Abstract
In older adults, chronic oxidative and inflammatory stresses are associated with an impaired increase in skeletal muscle protein synthesis after acute anabolic stimuli. Conjugated linoleic acid (CLA) and Protandim have been shown to activate nuclear factor erythroid-derived 2-like 2 (Nrf2), a transcription factor for the antioxidant response element and anti-inflammatory pathways. This study tested the hypothesis that compared to a placebo control (CON), CLA and Protandim would increase skeletal muscle subcellular protein (myofibrillar, mitochondrial, cytoplasmic) and DNA synthesis in older adults after 6 weeks of milk protein feeding. CLA decreased oxidative stress and skeletal muscle oxidative damage with a trend to increase messenger RNA (mRNA) expression of a Nrf2 target, NAD(P)H dehydrogenase quinone 1 (NQO1). However, CLA did not influence other Nrf2 targets (heme oxygenase-1 (HO-1), glutathione peroxidase 1 (Gpx1)) or protein or DNA synthesis. Conversely, Protandim increased HO-1 protein content but not the mRNA expression of downstream Nrf2 targets, oxidative stress, or skeletal muscle oxidative damage. Rates of myofibrillar protein synthesis were maintained despite lower mitochondrial and cytoplasmic protein syntheses after Protandim versus CON. Similarly, DNA synthesis was non-significantly lower after Protandim compared to CON. After Protandim, the ratio of protein to DNA synthesis tended to be greater in the myofibrillar fraction and maintained in the mitochondrial and cytoplasmic fractions, emphasizing the importance of measuring both protein and DNA synthesis to gain insight into proteostasis. Overall, these data suggest that Protandim may enhance proteostatic mechanisms of skeletal muscle contractile proteins after 6 weeks of milk protein feeding in older adults.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9968-8) contains supplementary material, which is available to authorized users.
Keywords: Sarcopenia, Proteostasis, Oxidative stress, Inflammation, Anabolic resistance
Introduction
For both geriatricians and basic scientists, it is important to understand how to translate interventions that prolong health span in experimental animals into human clinical application (Kirkland 2016). Decreased health span, as evident by a reduction in independence and increased risk for several chronic diseases, is linked with the age-related loss of skeletal muscle (i.e., sarcopenia) (Kirkland 2016). The synthesis, folding, modification, and transport of new skeletal muscle proteins are involved in maintaining protein homeostasis (i.e., proteostasis) (Orgel 1963; Schwanhäusser et al. 2011; Miller et al. 2014) and preserving skeletal muscle mass and function with advancing age. Since the aging population is rapidly expanding, it is imperative to identify additional strategies to maintain skeletal muscle proteostasis to counter sarcopenia and prolong health span.
In skeletal muscle, the response to acute stress involves increased protein synthesis to maintain protein structures and cellular homeostasis. Cell proliferation also requires increased protein synthesis rates but to ensure an equal complement of proteins for new cells rather than maintenance of protein structures. Skeletal muscle consists of post-mitotic, multi-nucleated myofibers and proliferative supportive cells (e.g., satellite, pericyte, endothelial, etc.), and measures of DNA synthesis are reflective of proliferation of the supportive cell types. Therefore, increased protein synthesis maintains existing protein structures in the former scenario, while in the latter, the increased protein synthesis is allocated toward proliferation of supportive cells. To understand if cellular priorities are dedicated to maintaining existing protein structures or making new cells, we simultaneously assess both protein synthesis and cell proliferation from the incorporation of the stable isotope deuterium oxide (D2O) into protein and DNA. We then use the ratio of protein to DNA synthesis to gain insight into the proportion of proteins synthesized to maintain existing cells versus proliferation of new cells. We have demonstrated an increased ratio of skeletal muscle protein to DNA synthesis in several dietary and genetic interventions shown to slow aging in mice (Miller et al. 2012, 2014; Drake et al. 2013, 2014, 2015). These data suggest that slowed aging models preferentially direct cellular resources toward maintaining existing structures rather than making new cells. It remains unknown if these shared proteostatic characteristics of slowed aging animal models are replicated by similar interventions applied to humans.
One process contributing to sarcopenia in older adults may be the blunted response of skeletal muscle protein synthesis after dietary protein feeding (Dickinson et al. 2013). Chronically elevated levels of oxidative stress and inflammation are associated with the anabolic resistance of mixed muscle protein synthesis after an acute protein bolus (Shenton et al. 2006; Zhang et al. 2009; Balage et al. 2010) and the age-related loss of muscle mass and function (Howard et al. 2007; Semba et al. 2007a, b; Schaap et al. 2009). Decreasing oxidative stress and inflammation through antioxidant or ibuprofen treatment has been shown to restore the acute anabolic effects of leucine-stimulated mixed muscle protein synthesis in animals (Marzani et al. 2008; Rieu et al. 2009). However, supplementing with exogenous antioxidants may have negative outcomes in patients with disease risk (Knekt et al. 2004; Nightingale et al. 2007). Additionally, non-steroidal anti-inflammatory drugs (NSAIDs) have noted long-term gastrointestinal and renal side effects that may be accentuated with concomitant drug consumption common in older adults (Doyle et al. 1999).
Due to the potential shortcomings of exogenous antioxidant or NSAID supplementation, we chose two compounds, Protandim and conjugated linoleic acid (CLA), because they have each been shown to activate nuclear factor erythroid-derived 2-like 2 (Nrf2) (Bergamo et al. 2007; Velmurugan et al. 2009; Bergamo et al. 2011; Donovan et al. 2012; Reuland et al. 2013), a transcription factor that regulates the expression of the endogenous antioxidant network and anti-inflammatory pathways. Both oxidants and non-oxidants can stimulate Nrf2 (Hybertson et al. 2011; Bruns et al. 2015). The induction of Nrf2 by oxidants acts as a compensatory response whereas the stimulation of Nrf2 signaling by non-oxidants like Protandim and CLA may impart a cytoprotective adaptation to resist or decrease chronic oxidative and/or inflammatory stresses (Hybertson et al. 2011). Indeed, Nrf2 signaling has been shown to contribute to the stress-resistant phenotype observed in long-lived species (Leiser and Miller 2010). We recently showed within the National Institute on Aging Interventions Testing Program (ITP) that Protandim extended median life span by 7% in heterogeneous male mice (Strong et al. 2016). The increase in median life span, but not maximal life span, is potentially indicative of a positive effect of Protandim on health span. However, the influence of dietary supplementation of Nrf2 activators Protandim or CLA has yet to be tested as a treatment in humans to improve skeletal muscle proteostasis, a shared characteristic of slowed aging.
We tested whether CLA or Protandim could enhance the skeletal muscle protein synthesis response to 6 weeks of protein feeding in older adults. We hypothesized that after 6 weeks of milk protein feeding, CLA and Protandim would (1) result in greater rates of skeletal muscle protein synthesis and have a greater ratio of protein to DNA synthesis when compared to a placebo control (CON); (2) increase the expression of downstream Nrf2 targets; and (3) decrease inflammation, oxidative stress, and oxidative damage.
Methods
This study was approved by the Institutional Review Board at Colorado State University (13-4191H). Prior to beginning participation, each participant was informed of the study design, potential risk, and benefits and provided written consent. Forty-six male and female individuals participated in this study after a detailed physical examination, including a medical history and physical activity questionnaire, resting ECG, and fasting blood draw. Subjects were excluded if lactose intolerant or recent/current tobacco users. Since many interested volunteers were taking multi-vitamins, antioxidant supplements, or medications known to promote anti-inflammation, participants were asked to refrain from using these products for a minimum of 4 weeks prior to data collection and throughout the 6-week intervention. Participants were sedentary to recreationally active based on physical activity questionnaires.
Study overview
In a randomized, double-blinded, placebo-controlled study design, participants completed one of three 6-week nutritional interventions. In efforts to stimulate protein anabolism, all three groups received protein in the form of 240 ml (8 fluid oz) of fat-free milk, three times daily (daily macronutrient composition: 240 kcal; fat 0 g; carbohydrate 36 g; protein 24 g). The investigative team provided fat-free milk to each participant on a weekly basis.
The control (CON) group consumed placebo pills (high oleic sunflower oil; 4 g/day) while the other groups were supplemented with either the phytochemical compound Protandim® (LifeVantage) at the commercially available dose (one pill/day) or conjugated linoleic acid (CLA; 4 g/day). Protandim is a mixture of five botanical extracts, including milk thistle extract seed, bacopa extract whole herb, ashwagandha extract root, green tea extract leaf, and turmeric extract rhizome (Nelson et al. 2006). The CLA supplement (Clarinol A-80; Stepan Lipid Nutrition) contained a 50:50 ratio of cis-9, trans-11 and trans-10, and cis-12 octadecadienoic acid since these isomers account for the majority of the total CLA isomers in dairy products. Dairy products are the best-known source for CLA providing 3–5.5 mg/g of fat (Dhiman et al. 1999). Since the CLA content of milk is known to vary from batch to batch and dairy to dairy, the milk protein feeding in the current study was in the form of fat-free milk devoid of CLA such that a controlled dose of CLA could be provided in supplement form. Participants were provided extra pills, and compliance was estimated based on returned pill count. Participants were instructed to not change their normal dietary or physical activity habits. Body composition was assessed by dual energy x-ray absorptiometry (DEXA) before and after the 6-week intervention.
Deuterium labeling
Participants orally consumed D2O (70%; Sigma-Aldrich) to achieve an isotopic steady state of 1–2% to label newly synthesized skeletal muscle protein and DNA. D2O was provided during the last 4 weeks of the intervention (weeks 3–6) starting with a priming stage in week 3 (50 ml 3×/day) followed by maintenance during weeks 4–6 (50 ml, 2×/day). Body water enrichment was determined from plasma collected at week 6 as we have previously described (Robinson et al. 2011; Scalzo et al. 2014).
Tissue sampling
Participants arrived to the laboratory after an overnight fast for blood and skeletal muscle sample collection before and after the 6-week nutritional interventions using procedures as we have previously described (Robinson et al. 2011; Scalzo et al. 2014). Venous blood was collected in EDTA vacutainers and centrifuged for 10 min (3500 rpm, 4 °C). Subsequently, plasma was aliquoted and stored at −80 °C until analysis. Muscle samples were obtained from the vastus lateralis under local anesthesia (1% lidocaine) with a modified 5-mm Bergstrom needle with manual suction and immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
Tissue and analyte preparation
Body water enrichment was determined from plasma as we have previously described (Robinson et al. 2011; Scalzo et al. 2014). Using differential centrifugation, skeletal muscle was fractionated to measure protein synthesis rates of subcellular fractions enriched with myofibrillar, cytoplasmic, and mitochondrial proteins according to our previously published standard operating procedures (Robinson et al. 2011; Miller et al. 2012, 2013; Drake et al. 2013; Scalzo et al. 2014). Following tissue fractioning, analytes were prepared for analysis on a 7890A gas chromatograph coupled to a 5975C mass spectrometer with a DB-5MS GC column (30 m × 0.25 mm × 0.25 μm; all from Agilent) (Robinson et al. 2011; Miller et al. 2012, 2013; Drake et al. 2013; Scalzo et al. 2014).
Total DNA (~8 μg) was extracted from approximately 15–20 mg tissue (QiAamp DNA mini kit Qiagen, Valencia, CA). Determination of deuterium oxide incorporation into purine deoxyribose of DNA was performed as described previously (Busch et al. 2007; Robinson et al. 2011; Miller et al. 2012). Deuterium labeling of the deoxyribose moiety of DNA occurs exclusively through de novo nucleotide synthesis and allows calculation of the rate of newly synthesized DNA over extended periods (Neese et al. 2002; Busch et al. 2007). The newly synthesized fraction (f) of muscle proteins and DNA was calculated from the enrichment of labeled alanine bound to muscle proteins and purine deoxyribose of DNA over the entire labeling period, divided by the true precursor enrichment (p), using plasma analyzed for D2O enrichment and then adjusted by using MIDA calculations (Busch et al. 2006). Fraction new was divided by time and multiplied by 100 to obtain the fractional synthesis rate (FSR).
Western blotting
The cytoplasmic muscle fraction was used for Western blot analysis of the Nrf2 signaling pathway and protein carbonyls following previously described procedures (Drake et al. 2013; Bruns et al. 2015). We chose to assess heme oxygenase 1 (HO-1, Abcam 13243) as a representation of Nrf2 activation since HO-1 is a known, downstream target of Nrf2 (Bruns et al. 2015). MagicMark XP (Invitrogen) and Color Burst (Sigma) were used as molecular weight markers. Blots were incubated with primary antibody (1:500) overnight, washed with TBST (3 × 10 min), and incubated with anti-rabbit, HRP-conjugated secondary antibody (1:2000; Santa Cruz Biotechnologies sc2004) with subsequent chemiluminescent detection (West Dura, Thermo Scentific). Protein carbonyls were measured by following the protocol in the commercially available OxiSelect Protein Carbonyl Immunoblot Kit (Cell Biolabs STA-308) as previously performed (Konopka et al. 2015). Images were obtained, and densitometry was analyzed with a UVP bioimaging system. HO-1 and protein carbonyls were expressed relative to tubulin (1:1000, Santa Cruz Biotechnologies sc5274). The entire lane of each sample was analyzed as a representation of global protein carbonyl content in the cytoplasmic fraction of skeletal muscle.
mRNA expression
Expressions of HO-1, NAD(P)H dehydrogenase quinone 1 (NQO1), and Gpx1 were analyzed by real-time RT-PCR as previously described (Robinson et al. 2010) with some modifications. Briefly, total RNA was extracted from whole muscle samples (15 mg) using the standard chloroform-phenol extraction methods (TRIzol, Thermo Fisher Scientific). RNA was reverse transcribed to cDNA using TaqMan reverse transcription reagents (Thermo Fisher Scientific). Target sequences (Table 1) were amplified using standard SYBR green reagents (Thermo Fisher Scientific) and RT-PCR procedures (LightCycler 480 System; Roche) with the following conditions: 95 °C for 5 min and 45 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s. Dissociation curve analysis was conducted to confirm amplification specificity. The relative quantification of each target gene was normalized to an endogenous control (β2-microglobulin (B2M)) and compared against the baseline sample using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Table 1.
Sequences used for RT-PCR
| Forward | Reverse | |
|---|---|---|
| B2M | 5′-CAGCAAGGACTGGTCTTTCTAT-3 | 5′-ACATGTCTCGATCCCACTTAAC-3 |
| HO-1 | 5′-CCAGCAACAAAGTGCAAGATTC-3 | 5′-CCACCAGAAAGCTGAGTGTAAG-3′ |
| GPX1 | 5′-CATCAGGAGAACGCCAAGAA-3′ | 5′-GCACTTCTCGAAGAGCATGA-3′ |
| NQO1 | 5′-TCCCAAAGTGCTGGGATTAC-3′ | 5′-GCAGATGTACGGTGTGGATTTA-3′ |
B2M β2-microglobulin, HO-1 hemoxygenase-1, GPX1 glutathione peroxidase 1, NQO1 NAD(P)H dehydrogenase quinone 1
Systemic oxidative stress and inflammation
Plasma samples were evaluated for oxidative stress via the commercially available thiobarbituric acid reactive substances (TBARS) kit (Cayman Chemical). TBARS monitors malondialdehyde (MDA), a product of lipid peroxidation. Two participants, one each in CON and PRO, had samples that were hemolyzed and therefore discolored. Since the TBARS assay is a colorimetric assay, these samples were >2 SD away from the mean and therefore considered outliers and removed from data analysis.
Markers of systemic inflammation were evaluated using the Human Adipokine Magnetic Bead Panel 2 (Millipore) to measure IL-6, IL-8, IL-1β, and TNF-α. Nearly all samples had levels of IL-6 and IL-1β lower than detectable limits and therefore are not included in analysis. We also used immunoturbidimetric determination (Beckman Coulter) of high-sensitivity C-reactive protein (CRP) in plasma samples as an additional indicator of systemic inflammation.
Plasma CLA
Fatty acid methyl esters (FAMEs) were prepared following a method described by Nuernberg et al. (2007). Briefly, 20 μl of plasma was dried for 2 h at 25 °C under vacuum using a Savant SpeedVac (AES2010). Then, 40 μl toluene was added to the dry samples to solubilize lipids. Following a brief vortex, 60 μl of sodium methoxide in methanol (0.5 M, Sigma-Aldrich, 403067) was added to methylate the esterified fatty acids, at 60 °C for 10 min. After the samples were cooled, 1 ml of 1% (v/v) sulfuric acid in methanol was added to esterify the remaining free fatty acids. The mixture was shaken at 22 °C for 10 min followed by another 10-min incubation at 60 °C. The acid catalyst was then neutralized with 4 ml of 5% aqueous potassium carbonate (4 °C), and FAMEs were extracted twice with 500 μl of hexane. Four hundred microliters of hexane extract was evaporated under nitrogen at ambient temperature, and FAMEs were then redissolved in 200 μl of hexane and stored at −20 °C until analysis.
FAMEs (1 μl, in hexane) were injected into a Thermo Tracel 1310 GC with a BPX70 column (30 m × 0.25 mm × 0.25 μm, SGE). The injector temperature was 255 °C, and split ratio was 10. Constant flow rate of the carrier gas was controlled at 1.2 ml/min, to 200 °C at 2 °C/min and to final temperature 250 °C at 15 °C/min. FAMEs were detected with Thermo ISQ-LT-MS under EI mode. MS transfer line and ion source temperatures were 250 and 260 °C, respectively. Data acquisition started at 2.5 min. The mass scan range was 50–650 amu, and the scan time was 0.2 s.
Statistical analysis
A two-way ANOVA with repeated measures was performed with a Holm-Sidak multiple comparison post hoc test for all variables except protein synthesis, DNA synthesis, and the ratio of protein to DNA synthesis. For these variables, a one-way ANOVA was used to compare Protandim and CLA versus CON with an uncorrected Fisher’s LSD post hoc analysis. A two-way ANOVA was performed with a Holm-Sidak multiple comparison post hoc test to explore sexual dimorphism for all variables. All data analysis was performed with investigators remaining blinded to the intervention. Statistical significance was set at P < 0.05. Statistical analysis was performed with Prism 6, and data are presented as mean ± SEM.
Results
Participant characteristics
Forty-six participants (CON, n = 15; Protandim, n = 15; CLA, n = 16) completed the 6-week study. Concentration of plasma CLA was increased with supplementation in all but four participants, as shown in Online Resource 1. Because of non-detectable levels of CLA supplementation and/or low body water deuterium enrichment, data from four subjects were not included in the analyses. All physical characteristics, presented in Table 2, were not different between groups and were maintained after each intervention.
Table 2.
Physical characteristics
| CON | PRO | CLA | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| N | 15 (10F/5M) | 15 (10F/5M) | 12 (10F/2M) | |||
| Age (years) | 67 ± 6 | 65 ± 5 | 71 ± 6 | |||
| Weight (kg) | 71 ± 8 | 71 ± 9 | 77 ± 15 | 77 ± 15 | 68 ± 11 | 69 ± 11 |
| BMI (kg/m2) | 26 ± 3 | 26 ± 3 | 26 ± 4 | 26 ± 4 | 25 ± 4 | 26 ± 4 |
| Fat mass (kg) | 26 ± 7 | 26 ± 6 | 29 ± 9 | 28 ± 8 | 25 ± 7 | 26 ± 7 |
| FFM (kg) | 42 ± 7 | 42 ± 7 | 45 ± 9 | 46 ± 9 | 40 ± 6 | 41 ± 7 |
BMI body mass index, FFM fat-free mass
Skeletal muscle protein and DNA synthesis
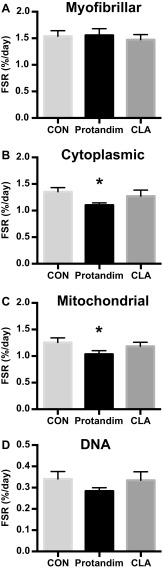
Contrary to our hypothesis, mitochondrial and cytoplasmic protein synthesis rates were lower (P < 0.05) in Protandim compared to CON while myofibrillar protein and DNA synthesis rates were unaltered (Fig. 1). In CLA, the synthesis rates for all subcellular protein fractions and DNA were not different compared to CON.
Fig. 1.
Myofibrillar (a), cytoplasmic (b), and mitochondrial (c) proteins and DNA (d) synthesis rates in response to 6 weeks of milk protein feeding with placebo control (CON, n = 13–15), Protandim (n = 15), or conjugated linoleic acid (CLA, n = 12). Data presented as mean ± SEM. *P < 0.05 vs. CON
Ratio of protein to DNA synthesis
Protandim tended (P = 0.07) to have a greater ratio of myofibrillar protein synthesis to total DNA synthesis (Fig. 2) while the ratio of mitochondrial and cytoplasmic protein syntheses to DNA synthesis was maintained. CLA did not alter the ratio of any subcellular protein synthesis to DNA synthesis. The ratio of myofibrillar protein to DNA synthesis was greater after Protandim in men compared to women (P < 0.05) and greater in men after Protandim versus CON (P = 0.06) (Online Resource 2).
Fig. 2.
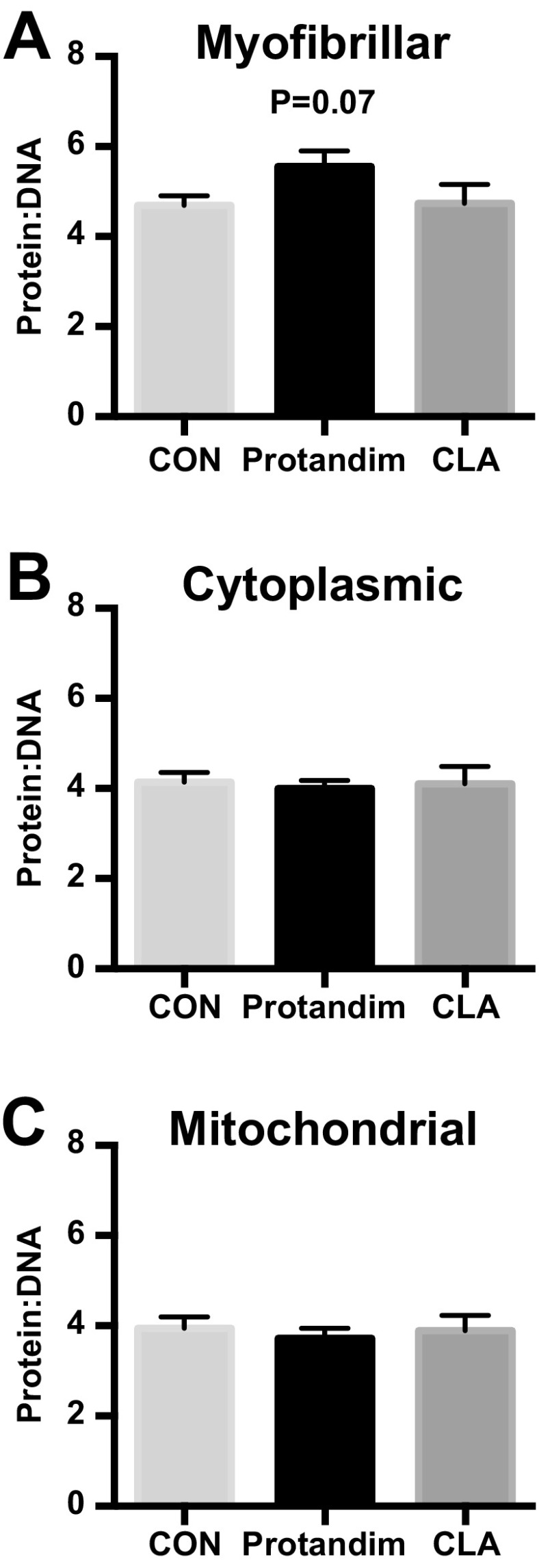
The ratio of myofibrillar (a), cytoplasmic (b), and mitochondrial (c) proteins to DNA synthesis rates in response to 6 weeks of milk protein feeding with placebo control (CON, n = 12–15), Protandim (n = 15), or conjugated linoleic acid (CLA, n = 12). Data presented as mean ± SEM. P = 0.07 vs. CON
Downstream targets of Nrf2
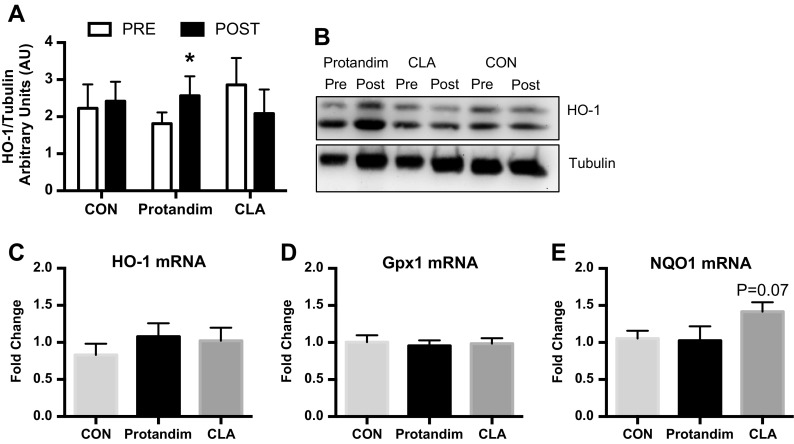
We evaluated if 6 weeks of protein supplementation (CON) plus Protandim or CLA activated select targets of Nrf2 in human skeletal muscle by measuring HO-1 messenger RNA (mRNA) expression and protein content. HO-1 protein content but not mRNA expression was increased (P < 0.05) after Protandim (Fig. 3). HO-1 protein content and mRNA were unaltered after CON and CLA. In addition to HO-1, we measured mRNA expression of additional Nrf2 targets Gpx1 and NQO1. Compared to CON, NQO1 mRNA expression tended (P = 0.07) to be greater after CLA while Gpx1 was unaltered after all treatments.
Fig. 3.
Heme oxygenase-1 (HO-1) protein content (a) pre and post 6 weeks of milk protein feeding with placebo control (CON, n = 14), Protandim (n = 14), or conjugated linoleic acid (CLA, n = 9). *P < 0.05 vs. PRE. Representative image of HO-1 and tubulin (b). Fold change of HO-1 (c), Gpx1 (d), and NQO1 (e) after 6 weeks of milk protein feeding with placebo control (CON, n = 11), Protandim (n = 9), and conjugated linoleic acid (CLA, n = 11). Data presented as mean ± SEM. *P < 0.05 vs. PRE
Oxidative stress and oxidative damage
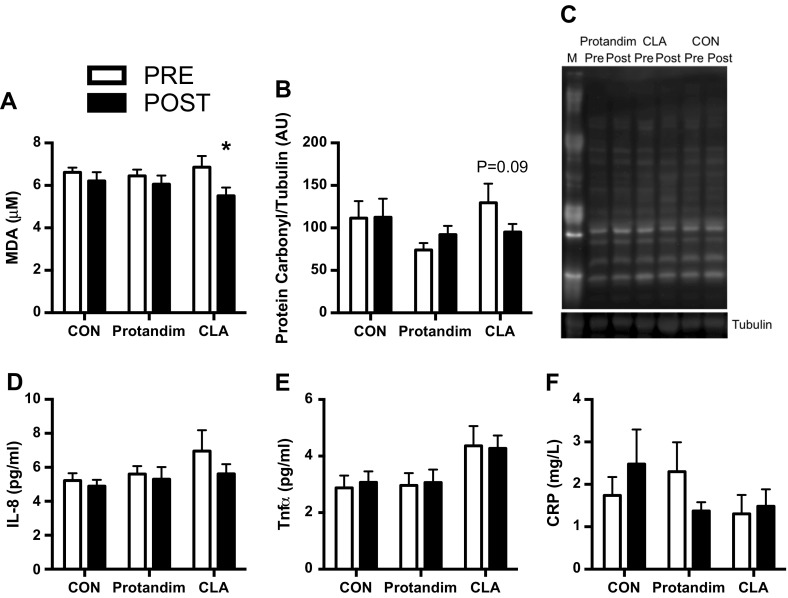
We also determined if Protandim or CLA influenced systemic oxidative stress or skeletal muscle oxidative damage (Fig. 4a–c). First, lipid peroxidation was measured by the concentration of MDA formation in plasma, which is an indicator of systemic oxidative stress. Overall, there was an effect for time (P < 0.05) that was primarily driven by decreased MDA in the CLA group (P < 0.05) with no significant changes in CON or Protandim. Protein oxidative damage was determined by the abundance of protein carbonyl residues in the cytoplasmic fraction of skeletal muscle. Similar to the systemic oxidative stress findings, CLA demonstrated a tendency (P = 0.09) for decreased oxidative damage with no changes in CON or PRO.
Fig. 4.
Markers of systemic oxidative stress (a), skeletal muscle oxidative damage (b, c), and systemic inflammation (d–f) pre and post 6 weeks of milk protein feeding with placebo control (CON, n = 13–14), Protandim (n = 14), and conjugated linoleic acid (CLA, n = 9–11). Representative image of protein carbonyl and tubulin (c). M molecular marker. Data presented as mean ± SEM. *P < 0.05 (a), P = 0.09 (b) vs. PRE
Systemic inflammation
To evaluate if Protandim or CLA could decrease circulating markers of inflammation, we measured IL-8, TNF-α, and CRP. Plasma levels of IL-8, TNF-α, and CRP were unaltered (Fig. 4d–f) suggesting that CON, Protandim, or CLA did not influence these select markers of inflammation.
Discussion
This study shows that myofibrillar protein synthesis was maintained despite lower rates of mitochondrial and cytoplasmic protein synthesis after Protandim supplementation compared to CON. However, when protein synthesis is expressed relative to DNA synthesis, the ratio of mitochondrial and cytoplasmic protein to DNA synthesis is maintained while the ratio of myofibrillar protein to DNA synthesis tended to be greater than CON. Furthermore, HO-1 protein content, a downstream target of Nrf2, was increased while mRNA expression of downstream Nrf2 targets (HO-1, Gpx1, NQO1) remained unaltered after Protandim. In contrast, CLA decreased oxidative stress and oxidative damage and tended to increase NQO1 mRNA expression without changes in protein synthesis or DNA synthesis rates. These data indicate the importance of taking into consideration the rates of both protein and DNA syntheses to understand skeletal muscle proteostasis in response to anabolic stimuli. Moreover, these data suggest that Protandim may improve proteostatic mechanisms of contractile proteins after 6 weeks of milk protein feeding and future research is warranted to determine if this could lead to an enhanced ability to counter sarcopenia.
Skeletal muscle protein and DNA synthesis
An expanding body of work has demonstrated that older adults have a dampened or slower increase in the rate of mixed muscle protein synthesis after an acute protein bolus (Dickinson et al. 2013). Chronic oxidative and inflammatory stresses are implicated as mechanisms that contribute to acute anabolic resistance and the loss of skeletal muscle mass with age. We tested if compounds that have been previously shown to activate Nrf2 signaling (Bergamo et al. 2007; Velmurugan et al. 2009; Bergamo et al. 2011; Donovan et al. 2012; Reuland et al. 2013) could improve both the cumulative protein and DNA synthesis responses to longer durations of milk protein feeding (i.e., 6 weeks). We found that despite decreasing a marker of oxidative stress, CLA did not affect protein synthesis in any subcellular skeletal muscle fraction nor DNA.
We found that after supplementing older adults with Protandim, skeletal muscle myofibrillar protein synthesis rates were maintained while mitochondrial and cytoplasmic protein syntheses were lower compared to CON with a parallel non-significant decrease (P = 0.18) in DNA synthesis. Although maintained or decreased protein synthesis opposes the dogma for reversing sarcopenia in older adults, when subcellular protein synthesis is expressed relative to DNA synthesis, a different conclusion emerges. We show that the ratios of mitochondrial and cytoplasmic protein to DNA synthesis were not different while the myofibrillar protein to DNA synthesis rates tended to be greater after Protandim versus CON. The tendency for increased myofibrillar protein to DNA synthesis ratio may be due to the notion that proliferating supportive cell types do not contain contractile proteins and DNA does not appear to replicate in myofibers (a post-mitotic cell type). Therefore, an increased ratio of myofibrillar protein to DNA synthesis may reflect a shift in metabolic priorities where supportive cell types decrease proliferation to allow cellular resources to maintain the synthesis of contractile proteins within myofibers. A potential limitation of the current study is we measured protein and DNA syntheses during the last 4 weeks of the intervention and may not have captured the robust changes of rapidly turning over proteins within the first 2 weeks of the investigation. Although we previously observed an improvement in the myofibrillar protein to DNA synthesis rate in skeletal muscle of slowed aging models, it was also accompanied by improvements in cytosolic and mitochondrial proteins (Miller et al. 2014). This is the first time that we have observed a change in the ratio of protein to DNA synthesis in only the fraction enriched with myofibrillar proteins.
Moreover, there appears to be a sexual dimorphic response to Protandim as men have a greater myofibrillar protein to DNA synthesis ratio compared to women after Protandim and both men and women after CON (Online Resource 2). Therefore, the maintenance of proteostasis of contractile and structural proteins enriched in the myofibrillar fraction after Protandim seems to occur primarily in male participants. Although the current study had a low number of male participants and was not originally designed to detect gender differences, these preliminary observations of sexual dimorphism in myofibrillar proteostasis after Protandim are consistent with our recent findings from the Interventions Testing Program where Protandim extended median life span in male but not female mice (Strong et al. 2016). We have previously demonstrated that an increased ratio of protein to DNA synthesis rates is a shared characteristic in models of slowed aging (Miller et al. 2014). Collectively, these data suggest that an increased ratio of protein to DNA synthesis in men after Protandim may be indicative of increased proteostatic mechanisms consistent with slowed aging.
Energy balance considerations
When comparing the current study in human subjects to slowed aging murine models (Miller et al. 2012; Drake et al. 2013), there are distinct differences in which subcellular protein pools are preferentially synthesized. Besides species differences, the distinctions in preferential synthesis of specific protein pools (myofibrillar vs. mitochondrial) may be due to energy status. For example, the energetic stress in long-lived chronically calorie-restricted (Miller et al. 2012) or rapamycin-treated (Drake et al. 2013) mice appears to preferentially maintain mitochondrial protein synthesis. We have proposed that this represents the dedication of resources to mitochondrial biogenesis for maintaining energy production while lowering energetically expensive processes like the synthesis of proteins enriched in the myofibrillar and cytoplasmic subcellular fractions. In the current study, participants consumed 24 oz. of fat-free milk per day and remained in energy balance as evident by maintenance of body weight and composition over the 6-week intervention. Protandim plus milk protein feeding appeared to preferentially dedicate resources to maintain myofibrillar protein synthesis rate. Our previous data demonstrated that when older adults were in energy balance or positive energy balance, there was an anabolic effect of post-exercise protein feeding that was not evident during negative energy balance (Minor et al. 2012). These data may indicate that when in energy balance or positive energy balance, cellular resources can be utilized for maintaining skeletal muscle contractile and structural proteins in the myofibrillar protein fraction rather than being diverted to maintain mitochondrial biogenesis during an energetic stress. The mechanisms that regulate the preferential synthesis of proteins remain unknown, and the contribution of specific proteins to slowed aging warrants further research.
Nrf2 and oxidative stress
Previous studies have reported diminished Nrf2 signaling, increased oxidative stress, and macromolecular damage in sedentary older individuals (Safdar et al. 2010). Consistent with these findings, Nrf2 knockout mice increased oxidant production and decreased skeletal muscle mitochondrial and contractile function (Crilly et al. 2016). Furthermore, several gene targets of Nrf2 are upregulated in models of slowed aging (Steinbaugh et al. 2012) and Nrf2 signaling may be a mechanism involved in the stress-resistant phenotype observed in long-lived species (Leiser and Miller 2010). Therefore, the activation of Nrf2 by Protandim and CLA may be an important therapeutic signaling pathway in older adults to impart resistance to oxidative stress and prevent the accumulation of oxidative damage.
In the current study in humans, we found the detectable effects of Protandim and CLA on select Nrf2 gene targets to be minimal. Protandim appeared to increase HO-1 protein content without changes in mRNA expression (HO-1, Gpx1, NQO1), systemic oxidative stress or inflammation, and skeletal muscle protein damage. We showed that after CLA, NQO1 tended to increase and was accompanied by decreased systemic oxidative stress and skeletal muscle oxidative damage. Previous reports have shown that Protandim and CLA have robust effects on Nrf2 signaling pathway in vitro (Bergamo et al. 2007; Velmurugan et al. 2009; Bergamo et al. 2011; Donovan et al. 2012; Reuland et al. 2013); however, our in vivo measurements of downstream targets of Nrf2 were made at 6 weeks; thus, there could be temporal effects on these variables that were not captured. While we expected to find greater activation of select Nrf2 targets after Protandim and CLA, it is possible that these compounds also act through additional pathways such as mitogen-activated protein kinases (MAPK) (Yu et al. 1999) and phosphatidylinositol-3 kinase (PI3K) (Kang et al. 2002).
Implications and conclusions
With increasing age, there is an increase in oxidative stress and inflammation and a decline in myofiber (Ochala et al. 2007) and mitochondrial (Coen et al. 2013; Santanasto et al. 2015) function. CLA decreased systemic oxidative stress and skeletal muscle oxidative damage despite no change in skeletal muscle protein or DNA synthesis rates. Therefore, CLA may have positive health effects in patients with high levels of oxidative stress and oxidative damage but warrants additional research. Interventions that can maintain both myofibrillar and mitochondrial muscle protein syntheses may be effective at delaying the onset of sarcopenia and maintaining physical function. Enhanced myofibrillar proteostasis after Protandim could have clinical implications in maintaining skeletal muscle fiber size and function with increasing age. Although this study examined skeletal muscle protein and DNA syntheses in response to 6 weeks of milk protein feeding, which is an extended timeframe compared to commonly used measurements of acute responses to a single meal, the long-term effects of Protandim or CLA remain unknown. Future inquiry should investigate if combining the effects of exercise, milk protein feeding, and potential slowed-aging treatments could have synergistic effects in improving proteostatic mechanisms associated with extended health span.
This study provides support for further examination into the mechanisms of how interventions that extend mammalian life span in experimental animals can be translated to humans. These studies will help facilitate the development of precision strategies to maintain proteostasis, slow sarcopenia, and extend human health span. Consistent with our previous findings that demonstrate that an increased ratio of protein to DNA synthesis, a contributor of proteostasis, is a shared characteristic of slowed aging (Miller et al. 2014), we have now shown that Protandim slows aging in male mice (Strong et al. 2016) and may also improve mechanisms of skeletal muscle proteostasis after 6 weeks of milk protein feeding in older humans.
Electronic supplementary material
Plasma concentration of C18:2 isomers (A) and cis-9, trans-11 and trans-10, cis-12 isomers (B). Cis-9, trans-11 and trans-10, cis-12 isomers co-eluted at the same retention time. Therefore, the overlapping CLA isomers were calculated using both standard curves and an average was taken. Undetectable levels were observed in CON and pre CLA. Therefore, individual data are presented. Detectable levels were observed in each participant post CLA. (PDF 28 kb)
Sexual dimorphism in the ratio of myofibrillar protein to DNA synthesis after 6 weeks of milk protein feeding with control (CON; n=4 Men, n=8 Women) and Protandim (n=5 men, n=10 women). Data presented as mean ± SEM. *P<0.05 vs. Protandim women; P=0.06 vs. CON Men. (PDF 35 kb)
Acknowledgements
This work was supported by the Dairy Research Institute and LifeVantage, Inc. (to KLH and BFM). The authors would like to thank the participants for their time and commitment to this study. We are grateful for the assistance of Gary Luckasen, M.D., and Jon Matthews, M.D., and their associates for providing medical oversight. We would also like to acknowledge the technical assistance provided by Gaia Bublitz, Kim Cox-York, PhD., and the Metabolomics and Proteomics Facility at Colorado State University. Analysis for C-reactive protein was performed at the University of Colorado Hospital Clinical and Translational Research Centers, which is a service within the Colorado Clinical and Translational Sciences Institute (CCTSI), supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082.
Compliance with ethical standards
This study was approved by the Institutional Review Board at Colorado State University (13-4191H). Prior to beginning participation, each participant was informed of the study design, potential risk, and benefits and provided written consent.
Footnotes
Karyn L. Hamilton and Benjamin F. Miller are co-senior authors.
References
- Balage M, Averous J, Rémond D, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2010;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Bergamo P, Maurano F, Rossi M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic Biol Med. 2007;43:71–79. doi: 10.1016/j.freeradbiomed.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Bergamo P, Gogliettino M, Palmieri G, et al. Conjugated linoleic acid protects against gliadin-induced depletion of intestinal defenses. Mol Nutr Food Res. 2011;55(Suppl 2):S248–S256. doi: 10.1002/mnfr.201100295. [DOI] [PubMed] [Google Scholar]
- Bruns DR, Drake JC, Biela LM, et al. Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxidative Med Cell Longev. 2015;2015:732596. doi: 10.1155/2015/732596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R, Kim Y-K, Neese RA, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006;1760:730–744. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Busch R, Neese RA, Awada M, et al. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crilly MJ, Tryon LD, Erlich AT, Hood DA (2016) The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol (1985) jap.00042.2016. doi: 10.1152/japplphysiol.00042.2016 [DOI] [PMC free article] [PubMed]
- Dhiman TR, Anand GR, Satter LD, Pariza MW. Conjugated linoleic acid content of milk from cows fed different diets. J Dairy Sci. 1999;82:2146–2156. doi: 10.3168/jds.S0022-0302(99)75458-5. [DOI] [PubMed] [Google Scholar]
- Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41:216–223. doi: 10.1097/JES.0b013e3182a4e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan EL, McCord JM, Reuland DJ, et al. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxidative Med Cell Longev. 2012;2012:132931. doi: 10.1155/2012/132931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle G, Furey S, Berlin R, et al. Gastrointestinal safety and tolerance of ibuprofen at maximum over-the-counter dose. Aliment Pharmacol Ther. 1999;13:897–906. doi: 10.1046/j.1365-2036.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- Drake JC, Peelor FF, Biela LM, et al. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci. 2013;68:1493–1501. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JC, Bruns DR, Peelor FF, et al. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol - Endocrinol Metab. 2014;307:E813–E821. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JC, Bruns DR, Peelor FF, et al. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14:474–482. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C, Ferrucci L, Sun K, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol Bethesda Md 1985. 2007;103:17–20. doi: 10.1152/japplphysiol.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Asp Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- Kirkland JL. Translating the science of aging into therapeutic interventions. Cold Spring Harb Perspect Med. 2016;6:a025908. doi: 10.1101/cshperspect.a025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Asante A, Lanza IR, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015 doi: 10.2337/db14-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marzani B, Balage M, Vénien A, et al. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr. 2008;138:2205–2211. doi: 10.3945/jn.108.094029. [DOI] [PubMed] [Google Scholar]
- Miller BF, Robinson MM, Bruss MD, et al. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11:150–161. doi: 10.1111/j.1474-9726.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Robinson MM, Reuland DJ, et al. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci. 2013;68:530–538. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Drake JC, Naylor B, et al. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev. 2014;18:106–111. doi: 10.1016/j.arr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor BD, Heusinger DE, Melanson EL, et al. Energy balance changes the anabolic effect of postexercise feeding in older individuals. J Gerontol A Biol Sci Med Sci. 2012;67:1161–1169. doi: 10.1093/gerona/gls080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese RA, Misell LM, Turner S, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–15350. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SK, Bose SK, Grunwald GK, et al. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 2006;40:341–347. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Nightingale AK, Crilley JG, Pegge NC, et al. Chronic oral ascorbic acid therapy worsens skeletal muscle metabolism in patients with chronic heart failure. Eur J Heart Fail. 2007;9:287–291. doi: 10.1016/j.ejheart.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Nuernberg K, Dannenberger D, Ender K, Nuernberg G. Comparison of different methylation methods for the analysis of conjugated linoleic acid isomers by silver ion HPLC in beef lipids. J Agric Food Chem. 2007;55:598–602. doi: 10.1021/jf061865k. [DOI] [PubMed] [Google Scholar]
- Ochala J, Frontera WR, Dorer DJ, et al. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland DJ, Khademi S, Castle CJ, et al. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–111. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MM, Richards JC, Hickey MS, et al. Acute {beta}-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol. 2010;298:R25–R33. doi: 10.1152/ajpregu.00524.2009. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Turner SM, Hellerstein MK, et al. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011;25:3240–3249. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, deBeer J, Tarnopolsky MA. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic Biol Med. 2010;49:1487–1493. doi: 10.1016/j.freeradbiomed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:1379–1385. doi: 10.1093/gerona/glu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo RL, Peltonen GL, Binns SE, et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J Off Publ Fed Am Soc Exp Biol. 2014;28:2705–2714. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhäusser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Semba RD, Ferrucci L, Sun K, et al. Oxidative stress is associated with greater mortality in older women living in the community. J Am Geriatr Soc. 2007;55:1421–1425. doi: 10.1111/j.1532-5415.2007.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Ferrucci L, Sun K, et al. Oxidative stress and severe walking disability among older women. Am J Med. 2007;120:1084–1089. doi: 10.1016/j.amjmed.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton D, Smirnova JB, Selley JN, et al. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem. 2006;281:29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–E495. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016 doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan K, Alam J, McCord JM, Pugazhenthi S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic Biol Med. 2009;46:430–440. doi: 10.1016/j.freeradbiomed.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Yu R, Lei W, Mandlekar S, et al. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46:1500–1509. doi: 10.1016/j.freeradbiomed.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma concentration of C18:2 isomers (A) and cis-9, trans-11 and trans-10, cis-12 isomers (B). Cis-9, trans-11 and trans-10, cis-12 isomers co-eluted at the same retention time. Therefore, the overlapping CLA isomers were calculated using both standard curves and an average was taken. Undetectable levels were observed in CON and pre CLA. Therefore, individual data are presented. Detectable levels were observed in each participant post CLA. (PDF 28 kb)
Sexual dimorphism in the ratio of myofibrillar protein to DNA synthesis after 6 weeks of milk protein feeding with control (CON; n=4 Men, n=8 Women) and Protandim (n=5 men, n=10 women). Data presented as mean ± SEM. *P<0.05 vs. Protandim women; P=0.06 vs. CON Men. (PDF 35 kb)