Abstract
Background
Patients with HIV mono-infection may develop chronic liver disease due to a number of factors including hepatic steatosis. We estimated the prevalence and predictors of hepatic steatosis and fibrosis in a cohort of HIV-mono-infected patients with persistently deranged liver function tests.
Methods
Of 2398 consecutive patients at one UK clinical center, 156 (6.5%) had persistently abnormal transaminases in at least two measurements six months apart. We used APRI and FIB4 scores to determine the presence of significant and/or advanced fibrosis in this group as well as its potential associations.
Results
Mean age was 47.5±8.5 years and 91% (142/156) were males. Diabetes mellitus was present in 11% of patients; hypertension in 18%; and dyslipidemia in 52%. Almost all were on antiretroviral therapy (ART) (97%) and most were virologically suppressed (94%). Steatosis was detected by ultrasound in 71% of patients. The prevalence of FIB4≤1.45, 1.46-3.24 and >3.25 was 67%, 29% and 4%, respectively, and that of APRI≤0.5, 0.51-1.49 and >1.5 was 52%, 45% and 3% respectively. In multivariate analysis, only cumulative ART exposure was associated with FIB4>1.45 (odds ratio [OR] 1.008, 95% confidence interval [CI] 1.000-1.016), while APRI>0.5 was associated with higher alanine aminotransferase levels (OR 1.033, 95%CI 1.015-1.510). Twenty patients had a liver biopsy, of whom 13 had non-alcoholic fatty liver disease (NAFLD).
Conclusions
Elevated transaminases are often present in HIV-mono-infected patients and this may be associated with NAFLD and/or ART. Non-invasive screening for the presence of NAFLD and fibrosis in all HIV-mono-infected patients as part of their routine clinical management should be further explored.
Keywords: Nonalcoholic fatty liver disease, FibroScan, FIB4, APRI, antiretroviral therapy
Introduction
In the era of effective antiretroviral therapy (ART), chronic liver disease is an important cause of morbidity and mortality among HIV-positive individuals, even in the absence of hepatitis C (HCV) and B (HBV) co-infections [1,2]. Mild elevation of transaminases in HIV-positive individuals on ART is frequently reported [3], although the etiology is often indeterminate. In a study by Crum-Cianflone, elevated transaminases were found in 80 (27%) of 299 HIV-mono-infected patients on ART and steatosis was detected on ultrasound (US) in 30%, though in 51% the cause of liver dysfunction remained unexplained [4].
Fatty liver due to metabolic syndrome and/or ART exposure is an increasingly recognized cause of liver test abnormality in HIV-mono-infected patients [3]. Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome [5], but could also represent a long-term toxicity of ART as nucleoside reverse-transcriptase inhibitors (NRTIs) and to a lesser extent protease inhibitors (PIs), are associated with insulin resistance and mitochondrial toxicity [6]. Moreover, specific HIV-related factors, such as lipodystrophy or the HIV virus per se, may predispose to NAFLD [7,8]. NAFLD has a prevalence of 20% in the general population of industrialized countries, which rises to more than 50% among those with HIV infection [9,10].
Since NAFLD encompasses a wide spectrum of disease, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), fibrosis and eventually cirrhosis [11], the early detection of liver fibrosis prior to the onset of complications associated with decompensated liver disease is crucial. Noninvasive fibrosis tests are increasingly used for the assessment of fibrosis in patients with liver disease [12], including those who are HIV positive [13].
In this study, we retrospectively evaluated the prevalence and predictors of liver fibrosis in a cohort of HIV-mono-infected patients with persistently elevated transaminases, using simple serum noninvasive fibrosis panels, namely the FIB4 and APRI scores. In addition, we assessed the presence of hepatic steatosis and associated features in this cohort.
Patients and methods
Study population
This was a retrospective cross-sectional audit of consecutive HIV-mono-infected patients who attended the HIV dedicated outpatient service at Royal Free Hospital, London, UK, between January and December 2014. A clinical database was used to identify patients with persistently elevated transaminases in at least two measurements six months apart. Serum levels more than 39 IU/L and 41 IU/L were considered as abnormal for aspartate aminotransferase (AST) and alanine aminotransferase (ALT), respectively. Patients with evidence of HCV or HBV co-infection, as well as other documented causes of liver disease other than NAFLD were excluded. Further information for patients with persistently abnormal transaminases was retrieved from their clinical notes.
Laboratory investigations and epidemiological and clinical features were retrieved from the clinical database for all included patients. The presence of diabetes, hypertension and dyslipidemia was documented, as well as relevant medications.
HIV specific information was also obtained; this included time since diagnosis, duration of ART, most recent CD4+ count (n/mm3) and HIV viral load (RNA copies/mm3). HIV RNA copies <40/mL defined virologic suppression.
Liver fibrosis and steatosis assessment
We obtained the results of abdominal US scans and transient elastography (TE) with FibroScan®, which were performed as part of routine clinical care, to further investigate the liver test abnormalities and stage of fibrosis, respectively. US and TE findings were included if within 6 months of the date of transaminase measurement.
The presence of significant (≥F2 METAVIR stage) or advanced (≥F3 METAVIR stage) liver fibrosis was evaluated by APRI and FIB4 scores, respectively. APRI consists of AST and platelet count in the formula:
APRI = [AST levels (IU/L)/AST upper limit of normal (ULN) (IU/L)/Platelets (109/L)] × 100
It has dual cutoffs for ruling out (<0.5) or diagnosing (>1.5) significant fibrosis [14]. FIB4 consists of age, AST, ALT and platelet count in the formula:
FIB4 = [Age (years) × AST (IU/L)]/[Platelets (109/L) × ALT (IU/L)]
It also has dual cutoffs for ruling out (<1.45) or diagnosing (>3.25) advanced fibrosis [15]. These tests usually have dual cutoffs: a high cutoff with high specificity and a low cutoff with high sensitivity. Depending on the clinical scenario and the disease prevalence, the low or high cutoff is used at the expense of increased false positives and false negatives respectively. If these cutoffs are combined, then the false positives and false negatives are minimized; however, a number of patients will fall in the indeterminate range of fibrosis (i.e., their score will be indeterminate, between the low and the high cutoff) and they will need either further noninvasive testing or a liver biopsy [16].
In a small subgroup of 19 patients, TE was performed with a FibroScan® system (Echosens; Paris, France), after at least 6 hours of fasting. Results in kilopascals (kPa) were calculated as the mean of ten valid measurements, obtained by placing the probe on the patient’s skin between the ribs at the level of the right lobe of the liver in a dorsal decubitus position. Only determinations of liver stiffness with an interquartile range for measurements within 30% and a ratio of success rate of measurements (number of total measurements/number of valid acquisition) >60% were considered reliable. Liver stiffness values >7.4 kPa were considered suggestive of significant fibrosis.
Finally, 20 patients in the same cohort had previous liver biopsies as part of their routine clinical care. The presence and severity of steatosis, as well as the histological grading and staging were assessed according to the Brunt score [17].
Statistical analysis
All data were analyzed using the statistical package SPSS (version 22.0, IBM, New York, NY, USA). Statistical analysis was performed using t-test, ANOVA, Mann-Whitney test or Kruskal-Wallis test for comparisons of continuous variables between or among groups, the corrected chi-square method or two-tailed Fisher’s exact test was used for comparisons of qualitative data, and Spearman’s coefficient for correlations of quantitative data, when appropriate. Multivariate analysis was performed using logistic regression models to assess predictors of steatosis and fibrosis with APRI and FIB-4 values of >0.5 and >1.45 respectively. Only variables with a P-value ≤0.10 in the univariate analysis were entered in the multivariate analysis models. A two-tailed P-value <0.05 was considered to indicate a statistically significant difference between groups.
Results
Baseline characteristics
Of 2398 consecutive patients with HIV mono-infection who were evaluated between January and December 2014, 156 (6.5%) had persistently elevated transaminases in at least two measurements six months apart and were included in the analysis. The mean age of those included was 47.5 years and 91% were male. Clinical, biochemical and virological characteristics are reported in Table 1. In particular, 28 patients (17.9%) had hypertension, 17 (10.9%) had diabetes and 80 (51.9%) had hyperlipidemia, while 47 (30.5%) were on lipid-lowering medication.
Table 1.
Characteristics of the cohort of HIV-mono-infected patients with deranged transaminases
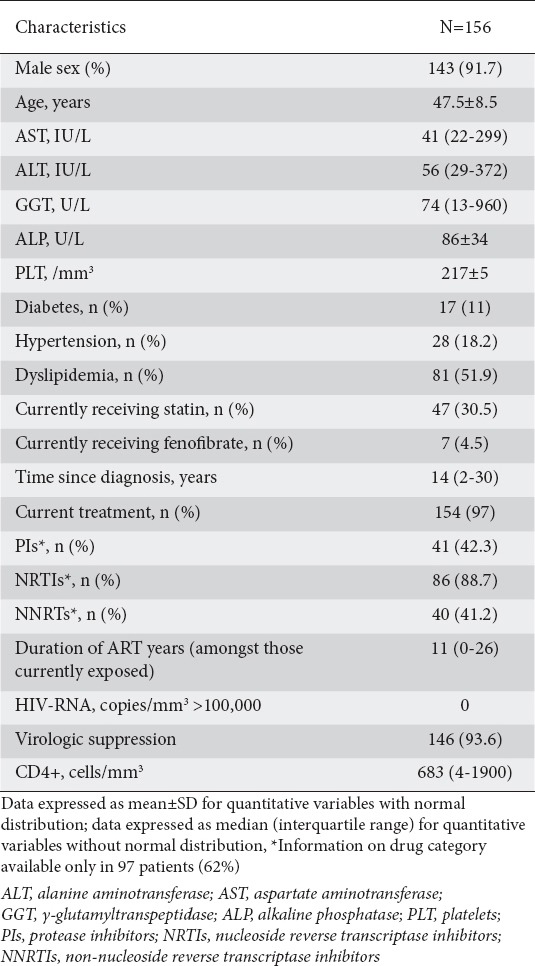
AST and ALT values <2 times ULN were found in 93.6% and 81.4% of patients, respectively, 2-3 times ULN in at least one measurement in 3.8% and 14.1%, and >3 times ULN in at least one measurement in 2.6% and 4.5% of the cohort.
Almost all patients were on ART (n=154, 97%), with a median length of treatment of 11 years (interquartile range [IQR] 0-26 years), and the majority of them had virological suppression (n=146, 93.6%). Mean time since diagnosis was 14 years (IQR 2-30 years). CD4 counts <200/mm3 were present only in 3 (1.9%) patients and median viral load was 39 copies/mm3 (IQR 39-2622).
Information about the specific treatment regimen was available for only 97 patients. The most common drugs used were NRTIs in 86 (88.7%) of treated patients, while PIs and non-NRTIs were prescribed in 41 (42.3%) and 46 (47.4%) of patients, respectively.
Prevalence and predictors of hepatic steatosis
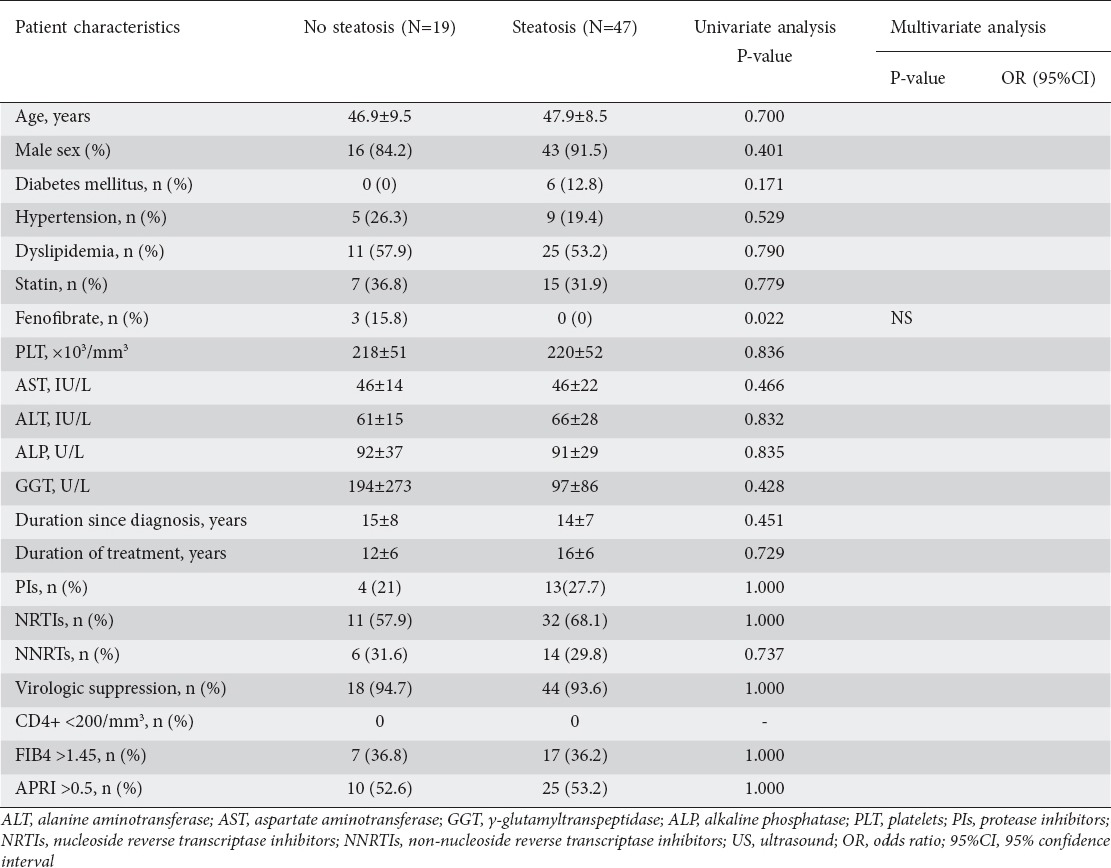
US examination was performed in 66 (42.3%) patients and fatty liver was detected in the majority of these (n=47, 71.2%), as shown in Table 2. Nevertheless, no association was found between the presence of US steatosis and patients’ clinical features, as shown in Table 3. In particular, there was no association between the presence of steatosis and metabolic features, namely hypertension, diabetes and dyslipidemia. Importantly, fatty liver was not significantly associated with the time since diagnosis, the length of ART, or the category of antiviral drugs used. On univariate analysis, fenofibrate use was significantly associated with the absence of steatosis on US (P=0.022), but this association was lost in the multivariate model.
Table 2.
Prevalence of liver disease in cohort of HIV-mono-infected patients with deranged transaminases
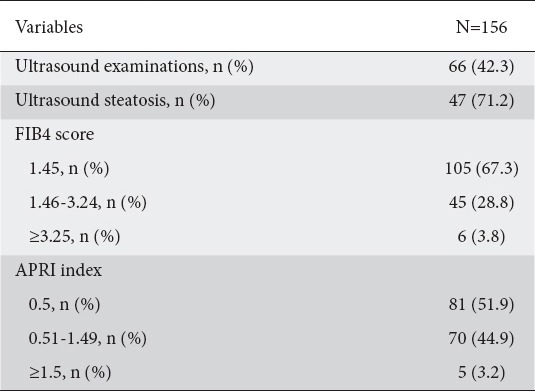
Table 3.
Variables associated with the presence of US liver steatosis in the cohort of HIV-mono-infected patients
Prevalence and predictors of significant and advanced liver fibrosis
Considering the high cutoff of >1.5 for APRI, we categorized 5 (3.2%) patients as having significant fibrosis (≥F2), whereas values <0.5 excluded this diagnosis in 51.9% of them. On the other hand, FIB4>3.25 suggested advanced fibrosis (≥F3) in 6 (3.8%) patients, while values ≤1.45 (<F3) were found in 67.3% of the cohort. Forty-five (28.8%) and 70 (44.9%) patients fell into the “indeterminate” diagnostic zone of FIB4 and APRI, respectively, as shown in Table 2.
For further analysis, and in keeping with the use of noninvasive tests for screening, we considered a threshold of 1.45 for FIB4 and of 0.5 of APRI to rule out the presence of advanced or significant fibrosis, respectively, in our cohort of HIV-mono-infected patients.
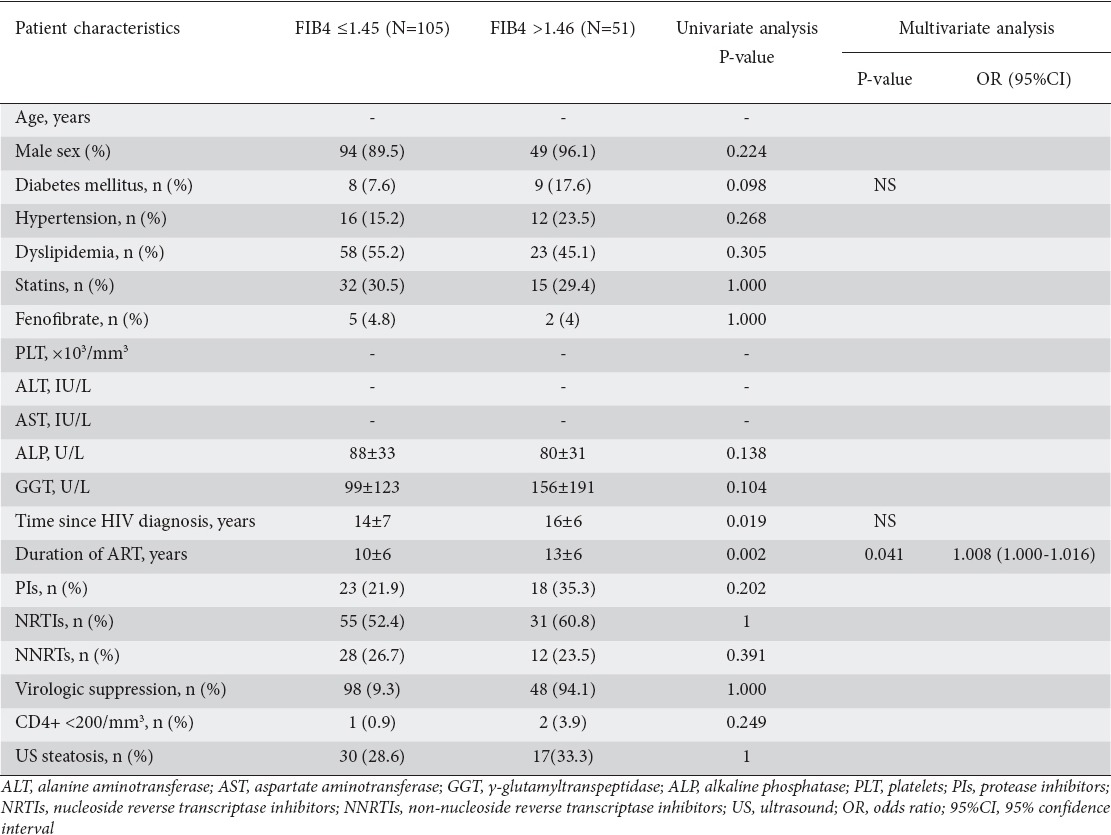
In univariate analysis, FIB4 >1.45 (n=51, 32.7%), was significantly associated with both time since HIV diagnosis (14±7 vs. 16±6 years, P=0.019) and duration of ART (10±6 vs. 13±6 years, P=0.002). Moreover, diabetes was significantly more prevalent in patients with a FIB4 score >1.45 compared to those with values <1.45 (17.6% vs. 7.6%, P=0.098). Nevertheless, in the multivariate analysis, only duration of ART maintained this association (OR 1.008, 95%CI 1.000-1.016; P=0.04) (Table 4).
Table 4.
Variables associated with the presence of liver fibrosis assessed by FIB4 in the whole cohort of HIV-mono-infected patients
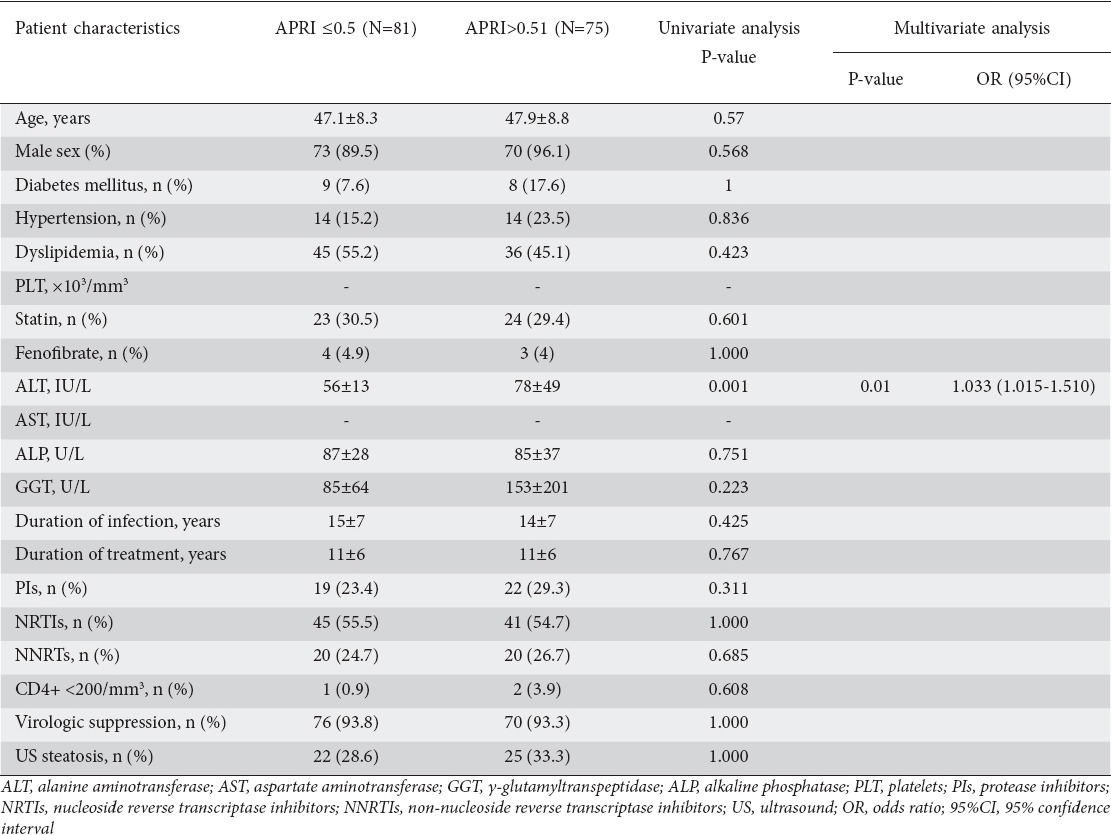
On the other hand, APRI values >0.5 were found in 75 (48%) patients and only ALT levels were significantly increased in patients with evidence of significant fibrosis (78±49 vs. 56±13; OR 1.033, 95%CI 1.015-1.510, P=0.01). In contrast to FIB4, neither the time since HIV diagnosis nor the use of ART or the presence of diabetes was associated with an increased APRI score. As with FIB4, no association with demographic or metabolic features was noted (Table 5). Interestingly, no relation was found between the presence of liver fibrosis assessed by both FIB4 and APRI scores and evidence of steatosis on US.
Table 5.
Variables associated with the presence of liver fibrosis assessed by APRI index in the whole cohort of HIV-mono-infected patients
A small subset of patients also underwent TE (n=19, 12.1%) and a liver biopsy (n=20, 12.8%). Considering a threshold stiffness value of 7.4 kPa, 4 (21.1%) patients were classified as having significant fibrosis, while stiffness values more than 10 kPa were found in only 1 patient, suggesting more advanced liver disease.
Despite the small number of liver biopsies, histology showed NAFLD in 13 (65%) patients, one of whom was also diagnosed with cirrhosis. Moreover, features of drug reaction were present in 2 cases, while 4 patients had non-specific histological changes and 1 biopsy was normal.
Discussion
In this study, we demonstrated that persistently abnormal transaminases are fairly prevalent in unselected HIV-mono-infected patients (156/2398, 6.5%). More importantly, 4% of these patients had evidence of advanced fibrosis based on simple noninvasive fibrosis tests. Despite the presence of persistently deranged liver function tests (LFTs) in this group, there was inconsistent further investigation of these abnormalities; just 66 (42%) patients had undergone a US examination and only 19 (12%) had a TE, despite the noninvasiveness and relatively low cost of these techniques.
A rise in liver enzymes in HIV-mono-infected individuals on ART has been previously reported. A cross-sectional study by Sterling et al reported elevated transaminases in 20% of 679 patients and a positive association with high viral load and features of metabolic syndrome [18]. Maida et al studied a cohort of 3200 individuals with a long history of HIV infection and ART exposure and found abnormal LFTs in only 17 (0.5%) patients, of whom 10 (58.8%) had histologically advanced fibrosis (F3-F4) and clinically advanced liver disease. However, in this study harmful alcohol use and medications predisposing to steatosis were excluded [19]. On the other hand, in a prospective study of 2365 HIV-mono-infected individuals who were followed for a 5-year period, 385 (16%) developed persistent ALT elevation, which was associated with increased body mass index, high HIV viral load and exposure to NRTIs, in particular zidovudine and stavudine [20].
The underlying etiology of increased LFTs in people who are HIV positive often remains unrecognized, as liver biopsy is not routinely performed. Nevertheless, many studies have indicated that NAFLD is more prevalent in this category of patients, potentially progressing to NASH and liver fibrosis.
A histological evaluation of a cohort of 20 HIV-mono-infected individuals with chronically increased LFTs demonstrated the presence of steatosis in 18 (60%) patients, of whom 16 (88.9%) had NASH and 13 (72.2%) had fibrosis [21]. Morse et al biopsied a cohort of 62 patients with similar features and found steatosis in 45 (73%), NASH in 34 (55%) and bridging fibrosis in 11 (17%) patients [22].
These data are similar to those of our study and indicate the necessity of screening HIV positive individuals for the presence of steatosis and fibrosis, especially when LFTs are deranged. In fact, in the subgroup of patients who underwent a liver US, NAFLD was present in the majority (47/66, 71.2%). Moreover, even though a liver biopsy was performed in only in 20 patients, histology showed NAFLD in 13 (65%), and NASH in 5 (25%) patients, of whom one had cirrhosis.
The prevalence of steatosis is likely to be even higher than 70%, as US can detect steatosis only when more than 20-30% of hepatocytes are involved. Importantly, in our study there was no significant association with metabolic, anthropometric and either infection- or ART-related features. However, as US reports were available for less than half of our cohort, this likely reflects under-powering of the study to detect any significant associations.
Conversely, two other studies reported a lower prevalence of NAFLD assessed by imaging techniques. Guaraldi et al detected steatosis by CT scan in 37% of 225 HIV-mono-infected patients, and found an association with cumulative NRTI exposure (OR 1.12, 95%CI 1.03-1.22; P=<0.001) and obesity (OR 1.07, 95%CI 1.03-1.11; P<0.001) [23]. In contrast, in another study, though steatosis was present on US in 31% and by histology in 33% of 216 HIV-mono-infected patients, there was no association with either duration of HIV infection or ART, as only obesity (OR 2.1, 95%CI 1.6-2.8; P<0.001) and hypertriglyceridemia (OR 1.2, 95%CI 1-1.5; P=0.03) reached statistical significance [24]. Nevertheless, in both studies less than 30% of patients had deranged LFTs; this could possibly explain the lower prevalence of steatosis compared to our findings.
Using the APRI and FIB4 scores, we classified 5 (3.2%) and 6 (3.8%) patients as potentially having significant and advanced fibrosis, respectively. Although TE appears to be the best noninvasive tool in HIV infected patients, showing excellent accuracy for the detection of liver cirrhosis and moderate accuracy for significant fibrosis [25,26], it was available in only a small subset of the cohort, where it suggested significant fibrosis in 4 patients. On the other hand, our data suggest that 81 (51.2%) and 105 (67.3%) patients could be excluded from having significant and advanced fibrosis according to APRI and FIB4, respectively. Indeed, APRI and FIB4 have a higher diagnostic accuracy in ruling out rather than ruling in the presence of significant/advanced fibrosis and are ideal screening tests, as they have a negative predictive value of >90% [15,27]. Low FIB4 scores are associated with excellent liver-related outcomes in patients with NAFLD over a 10-year period of follow up; therefore, these scores can be used in the HIV mono-infection setting [28].
The low prevalence of significant and advanced fibrosis based on simple noninvasive tests is compatible with previously published data. In a cohort of 818 HIV positive women, the majority of patients (86.6%) had FIB-4 values ≤1.45, while increasing FIB4 values were associated with higher HIV viral load [29]. Similarly, in a cohort of 225 HIV-mono-infected patients, 28 were classified as not having fibrosis on FIB-4 [23], while in another cohort of 62 patients FIB4 ≤1.45 and >3.25 were found in 37 (59.7%) and 5 (8%) patients, respectively [22]. Conversely, a slightly higher prevalence of 8% of APRI values >1.5 was found in 432 HIV-mono-infected patients, and both diabetes and detectable HIV viremia were confirmed as risk factors for significant fibrosis [30]. However, the presence of obesity in 50% of the cohort could have contributed to the higher prevalence of significant fibrosis.
In our study, the duration of ART was the only factor independently associated with the presence of fibrosis based on FIB-4. The impact of ART on liver fibrosis is controversial, as highlighted by conflicting reports on worsening with long-term use of ART, especially didanosine or stavudine [9,31], absence of significant effect [22], or even improvement following commencement of PIs [32,33]. Furthermore, Mendeni et al followed a cohort of 1112 HIV-mono-infected patients for approximately 6 years and found that progression of fibrosis, assessed by APRI and FIB4, was prevented by viral control with early ART, provided that didanosine use was avoided [33].
Interestingly, in this study neither FIB4 nor APRI showed any association with metabolic features or increasing age. Diabetes was significantly associated with increased FIB4 values, but only in the univariate analysis. These data reflect the characteristics of our population, as the HIV patients were relatively young (mean age 47 years) and both diabetes and hypertension had a low prevalence, thus limiting the power of this study to detect any associations with fibrosis.
This study has limitations. First, it was a cross-sectional study, so it was not possible to define the dynamic process underlying the development of both steatosis and fibrosis in HIV positive patients. Therefore, these data should be assessed prospectively, in order to evaluate the true prognostic impact of hepatic steatosis and fibrosis in HIV. Second, the selection of patients on the basis of elevated transaminases could have led to an underestimation of fatty liver disease, given their lack of sensitivity in diagnosing NAFLD or NASH [34]. Third, detection of steatosis by US could also have underestimated the prevalence of NAFLD, as the accuracy of this technique is suboptimal. In addition, an element of undisclosed alcohol abuse could also have contributed to the high steatosis prevalence in this cohort.
In conclusion, the presence of liver disease is under-investigated in HIV-mono-infected patients, despite deranged LFTs. Such abnormalities are often associated with the presence of NAFLD, and could potentially evolve into progressive liver fibrosis. A potential role for ART in this scenario has yet to be determined, but it is likely that NAFLD could represent a long-term toxicity of antiviral therapy, as suggested by the association of the length of antiviral treatment and liver fibrosis and evidence from other studies. A wide range of noninvasive tools for the assessment of liver fibrosis are currently available; therefore, noninvasive screening for the presence of NAFLD and fibrosis should be a part of the routine clinical management of HIV-mono-infected patients receiving antiretroviral treatment. Moreover, aggressive treatment of the metabolic syndrome components should be undertaken in HIV positive patients with steatosis, given the well-established association of NAFLD with increased cardiovascular morbidity and mortality [35].
Summary Box.
What is already known:
Liver disease is an important cause of morbidity and mortality among HIV-infected patients, even in the absence of hepatitis C (HCV) and B (HBV) co-infections
Abnormal liver tests in HIV mono-infection are frequently reported and nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as potential cause
Noninvasive fibrosis tests are widely used for the assessment of fibrosis in patients with liver disease, including HIV-positive individuals
What the new findings are:
Despite the high prevalence of liver test abnormalities in HIV patients, liver disease is frequently under-investigated
The duration of antiretroviral therapy seems to have an impact on the onset of hepatic steatosis and its progression to fibrosis. Therefore, NAFLD could represent a long-term toxicity of this pharmacological treatment
Simple noninvasive tests such as APRI and FIB-4 can be used for the triaging of patients for dedicated hepatological follow up and/or liver biopsy
Biography
Royal Free Hospital and UCL, London, UK
Footnotes
Conflict of interest: None
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 3.Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis. 2004;38(Suppl 2):pS65–S72. doi: 10.1086/381499. [DOI] [PubMed] [Google Scholar]
- 4.Crum-Cianflone N, Collins G, Medina S, et al. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183–191. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsochatzis EA, Manolakopoulos S, Papatheodoridis GV, Archimandritis AJ. Insulin resistance and metabolic syndrome in chronic liver diseases: old entities with new implications. Scand J Gastroenterol. 2009;44:6–14. doi: 10.1080/00365520802273058. [DOI] [PubMed] [Google Scholar]
- 6.Núñez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–S139. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 7.McGovern BH, Ditelberg JS, Taylor LE, et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365–372. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 8.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88:1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 9.Blanco F, Barreiro P, Ryan P, et al. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18:11–16. doi: 10.1111/j.1365-2893.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi R, Sambatakou H, Mariolis I, Cokkinos D, Papatheodoridis GV, Tsochatzis EA. Prevalence and predictors of liver steatosis and fibrosis in unselected patients with HIV mono-infection. Dig Liver Dis. 2016;48:1471–1477. doi: 10.1016/j.dld.2016.08.117. [DOI] [PubMed] [Google Scholar]
- 11.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzzetti E, Lombardi R, De Luca L, Tsochatzis EA. Noninvasive assessment of fibrosis in patients with nonalcoholic fatty liver disease. Int J Endocrinol. 2015;2015:343828. doi: 10.1155/2015/343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsochatzis EA, Castera L. Assessing liver disease in HIV-HCV coinfected patients. Curr Opin HIV AIDS. 2015;10:316–322. doi: 10.1097/COH.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 16.Crossan C, Tsochatzis EA, Longworth L, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19:1–409. doi: 10.3310/hta19090. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 18.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maida I, Núñez M, Ríos MJ, et al. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177–182. doi: 10.1097/01.qai.0000221683.44940.62. [DOI] [PubMed] [Google Scholar]
- 20.Kovari H, Ledergerber B, Battegay M, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis B or C virus co-infection. Clin Infect Dis. 2010;50:502–511. doi: 10.1086/649922. [DOI] [PubMed] [Google Scholar]
- 21.Ingiliz P, Valantin MA, Duvivier C, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436–442. doi: 10.1002/hep.22665. [DOI] [PubMed] [Google Scholar]
- 22.Morse CG, McLaughlin M, Matthews L, et al. Nonalcoholic steatohepatitis and hepatic fibrosis in HIV-1-monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis. 2015;60:1569–1578. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 24.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lédinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 26.Vergara S, Macías J, Rivero A, et al. Grupo para el Estudio de las Hepatitis Viricas de la SAEI. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969–974. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 27.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 28.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789.e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackard JT, Welge JA, Taylor LE, et al. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis. 2011;52:674–680. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44:463–469. doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 32.Han SH, Kim SU, Kim CO, et al. Abnormal liver stiffness assessed using transient elastography (Fibroscan®) in HIV-infected patients without HBV/HCV coinfection receiving combined antiretroviral treatment. PLoS One. 2013;8:e52720. doi: 10.1371/journal.pone.0052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendeni M, Focà E, Gotti D, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis. 2011;52:1164–1173. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 34.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 35.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]


