Summary
The relationship between drug concentrations, minimum inhibitory concentrations, and sputum culture conversion in tuberculosis is unclear. We identified concentration-dependent antagonism between isoniazid and rifampicin that was associated with poorer 2-month sputum conversion.
Keywords: tuberculosis treatment outcomes, pharmacokinetic-pharmacodynamic variability, Mycobacterium tuberculosis, minimum inhibitory concentrations, drug–drug antagonism.
Abstract
Background.
There is scant evidence to support target drug exposures for optimal tuberculosis outcomes. We therefore assessed whether pharmacokinetic/pharmacodynamic (PK/PD) parameters could predict 2-month culture conversion.
Methods.
One hundred patients with pulmonary tuberculosis (65% human immunodeficiency virus coinfected) were intensively sampled to determine rifampicin, isoniazid, and pyrazinamide plasma concentrations after 7–8 weeks of therapy, and PK parameters determined using nonlinear mixed-effects models. Detailed clinical data and sputum for culture were collected at baseline, 2 months, and 5–6 months. Minimum inhibitory concentrations (MICs) were determined on baseline isolates. Multivariate logistic regression and the assumption-free multivariate adaptive regression splines (MARS) were used to identify clinical and PK/PD predictors of 2-month culture conversion. Potential PK/PD predictors included 0- to 24-hour area under the curve (AUC0-24), maximum concentration (Cmax), AUC0-24/MIC, Cmax/MIC, and percentage of time that concentrations persisted above the MIC (%TMIC).
Results.
Twenty-six percent of patients had Cmax of rifampicin <8 mg/L, pyrazinamide <35 mg/L, and isoniazid <3 mg/L. No relationship was found between PK exposures and 2-month culture conversion using multivariate logistic regression after adjusting for MIC. However, MARS identified negative interactions between isoniazid Cmax and rifampicin Cmax/MIC ratio on 2-month culture conversion. If isoniazid Cmax was <4.6 mg/L and rifampicin Cmax/MIC <28, the isoniazid concentration had an antagonistic effect on culture conversion. For patients with isoniazid Cmax >4.6 mg/L, higher isoniazid exposures were associated with improved rates of culture conversion.
Conclusions.
PK/PD analyses using MARS identified isoniazid Cmax and rifampicin Cmax/MIC thresholds below which there is concentration-dependent antagonism that reduces 2-month sputum culture conversion.
The rationale for multidrug antituberculosis therapy (ATT) administered over 6 months is to ensure sterilization of both actively and slow/nonreplicating bacilli, and to prevent selection of resistant mutants. A treatment success rate of 86% has been reported in new tuberculosis (TB) cases [1]. The rate of relapse in drug-susceptible TB have been reported to be approximately 5% [2]. Interim treatment outcomes such as culture conversion by 2 months of treatment and time to culture conversion [3, 4] have been used as surrogates of outcome although, arguably, these are suboptimal measures of sterilizing activity against drug-tolerant persisting bacillary subpopulations and subsequent relapse [5, 6].
There have been hypothesis-generating in vitro studies [7–9], animal models [10, 11], and Monte Carlo simulation analyses [12, 13] predicting that variability of drug concentrations both in plasma and at the site of disease significantly affects treatment outcome [14, 15]. The relationship between bacterial growth and different antibiotic concentrations can be obtained from the pharmacodynamic parameter, the minimum inhibitory concentration (MIC). In the context of Mycobacterium tuberculosis (MTB), this is the lowest of a series of drug dilutions, which will limit growth of <1% (<10% for pyrazinamide) of the bacterial population under defined in vitro conditions. The pharmacokinetic/pharmacodynamic (PK/PD) parameter that best predicts microbial kill in murine and hollow fiber models for isoniazid, rifampicin, and pyrazinamide is the ratio of the 0- to 24-hour area under the PK concentration-time curve (AUC0-24) to MIC of the MTB strain consistent with data from clinical studies [16, 17]. Studies evaluating PK/PD predictors of 2-month culture conversion and treatment outcomes are conflicting [18–22]. This could be due, in part, to heterogeneity in geographical populations studied, the prevalence of human immunodeficiency virus type 1 (HIV-1) coinfection, dose in milligrams per kilogram, dose frequency, pharmacokinetic sampling methodology, and methods of PK/PD analysis.
Few studies have MIC data on the infecting MTB strain, necessary to calculate AUC0-24/MIC, maximum concentration (Cmax)/MIC, and percentage of time that concentrations persisted above the MIC (%TMIC). Moreover, due to the retrospective nature of many studies, not all studies had comparator pharmacokinetic data available from control patients with successful outcomes [23, 24]. Furthermore, these studies relied on concentration target ranges derived from healthy volunteers in phase 1 studies with no tuberculosis response data [25].
We assessed the role of the PK measures Cmax and AUC0-24, as well as the PK/PD exposures Cmax/MIC, AUC0-24/MIC, and %TMIC for rifampicin, isoniazid, and pyrazinamide in predicting the outcome of sputum culture conversion at 2 months in a cohort including HIV-1–uninfected and HIV-1–coinfected tuberculosis patients.
MATERIALS AND METHODS
Patients
Patients with GeneXpert MTB/RIF–confirmed rifampicin-susceptible pulmonary tuberculosis were recruited at Ubuntu HIV/tuberculosis clinic (site B), Khayelitsha, South Africa, as part of a prospective study (Human Research Ethics Committee approval 568/2012) assessing frequency and determinants of acquired drug resistance. The study was carried out during March 2013–July 2014, with clinical follow-up until November 2015. A subset of the patients was invited to participate in this nested pharmacokinetic study. All patients provided written consent prior to participation.
Detailed data on sociodemographic factors, past tuberculosis treatment history, and comorbidities were collected. On a single baseline sputum, bacterial load was estimated via smear grade and days to culture positivity in liquid culture media liquid (mycobacterial growth indicator tube [MGIT]). Chest radiographs were graded as extensive radiological disease in the presence of disease in both lung fields or ≥2 of 3 zones per lung, and the presence of cavitation >1 cm was also noted. Participants underwent HIV testing, CD4 lymphocyte count, and HIV-1 viral load quantification.
Antituberculosis therapy was provided as a fixed-dose combination supplied by the National Tuberculosis Control Programme (Rifafour e-275, Sanofi-Aventis; or Ritib, Aspen South Africa). Each tablet contained rifampicin at 150 mg, isoniazid at 75 mg, pyrazinamide at 400 mg, and ethambutol at 275 mg.
Weight band–based dosing was used in line with World Health Organization guidelines [26] (Supplementary Methods). Antituberculosis therapy was administered 7 days/week. At the 7- to 8-week follow-up, participants had sputum induction to ascertain culture conversion. They were classed as poorly adherent if they missed 5 or more doses of TB medication in the previous month based on either self-report and/or pill counts. They were clinically reviewed at 5–6 months and induced sputum was sent for culture to ascertain treatment completion/cure.
Pharmacokinetics
On the day of the PK study, participants were fasting, the time of the previous dose was recorded, and all participants were observed swallowing their dose of medication. Blood draws were taken before and at 1, 2, 3, 4, 6, and 8 hours after drug ingestion after 7–8 weeks of ATT. Plasma samples were assayed for rifampicin, isoniazid, and pyrazinamide using liquid chromatography–tandem mass spectrometry methods, and plasma concentration-time data from all subjects were analyzed with nonlinear mixed-effects modeling as previously described [27] (Supplementary Methods). The free PK measures for rifampicin, isoniazid, and pyrazinamide were calculated assuming unbound fractions (fu) of 0.2 [28], 0.95, and 0.9 [29], respectively.
MIC Determination
In the study cohort, the MIC for rifampicin (using concentrations of 0.03, 0.06, 0.12, 0.25, 0.5, 1 mg/L), isoniazid (0.025, 0.05, 0.1, 0.4, 1 mg/L), and pyrazinamide (12.5, 25, 50, 100, 150 mg/L) were determined in triplicate in the MGIT system with EpiCenter software (Supplementary Methods). The MICs were performed on baseline MTB isolates.
Statistical Analyses
Sample size for this study assumed a coefficient of variation for rifampicin Cmax (drug with greatest pharmacokinetic variability) of 40% [30]. Estimating a primary outcome rate (culture conversion at 2 months) of 70% [31], a sample size of 94 was required to detect a 25% difference in Cmax between 2-month culture converters and nonconverters.
The median PK values for pyrazinamide were imputed for 2 patients who took only rifampicin/isoniazid on the day of PK sampling. The Kruskal-Wallis test was used to compare groups of independent variables. Unadjusted PK measures, PK measures adjusted by MIC, and clinical covariates were considered in univariate logistic regression analyses to determine predictors of 2-month culture conversion. P values from the univariate analyses were used to guide variable selection for the multivariate model and should be interpreted with caution in light of the potential for multiple comparisons. Clinical covariates were tested for pairwise interactions with PK parameters and the outcome of 2-month culture conversion. It was decided a priori, to include AUC0-24/MIC for rifampicin, isoniazid, and pyrazinamide in the multivariate model, along with the clinical covariates which were significant (P ≤ .2) in the univariate analysis. Stata software version 13.1 (StataCorp, College Station, Texas) was used for these analyses.
Machine Learning
Multivariate analysis regression splines (MARS) were used to identify predictors of the probability of 2-month culture conversion. Unlike logistic regression, MARS breaks up co-linearity and complex nonlinear relationships in distinct ranges or regions of the data set, to perform an operation akin to piecewise regression with automatic examination of high-order (ie, both 1-way and 2-way) interactions [32]. The data ranges are delineated by hinges/knots, and the relationships are given as slopes in basis functions (BFs) that specify the hinges or data range reported. The receiver operating characteristic (ROC) values of the learning and test set models after 10-fold cross-validation were used to select the best model. All demographic, clinical, laboratory, and PK/PD exposure variables (Cmax, fu.Cmax, %TMIC, %TMIC (free), Cmax /MIC, fu.Cmax/MIC, AUC0-24, fu.AUC0-24, and AUC0-24 /MIC) were included as potential predictors in initial stepwise modeling exercises, and parameters were arbitrarily set at a maximum of 15 BFs. Thereafter, parameters in basis function format were pruned back to increase prediction on the test sample as well as to improve interpretability and parsimony (Supplementary Methods). Salford Predictive Modeler version 7.0 was used for MARS [32].
Finally, we used logistic regression to compute an adjusted odds ratio (OR) with a 95% confidence interval (CI) using thresholds identified by the MARS model.
RESULTS
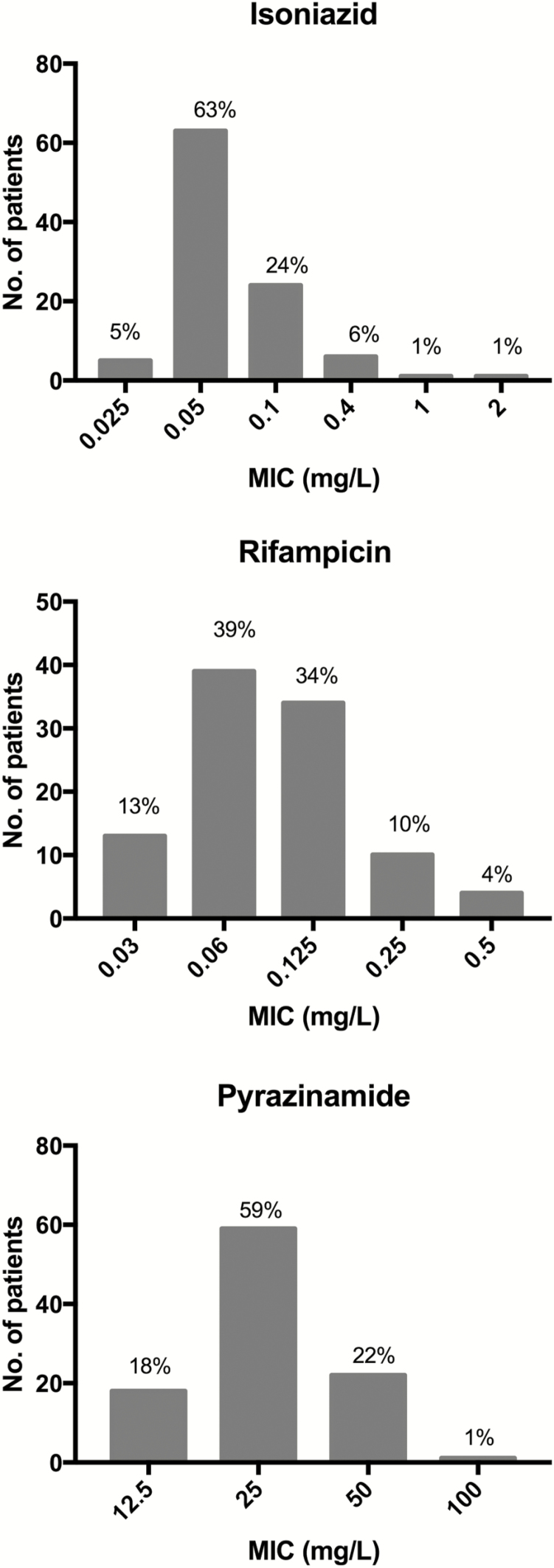
Table 1 provides the clinical characteristics and treatment outcomes of the PK cohort. The median dose for rifampicin, isoniazid, and pyrazinamide was 10 (range, 7–11) mg/kg, 5 (range, 3.5–6) mg/kg, and 26 (range, 19–31) mg/kg. Of the 100 study participants, 65% were HIV-1 infected with a median CD4 lymphocyte count of 233 (interquartile range [IQR], 106–386) cells/mm3. The proportion on ART increased from 27 of 65 (42%) at baseline to 50 of 65 (77%) at the time of the PK study. Fifty-two percent had lung cavities present at baseline and 66% were smear positive (grading 1 to 3+), of whom 24 of 66 (36%) were graded 3+. All participants were culture positive at baseline. MIC distributions are shown in Figure 1. Culture conversion at 2 months was 77%. At the end of study follow-up, there was 1 death in the PK cohort and 3 failures and 4 relapses.
Table 1.
Clinical Characteristics of Cohort and Outcomes
| Characteristic | Whole PK Cohort (N = 100) | Culture Negative at 2 mo (n = 77) | Culture Positive at 2 mo (n = 23) |
|---|---|---|---|
| Clinical covariatesa | |||
| Male sex | 57 (57) | 43 (56) | 14 (61) |
| Xhosa ethnicity | 98 (98) | 76 (99) | 23 (100) |
| Median age, y (IQR) | 33 (29–40) | 32 (29–38) | 40 (30–48) |
| Smoking history | |||
| Current | 24 (24) | 17 (22) | 7 (30) |
| Previous | 27 (27) | 23 (30) | 4 (17) |
| Never | 49 (49) | 37 (48) | 12 (52) |
| Alcohol consumption | 37 (37) | 27 (35) | 10 (43) |
| Recreational drug use | 5 (5) | 5 (6) | 0 (0) |
| Previously in prison | 14 (14) | 10 (13) | 4 (17) |
| Previous mining history | 5 (5) | 2 (3) | 3 (13) |
| Re-treatment | 39 (39) | 26 (34) | 13 (57) |
| Type 2 diabetes mellitus | 4 (4) | 2 (3) | 2 (15) |
| Median BMI at baseline (IQR), kg/m2 | 21 (19–23) | 21 (19–23) | 21 (20–23) |
| Median BMI at PK study (IQR), kg/m2 | 21.5 (20–23) | 21.5 (20–23) | 21 (20–23) |
| Median FFM at PK study (IQR), kg | 45 (38–49) | 44 (38–49) | 47 (39–50) |
| HIV-1 coinfected | 65 (65) | 53 (69) | 12 (52) |
| Baseline median CD4 count (IQR), cells/mm3 | 233 (106–386) | 224 (101–355) | 397 (216–466) |
| % VL <40 copies/mL at baseline | 26 | 23 | 22 |
| Median albumin at PK study (IQR), g/L | 38 (34–40) | 38 (34–40) | 38 (34–40.5) |
| Median total protein at PK study (IQR), g/L | 86 (79–92) | 86 (78–92) | 86 (83–91) |
| Months on ART by day of PK study (IQR) | 1.32 (0–15.5) | 1.3 (0.52–13.6) | 14.3 (0–59.1) |
| Smear grading at baseline | |||
| 3+ | 24 (24) | 13 (17) | 11 (48) |
| 2+ | 22 (22) | 19 (25) | 3 (13) |
| 1+ | 20 (20) | 16 (21) | 4 (17) |
| Scanty/negative | 34 (34) | 29 (38) | 5 (22) |
| Median TTD, days (IQR) | 10 (7–14) | 12 (7–14) | 7 (6–10.5) |
| Extensive radiological disease at baseline | 71 (71) | 53 (69) | 17 (74) |
| Cavities at baseline | 52 (52) | 38 (49) | 14 (61) |
| Baseline isoniazid monoresistance | 8 (8) | 6 (8) | 2 (9) |
| Median dose administered at PK study in mg/kg (range) | |||
| Rifampicin | 10 (7–11.5) | 10 (9–10) | 10 (9–10) |
| Isoniazid | 5 (3.5–6) | 5 (4–5) | 5 (4–5) |
| Pyrazinamide | 26 (19–31) | 26 (23.5–28) | 26 (23–27) |
| Side effects of TB treatment at 2 month review | 35 (35) | 24 (31) | 12 (52) |
| Poor adherence at 2 month review as per pill counts/self-report | 10 (10) | 7 (9) | 3 (13) |
| Outcomes | |||
| 5 month culture conversion (out of 83 patients who produced sputum)b | 80/83 (96) | ||
| Treatment failures over study duration | 3 (3) | ||
| Treatment relapse | 4 (4)c | ||
| Overall successful outcome (treatment cure/completion without relapse)d | 86/99 (87) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; FFM, fat-free mass; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; PK, pharmacokinetic; TB, tuberculosis; TTD, time to culture positivity at baseline; VL, viral load.
aAt TB diagnosis (ie, baseline) unless otherwise specified as day of PK study or 2-month review.
bSeven defaulters, 1 transfer of care, 9 treatment completers (no sputum produced at 5 months).
cOne relapse died and had acquired drug resistance.
dDefaulters assumed to have unsuccessful outcome. Transfer of care with unknown outcome not included in denominator.
Figure 1.
Histograms showing distributions of minimum inhibitory concentrations (MICs) in baseline Mycobacterium tuberculosis isolates.
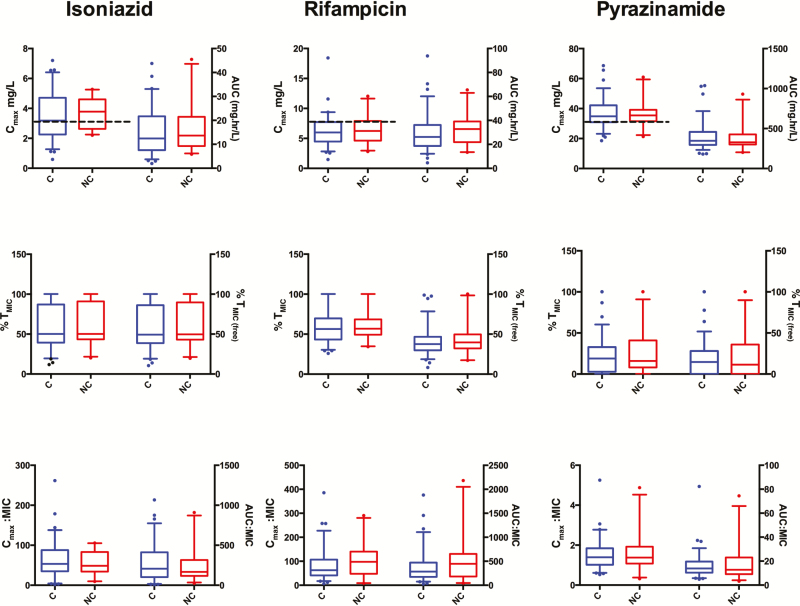
While there was considerable interindividual variability of Cmax, AUC0-24, Cmax/MIC, AUC0-24/MIC, and %TMIC (unbound and free), for all 3 drugs (Figure 2), on logistic regression analysis there were no statistically significant relationships between the above PK/PD parameters and the proportion culture converting at 2 months. The proportion of all patients with estimated free drug in plasma above the MIC for at least 12 hours (ie, 50% of dosing interval) was 49%, 21%, and 4% for rifampicin, isoniazid, and pyrazinamide, respectively.
Figure 2.
The pharmacokinetic (PK) measures maximum concentration (Cmax), 0- to 24-hour area under the curve (AUC0-24; with and without adjustment for minimum inhibitory concentration [MIC]) and percentage of time above the MIC (%TMIC), stratified by culture converter status. The box-and-whisker plots show model-derived PK measures. Cmax and AUC0-24 (with and without adjustment for MIC) are plotted on the left and right y-axes. The boxes show median PK and PK/pharmacodynamic measures (and interquartile range) and the whiskers show 5th–95th percentile and illustrate considerable variability within converter (C) and nonconverter (NC) groups. The proportion of 2-month culture conversion is also shown stratified by AUC0-24/ MIC quartile for isoniazid (INH), rifampicin (RIF), and pyrazinamide (PZA). The dotted black line indicates current recommended thresholds for Cmax of 3 mg/L, 8 mg/L, and 30 mg/L for INH, RIF, and PZA, respectively. There were 2 patients in whom pyrazinamide values were missing and in whom the median AUC0-24 and Cmax values for pyrazinamide were imputed in the PK analysis. %TMIC is the proportion of time between dosing intervals that drug concentration is above the MIC; %TMIC(free) is the proportion of time between dosing intervals that unbound drug concentration is above MIC.
For both converters and nonconverters, a significant proportion of patients had a Cmax lower than the currently recommended guidelines for all drugs [25]. For isoniazid, 43% patients had a low Cmax (<3 mg/L) and 6% had very low maximum concentrations (<1.5 mg/L). For rifampicin 80% had a low Cmax (<8 mg/L) and 17% had a very low Cmax (<4 mg/L). None of these Cmax cutoff values for isoniazid or rifampicin predicted 2-month culture conversion and/or failure/relapse. For pyrazinamide, 53% of patients had Cmax <35 mg/L [33] and 1% had Cmax <20 mg/L. The cutoff of pyrazinamide <35 mg/L was not predictive of 2-month culture conversion, but did predict failure/relapse (OR, 0.16; P = .03). Twenty-six of 31 patients (84%) with low concentrations of all 3 drugs had culture converted at 2 months, and 4 of 31 (13%) had treatment failure/relapse compared with 3 of 69 (4%) who did not have low concentrations of all 3 drugs (P = .12).Table 2 shows the significant clinical predictors of culture conversion at 2 months. On multivariate analyses, 10-year increment in age (OR, 0.44 [95% CI, .24–.81]; P = .01), smear 3+ positivity (OR, 0.09 [95% CI, .02–.35]; P = .001) and drug side effects (OR, 0.17 [95% CI, .05–.63]; P = .01) were the only significant predictors of 2-month culture conversion.
Table 2.
Multivariate Analysis of Clinical Risk Factors for Culture Conversion at 2 Months
| Variablea | Univariate Analysis OR (95% CI) |
P Value | Multivariate Analysisb OR (95% CI) |
P Value |
|---|---|---|---|---|
| Male sex | 0.81 (.31–2.10) | .67 | ||
| 10-y increment in age | 0.56 (.35–.91) | .02 | 0.44 (.24–.81) | .01 |
| BMI | 0.97 (.89–1.06) | .48 | ||
| Re-treatment status | 0.39 (.15–1.01) | .05 | 0.45 (.12–1.64) | .23 |
| Smoker status | ||||
| Never | Referent | |||
| Ex | 1.86 (.54–6.48) | .33 | ||
| Current | 0.79 (.26–2.35) | .67 | ||
| Alcohol use | 0.70 (.27–1.81) | .46 | ||
| Ex-prisoner | 0.71 (.20–2.52) | .60 | ||
| Ex-miner | 0.18 (.03–1.13) | .07 | ||
| Diabetes | 0.28 (.04–2.11) | .22 | ||
| Drug side effects at 2-mo review | 0.39 (.15–1.01) | .05 | 0.17 (.05–.63) | .01 |
| Poor adherence at 2-mo review as per pill counts/self-report | 0.67 (.16–2.81) | .58 | ||
| INH resistance | 0.89 (.17–4.73) | .89 | ||
| Smear gradingc | ||||
| Negative/scanty | Referent | |||
| 1+ | 0.69 (.16–2.93) | .61 | 1.17 (.21–6.53, 0.85) | |
| 2+ | 1.09 (.23–5.11) | .91 | 0.74 (.14–3.89, 0.72) | |
| 3+ | 0.20 (.06–.71) | .01 | 0.09 (.02–.35) | .001 |
| Time to culture positivity at baselinec | 1.14 (1.01–1.28) | .04 | 1.16 (1.02–1.33) | .02 |
| HIV status | 2.02 (.78–5.23) | .15 | ||
| Log10 CD4 | 0.70 (.20–2.42) | .57 | ||
| Log10 VL | 1.50 (1.02–2.19) | .04 | 1.51 (.86–2.67) | .15 |
| ART at baseline | 0.5 (.14–1.67) | .25 | ||
| Extensive radiological disease | 0.61 (.20–1.85) | .39 | ||
| Cavitary disease | 0.62 (.24–1.62) | .33 |
The significance for bold values are P < .05. Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; INH, isoniazid; OR, odds ratio; TB,tuberculosis; VL, viral load.
aAt TB diagnosis (ie, baseline) unless otherwise specified as day of pharmacokinetics study or 2-month review.
bModel executed inclusive of 0- to 24-hour area under the curve/minimum inhibitory concentration for rifampicin, isoniazid, and pyrazinamide as variables.
cTested separately due to co-linearity.
Patients with rifampicin or pyrazinamide AUC0-24/MIC quartile in the second and third quartiles were more likely, on average, to achieve culture conversion at 2 months as shown in Supplementary Figure 1. Conversely, culture conversion rates were lowest in the second and third AUC0-24/MIC quartiles for isoniazid. Table 3 shows potential clinical predictors of conversion within the different AUC0-24/MIC quartiles for isoniazid, rifampicin, and pyrazinamide. A higher percentage of side effects was reported by patients with isoniazid AUC0-24/MIC in the highest quartile as previously reported [27]. No statistically significant interaction was found between isoniazid exposures, drug side effects, and the outcome of culture conversion. There was no association seen between pyrazinamide AUC0-24/MIC quartile and side effects.
Table 3.
Distribution of Independent Variables in Patients Within Different Quartiles of 0- to 24-Hour Area Under the Curve/Minimum Inhibitory Concentration (AUC0-24)
| Covariate | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value |
|---|---|---|---|---|---|
| Isoniazid | 0–116 | >116–189.5 | >189.5–355.8 | >355.8 | |
| Median age, y | 32.6 | 31.1 | 32.3 | 33.7 | .95 |
| % Re-treatment | 36 | 44 | 40 | 36 | .92 |
| % Side effects | 24 | 32 | 20 | 64 | .004 |
| % Extensive radiological disease | 64 | 88 | 64 | 68 | .19 |
| % Cavities | 44 | 60 | 44 | 60 | .46 |
| % Smear 3+ | 28 | 24 | 20 | 24 | .93 |
| Median TTD | 10 | 9 | 11 | 11 | .94 |
| % Poor adherence | 4 | 16 | 4 | 16 | .26 |
| Median log10 VL | 4.8 | 4.5 | 3.4 | 5.16 | .22 |
| Median log10 CD4 | 2.1 | 2.3 | 2.5 | 2.4 | .68 |
| Rifampicin | 0–184 | >184–299 | >299–560 | >560 | |
| Median age, y | 32.6 | 32.6 | 32.4 | 33.7 | .68 |
| % Re-treatment | 40 | 44 | 32 | 40 | .85 |
| % Side effects | 32 | 28 | 32 | 48 | .46 |
| % Extensive radiological disease | 64 | 60 | 84 | 76 | .22 |
| % Cavities | 44 | 48 | 60 | 56 | .66 |
| % Smear 3+ | 16 | 12 | 28 | 40 | .09 |
| Median TTD | 11 | 13 | 10 | 9 | .20 |
| % Poor adherence | 8 | 4 | 12 | 16 | .53 |
| Median log10 VL | 4.6 | 4.7 | 4.5 | 5.2 | .22 |
| Median log10 CD4 | 2.1 | 2.3 | 2.5 | 2.4 | .32 |
| Pyrazinamide | 0–10 | >10–13.7 | >13.7–19.8 | >19.8 | |
| Median age, y | 31.15 | 32.47 | 34.8 | 33.1 | .75 |
| % Re-treatment | 48 | 36 | 28 | 44 | .48 |
| % Side effects | 28 | 44 | 48 | 20 | .12 |
| % Extensive radiological disease | 72 | 88 | 68 | 56 | .09 |
| % Cavities | 40 | 68 | 60 | 40 | .11 |
| % Smear 3+ | 20 | 24 | 24 | 28 | .93 |
| Median TTD | 11 | 8 | 9 | 11 | .87 |
| % Poor adherence | 12 | 8 | 12 | 8 | .93 |
| Median log10 VL | 4.9 | 4.8 | 4.8 | 4.1 | .88 |
| Median log10 CD4 | 2.5 | 2.5 | 2.3 | 2.2 | .13 |
The significance for bold values are P < .05. Abbreviations: TTD, time to culture positivity at baseline; VL, viral load.
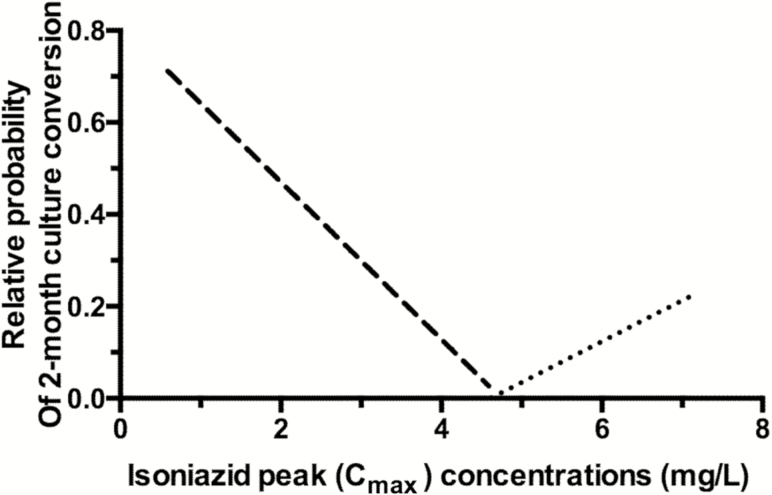
Next we used MARS, to identify and rank potential predictors of 2-month conversion. The findings are shown as BFs, and the meaning of each BF is explained in Table 4. The probability of culture conversion increased with an increase in isoniazid Cmax above 4.6 mg/L (n = 28) (as indicated by the positive coefficient of BF1). At an isoniazid Cmax of 4.6 mg/L, this effect was reversed, with the probability of culture conversion increasing as isoniazid Cmax decreased (as per positive coefficient of BF2). These are termed mirror BFs, the reason for which can be seen in Figure 3, where the BFs are characterized by a hinge on the value of Cmax 4.6 mg/L and show a V-shaped relationship of isoniazid Cmax vs the probability of culture conversion. BF6 shows the interaction on condition of an isoniazid Cmax <4.6 mg/L (n = 74) and rifampicin Cmax/MIC <28 (n = 12). Below the rifampicin Cmax/MIC ratio of 28, the probability of culture conversion decreased as rifampicin Cmax/MIC decreased from 28 to 0. This effect was modified by an interaction whereby the effect of increasing rifampicin Cmax/MIC was increased as isoniazid Cmax decreased from 4.6 to 0 mg/L. Hence, in this subset of patients, with isoniazid Cmax ≤4.6 mg/L and rifampicin Cmax/MIC <28 (n = 9), the antagonistic effect of isoniazid on culture conversion was counteracted by increasing rifampicin Cmax/MIC. The robustness of this finding was further verified via multivariate logistic regression analysis. Among patients with isoniazid Cmax ≤4.6 mg/L, isoniazid Cmax was associated with reduced culture conversion (adjusted OR, 0.35 for each 1 mg/L [95% CI, .15–.80]; P = .01) and patients with rifampicin Cmax/MIC >28 had adjusted odds of 6.44 (95% CI, 1.02–40.54; P = .04) for culture conversion at 2 months (Table 5).
Table 4.
Explanation of Basis Functions Identified in the Final Multivariate Adaptive Regression Splines Model
| Basis Function | Function | Coefficient in Model | Interpretation and No. of Patients Basis Function Applies to |
|---|---|---|---|
| BF0 | Constant/intercept | 0.652 | Baseline probability of sputum conversion |
| BF1 | max (0, INH Cmax – 4.6) | 0.24 | Where INH Cmax >4.6 mg/L (n = 26), the probability of culture conversion was increased as an additive effect (+0.24 * BF1). However, at or below 4.6 mg/L, the effect of BF1 became zero. |
| BF2 | max (0, 4.6 – INH Cmax) | 0.17 | The mirror image of BF1, basis function 2 (BF2), had a lower bound of 0 and was only retained when INH Cmax <4.6 mg/L (n = 74). As the value of INH Cmax decreased, the value of the function included increased as an additive effect (+0.17 * BF2). There were also some interactions with BF3, BF6 (ie, HIV-1 status and RIF Cmax/ MIC. |
| BF3 | max(subset = HIV infected) * BF2 | Nil | Basis function 3 was a dummy variable for HIV-infected patients and solely existed to interact with BF2 and only applied to HIV-1–infected patients with INH Cmax <4.6 mg/L (n = 46). |
| BF4 | max (0, CD4 – 190) * BF3 | –0.001 | BF4 retained its function only in HIV-infected patients with CD4 lymphocyte count >190 (n = 40). For these patients, probability of culture conversion was reduced by a small factor (–0.001 * BF4) as CD4+ lymphocyte count increased from 190 upwards. This function was modified by an interaction with BF2 where the effect size increased as INH Cmax decreased from 4.6 to 0 mg/L. |
| BF5 | max(subset = smear grade 3+) | –0.33 | In those with an initial sputum smear grade of 3+, the average probability of culture conversion was decreased (–0.33 * BF5) (n = 24). |
| BF6 | max (0, 28.00 – RIF Cmax /MIC) * BF2 | –0.016 | For patients with RIF Cmax/MIC <28 (n = 12), probability of culture conversion decreased as per negative coefficient (–0.016) as RIF Cmax/MIC decreased from 28 to 0 (–0.016 * BF6). This function was modified by an interaction with BF2, where the negative effect increased as INH Cmax increased from 0 to 4.6 mg/L. |
Abbreviations: BF, basis function; Cmax, maximum concentration; HIV, human immunodeficiency virus; INH, isoniazid; MIC, minimum inhibitory concentration; RIF, rifampicin.
Figure 3.
V-shaped relationship between 2-month sputum conversion and isoniazid maximum concentration (Cmax). The figure depicts the “mirror” basis function identified by multivariate adaptive regression splines with hinge at isoniazid Cmax of 4.6 mg/L such that for patients with concentration above the threshold have an increase in probability of sputum conversion. On the other hand, for patients below the same threshold, the probability for sputum conversion increased as isoniazid Cmax concentration decreased and has interactions with other factors (such as human immunodeficiency virus and rifampicin concentration).
Table 5.
Multivariate Logistic Regression Analysis in Subset of Patients With Isoniazid Maximum Concentration <4.6 mg/L
| Variable | Multivariate Analysisa Adjusted OR (95% CI) |
P Value |
|---|---|---|
| Isoniazid Cmax. | 0.35 (.15–.80) | .01 |
| Rifampicin Cmax/MIC ≤28 Cmax/MIC>28 |
Referent 6.44 (1.02–40.54) |
.04 |
The significance for bold values are P < .05. Abbreviations: CI, confidence interval; Cmax, maximum concentration; MIC, minimum inhibitory concentration; OR, odds ratio.
aAdjusted for all significant determinants of 2-month culture conversion in Table 2.
The optimized MARS model after stepwise elimination represented the probability of culture conversion by the equation:
Overall, the ROC for the selected model was 87% in the learn model and 66% on cross-validation, while the misclassification rates were 14% and 28%, respectively. These model performance figures are reassuring, suggesting that similar estimates could be expected in an independent sample of patients.
DISCUSSION
The MARS model identified a potential interaction of concentration-dependent antagonism between isoniazid and rifampicin affecting outcome at the 2-month time point. This finding of concentration-dependent antagonism at the lower concentration range of rifampicin and isoniazid in the current study are consistent with murine and hollow fiber preclinical model data and antagonism in sterilizing effect rates in patients.
Chigutsa et al [19] showed in an adult South African population that an increase in isoniazid Cmax was antagonistic when rifampicin AUC <35.4 mg × hour/L for rates of sterilizing effect based on TTD, which supported our finding of potential isoniazid antagonism in the MARS model below thresholds of rifampicin Cmax/MIC <28 and isoniazid Cmax <4.6 mg/L. Similarly, Swaminathan et al [34] showed in Indian children with pyrazinamide Cmax ≤38.10 and rifampin Cmax ≤6.20 mg/L, isoniazid AUC0–24 >31.80 mg × hour/L led to higher proportions of children with poor outcomes. Supplementary Figure 1 shows the percentage of culture conversion stratified by AUC0–24 /MIC quartile for isoniazid, rifampicin, and pyrazinamide. This is consistent with findings of Almeida et al, in a mouse model of tuberculosis that showed dose-dependent antagonistic response of isoniazid on rifampicin/pyrazinamide activity, measured by colony-forming units in mouse lung [35]. The antagonistic relationship was narrowed down to the dual combination of isoniazid and pyrazinamide, which are both structural analogues of nicotinamide [35]. Grosset et al found that discontinuation of isoniazid after the first 2 days improved bactericidal activity over days 3–14 of antituberculosis treatment in mice [36]. In the hollow fiber system, coadministration of isoniazid and rifampicin at both drugs’ highest Cmax/MIC was associated with inferior microbial kill compared to administration of rifampicin after a delay of 6 hours, 12 hours, and 24 hours (coinciding with progressive fall in isoniazid concentrations), hence consistent with concentration-dependent antagonism [37].
Although this data set does not lend itself to further in depth analysis of drug–drug antagonism and synergism, the finding of drug–drug antagonism at the lower range of isoniazid and rifampicin may be a contributory factor to treatment outcomes and must be studied in further clinical studies and simulation analyses which encompass further dosing ranges of both isoniazid and rifampicin. The efficacy of isoniazid beyond its initial early bactericidal activity, should be evaluated further in the context of randomized controlled studies with appropriate follow-up and long-term treatment outcomes. Further research questions include determination of efficacy and tolerability of increased isoniazid concentrations in patients with slow N-acetyltransferase 2 status and the potential for staggered dosing—for example, 12-hour difference in dosing time between rifampicin/pyrazinamide and isoniazid in light of potential drug–drug antagonism. Whilst the MIC distributions were representative of populations reported elsewhere [38, 39], our cohort had good long-term outcomes and culture conversion at 2 months was 77% in liquid culture. With only 3 treatment failures and 4 relapses, the study was underpowered to study clinical and PK/PD predictors of long-term treatment outcomes. However, we did find that a pyrazinamide Cmax <35 mg/L was predictive of unfavorable treatment outcome, consistent with findings from other groups [33, 34].
Despite significant variability of AUC/MIC for rifampicin, isoniazid, and pyrazinamide, the range of percentage of culture conversion over different AUC/MIC quartiles was limited: 64%–88% for rifampicin, 64%–84% for isoniazid, and 72%–88% for pyrazinamide. Logistic regression failed to identify a relationship between Cmax/MIC, AUC/MIC, or %TMIC and the probability of culture conversion. As an example, these would average out outcomes on either side of the “V-shaped” relationship we identified, so that measures of central tendency would not differ for the range of exposures. We also note clinical covariates, which may contribute to the PK/PD trends observed. For example, there was a nonsignificant trend for patients with rifampicin AUC0-24/MIC >75th percentile to have a baseline sputum smear grading of 3+, suggesting that confounding by severity of the pulmonary disease may explain reduced culture conversion at this top quartile of rifampicin exposure. Although increased side effects, perhaps via reduced adherence, contributed to decreased likelihood culture conversion, this would not explain the increase in culture conversion above a certain isoniazid threshold. The latter is likely to be secondary to reversal of isoniazid-rifampicin antagonism above isoniazid Cmax >4.6 mg/L.
This is the largest study to date reporting the effect of first-line antituberculosis drug exposures, measured by intensive sampling and inclusive of adjustment for MIC of infecting MTB strain, on the interim outcome of 2-month culture conversion in a predominantly HIV-1–coinfected cohort. There were several limitations to this study. Ethambutol exposures, which could have contributed both to the sterilizing activity of the quadruple drug regimen and also to drug–drug antagonism/synergism, were not measured. Multiple cultures were not sent during the first 2 months and, hence, time to culture conversion could not be ascertained. The binary outcome of culture conversion was via a single optimized volume induced sputum sample expectorated at week 7–8 of treatment. Lack of multiple cultures may have decreased sensitivity to determine culture conversion. However, in the context of baseline smear negative/scanty rates of 34%, the ascertained rates of conversion in MGIT cultures are unlikely to be overestimated and are comparable to populations with similar HIV-1 coinfection rates [31]. The calculated PK exposures in plasma do not necessarily equate to penetration in diseased tissue [15]. There may have been unmeasured confounders in this observational study and we may have underestimated interoccasional PK variability secondary to drug side effects and fluctuating adherence. Last, while machine-learning models are very good for generating precise hypotheses, the derived antagonistic interactions need to be tested in larger prospective studies with appropriate designs.
In summary, in this outpatient setting with a high prevalence of HIV-1/TB–coinfected patients, the majority had plasma drug exposures below accepted thresholds but nevertheless had good treatment outcomes. This was not explained by any measured clinical or programmatic factors, nor by adjusting for MIC of infecting MTB strain. We found concentration-dependent antagonism of isoniazid at the lower range of rifampicin affecting the interim outcome of 2-month culture conversion. Large studies with better biomarker models of disease response, detailed accounting for day-to-day PK variability, and further analyses evaluating the nonlinear effects of drugs in combination may further the evidence base for treatment monitoring using PK/PD measures.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. Design and conception of experiments: N. R., H. M., R. J. W., and G. M. Acquisition of clinical, MTB strain, and drug concentration data: N. R. MIC work: F. S. Compartmental PK analyses: P. D. and N. R. MARS analyses: J. P. and T. G. Analysis of data and interpretation: N. R. , H. M., J. P., T. G., M. L., P. D., R. J. W., and G. M. Writing of manuscript: N. R., H. M., J. P., T. G., R. J. W., and G. M. All authors approved the final version of the manuscript.
Acknowledgments. We acknowledge the patients who participated in the study, and the clinical and administrative staff of the Western Cape Department of Health. The Division of Clinical Pharmacology at the University of Cape Town would like to acknowledge Novartis Pharma for their support of the development of pharmacometrics skills in Africa.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings, and conclusions expressed in this manuscript reflect those of the authors alone.
Financial support. This work was supported by the Wellcome Trust (grant numbers WT104803 and WT084323 to R. J. W. and WT098316 to G. M.). R. J. W. is supported by the Francis Crick Institute ,which receives its core funding from Cancer Research UK (FC00110218), the UK Medical Research Council (FC00110218), and the Wellcome Trust (FC00110218); there was additional support from the European Union (grant number EU FP7 HEALTH-F3-2012–305578) to R. J. W., the National Research Foundation of South Africa (grant numbers 96841 to R. J. W., 64787 and 85858 to G. M., and 90729 to H. M.), and the South African Medical Research Council (grant number RFA SAMRC-RFA-CC: TB/HIV/AIDS-01-2014 to G. M.).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2015. Available at: http://www.who.int/tb/publications/global_report/gtbr2015_executive_summary.pdf?ua=1 Accessed 6 June 2016. [Google Scholar]
- 2. Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis 1986; 133:423–30. [DOI] [PubMed] [Google Scholar]
- 3. Phillips PP, Fielding K, Nunn AJ. An evaluation of culture results during treatment for tuberculosis as surrogate endpoints for treatment failure and relapse. PLoS One 2013; 8:e63840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallis RS, Wang C, Meyer D, Thomas N. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One 2013; 8:e71116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burman WJ. The hunt for the elusive surrogate marker of sterilizing activity in tuberculosis treatment. Am J Respir Crit Care Med 2003; 167:1299–301. [DOI] [PubMed] [Google Scholar]
- 6. Nimmo C, Lipman M, Phillips PP, McHugh T, Nunn A, Abubakar I. Shortening treatment of tuberculosis: lessons from fluoroquinolone trials. Lancet Infect Dis 2015; 15:141–3. [DOI] [PubMed] [Google Scholar]
- 7. Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 2009; 53:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 2007; 51:3781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 2007; 51:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2003; 47:2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kjellsson MC, Via LE, Goh A, et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother 2012; 56:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goutelle S, Bourguignon L, Maire PH, Van Guilder M, Conte JE, Jr, Jelliffe RW. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother 2009; 53:2974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 2011; 204:1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziglam HM, Baldwin DR, Daniels I, Andrew JM, Finch RG. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 2002; 50:1011–5. [DOI] [PubMed] [Google Scholar]
- 15. Prideaux B, Via LE, Zimmerman MD, et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 2015; 21:1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61suppl 1:S25–31. [DOI] [PubMed] [Google Scholar]
- 17. Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 2011; 55:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chigutsa E, Pasipanodya JG, Visser ME, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burhan E, Ruesen C, Ruslami R, et al. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 2013; 57:3614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang KC, Leung CC, Yew WW, et al. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur J Clin Microbiol Infect Dis 2008; 27:467–72. [DOI] [PubMed] [Google Scholar]
- 22. Park JS, Lee JY, Lee YJ, et al. Serum levels of anti-tuberculosis drugs and their effect on tuberculosis treatment outcome. Antimicrob Agents Chemother 2015; 60:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 1998; 113:1178–83. [DOI] [PubMed] [Google Scholar]
- 24. Heysell SK, Moore JL, Keller SJ, Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 2010; 16:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74:839–54. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization. Treatment of tuberculosis guidelines. 4th ed, 2010. Available at: http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1 Accessed 16 May 2016. [PubMed] [Google Scholar]
- 27. Rockwood N, Meintjes G, Chirehwa M, et al. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother 2016; 60:6050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woo J, Cheung W, Chan R, Chan HS, Cheng A, Chan K. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin Biochem 1996; 29:175–7. [DOI] [PubMed] [Google Scholar]
- 29. Wishart DS, Knox C, Guo AC, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 2006; 34(database issue):D668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 2007; 51:2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sloan DJ, Mwandumba HC, Garton NJ, et al. Pharmacodynamic modeling of bacillary elimination rates and detection of bacterial lipid bodies in sputum to predict and understand outcomes in treatment of pulmonary tuberculosis. Clin Infect Dis 2015; 61:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res 1995; 4:197–217. [DOI] [PubMed] [Google Scholar]
- 33. Chideya S, Winston CA, Peloquin CA, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 2009; 48:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swaminathan S, Pasipanodya JG, Ramachandran G, et al. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63(suppl 3):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almeida D, Nuermberger E, Tasneen R, et al. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother 2009; 53:4178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grosset J, Almeida D, Converse PJ, et al. Modeling early bactericidal activity in murine tuberculosis provides insights into the activity of isoniazid and pyrazinamide. Proc Natl Acad Sci U S A 2012; 109:15001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srivastava S, Sherman C, Meek C, Leff R, Gumbo T. Pharmacokinetic mismatch does not lead to emergence of isoniazid- or rifampin-resistant Mycobacterium tuberculosis but to better antimicrobial effect: a new paradigm for antituberculosis drug scheduling. Antimicrob Agents Chemother 2011; 55:5085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suo J, Chang CE, Lin TP, Heifets LB. Minimal inhibitory concentrations of isoniazid, rifampin, ethambutol, and streptomycin against Mycobacterium tuberculosis strains isolated before treatment of patients in Taiwan. Am Rev Respir Dis 1988; 138:999–1001. [DOI] [PubMed] [Google Scholar]
- 39. Schön T, Juréen P, Giske CG, et al. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 2009; 64:786–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.