We found that early-life GH/IGF-1 deficiency in donor animals was associated with significant alterations (as determined by one-way ANOVA) in cellular expression of Gadd45a, Xrcc5, Ercc6, and Ddit3 (Fig. 3). Importantly, short-term early-life GH treatment of donor animals prevented these gene expression changes (Fig. 3). We analyzed the time course of changes in expression of known γ-radiation-induced common stress response genes. We found that many of these genes (including Gadd45b, Bbc3, Mdm2) were upregulated (as determined by t test) in Lewis dwarf fibroblasts post-irradiation. The radiation-induced expression of Gadd45b, Bbc3, and Mdm2 (but not baseline expression of these targets) was significantly greater in Lewis dwarf fibroblasts as compared to that in cells derived from control animals or Lewis dwarf rats with early-life GH treatment (one-way ANOVA; Fig. 3).
Fig. 3.
qPCR data showing expression of genes involved in regulation of DNA repair pathways, including γ-radiation-induced common stress response genes, in fibroblasts derived from control rats, Lewis dwarf rats, and GH-replete dwarf rats. Expression of target genes was compared under basal conditions and at 2, 4, and 6 h post-irradiation. Data are mean ± SEM (n = 3–6 for each data point). *p < 0.05 Lewis dwarf vs control, #p < 0.05 Lewis dwarf vs Lewis dwarf + GH
Discussion
This is the first study to demonstrate that GH/IGF-1 status during a critical developmental time-window elicits persisting alterations in cellular DNA repair pathways. Proficient cellular DNA repair systems substantially reduce cancer incidence, and thus, upregulation of DNA repair pathways by peripubertal GH/IGF-1 deficiency could well have relevance for decreased organismal susceptibility to carcinogenesis later in life. A critical role for pubertal IGF-1 levels in determining cancer risk is supported by both clinical studies (Guevara-Aguirre et al. 2011) and experimental findings (Panici et al. 2010; Ramsey et al. 2002). In Lewis dwarf rats, the reductions in IGF-1 around puberty profoundly influence late-life pathologies (Sonntag et al. 2005b, 2013), including a decrease in cancer risk at old age (Guevara-Aguirre et al. 2011; Pollak et al. 2004). Importantly, in these animals, restoration of IGF-1 levels by GH treatment early in life dramatically increases susceptibility to cancer (Ramsey et al. 2002). Similar evidence was obtained in IGF-1-deficient Ames dwarf mice supporting the concept that a critical time window exists during postnatal development when circulating IGF-1 levels regulates cellular mechanisms that maintain genomic integrity and thus determine resistance to cancer later in life (Panici et al. 2010). In addition, IGF-1 is also known to exert growth-promoting and antiapoptotic effects (Pollak et al. 2004; Yakar et al. 2004), which may contribute to the delayed growth of tumors and metastases associated with IGF-1 deficiency (Sonntag et al. 2012; Pollak et al. 2004; Yakar et al. 2004; Wu et al. 2003; Burgers et al. 2011; Renehan et al. 2004).
Our findings have important clinical significance. Circulating levels of IGF-1 are highly variable during puberty (range: from ∼100 to 800 ng/mL) (Sorensen et al. 2012; Bidlingmaier et al. 2014). The significant variability in peripubertal IGF-1 levels, in addition to genetics, can be largely attributed to differences in nutritional status and increasing prevalence of childhood obesity. In the USA among children, 17% are estimated to be obese. Several studies have demonstrated that childhood obesity is associated with increased basal IGF-1 levels (Ong et al. 2002; Garnett et al. 2004), increased GH-induced IGF-1 generation, and increased IGF-1 bioavailability via reductions in IGF binding proteins (Ballerini et al. 2004; Bouhours-Nouet et al. 2007; Burt Solorzano and McCartney 2010). Because childhood obesity increases cancer predisposition, future studies are warranted to determine the effects of this obesity epidemic on cellular DNA repair pathways. There are also important ethnic differences; for example, African American prepubertal children have higher circulating IGF-1 levels as compared to their Latino and Caucasian peers (Alderete et al. 2011). It remains to be determined how these differences affect cellular DNA repair pathways.
The mechanisms by which developmental/peripubertal GH/IGF-1 status regulates cellular DNA repair processes are likely multifaceted. Our findings support the concept that peripubertal IGF-1 levels affect the expression of multiple DNA repair genes later in life. As shown in Fig. 3a, we found that in fibroblasts of rats, lacking the peripubertal IGF-1 surge expression of Gadd45a is significantly upregulated. This is a potentially important finding, because Gadd45a is an important component of the p53 pathway that contributes to the maintenance of genomic stability (Hollander et al. 1999, 2001; Jung et al. 2007). Mice lacking the Gadd45a gene are more prone to tumors induced by ionizing radiation and genotoxin exposure (Hollander et al. 2001), and their fibroblasts have defective NER (Hollander et al. 1999) and BER (Jung et al. 2007). Furthermore, Gadd45a was also shown to have a key role in active DNA de-methylation (Barreto et al. 2007). It has been proposed that Gadd45a may regulate promoter methylation and thereby activation of various tumor suppressor genes such as Mlh1. Importantly, early-life IGF-1 deficiency is also associated with upregulation of Gadd45a in tail-derived fibroblasts of the Snell dwarf mouse model as well (Ungvari, Miller and Csiszar, 2016, unpublished data). Another potentially interesting target regulated by early-life IGF-1 status is Xrcc5. The Ku80 protein, which is encoded by the Xrcc5 gene, is known to bind to DNA double-strand break ends and is required for the non-homologous end joining pathway of DNA repair. Peripubertal IGF-1 levels also elicit persisting changes in the expression of Ercc6 encoding the DNA excision repair protein ERCC-6/CSB. Further studies are evidently warranted to evaluate potential causal links among these gene expression changes, alterations in DNA repair efficiency, mutagenicity, and susceptibility for carcinogenesis. Importantly, our data show that peripubertal IGF-1 status determines the upregulation of the known p53-dependent tumor suppressor gene Mdm2 and the p53 upregulated modulator of apoptosis Bbc3 upon γ-irradiation. p53 (encoded by the Tp53 gene) is a critically important tumor suppressor, which protects the genome from mutations and genetic alterations. Tp53 is one of the most commonly mutated genes in human cancers, and genetic deficiency of p53 was shown to promote the development of a variety of different cancers (Harvey et al. 1993). p53 functions as a transcription factor, which is activated in response to DNA damage, initiating the transcription of a number of genes. Importantly, Gadd45a is also a target of p53. Although there is ample evidence in the literature suggesting that a link exists between IGF-1 signaling and p53-regulated pathways, it is currently unknown how peripubertal IGF-1 deficiency affects this aspect of the regulation of cancer susceptibility. In addition to Lewis dwarf rats and Snell dwarf mice (Carter et al. 2002a; Flurkey et al. 2001), there are many mouse models in which early-life disruption of GH/IGF-1 signaling is associated with significant lifespan extension and/or reduced cancer burden later in life (e.g., Ames (Brown-Borg et al. 1996) dwarf mice, GHRHR-deficient lit/lit mice (Flurkey et al. 2001), GHR knockout mice (Berryman et al. 2008), and mice lacking both GH and GHR (Gesing et al. 2016)). It will be informative to determine how DNA repair pathways are altered in the tissues of these models in vivo. Initial evidence shows that two proteins involved in DNA repair (MGMT, NDRG1) are upregulated in Snell dwarf mice, growth hormone receptor gene disrupted mice (GHRKO), and in mice deficient in the pregnancy-associated protein-A (PAPPA-KO) (Dominick et al. 2016).
The molecular mechanisms by which peripubertal GH/IGF-1 status regulates expression of genes involved in cellular resilience and DNA repair processes remain obscure. There is growing evidence linking silencing of tumor suppressor genes by DNA methylation to carcinogenesis. Previous results (Murakami et al. 2003; Salmon et al. 2005; Ungvari et al. 2011; Harper et al. 2007; Hsieh and Papaconstantinou 2009) showing that rodent fibroblasts retain their unique stress resistance signatures against DNA damaging agents in culture through many rounds of mitosis are consistent with the presence of epigenetic control mechanisms regulating DNA repair pathways that are induced in vivo by neurohormonal factors and maintained in extended culture. Thus, future studies should elucidate cellular epigenetic changes as a result of differing IGF-1 histories (Dominick et al. 2016). Studies correlating IGF-1-dependent alterations in DNA methylation status and expression profiles of DNA repair genes should be quite revealing. In addition to the putative role of DNA methylation, translational control of proteins involved in DNA repair may also be important (Dominick et al. 2016). We have recently showed that peripubertal IGF-1 deficiency also influences the cellular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation (Tarantini et al. 2016a).
Limitations of the study
There are important limitations of our study, including the limited endpoints tested. We have explored how DNA repair and gene expression are coordinated only in response to γ-irradiation. Future studies should test responses to multiple types of DNA damage and how specific DNA repair pathways are affected by early-life IGF-1 deficiency (Leiser et al. 2006; Murakami et al. 2003; Salmon et al. 2005; Ungvari et al. 2013b). Importantly, previous studies by the Miller laboratory show that UV-induced DNA damage is attenuated in fibroblasts from Snell dwarf mice (Salmon et al. 2008). It will also be interesting to determine whether transcription patterns reflect the relative cellular sensitivities to DNA-damaging stressors across multiple species. It is also a limitation of our studies that we do not have data on DNA repair in cells of GH replete Snell dwarf mice. Importantly, an earlier study found that Snell dwarf mice treated with GH starting at 4 weeks of age had no effect on lifespan (Vergara et al. 2004). Because in these studies, GH treatment did not reverse the dwarf phenotype, one may speculate that starting the GH treatment at 4 weeks may be too late to interfere with cellular stress resistance pathways. We found that in white blood cells, adult onset IGF-1 deficiency did not affect DNA repair efficiency. It would be also informative to assess DNA repair efficiency in white blood cells derived from Snell dwarf mice in future studies. Finally, because there is emerging evidence that there are important sex differences in the longevity phenotypes in models of early-life disruption of GH/IGF-1 axis (e.g., in mice heterozygous for the deletion of the IGF-1 receptor (Holzenberger et al. 2003) and in mice with early-life knockdown of circulating IGF-1 (Sonntag et al. 2016, submitted)), future studies should determine how sex influences epigenetic regulation of DNA repair pathways by peripubertal GH/IGF status.
Future studies should also determine whether levels of IGF-1 or GH early in life are more important in regulation of cellular resilience and DNA repair pathways. On the one hand, mice deficient in the pregnancy-associated protein-A (PAPPA-KO) exhibit a longevity phenotype and upregulation of DNA repair proteins without any change in GH (Dominick et al. 2016). On the other hand, liver-specific GHRKO mice have no lifespan increase despite significant decline in circulating IGF-1 (List et al. 2014).
Previous studies suggest that paracrine IGF-1 signaling may compensate for circulating IGF-1 deficiency in certain tissues (e.g., in the brain (Sun et al. 2005; Sun and Bartke 2007)). Thus, it would be of great interest to determine whether the effects of early-life IGF-1 status on cellular resilience and DNA repair pathways show organ/tissue specificity. It would also be interesting to know whether local GF-1 production in individual tissues/organs can be modified by circulating IGF-1/GH status during development.
In conclusion, GH/IGF-1 levels during a critical period during early life determine cellular DNA repair capacity in rodents likely by post-transcriptional control of genes involved in DNA repair. Because lifestyle factors (e.g., nutrition, childhood obesity) cause huge variation in peribubertal GH/IGF-1 levels in children, further studies are warranted to determine their persisting influence on cellular cancer resistance pathways.
Acknowledgement
This work was supported by grants from the American Heart Association (to MNVA, AC and ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; 3P30AG050911-02S1 to AC, WES and ZU, AG019899 and AG024824 to RAM), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC), the National Institute of General Medical Sciences (UL1GM118991, TL4GM118992, and RL5GM118990 to AP and VP), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to ZU; P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU), the Oklahoma IDeA Network for Biomedical Research Excellence (to AC), and the Reynolds Foundation (to ZU and AC) and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395 (to AP and KY). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Author contribution
AP, AC, and ZU designed research; MNVA, AP, KV, VP, EN, TG, and RAM performed experiments; AP, MNVA, AC, RAM, WES, and ZU analyzed and interpreted data; AP, AC, and ZU wrote the paper; MNVA, WES, RAM revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alderete TL, Byrd-Williams CE, Toledo-Corral CM, Conti DV, Weigensberg MJ, Goran MI. Relationships between igf-1 and igfbp-1 and adiposity in obese african-american and latino adolescents. Obesity (Silver Spring) 2011;19:933–938. doi: 10.1038/oby.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and igf-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini MG, Ropelato MG, Domene HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (igf)-igf binding proteins axis. J Pediatr Endocrinol Metab. 2004;17:749–757. doi: 10.1515/JPEM.2004.17.5.749. [DOI] [PubMed] [Google Scholar]
- Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, Niehrs C. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the gh/igf-1 axis in lifespan and healthspan: lessons from animal models. Growth Hormon IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Korner A, Obermayer-Pietsch B, Hubener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence igf-i immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99:1712–1721. doi: 10.1210/jc.2013-3059. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours-Nouet N, Gatelais F, Boux de Casson F, Rouleau S, Coutant R. The insulin-like growth factor-i response to growth hormone is increased in prepubertal children with obesity and tall stature. J Clin Endocrinol Metab. 2007;92:629–635. doi: 10.1210/jc.2005-2631. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp Gerontol. 2009;44:10–19. doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers AM, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, Dekkers OM. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor i (igf-i) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and igf-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Ingram RL, Cashion AB, Cefalu WT, Wang ZQ, Sonntag WE. Models of growth hormone and igf-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57:B177–B188. doi: 10.1093/gerona/57.5.B177. [DOI] [PubMed] [Google Scholar]
- Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and sirt 1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE, Ungvari ZI. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and ad. Am J Physiol Heart Circ Physiol. 2013 [DOI] [PMC free article] [PubMed]
- D'Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil Suppl. 1993;46:87–98. [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (igf)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominick G, Bowman J, Li X, Miller RA, Garcia GG. Mtor regulates the expression of DNA damage response enzymes in long-lived snell dwarf, ghrko, and pappa-ko mice. Aging Cell 2016 [DOI] [PMC free article] [PubMed]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, Cowell CT. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80:966–972. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- Gesing A, Wiesenborn D, Do A, Menon V, Schneider A, Victoria B, Stout MB, Kopchick JJ, Bartke A, Masternak MM. A long-lived mouse lacking both growth hormone and growth hormone receptor: A new animal model for aging studies. J Gerontol A Biol Sci Med Sci 2016 [DOI] [PMC free article] [PubMed]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Schwartz RC. Is there a link between a high-fat diet during puberty and breast cancer risk? Women's Health (Lond Engl) 2011;7:1–3. doi: 10.2217/whe.10.83. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Deng CX, Fornace AJ., Jr Genomic instability in gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Kovalsky O, Salvador JM, Kim KE, Patterson AD, Haines DC, Fornace AJ., Jr Dimethylbenzanthracene carcinogenesis in gadd45a-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 2001;61:2487–2491. [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. Igf-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Dermal fibroblasts from long-lived Ames dwarf mice maintain their in vivo resistance to mitochondrial generated reactive oxygen species (ros) Aging (Albany NY) 2009;1:784–802. doi: 10.18632/aging.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.B291. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Springer NM, Bielinsky AK, Largaespada DA, Ross JA. Developmental origins of cancer. Cancer Res. 2009;69:6375–6377. doi: 10.1158/0008-5472.CAN-09-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Kim EH, Mun JY, Park S, Smith ML, Han SS, Seo YR. Base excision DNA repair defect in gadd45a-deficient cells. Oncogene. 2007;26:7517–7525. doi: 10.1038/sj.onc.1210557. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009;296:H946–H956. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Salmon AB, Miller RA. Correlated resistance to glucose deprivation and cytotoxic agents in fibroblast cell lines from long-lived pituitary dwarf mice. Mech Ageing Dev. 2006;127:821–829. doi: 10.1016/j.mad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific gh receptor gene-disrupted (lighrko) mice have decreased endocrine igf-i, increased local igf-i, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Carbajal S, Beltran L, Perkins SN, Yakar S, Leroith D, Hursting SD, Digiovanni J. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor i levels. Cancer Res. 2008;68:3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Olivo-Marston SE, Hursting SD, Lavigne J, Perkins SN, Maarouf RS, Yakar S, Harris CC. Genetic reduction of circulating insulin-like growth factor-1 inhibits azoxymethane-induced colon tumorigenesis in mice. Mol Carcinog. 2009;48:1071–1076. doi: 10.1002/mc.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Tan Y, Zhao Y, Aupperlee MD, Haslam SZ. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int J Obes. 2010;34:1415–1426. doi: 10.1038/ijo.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K, Kratzsch J, Kiess W, Dunger D. Circulating igf-i levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab. 2002;87:1041–1044. doi: 10.1210/jcem.87.3.8342. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci U S A. 1976;73:4536–4540. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MM, Salmon AB, Leiser SF, Robb EL, Brown MF, Miller RA, Stuart JA. Mechanisms of stress resistance in snell dwarf mouse fibroblasts: enhanced antioxidant and DNA base excision repair capacity, but no differences in mitochondrial metabolism. Free Radic Biol Med. 2009;46:1109–1118. doi: 10.1016/j.freeradbiomed.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:1–7. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (dmba)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (igf)-i, igf binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Ljungman M, Miller RA. Cells from long-lived mutant mice exhibit enhanced repair of ultraviolet lesions. J Gerontol A Biol Sci Med Sci. 2008;63:219–231. doi: 10.1093/gerona/63.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (igf)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.B521. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and igf-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1017/S002187829900713X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (igf-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor i deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Csiszar A, de Cabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in cns and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum igf1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol. 2012;166:903–910. doi: 10.1530/EJE-12-0106. [DOI] [PubMed] [Google Scholar]
- Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci. 2007;62:117–125. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of gh and igf-1 in the hippocampus of gh-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. Igf-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering mirna-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares M, Toth P, Gautam T, Giles C, Ballabh P, Wei Y, Wren J, Ashpole N, Sonntag W, Ungvari Z, Csiszar A. Circulating igf-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin ii-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34(12):1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and alzheimer's disease J Gerontol Biol Med Sci. 2013: in press [DOI] [PMC free article] [PubMed]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal gh replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal gh treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A, Sonntag WE. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–1457. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Mason JB, Gruber H, Lee SW, Schwartz TS, Brown MK, Storm NJ, Fortney K, Sowa J, Byrne AB, Kurz T, Levy E, Sonntag WE, Austad SN, Csiszar A, Ridgway I. Resistance to genotoxic stresses in Arctica islandica, the longest living noncolonial animal: is extreme longevity associated with a multistress resistance phenotype? J Gerontol A Biol Sci Med Sci. 2013;68:521–529. doi: 10.1093/gerona/gls193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nat Rev Cancer. 2012;12:479–486. doi: 10.1038/nrc3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300:H736–H744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE (2012) Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One 7:–e30444 [DOI] [PMC free article] [PubMed]
- Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor i levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388. [PubMed] [Google Scholar]
- Yakar S, Pennisi P, Zhao H, Zhang Y, LeRoith D. Circulating igf-1 and its role in cancer: lessons from the igf-1 gene deletion (lid) mouse. Novartis Found Symp. 2004;262:3–9. doi: 10.1002/0470869976.ch2. [DOI] [PubMed] [Google Scholar]
- Yan H, Mitschelen M, Toth P, Ashpole NM, Farley JA, Hodges EL, Warrington JP, Han S, Fung KM, Csiszar A, Ungvari Z, Sonntag WE. Endothelin-1-induced focal cerebral ischemia in the growth hormone/igf-1 deficient Lewis dwarf rat. J Gerontol A Biol Sci Med Sci. 2014;69:1353–1362. doi: 10.1093/gerona/glu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tan YS, Aupperlee MD, Langohr IM, Kirk EL, Troester MA, Schwartz RC, Haslam SZ. Pubertal high fat diet: effects on mammary cancer development. Breast Cancer Res. 2013;15:R100. doi: 10.1186/bcr3561. [DOI] [PMC free article] [PubMed] [Google Scholar]