Abstract
Background
Compliance rates for colorectal cancer (CRC) screening are much lower than those desired. Appropriate information on CRC risks and screening methods is supposed to stimulate motivation for screening. We aimed to identify parameters associated with the decision for CRC screening and colonoscopy in a population expected to have high awareness of disease prevention.
Methods
In a single-center, cross-sectional study, we used an anonymous questionnaire (AQ) to record the demographics, habits and screening behavior for cancers and other common diseases of all employees older than 50 years in our hospital.
Results
Among 287 active employees, 83% (n=237) answered the AQ (age 55±4 years). Thirty percent (n=70) underwent colonoscopy while 17% (n=40) underwent CRC screening (39/40) colonoscopy). Comparatively, among women 97% had a Pap-smear, 92% a mammography, while among men 83% had been tested for serum prostate-specific antigen. Age, male sex, alcohol consumption and university education correlated positively with CRC screening (P<0.05 for all). After multivariate analysis, university education remained an independent determinant of CRC screening (OR 2.488, 95%CI 1.096-5.648; P=0.029). Among subjects who had not undergone colonoscopy in the past, ignorance of the need for CRC screening (OR 0.360, 95%CI 0.150-0.867; P=0.023) and indifference to undergo such a procedure (OR 0.188, 95%CI 0.066-0.537; P=0.002) were independent determinants for not planning a future screening colonoscopy.
Conclusions
Education was the most important factor in the decision to undergo CRC screening. Colonoscopy was the preferred screening method. Ignorance of and indifference to CRC risks were the major obstacles for a future screening colonoscopy.
Keywords: Colorectal cancer, screening, colonoscopy, behavior, education
Introduction
Colorectal cancer (CRC) is an important health problem worldwide. According to an estimate of cancer incidence and mortality in Europe 436,000 new cases and 212,000 deaths were attributed to CRC [1]. In the United States, CRC is the second leading cause of cancer death and accounts for approximately 9% of cancer deaths overall [2]. Screening aims to diagnose premalignant conditions (adenomas) or early stages of disease and it appears to have had a considerable impact on reducing CRC incidence and mortality [3]. MISCAN-colon, a microsimulation model, suggests that screening may account for 53% of the observed reduction in CRC mortality [4]. The frequency of CRC screening is increasing, but remains below the desirable rates in most countries [5-7].
Most scientific societies recommend the age of 50 to begin CRC screening in an asymptomatic population, but there is uncertainty concerning the most cost-effective strategy [8-10]. Colonoscopy is a key tool in all CRC screening programs, either as the initial method or as a method to complement another positive screening test. The American College of Gastroenterologists recommends that quality colonoscopy should be offered first to average-risk population aged ≥50 years and other screening tests should only be used in cases of unavailability or patient’s unwillingness [11]. It is estimated that in the USA the contribution of screening to the decline of CRC mortality in the examined population is greater than 50%, while the majority of CRC deaths are attributed to non-screening [12,13]. Recently, a Canadian study confirmed that undergoing a colonoscopy within the previous 10 years provides substantial protective benefit for average-risk individuals aged over 60 years [14].
Worldwide, there are different approaches towards CRC screening that can be summarized as either organized or opportunistic methods or not screening at all [15]. Greece is classified among the countries with an opportunistic screening approach delivered outside an organized screening program. However, colonoscopy is an acceptable method reimbursed by the National Health System for those willing to be screened.
The efficacy of screening depends on many parameters, but for a common disease the main parameter for a good test is the compliance of the population. Data concerning CRC screening in Greece are scarce. In a self-reported questionnaire among a sample of Greek primary care physicians, the rate of recommending CRC screening to their patients was very low, while the presence of a regular primary physician and knowledge of CRC risks were very important discriminators influencing screening status [16,17]. In addition, financial considerations can be a significant barrier to screening [18]. In the USA, endoscopic CRC screening has increased during the last decade in the higher socioeconomic group, while this is not the case for those with low educational and socioeconomic level [19]. However, there are data showing that physicians (as patients) are less likely than the general population to adhere to specific guidelines [20].
The aim of our study was to examine whether a population supposed to be aware of the CRC problem had had any type of CRC screening. In this setting, we aimed to assess the particular place of colonoscopy and to compare characteristics between those who participated in the screening procedures and those who did not.
Patients and methods
This cross-sectional study was conducted during a three-month period (September-November 2014). The targeted population was the staff aged more than 50 years of a tertiary hospital, the “Alexandra” University Hospital. The list of employees was provided by the hospital personnel office after approval of the protocol by the local Scientific and Ethical committee and with the final permission of the Hospital Board. An anonymous questionnaire (AQ) was distributed on a personal basis and each person receiving the AQ was marked on the list. The AQs were collected in a ballot box and a second mark was added beside the same name on the list when an employee dropped the AQ in the box.
Questionnaire
To evaluate whether the population under evaluation had different characteristics influencing its preference to be screened for CRC or not, we developed a questionnaire which consisted of two parts: 1) asking for demographic characteristics, educational level, professional activities, as well as medical history information; and 2) evaluating whether the patient participated in screening programs for common diseases. This second part had an additional section addressing questions separately for performing screening tests specific to men and women. A special part asked if the person has had CRC screening, as well as the screening method used, and finally for those who did not have a colonoscopy the reasons for this declination. All subjects were asked if they planned to have a colonoscopy in the future. The questionnaire was written in Greek and printed on two sides of an A4 sheet. It was decided to make the survey anonymous in order to ease and encourage most of the working personnel in the hospital to participate.
Statistical analysis
Statistical comparisons were performed between subjects who had undergone a screening colonoscopy and those who had not, as well as between individuals who were willing or not to undergo a screening colonoscopy in the future. Dichotomous variables were compared using the χ2-test and continuous variables using the independent t-test. Multivariate analyses were performed with the use of logistic regression to identify predictor variables for subjects who performed a screening colonoscopy and for those who were willing to perform it in the future. In the initial univariate analysis, a threshold of P<0.1 (because of the risk of developing a type 2 error due to low statistical power in such an analysis) was used to identify candidate variables for inclusion in the final model. All covariates included in the final models were tested for interactions with each other. Because the tolerance values for each covariate were less than 0.5, no correction for the collinearity of data was necessary. In the final multivariate analysis, statistical significance was achieved if P was <0.05. The Statistical Package for Social Science, version 23.0 for Windows (SPSS Inc., Chicago, Illinois, USA), was used for the statistical analyses.
Results
Three hundred seventy employees older than 50 years were found on the administrative list. This list included employees in a pre-retirement period or on long sick leave and therefore impossible to reach. Two hundred eighty-seven active employees finally collected an AQ and 237 (83%) dropped it in the “ballot box”. The group consisted of 81 men and 156 women. The mean age was 55±4 (50-67) years; 59% of the population were in the range 50-55 years. Divided by profession, they consisted of 30% physicians, 43% nurses and 27% administrative employees, technical workers and other subspecialties. Thirty-six percent were smokers, 64% did not drink at all or drank occasionally, 43% consumed red meat more than twice per week, while 17% reported regular use of aspirin or nonsteroidal anti-inflammatory drugs.
Overall, 70 (30%) reported having undergone colonoscopy (37% of men vs. 26% of women, P=0.073). Thirty-nine (56%) of the 70 persons who had undergone colonoscopy responded that this was for preventive reasons, while only 1 was tested with a fecal occult blood test and performed an additional CT-colonography. Those results led to a total of 17.0% (40 patients) who had undergone CRC screening with any method, while colonoscopy was the strong preference of this population as CRC screening method.
Cholesterol and triglycerides were evaluated at least once by 88% of the population, blood glucose by 83%, while 87% had had a measurement of their arterial pressure. Among women, 97% had a Pap-smear, 92% a mammography and 51% a breast ultrasound, while 83% of males had been tested for serum prostate-specific antigen (PSA). There was no statistical difference between men and women for the common tests.
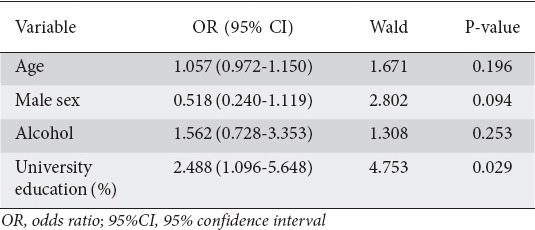
The main characteristics of those who had undergone a screening colonoscopy or not are summarized in Table 1. In the univariate analysis, the parameters with a positive influence on the performance of screening colonoscopy in the past were age (P=0.003), male sex (P=0.009), alcohol consumption (P=0.026) and university education (P=0.001). It is notable that 70 of the 87 (81%) subjects with a university education were physicians (Table 1). The aforementioned variables were included in the multivariate logistic regression analysis (Table 2). The only significant independent predictor for undergoing screening colonoscopy was university education (OR 2.488, 95%CI 1.096-5.648; P=0.029).
Table 1.
Baseline characteristics of the study population, separated into those who had and those who had not previously undergone a screening colonoscopy
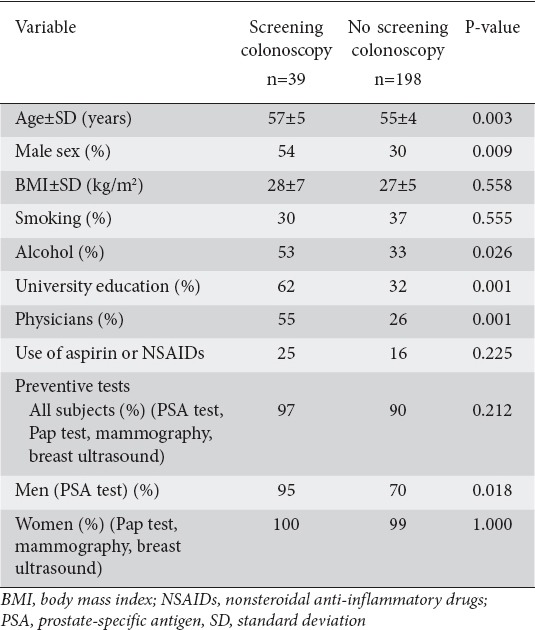
Table 2.
Multivariate logistic regression analysis of factors associated with the performance of screening colonoscopy
The 167 subjects who had never undergone a colonoscopy (70%) answered the part of the questionnaire concerning the reasons why they had not undergone screening. Twenty-seven percent of them reported that they were unaware of the need to be screened for CRC. Other reasons for not undergoing colonoscopy were fear (17%), shame (10%), indifference (16%), other priorities (23%) and other (7%). Fear and shame were more prevalent among women, while more men were indifferent, and stated that they had “other priorities” and they “didn’t know”. There were no statistical differences between men and women regarding the reasons leading them not to undergo a screening colonoscopy (Fig. 1). Among the 39 screening colonoscopies, 12 polyps (31%) and no CRC were found, while the 31 colonoscopies performed for non-preventive reasons found 9 polyps (29%) and 2 CRCs (6%).
Figure 1.
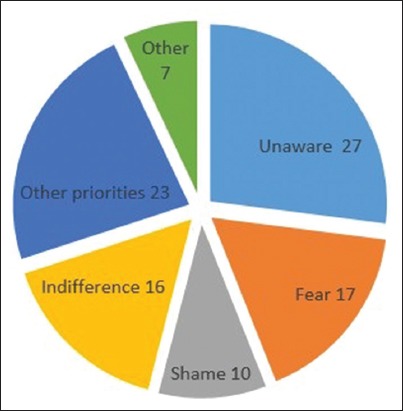
Schematic representation of reported reasons among those who had not undergone colorectal cancer (CRC) screening (%): 27% reported that they were “unaware” of the need to be screened for CRC. Other reasons were “fear” (17%), “shame” (10%), “indifference” (16%), “other priorities” (23%) and “other” (7%). Fear and shame were more prevalent among women, while more men were indifferent, and stated that they had “other priorities” or that they “didn’t know”. However, no statistically significant difference was found between the sexes for any specific reason
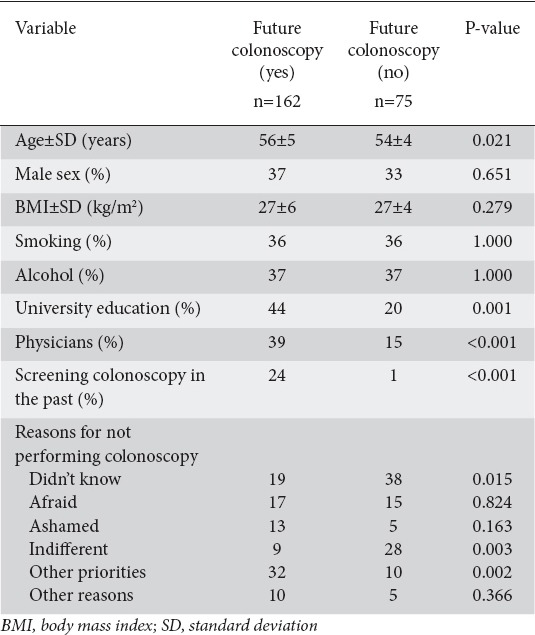
All subjects completed the question regarding a possible future colonoscopy. One hundred sixty-two answered positively (68%). The main characteristics of those who were willing to undergo a screening colonoscopy or not are summarized in Table 3. In the univariate analysis, the parameters with a positive influence on the performance of a future screening colonoscopy were age (P=0.021), university education (P=0.001), being a physician (P<0.001) and having had a screening colonoscopy in the past (P<0.001). Concerning the reasons for not performing colonoscopy in the past and the attitude to a future colonoscopy, indifference (P=0.003) and the fact that “I didn’t know about it” (P=0.015) were negatively associated, whereas the fact that “I knew about it but I had other priorities” (P=0.002) was positively associated with the possibility of performing a future colonoscopy. Among the 39 screening colonoscopies, 36 (92%) answered positively regarding a future colonoscopy (2 negatively and 1 did not answer), while among the 31 colonoscopies performed for non-preventive reasons only 21 (68%) answered positively (6 negatively and 3 did not answer), the latter group responding similarly to the average population. Twenty-four percent of those who answered positively concerning a future colonoscopy had had a screening colonoscopy while only 1% with a past screening colonoscopy answered negatively regarding a future colonoscopy (Table 3).
Table 3.
Baseline characteristics of the study population according to the decision to perform a future screening colonoscopy
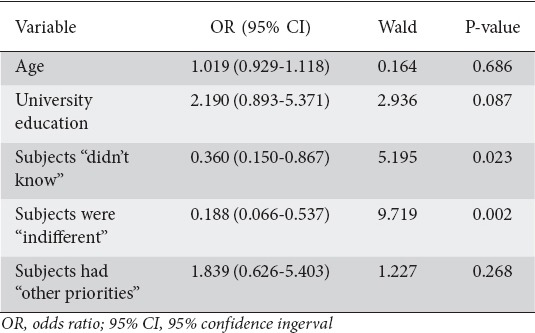
In the multivariate logistic regression analysis of factors associated with the performance of a future screening colonoscopy among subjects who had not undergone colonoscopy in the past, independent predictors were ignorance of the need for CRC screening (OR 0.360, 95%CI 0.150-0.867; P=0.023) and indifference to undergo such a procedure (OR 0.188, 95%CI 0.066-0.537; P=0.002) (Table 4).
Table 4.
Multivariate logistic regression analysis of factors associated with the performance of future screening colonoscopy in subjects who had not previously undergone colonoscopy
Discussion
The population included in our study does not represent a typical sample of the Greek population. Instead, we studied a specific population with characteristics expected to positively influence a predilection for CRC screening and the practice of screening colonoscopy in particular. This sample consisted of employees in a tertiary hospital where screening colonoscopy under conscious sedation has been practiced for many years. The large majority among them are physicians or nurses, who are consequently aware of the dangers of CRC and of the screening methods applied for its prevention. In addition, our hospital operates two big university clinics, an oncology and a gynecology-oncology clinic, which occupy a large part of the personnel, thus rendering the familiarity with screening programs even more powerful. The age was appropriate for the initiation of CRC screening (50-67 years), with more than half in the range of 50-55 years. A large percentage (83%) agreed to complete the AQ and drop it into the “ballot box”. In a 2-year study (2009-10) conducted in a semi-rural area in central Greece, 6536 subjects aged 45-80 years were called after intense advertisement to participate in a free screening colonoscopy program. Only 402 (6.2%) responded positively to this call and underwent colonoscopy (data published on the internet site of the Hellenic Foundation of Gastroenterology and Nutrition, Patroklos Study, eligast.gr). In our population, 17% had undergone CRC screening, all but 1 with colonoscopy. This is much better than the above mentioned disappointing percentage in Central Greece, but it remains low for a very sensitive and informed population, far below the minimum 45% and the desirable 65% recommended by the European Commission [21]. Surprisingly, a large percentage reported that they did not know that they had to undergo a CRC screening test after the age of 50 (24%), 12% among them were physicians. Comparatively, almost all women (97%) had had a Pap-smear and a substantial percentage (93%) one or two tests for breast cancer. In addition 83% of men had performed a PSA blood level examination, a test much less well validated for prostatic cancer than the screening tests for CRC. However, in contrast to women’s behavior regarding CRC screening, which was not influenced by having undergone another screening test, we found that men who had been tested for PSA were more willing to undergo CRC screening. The same result was found in another earlier study examining the relation between prostate and CRC screening [22]. In contrast, an uptake to CRC screening related to the adherence with either cervical cancer or breast cancer screening in women was not found in our study [23]. An additional advantage of our population was that all were living in the same big city. Living in a rural area or not could influence access to CRC screening, probably reflecting the great diversities among different health systems [24,25]. Non-insurance, cost and general lack of access to health care were reported as main reasons for non-access to CRC screening [26,27]. None of the abovementioned reasons was valid for our population. A university education was the only significant independent predictor for undergoing screening colonoscopy in the multivariate logistic regression analysis. Education has also been found to be an important factor influencing CRC screening in several studies: the lower the education level the lower the participation [28-30]. Income was not measured directly, but because all the participants were employees we can deduce with considerable accuracy that the higher the education, the higher the income. Age and male sex were found in the univariate analysis to be important factors influencing screening colonoscopy. Older people (>65 years) are more likely to undergo CRC screening both in the USA and in Europe [31,32]. In our population, most patients were aged between 50-55 years. However, this tendency for older individuals to have undergone screening colonoscopy more often than younger was reproduced in our study. Male sex was also found to be a predictive factor for CRC screening in other studies, but less often than age [33,34]. We have no satisfactory explanation for our finding: for example physicians and persons with higher education were equally distributed as to sex. In addition, because almost all women had had a Pap-test and screening for breast cancer, we would expect that there would be no sex-related difference; however, this was not the case.
Interpretation of individual or collective behaviors for subjects participating or not in screening programs is a difficult and sometimes slippery task. Different strategies to make screening more attractive have been used with mixed results. In a meta-analysis examining the effectiveness of approaches for improving adherence to adult immunization and cancer screening, organizational change interventions were the most potent to achieve the best results [35]. Many speculations have been formulated concerning either the reasons for non-participation or the methods of attracting people into CRC screening and screening colonoscopy in particular. For example, different types of financial incentives seem not to improve CRC screening participation, unlike the screening uptake for other cancers [36,37].
In our study, among those who had never undergone a colonoscopy 27% reported that they were unaware that CRC screening should be performed after the age of 50. This percentage was lower than that previously reported even among previously screened populations, probably reflecting the high level of information on medical subjects among our population as a result of their working environment [17]. All subjects were questioned about their willingness to perform a future colonoscopy. Age, university education, being a physician and having had a screening colonoscopy in the past positively influenced the decision for a future screening colonoscopy in the univariate analysis. Among subjects who had not undergone colonoscopy, ignorance of and indifference to perform a CRC screening colonoscopy were negatively associated with a future colonoscopy in the multivariate logistic regression analysis.
In the USA, the prevalence of CRC screening has increased since 2000. This was due almost exclusively to an increase in colonoscopies, which tripled during the past decade from 19% to 55% [38,39]. Patients with previous experience of colorectal screening preferred future screening, although patients who should be very motivated, such as those with previously detected adenoma, were not always compliant with follow-up colonoscopy [40,41].
In our study, those who had had a screening colonoscopy were much more positive about a future colonoscopy (92%), while among those who had had a colonoscopy for non-preventive reasons the willingness to undergo another procedure reached only the same percentage as in the overall population (68%). Although there are no data to explain this behavior, it probably reflects the differences between those who have the willingness and the conviction to engage in prevention and those who have not. This underlines the need for better promotion in order to spotlight the benefits of CRC screening and to motivate people to enter screening programs. In addition, a previous non-negative experience should help maintain the motivation to be screened for CRC, but we have no data to support this. Interestingly, in a Greek study addressing a questionnaire to medical students in their fourth study year, only 69% considered CRC as an important public health problem, 85% would prefer a method other than colonoscopy for screening, and 53% believed that colonoscopy is painful [42]. These results, combined with ours from informed adults over 50 years old, underpin the need for better education about CRC as a public health problem and the usefulness of CRC screening as part of the very basic phase of physicians’ studies, as well as a policy promoting painless colonoscopy.
In summary, in our cross-sectional study of a specific population older than 50 years, working in a tertiary hospital, we found that a non-negligible percentage declared that they were not aware of the need for CRC screening, which was practiced much less than screening for other common diseases or cancers. However, among the screened population almost all had preferred colonoscopy as screening method. Education was the most important factor influencing willingness to undergo CRC screening. Ignorance and indifference to the CRC risks were the major obstacles to a future screening colonoscopy, while experience with a previous colonoscopy facilitated the decision for a future screening colonoscopy. Further studies aiming to effectively intervene in modifiable behavioral factors must be undertaken in order to make CRC screening and colonoscopy more attractive to populations who are ignorant of or indifferent to the dangers of CRC.
Summary Box.
What is already known:
Colorectal cancer (CRC) screening substantially reduces mortality
Implementation of CRC screening is suboptimal, even in well-organized societies
Screening for other cancers shows better compliance compared to CRC
Decisions about screening and adherence to screening programs are multifactorial
What the new findings are:
CRC screening is much less well implemented in a well-informed Greek population compared to screening for other cancer and common diseases
Education is the major factor influencing decisions about CRC screening
Colonoscopy is the preferred screening method in this population
Ignorance and indifference to CRC risks are the major barriers to a future screening colonoscopy
Biography
Alexandra Hospital; National and Kapodistrian University of Athens, Medical School, Alexandra Hospital, Athens, Greece
Footnotes
Conflict of interest: None
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U. S. Preventive Services Task Force. Ann Intern Med. 2008;149:659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahidi NC, Homayoon B, Cheung WY. Factors associated with suboptimal colorectal cancer screening in US immigrants. Am J Clin Oncol. 2013;36:381–387. doi: 10.1097/COC.0b013e318248da66. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening test use--United States 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 7.Hoff G, Dominitz JA. Contrasting US and European approaches to colorectal cancer screening: which is best? Gut. 2010;59:407–414. doi: 10.1136/gut.2009.192948. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, et al. American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps. 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Leddin D, Hunt R, Champion M, et al. Canadian Digestive Health Foundation. Canadian Association of Gastroenterology and the Canadian Digestive Health Foundation: Guidelines on colon cancer screening. Can J Gastroenterol. 2004;18:93–99. doi: 10.1155/2004/983459. [DOI] [PubMed] [Google Scholar]
- 10.von Karsa L, Patnick J, Segnan N, et al. European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45:51–59. doi: 10.1055/s-0032-1325997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 12.Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60:681–691. doi: 10.1007/s10620-015-3600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol. 2015;25:208–213. doi: 10.1016/j.annepidem.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock D, Paszat LF, Rabeneck L. Colorectal cancer mortality reduction is associated with having at least 1 colonoscopy within the previous 10 years among a population-wide cohort of screening age. Gastrointest Endosc. 2016;84:133–141. doi: 10.1016/j.gie.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 16.Xilomenos A, Mauri D, Kamposioras K, et al. Panhellenic Association for Continual Medical Research (PACMeR) Colorectal cancer screening awareness among physicians in Greece. BMC Gastroenterol. 2006;6:18. doi: 10.1186/1471-230X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harewood GC, Wiersema MJ, Melton LJ., 3rd A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97:3186–3194. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 18.Perkins A, Nicholls K, Shaw T, Liu G, Molokhia E. Attitudes toward colorectal cancer screening in the digital age: a survey of practices and attitudes among screening-eligible Alabamians. South Med J. 2013;106:462–467. doi: 10.1097/SMJ.0b013e3182a0e7be. [DOI] [PubMed] [Google Scholar]
- 19.Bandi P, Cokkinides V, Smith RA, Jemal A. Trends in colorectal cancer screening with home-based fecal occult blood tests in adults ages 50 to 64 years 2000-2008. Cancer. 2012;118:5092–5099. doi: 10.1002/cncr.27529. [DOI] [PubMed] [Google Scholar]
- 20.Berk J, Mills B, Varma S. Physician, heal thyself: health maintenance behaviors among physicians. Tex Med. 2014;110:e1. [PubMed] [Google Scholar]
- 21.Moss S, Ancell-Park R, Brenner H. Evaluation and interpretation of screening outcomes. In: Segnan N, Patnick J, von Karsa L, editors. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis, European Union. 1st edition. 2010. pp. 71–102. [Google Scholar]
- 22.Carlos RC, Underwood W, 3rd, Fendrick AM, Bernstein SJ. Behavioral associations between prostate and colon cancer screening. J Am Coll Surg. 2005;200:216–223. doi: 10.1016/j.jamcollsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Carlos RC, Fendrick AM, Ellis J, Bernstein SJ. Can breast and cervical cancer screening visits be used to enhance colorectal cancer screening? J Am Coll Radiol. 2004;1:769–776. doi: 10.1016/j.jacr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Blom J, Yin L, Lidén A, et al. Toward understanding nonparticipation in sigmoidoscopy screening for colorectal cancer. Int J Cancer. 2008;122:1618–1623. doi: 10.1002/ijc.23208. [DOI] [PubMed] [Google Scholar]
- 25.Faruque FS, Zhang X, Nichols EN, et al. The impact of preventive screening resource distribution on geographic and population-based disparities in colorectal cancer in Mississippi. BMC Res Notes. 2015;8:423. doi: 10.1186/s13104-015-1352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 27.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 28.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 29.von Wagner C, Knight K, Steptoe A, Wardle J. Functional health literacy and health-promoting behaviour in a national sample of British adults. J Epidemiol Community Health. 2007;61:1086–1090. doi: 10.1136/jech.2006.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gimeno-García AZ, Quintero E, Nicolás-Pérez D, Parra-Blanco A, Jiménez A. Colorectal cancer screening in a Spanish population. Med Clin (Barc) 2009;133:736–740. doi: 10.1016/j.medcli.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Thrasher JF, Cummings KM, Michalek AM, Mahoney MC, Moysich KB, Pillittere DM. Colorectal cancer screening among individuals with and without a family history. J Public Health Manag Pract. 2002;8:1–9. doi: 10.1097/00124784-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Segnan N, Senore C, Andreoni B, et al. SCORE2 Working Group-Italy. Randomized trial of different screening strategies for colorectal cancer: patient response and detection rates. J Natl Cancer Inst. 2005;97:347–357. doi: 10.1093/jnci/dji050. [DOI] [PubMed] [Google Scholar]
- 33.Zorzi M, Da Re F, Mantellini P, et al. Italian colorectal cancer screening survey group. Screening for colorectal cancer in Italy 2011-2012 survey. Epidemiol Prev. 2015;39:93–107. [PubMed] [Google Scholar]
- 34.Rim SH, Joseph DA, Steele CB, Thompson TD, Seeff LC Centers for Disease Control and Prevention (CDC) Colorectal cancer screening—United States 2002, 2004, 2006, and 2008. MMWR (Suppl) 2011;60:42–46. [PubMed] [Google Scholar]
- 35.Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–651. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kullgren JT, Dicks TN, Fu X, et al. Financial incentives for completion of fecal occult blood tests among veterans: a 2-stage, pragmatic, cluster, randomized, controlled trial. Ann Intern Med. 2014;161:S35–S43. doi: 10.7326/M13-3015. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Miller S, Koch M, et al. Financial incentives for promoting colorectal cancer screening: a randomized, comparative effectiveness trial. Am J Gastroenterol. 2016;111:1630–1636. doi: 10.1038/ajg.2016.286. [DOI] [PubMed] [Google Scholar]
- 38.Schenck AP, Peacock SC, Klabunde CN, Lapin P, Coan JF, Brown ML. Trends in colorectal cancer test use in the medicare population 1998-2005. Am J Prev Med. 2009;37:1–7. doi: 10.1016/j.amepre.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 39.National Center for Health Statistics. Health, United States 2012: With Special Feature on Emergency Care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- 40.Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: a patient demographic study. South Med J. 2004;97:224–230. doi: 10.1097/01.SMJ.0000078619.39604.3D. [DOI] [PubMed] [Google Scholar]
- 41.Murphy CC, Lewis CL, Golin CE, Sandler RS. Underuse of surveillance colonoscopy in patients at increased risk of colorectal cancer. Am J Gastroenterol. 2015;110:633–641. doi: 10.1038/ajg.2014.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papanikolaou IS, Sioulas AD, Kalimeris S, et al. Awareness and attitudes of Greek medical students on colorectal cancer screening. World J Gastrointest Endosc. 2012;4:513–517. doi: 10.4253/wjge.v4.i11.513. [DOI] [PMC free article] [PubMed] [Google Scholar]


