Summary
Patient interleukin 10 (IL-10) was higher in Staphylococcus aureus bacteremia (SaB) mortality and correlated to elevated bacterial burden in the bloodstream. Using IL-10 as an initial biomarker, clinicians may consider more aggressive antimicrobials for rapid bacterial load reduction in high-risk SaB patients.
Keywords: biomarker, Staphylococcus aureus, bacteremia, mortality, inoculums.
Abstract
Background.
Cell wall peptidoglycan stimulates interleukin 10 (IL-10) production in Staphylococcus aureus bacteremia (SaB) animal models, but clinical data are not available. This study evaluates the impact of intravascular bacterial cell numbers (ie, the level of bacteremia), in patients at the time of clinical presentation on IL-10 production and its association with S. aureus bacteremia (SaB) mortality.
Methods.
Blood and isolates were collected in 133 consecutive SaB patients. Serum IL-10 was quantified by an electrochemoluminescence assay. Bacterial inoculum was measured in patient sera with elevated (n = 8) or low (n = 8) IL-10 using a magnetic bacterial capture assay. Staphylococcus aureus from these 2 groups were introduced into whole blood ex vivo to determine IL-10 production with variable inocula.
Results.
IL-10 serum concentration was higher in SaB patient mortality (n = 27) vs survival (n = 106) (median, 36.0 pg/mL vs 10.4 pg/mL, respectively, P < .001). Patients with elevated IL-10 more often had endovascular SaB sources. The inoculum level of SaB was higher in patients with elevated serum IL-10 vs patients with low IL-10 (35.5 vs 0.5 median CFU/mL; P = .044). Ex vivo studies showed that 108 CFU/mL yielded greater IL-10 than did 103 CFU/mL (4.4 ± 1.8 vs 1.0 ± 0.6 pg/mL; P < .01).
Conclusions.
Elevated IL-10 serum concentrations at clinical presentation of SaB were highly associated with mortality. High intravascular peptidoglycan concentration, driven by a higher level of bacteremia, is a key mediator of IL-10 anti-inflammatory response that portends poor clinical outcome. Using IL-10 as an initial biomarker, clinicians may consider more aggressive antimicrobials for rapid bacterial load reduction in high-risk SaB patients.
Marked heterogeneity in the clinical presentation of Staphylococcus aureus bacteremia (SaB) suggests case-by-case variation in the host-pathogen relationship, with diverse inflammatory responses and cytokine signaling playing a significant role. Staphylococcus aureus virulence is multifactorial, dependent on numerous toxins, adhesins, and immune evasive determinants [1–7]. With regard to methicillin-resistant S. aureus (MRSA), hospital-associated strains correlate with higher risk of treatment failure, suggesting intrinsic strain-specific virulence properties [8, 9]. The relative contributions of host genetics and S. aureus virulence factors to the inflammatory/anti-inflammatory balance are poorly understood, especially related to therapeutic outcome. Despite available treatment options for methicillin-susceptible S. aureus (MSSA) and MRSA bacteremia, the morbidity and mortality attributed to this disease remain high.
In a recent preliminary study, we showed that elevated interleukin (IL) 10 serum concentrations collected upon hospital presentation of SaB (>7.8 pg/mL) identified all patients who died whereas there were no deaths in patients with IL-10 concentrations ≤7.8 pg/mL [10]. In the multivariate analysis, elevated IL-10 along with known risk factors of older age and duration of bacteremia were independent predictors of mortality. Another group corroborated the role of IL-10 in patient outcomes by identifying an elevated IL-10/tumor necrosis factor alpha (TNF-α) ratio up to 72 hours after presentation as a predictor of persistence and mortality [11].
IL-10 is a cytokine with important anti-inflammatory properties that regulates the immune response to pathogens. The innate immune response stimulates IL-10 production through Toll-like receptors (TLRs) 1, 2, 4, 6, and 9 [12–14]. IL-10 production by naive T cells is higher with increasing doses of antigen [15]. Recent evidence in animal models has increasingly recognized the role of cell wall peptidoglycan on host response immune dysregulation via TLR-2–mediated IL-10 induction [16, 17]. The objectives of this study were to validate IL-10 as a biomarker for patient mortality risk and to identify the association between the level of bacteremia (a reflection of bacterial burden and peptidoglycan quantity) and IL-10 production in patients with SaB.
MATERIALS AND METHODS
Patient and Isolate Identification and Collection
Consecutive patients with blood cultures growing MSSA or MRSA were prospectively identified from July 2010 through August 2012 at the University of Wisconsin Hospital (a 493-bed academic medical center in Madison) for this study. Patients were included if at least 2 positive blood cultures were identified, or 1 positive culture was congruent with a clinical diagnosis of SaB. The institutional review board approved the study.
Medical records were reviewed to collect age; sex; comorbid conditions; white blood cell count; Pitt bacteremia score as a validated marker of disease severity using temperature, blood pressure, mechanical ventilation, cardiac arrest, and mental status scoring system variables [18]; culture and sensitivity; duration and source of bacteremia; hospital length of stay; and in-hospital mortality. The bacteremia source was classified into 3 groups based on confirmed or suspected foci: (1) noncatheter primary endovascular, encompassing endocarditis (based on Duke criteria: either 2 major criteria or 1 major plus 3 minor criteria) and unknown or presumed endovascular sources; (2) secondary to another primary nonendovascular focus of infection (eg, lung, soft tissue, bone/joint); or (3) catheter source. Patient serum samples were obtained on the same day of initial presentation of SaB, before antibiotic therapy initiation and often within 1 hour of blood culture. The samples were stored at –80°C (typically within 48 hours of collection) until analysis.
In-hospital mortality was the primary clinical outcome. Additional clinical variables collected during the course of bacteremia included (1) the duration of bacteremia categorized as prolonged (>4 days’ duration) vs short (≤4 days’ duration) based on the previously identified optimal breakpoint for the impact of duration on patient mortality [10, 19] and (2) duration of hospitalization. Total duration of bacteremia included cases of persistent bacteremia (consecutive days of positive blood cultures) and in-hospital microbiologic relapse defined as recurrence of a positive blood culture after the first negative culture while receiving appropriate antibiotic. All subjects were followed prospectively and all clinical information was collected prior to cytokine measurements.
Analysis of IL-10 Concentrations in Patients at Clinical Presentation of Staphylococcus aureus Bacteremia
Patient IL-10 concentrations at presentation of bacteremia were measured using a validated electrochemiluminescence–based multiplex immunoassay on an MSD technology platform (Meso Scale Discovery, Gaithersburg, Maryland) [20]. In addition, the inflammatory cytokines TNF-α and interleukin 1β (IL-1β) were measured in patient sera with this platform. Cytokine concentrations were determined using standard curves. Internal positive and negative controls were used to assess for interday and intraday assay variability. The MSD multispot assay system is currently available for investigational use only and not for use in diagnostic procedures.
Quantification of Bacterial Burden in the Blood of Patients With Staphylococcus aureus Bacteremia
Bacterial density in patient sera was performed using a magnetic particle assay using magnetic nanoparticles capable of binding the bacterial membrane via a dibenzocyclooctyne-activated coated surface with azide-derivatized vancomycin (Supplementary Data). The samples analyzed using this assay represent a convenience sample of patients with elevated and low IL-10 due to limited samples and reagent available for testing. The range of IL-10 concentrations in healthy volunteers is 0–20 pg/mL with the MSD assay according to the manufacturer, so 20 pg/mL was used to stratify patients into high and low IL-10 groups. Plasma from heparinized patient blood collected at the day of SaB presentation and stored at –80°C. One milliliter of patient plasma was aliquoted into a low protein-binding microcentrifuge tube. Functionalized magnetic particles were added to each sample. The microcentrifuge tube with the plasma/particle mixture was placed on a rotary shaker for 90–100 minutes at room temperature to allow the particles to adhere to bacteria.
Tubes were placed on a neodymium magnet for 15 minutes to allow nanoparticles with bacteria to coalesce at the bottom of each tube. The magnet was then gently moved to force the pellet to the side of the tube while the plasma was removed, leaving the remaining particles/bacteria in the tube. The particles were washed twice in 1 mL phosphate-buffered saline with 0.1% Tween 20 (PBST) and then resuspended to a final volume of 0.1 mL PBST. The final volume was plated on Mueller Hinton II agar and incubated at 35°C for 18–24 hours. Bacterial colonies were quantified and burden expressed as colony-forming units per milliliter (CFU/mL). Healthy volunteer sera spiked with S. aureus ATCC 29213 were used as a positive control, whereas no bacteria were used for the negative control.
Ex Vivo Whole-Blood Assay to Evaluate Staphylococcus aureus Inoculum Effect on IL-10 Concentrations
Staphylococcus aureus isolates collected at presentation from patients in the 2 previously described IL-10 groups (elevated IL-10 and low IL-10; n = 8 for each group) were selected. Whole blood was collected from 5 healthy volunteers using heparinized serum separator tubes and pooled together in 50-mL conical tubes. Staphylococcus aureus from the elevated and low IL-10 producer groups were standardized to a 0.5 McFarland turbidity standard, heat-killed at 60°C for 1 hour, and serially diluted to introduce into 2-mL aliquots of healthy volunteer whole blood ex vivo at low (103 CFU/mL) and high (108 CFU/mL) inocula. IL-10 concentrations in blood were measured prior to bacteria exposure and after 4 hours of incubation at 35°C using a high sensitivity IL-10 enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota) with a range of 0.78–7.8 pg/mL in healthy volunteers. Heat-killed bacteria were used to study the inoculum impact to limit the potential confounding effects of variable S. aureus virulence expression on host cytokine production. The experiment was performed in duplicate for each isolate, and the IL-10 concentration from each replicate was evaluated in triplicate. Whole blood without the addition of S. aureus was used as a negative control.
Statistical Analysis
The primary clinical outcome for comparison was in-hospital mortality. Statistical analysis of continuous data was performed using with an unpaired t test or nonparametric Mann-Whitney U test if the markers did not meet criteria for Gaussian distribution and Fisher exact or χ2 test for categorical data where appropriate. Bacterial burden between high and low IL-10 groups as well as the ex vivo model results were compared by t test. Multivariate logistic regression analysis was conducted to evaluate the association between IL-10, collected patient variables, and mortality using previously described model criteria (see Supplementary Data) [10]. All statistical tests were 2-tailed, and P value of <.05 was considered significant. Statistical analyses were performed using STATA software version 14.1 (StataCorp LP, College Station, Texas) and Prism 6 (GraphPad, Inc, La Jolla, California).
RESULTS
During the 26-month study period, 135 consecutive patients presented with SaB and serum and bacterial strains were collected from 133 patients for inclusion in this study. Ninety-six patients (72%) were classified as community-onset defined by culture ≤48 hours after admission [21]. The comparison of characteristics between patients who survived vs died while in the hospital is presented in Table 1. Overall in-hospital mortality was 20.3% (27 of 133 patients); the most common source of bacteremia was from secondary sources (mostly pneumonia, skin and skin structure, and bone/joint) followed by primary endovascular source and catheter-related sources. MRSA accounted for 35.3% of cases, consistent with our prior study, with 44% MRSA in patients who died vs 32% MRSA who survived (P = .228). Duration of bacteremia was variable, with a mean and median duration of 13.5 days and 3 days in all patients, respectively. Higher Pitt bacteremia score at presentation, primary endovascular source of bacteremia, and persistent bacteremia (>4 days’ duration) were significantly associated with in-hospital mortality.
Table 1.
Comparison of Patient Characteristics According to Survival Outcome
| Characteristica | Survival (n = 106) | In-hospital Mortality (n = 27) | P Value |
|---|---|---|---|
| Age, y | 58 (48–67) | 61 (46–76) | .639b |
| Male, No. (%) | 60 (56.6) | 14 (51.9) | .659c |
| Comorbid conditions, No. (%) | |||
| Dialysis | 19 (17.9) | 6 (22.2) | .610c |
| Cardiovascular disease | 34 (32.1) | 9 (33.3) | .901c |
| Diabetes | 43 (40.6) | 10 (37.0) | .845c |
| Immunosuppression | 30 (28.3) | 6 (22.2) | .526c |
| White blood cells, 103/μL | 11.8 (7.7–15.3) | 14.5 (8.9–19.9) | .084b |
| Pitt bacteremia score | 0 (0–3) | 2 (1–5) | <.001b |
| Source of bacteremia | |||
| Primary endovasculard, No. (%) | 22 (20.7) | 14 (51.8) | .003c |
| Secondarye, No. (%) | 60 (56.6) | 10 (37.0) | .085c |
| Catheter-associated, No. (%) | 24 (22.6) | 3 (11.1) | .283c |
| Infection onset | |||
| Community | 75 | 21 | .631c |
| Hospital | 31 | 6 | |
| Persistent bacteremia ≥4 d, No. (%) | 32 (30.2) | 15 (55.5) | .014c |
| Hospital length of stay, d | 8 (6–15) | 8 (5–12) | .531b |
aAll characteristics data are presented as median (interquartile range) unless otherwise noted.
bMann-Whitney U test.
cPearson test.
dIncludes endovascular, endocarditis, or unknown presumed to be endovascular source.
eIncludes any site secondary to another primary nonendovascular focus of infection.
Association of Cytokine Concentrations at Initial SaB Presentation With Patient Mortality
In a previous study, we identified elevated IL-10 at clinical presentation as an independent predictor of mortality. Due to the small sample of that study, we aimed to validate this in a larger patient group. At the time of presentation (day 1), the mean IL-10 concentration for those patients who died during hospitalization was 115.9 pg/mL compared with 32.0 pg/mL for patients who were alive at discharge. The median IL-10 concentration in mortality was 36.0 pg/mL vs 10.4 pg/mL survival (Mann-Whitney U test, P < .001; Figure 1A). The inflammatory cytokine concentrations of TNF-α and IL-1β were also elevated in patients who died, although this was only significantly different from survivors for TNF-α (median, 27.3 pg/mL vs 14.4 pg/mL, respectively; Figure 1B). In comparing the anti-inflammatory to inflammatory cytokine ratios between death and survival, both the IL-10:TNF-α and IL-10:IL-1β ratios were significantly higher for patients who died (Figure 1D and 1E). Of the cytokines and ratios analyzed, the median difference between death and survival was greatest for IL-10 (25.6 pg/mL).
Figure 1.
Cytokine serum concentrations at patient presentation with Staphylococcus aureus bacteremia stratified by in-hospital mortality or survival. Median and interquartile ranges for death and survival were compared by Mann-Whitney U test. All data points are presented in the panels for patient death, while only those outside of the interquartile range are presented for survival. A, Interleukin 10 (IL-10). B, Tumor necrosis factor alpha (TNF-α). C, Interleukin 1β (IL-1β). D, Ratio of IL-10 to TNF-α. E, Ratio of IL-10 to IL-1β.
Increased Serum IL-10 Concentration Is Associated With a Higher Intravascular Inoculum
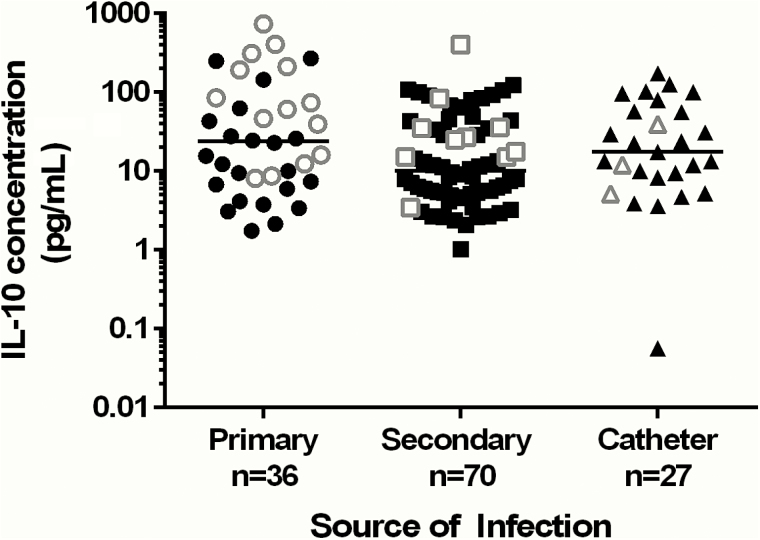
IL-10 concentrations according to source of SaB are presented in Figure 2. We hypothesized that higher intravascular peptidoglycan concentrations corresponding to a higher S. aureus intravascular inoculum may be, in part, responsible for stimulating IL-10 production. Traditionally, it has been difficult to quantify S. aureus in the blood of patients with bacteremia due to low sensitivity of traditional culture methods, and lack of precision for time to positivity for detection in blood culture bottles. Tables 2 and 3 present the results of all patients analyzed for bacterial inoculum in blood samples. The mean (median) IL-10 concentration in patients with high IL-10 was 308.1 pg/mL (290.9 pg/mL), compared with 2.2 pg/mL (2.3 pg/mL) in patients with low IL-10. Variability in bacterial numbers in blood was observed. Patients with elevated IL-10 serum concentrations had higher numbers of S. aureus in blood at day 1 of presentation, with a median bacterial concentration of 35.5 CFU/mL (interquartile range [IQR], 8.5–60.0 CFU/mL). In comparison, patients with low IL-10 serum concentrations had a median bacterial concentration in blood of 0.5 CFU/mL (IQR, 0.0–15.5; P = .044). Interestingly, 87.5% (7/8) of patients in the elevated IL-10 group had quantifiable S. aureus directly in blood, compared with only 50% (4/8) of patients in the low IL-10 group, but this was not statistically significant (P = .282).
Figure 2.
Distribution of patient interleukin 10 (IL-10) concentrations according to primary, secondary, or catheter-associated source of Staphylococcus aureus bacteremia. All patient data points are presented with the black symbols representing patients who survived and the white symbols as patient deaths. The bar in each category represents the median IL-10 concentration.
Table 2.
Bacterial Burden and Clinical Characteristics in Patients With High Interleukin 10 Serum Concentrations (>7.8 pg/mL) at Initial Presentation
| Study No. | IL-10 Concentration, pg/mL | Bacterial Burden in Bloodstream, CFU/mL | Bacteremia Source | Death |
|---|---|---|---|---|
| 1a | 402.98 | 0 | Abdominal abscess | Y |
| 2 | 270.41 | 33 | Endocarditis | N |
| 3a | 311.38 | 30 | Endovascular | Y |
| 4 | 83.93 | 5 | Pneumonia | Y |
| 5 | 213.06 | 19 | Endovascular | Y |
| 6a | 408.47 | 33 | Endocarditis | Y |
| 7 | 35.98 | 69 | Endovascular | Y |
| 8a | 738.28 | 510 | Endovascular | Y |
Abbreviations: CFU, colony-forming units; IL-10, interleukin 10.
aDenotes patients with corresponding isolates used in the ex vivo whole-blood inocula model.
Table 3.
Bacterial Burden and Clinical Characteristics in Patients With Low Interleukin 10 Serum Concentrations (≤7.8 pg/mL) at Initial Presentation
| Study No. | IL-10 Concentration, pg/mL | Bacterial Burden in Bloodstream, CFU/mL | Bacteremia Source | Death |
|---|---|---|---|---|
| 9 | 2.38 | 20 | Infected TKA | N |
| 10a | 1.03 | 1 | SSTI | N |
| 11 | 3.46 | 44 | Pneumonia | N |
| 12 | 2.63 | 0 | SSTI | N |
| 13a | 2.15 | 0 | UTI | N |
| 14a | 2.08 | 0 | Pyelonephritis | N |
| 15 | 3.76 | 2 | Endocarditis | N |
| 16a | 0.06 | 0 | Catheter | N |
Abbreviations: CFU, colony-forming units; IL-10, interleukin 10; SSTI, skin and soft tissue infection; TKA, total knee arthroplasty; UTI, urinary tract infection.
aDenotes patients with corresponding isolates used in the ex vivo whole-blood inocula model.
The sources of bacteremia in the high and low IL-10 groups displayed clear differences. Tables 2 and 3 present the bacteremia source, mortality outcome, and bacterial burden in these 2 groups. In this subset, 7 of 8 patients with elevated IL-10 concentrations died, while all patients with low IL-10 survived (P = .001). All patients with an endovascular source had quantifiable bacteria up to 510 CFU/mL in the bloodstream at presentation. Of the 7 patients with an endovascular source, 6 had elevated IL-10 serum concentrations. A correlation analysis of IL-10 concentrations and bacterial burden in patients with an endovascular source (n = 7) found a significant correlation between these variables (R2 = 0.648, P = .029). Interestingly, the only patient with an endovascular source and low IL-10 concentration had a bacterial burden of 2 CFU/mL in the bloodstream, the lowest among this group. Patients with no quantifiable S. aureus in the bloodstream typically had primary nonendovascular infection sources, such as skin and soft tissue, catheter, or urinary tract.
Staphylococcus aureus Inoculum Effect on IL-10 Production Ex Vivo
Based upon the above results, it was unclear if high endovascular bacterial inoculum triggered a high IL-10 response or whether the immunosuppressive effects of high IL-10 production allowed a high level of bacteremia to develop in the patient. To determine whether higher bacterial load triggers a higher IL-10 response in blood, an ex vivo cell culture model was performed using isolates from the patients. Bacteria isolated from the 4 patients presenting with the highest measured IL-10 concentration (study numbers 1, 3, 6, 8) and from the 4 patients presenting with the lowest measured IL-10 concentration (study numbers 10, 13, 14, 16) were selected to evaluate the effect of bacterial inoculum on IL-10 production. Staphylococcus aureus concentrations of 103 and 108 were used to represent low and high inocula, respectively. Overall, this assay provoked lower IL-10 concentrations in whole blood compared to those observed in patients. Among the isolates from the high IL-10 patient group, high-inoculum exposure ex vivo resulted in IL-10 concentrations of 8.1 ± 4.4 pg/mL compared to 0.9 ± 0.7 pg/mL with low inoculum exposure (P = .018). A mild increase in IL-10 production was noted at high inoculum using isolates from patients with low IL-10 concentrations, but this was not significant.
DISCUSSION
The rapid emergence of antimicrobial resistance has highlighted an urgent need for novel approaches and treatment paradigms for invasive bacterial infections. This high mortality rate of S. aureus persists despite a variety of treatments for SaB. Recent evidence suggests that antibiotic selection may not entirely explain the poor treatment outcomes associated with SaB and points toward a poorly understood pathogen immune-evasion phenotype [22, 23]. Precision medicine, recognized as an important area of investigation to improve treatment strategies for complex diseases, has shown recent success in laying foundations for shortening duration of antimicrobial therapy utilizing biomarkers such as procalcitonin [24]. However, for invasive S. aureus infections, there remains a significant gap in the identification of relevant biomarkers to improve antimicrobial utilization and individualize patient response. Most clinicians continue to rely on “cookie-cutter” antimicrobial regimens to treat SaB, frequently 1 drug, with more aggressive options reserved only for clinical and microbiological failure, often with dire consequences both in terms of adverse patient outcomes and progression to greater antimicrobial resistance [19].
We have previously identified 2 innate immunity biomarkers that correlate to patient outcomes in SaB: (1) elevated IL-10, which is independently associated with patient mortality and (2) IL-1β, which is reduced in patients with prolonged bacteremia [10]. In this current study, we expanded upon the IL-10 association, which validates its association with mortality and provides additional evidence that the quantity of intravascular S. aureus bacteria is related to elevated IL-10 in patients. We show that a higher inoculum of bacteria in healthy donor blood ex vivo resulted in higher IL-10 induction, particularly using isolates obtained from patients with elevated IL-10 concentrations in vivo. These studies establish that high bacterial numbers drive increased IL-10 production, rather than the immunomodulatory effects of increased IL-10 driving bacterial proliferation. One limitation of our study is that we did not evaluate the effect of inoculum or peptidoglycan stimulation in an animal model of bacteremia or infective endocarditis; however, other studies have explored this relationship and support this hypothesis [16]. In addition, we limited testing the inoculum in patients, both with the functionalized particle and ex vivo assays, in those with very high and low IL-10 concentrations to determine this correlation with available sample and assay. As such this included 7 of the 9 fatal cases with the highest IL-10 values and none of the 10 fatal cases with the lowest IL-10 values. Although the samples from the patients in this study were collected prior to antibiotic therapy on the day of SaB identification, prior antibiotic therapy could confound the results of this assay. Further studies are needed to identify the inoculum effect in patients with a range of IL-10 concentrations. Our data show variable anti-inflammatory response at patient presentation, with IL-10 serum concentration being approximately 4-fold higher in patients who ultimately died.
This current study confirms IL-10 at patient presentation as an independent predictor of SaB mortality (see Supplementary Data). The consistency of the IL-10 biomarker finding for mortality in our 2 separate studies now totaling almost 200 SaB patients, as well as the recent study by investigators at a separate institution [11], demonstrates that the inflammatory/anti-inflammatory response balance is of great clinical importance to patient outcome. More broadly, other studies have shown elevated IL-10 paralleling sepsis scores [25] and community-acquired pneumonia severity [26]. Although the inflammatory/anti-inflammatory cascade in humans is highly complex, a recent study by Minejima and colleagues identified IL-10 as an important marker for SaB outcomes among multiple cytokines tested including IL-8, IL-6, and IL-17A [11]. Collectively, these studies along with this current work point toward a potential role for IL-10 in the identification of patients at highest mortality risk, for targeting use of more aggressive therapeutic interventions.
Understanding the mechanisms involved in dysregulated inflammatory/anti-inflammatory response, particularly elevated IL-10, is critical to begin to understand its potential clinical utility in patient care. Circulating bacterial peptidoglycan is an important factor in increasing IL-10 concentrations and leads to an imbalance in the cytokine and complement cascades [16, 27]. An association between reduced S. aureus susceptibility to vancomycin, a primary antibiotic therapy that targets peptidoglycan synthesis, and attenuated host inflammatory response underscores the potential clinical consequences of altered immunologic function [28]. All these studies were done in animal or in vitro cell culture models rather than human patient data. As with our previous study, more patients with an endovascular source died compared with those patients with a secondary or catheter source. Endovascular infections are associated with high burdens at the vegetation source providing direct access to the bloodstream [29].
The embedded components of peptidoglycan including teichoic acid, lipoprotein, and lipoteichoic acid are recognized by TLR-2 and induce an IL-10 response from antigen-presenting cells [30]. Our findings are unique in that we have identified an immune biomarker that correlates to S. aureus bacterial numbers. As natural hosts, humans are much more susceptible to complications induced by SaB, perhaps because they are already primed by frequent interactions with the organism [31]. The highest bacterial burden in blood that was detected in this study was only 2.7 log10 CFU/mL, which is almost 4 orders of magnitude lower than typical challenge inoculum required to establish infection in animal models. The analysis of bacterial inocula in blood was limited in sample size due to availability of the novel functionalized particle assay, and therefore further studies of this effect are necessary for determining a more granular relationship. The immune dysregulation could be multifactorial but likely is a combination of dysregulated host recognition as well immune evasion characteristics that are increasingly recognized in S. aureus [31–34]. Further evidence for immune dysregulation comes from a staphylococcal vaccine trial that implicated immune dysregulation in the deaths of vaccine recipients who developed invasive S. aureus infections [35]. This suggests that host factor determinants may also play an important role in the human innate immune response, including IL-10, during SaB, and warrants further study to identify these key factors and impact of the inflammatory/anti-inflammatory cytokine balance.
A potential biomarker for stratifying high-risk patients with SaB will have important implications on patient management and antibiotic stewardship. It has been widely recognized that a delay in optimal therapy for complex infections leads to suboptimal outcomes [36, 37]. Increasingly, institutions are managing MRSA bacteremia with new antibiotics or β-lactams in combination with standard therapies. The results of our study indicate that higher intravascular bacterial inoculum may be responsible for elevated IL-10 concentrations, resulting in a predominantly anti-inflammatory response and increased mortality. Therefore, using highly bactericidal antibiotic therapy to reduce organism burden may be important in normalization of the anti-inflammatory response. However, some patients may do just as well clinically with conventional monotherapy. It will be important to identify the impact of duration of SaB symptoms on IL-10 productions after initial presentation. Based upon the emerging literature associating IL-10 with mortality, future studies evaluating antibiotic effectiveness for SaB should consider this biomarker to stratify patients at risk for clinical failure who may require more aggressive and pharmacodynamically potent therapeutic interventions that take advantage of both drug–drug synergy, as well as enhancement of innate immune-mediated bacterial clearance. These early, aggressive approaches include high-dose therapies, novel synergistic combinations such as vancomycin or daptomycin combined with a β-lactam [38–40], and/or earlier surgical management for improved infection control measures.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. The authors thank Debra Brunner, Trisha Robakowski, Alyson Nelson, Patti Anderson, and Tina Grindle at UW Health for their assistance with sample collection for this study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported in part by the Clinical and Translational Science Award program, previously through the National Center for Research Resources (grant number 1UL1RR025011) and now by the National Center for Advancing Translational Sciences (grant number 9U54TR000021).
Potential conflicts of interest. W. E. R. has received speaking honoraria from Theravance and The Medicines Company, grant funding from Merck (Cubist), and consulting fees from Theravance and Visante, Inc. M. S. H. has received grant funding from GlaxoSmithKline and speaking honoraria from Sanofi Pasteur. G. S. has received speaking honoraria from Merck (Cubist), Forest, and Novartis, consulting fees from Merck (Cubist) and Forest, and research grant support from Forest. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kreisel KM, Stine OC, Johnson JK, et al. USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn Microbiol Infect Dis 2011; 70:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hota B, Lyles R, Rim J, et al. ; CDC Prevention Epicenters Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis 2011; 53:757–65. [DOI] [PubMed] [Google Scholar]
- 3. Rooijakkers SH, Ruyken M, van Roon J, van Kessel KP, van Strijp JA, van Wamel WJ. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol 2006; 8:1282–93. [DOI] [PubMed] [Google Scholar]
- 4. Kempker RR, Farley MM, Ladson JL, Satola S, Ray SM. Association of methicillin-resistant Staphylococcus aureus (MRSA) USA300 genotype with mortality in MRSA bacteremia. J Infect 2010; 61:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, et al. ; International Collaboration on Endocarditis-Microbiology Investigators Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis 2011; 204:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popovich KJ, Hota B, Aroutcheva A, et al. Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin Infect Dis 2013; 56:1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shukla SK, Karow ME, Brady JM, et al. Virulence genes and genotypic associations in nasal carriage, community-associated methicillin-susceptible and methicillin-resistant USA400 Staphylococcus aureus isolates. J Clin Microbiol 2010; 48:3582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen SY, Liao CH, Wang JL, et al. Methicillin-resistant Staphylococcus aureus (MRSA) staphylococcal cassette chromosome mec genotype effects outcomes of patients with healthcare-associated MRSA bacteremia independently of vancomycin minimum inhibitory concentration. Clin Infect Dis 2012; 55:1329–37. [DOI] [PubMed] [Google Scholar]
- 9. Wang JT, Wang JL, Fang CT, et al. Risk factors for mortality of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection: with investigation of the potential role of community-associated MRSA strains. J Infect 2010; 61:449–57. [DOI] [PubMed] [Google Scholar]
- 10. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 2016; 44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teixeira-Coelho M, Guedes J, Ferreirinha P, et al. Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur J Immunol 2014; 44:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh C, Bishayi B. Toll-like receptor 2 and 6 interdependency in the erosive stage of Staphylococcus aureus induced septic arthritis mediated by IFN-γ and IL-6–A possible involvement of IL-17 in the progression of the disease. Immunobiology 2015; 220:910–23. [DOI] [PubMed] [Google Scholar]
- 14. Boonstra A, Rajsbaum R, Holman M, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol 2006; 177:7551–8. [DOI] [PubMed] [Google Scholar]
- 15. Gabrysová L, Nicolson KS, Streeter HB, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med 2009; 206:1755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frodermann V, Chau TA, Sayedyahossein S, Toth JM, Heinrichs DE, Madrenas J. A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J Infect Dis 2011; 204:253–62. [DOI] [PubMed] [Google Scholar]
- 17. Matsui K, Ikeda R. Peptidoglycan in combination with muramyldipeptide synergistically induces an interleukin-10-dependent T helper 2-dominant immune response. Microbiol Immunol 2014; 58:260–5. [DOI] [PubMed] [Google Scholar]
- 18. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001; 31:97–103. [PubMed] [Google Scholar]
- 19. Kullar R, McKinnell JA, Sakoulas G. Avoiding the perfect storm: the biologic and clinical case for reevaluating the 7-day expectation for methicillin-resistant Staphylococcus aureus bacteremia before switching therapy. Clin Infect Dis 2014; 59:1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods 2009; 340:55–64. [DOI] [PubMed] [Google Scholar]
- 21. David MZ, Daum RS, Bayer AS, et al. Staphylococcus aureus bacteremia at 5 US academic medical centers, 2008–2011: significant geographic variation in community-onset infections. Clin Infect Dis 2014; 59:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/ Accessed 13 March 2016. [Google Scholar]
- 23. Holmes NE, Turnidge JD, Munckhof WJ, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 2011; 204:340–7. [DOI] [PubMed] [Google Scholar]
- 24. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16:819–27. [DOI] [PubMed] [Google Scholar]
- 24. de Jong E, de Lange DW, Beishuizen A, van de Ven PM, Girbes AR, Huisman A. Neutrophil CD64 expression as a longitudinal biomarker for severe disease and acute infection in critically ill patients. Int J Lab Hematol 2016; 38:576–84. [DOI] [PubMed] [Google Scholar]
- 25. Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000; 181:176–80. [DOI] [PubMed] [Google Scholar]
- 26. Gallagher PM, Lowe G, Fitzgerald T, et al. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax 2003; 58:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spika JS, Peterson PK, Wilkinson BJ, et al. Role of peptidoglycan from Staphylococcus aureus in leukopenia, thrombocytopenia, and complement activation associated with bacteremia. J Infect Dis 1982; 146:227–34. [DOI] [PubMed] [Google Scholar]
- 28. Howden BP, Smith DJ, Mansell A, et al. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol 2008; 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:5631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chau TA, McCully ML, Brintnell W, et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat Med 2009; 15:641–8. [DOI] [PubMed] [Google Scholar]
- 31. Proctor RA. Recent developments for Staphylococcus aureus vaccines: clinical and basic science challenges. Eur Cell Mater 2015; 30:315–26. [DOI] [PubMed] [Google Scholar]
- 32. Shukla SK, Rose W, Schrodi SJ. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends Microbiol 2015; 23:529–36. [DOI] [PubMed] [Google Scholar]
- 33. Stapels DA, Kuipers A, von Köckritz-Blickwede M, et al. Staphylococcus aureus protects its immune-evasion proteins against degradation by neutrophil serine proteases. Cell Microbiol 2016; 18:536–45. [DOI] [PubMed] [Google Scholar]
- 34. Stapels DA, Ramyar KX, Bischoff M, et al. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci U S A 2014; 111:13187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McNeely TB, Shah NA, Fridman A, et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother 2014; 10:3513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42:1749–55. [DOI] [PubMed] [Google Scholar]
- 37. Kumar A. An alternate pathophysiologic paradigm of sepsis and septic shock: implications for optimizing antimicrobial therapy. Virulence 2014; 5:80–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 2011; 53:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36:1317–33. [DOI] [PubMed] [Google Scholar]
- 40. Davis JS, Sud A, O’Sullivan MV, et al. ; Combination Antibiotics for MEthicillin Resistant Staphylococcus aureus (CAMERA) study group; Australasian Society for Infectious Diseases Clinical Research Network Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 2016; 62:173–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.