Summary
Human immunodeficiency virus–infected Zambian adults had reduced liver stiffness after initiation of tenofovir-containing antiretroviral therapy, regardless of hepatitis B virus (HBV) coinfection. Despite good early virological and serological response to therapy, HBV coinfected patients had increased odds of significant fibrosis/cirrhosis at follow-up.
Keywords: Africa, HIV/AIDS, liver fibrosis, hepatitis B virus, transient elastography.
Abstract
Background.
We investigated changes in hepatic fibrosis, based on transient elastography (TE), among human immunodeficiency virus (HIV)–infected patients with and without hepatitis B virus (HBV) coinfection on antiretroviral therapy (ART) in Zambia.
Methods.
Patients’ liver stiffness measurements (LSM; kiloPascals [kPa]) at ART initiation were categorized as no or minimal fibrosis (equivalent to Metavir F0–F1), significant fibrosis (F2–F3), and cirrhosis (F4). TE was repeated following 1 year of ART. Stratified by HBV coinfection status (hepatitis B surface antigen positive at baseline), we described LSM change and the proportion with an increase/decrease in fibrosis category. Using multivariable logistic regression, we assessed correlates of significant fibrosis/cirrhosis at 1 year on ART.
Results.
Among 463 patients analyzed (61 with HBV coinfection), median age was 35 years, 53.7% were women, and median baseline CD4+ count was 240 cells/mm3. Nearly all (97.6%) patients received tenofovir disoproxil fumarate–containing ART, in line with nationally recommended first-line treatment. The median LSM change was −0.70 kPa (95% confidence interval, −3.0 to +1.7) and was similar with and without HBV coinfection. Significant fibrosis/cirrhosis decreased in frequency from 14.0% to 6.7% (P < .001). Increased age, male sex, and HBV coinfection predicted significant fibrosis/cirrhosis at 1 year (all P < .05).
Conclusion.
The percentage of HIV-infected Zambian adults with elevated liver stiffness suggestive of significant fibrosis/cirrhosis decreased following ART initiation—regardless of HBV status. This suggests that HIV infection plays a role in liver inflammation. HBV-coinfected patients were more likely to have significant fibrosis/cirrhosis at 1 year on ART.
Clinical Trials Registration.
Among human immunodeficiency virus (HIV)–infected individuals, liver-related mortality is primarily attributed to viral hepatitis coinfection [1]. However, HIV infection also induces hepatic inflammation and immune activation, and unsuppressed HIV infection is a risk factor for liver fibrosis progression [2–5]. The World Health Organization (WHO) and most national guidelines recommend that HIV–hepatitis B virus (HBV)–coinfected individuals receive an antiretroviral therapy (ART) regimen that contains tenofovir disoproxil fumarate (TDF) because the drug is highly potent against both viruses [6, 7]. Among HIV-negative individuals with chronic HBV infection (CHB), TDF not only suppressed HBV replication but also improved liver histology [8]. Among TDF-treated CHB patients in China, a biphasic pattern of fibrosis regression was described, reflected in both liver histology and transient elastography (TE), a noninvasive surrogate of fibrosis/cirrhosis [9].
We sought to describe the impact of HBV-active ART on liver stiffness in a prospective HIV cohort study in Zambia, including a subset of individuals with HIV–HBV coinfection. We hypothesized that liver stiffness, based on TE, would decrease in the year following ART initiation, particularly among those with HIV–HBV coinfection. Among HIV–HBV patients, we also aimed to establish the short-term HBV virological and serological outcomes of TDF-containing ART.
METHODS
IeDEA Hepatitis Cohort in Zambia
We established a prospective cohort of HIV-infected adults at 2 public sector outpatient primary care facilities in Zambia’s capital Lusaka under the framework of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) collaboration in southern Africa [10]. Any HIV-infected patient aged 18+ years, treatment-naive, and eligible to initiate ART under national guidelines met the criteria to join the cohort [11]. The preferred first-line ART regimen consisted of the fixed-dose combination TDF, emtricitabine (FTC), and efavirenz (EFV), with abacavir, lamivudine, nevirapine (NVP) and ritonavir-boosted lopinavir as alternative agents. Baseline was defined as the time of ART initiation, and cohort characteristics at baseline were previously described [12, 13]. The University of Zambia (Lusaka) and University of North Carolina at Chapel Hill ethics committees approved this study.
Study Procedures and Definitions
At baseline, we measured alanine aminotransferase (ALT; normal was <19 U/L for women and <30 for men), creatinine, and CD4+ count; tested for HBV and hepatitis C virus (HCV) coinfection; assessed alcohol consumption levels; and performed TE. HBV coinfection was defined as a positive hepatitis B surface antigen (HBsAg) test by finger prick (Determine, Alere, Waltham, MA) or in serum (Access2Analyzer, Beckman Coulter, Brea, CA); among those who were HBsAg positive, we measured serum hepatitis B e-antigen and HBV viral load (VL; COBAS AmpliPrep/COBAS Taqman HBV test, version 2.0, Roche Molecular Systems, Pleasanton, CA). We used Sanger sequencing to determine HBV genotypes [14] and screened HIV–HBV patients for hepatitis delta antibodies (ETI-AB-Delta-2, Diasorin, Brussels, Belgium). We used a rapid point-of-care antibody test for HCV testing (OraQuick, OraSure Technologies, Bethlehem, PA) and confirmed active infections by measuring HCV RNA in antibody positives [12]. We used the Alcohol Use Disorders Identification Test–Consumption (AUDIT–C) questions to establish alcohol consumption levels and defined hazardous drinking as AUDIT–C score ≥3 for women and ≥4 for men [15–17]. We measured liver stiffness (in kiloPascals) using TE (Fibroscan 402, Echosens, Paris, France) in nonpregnant patients according to the manufacturer’s guidelines [18, 19]. Fasting was not required before TE; however, study visits occurred in the mid-morning or early afternoon, and most patients had waited 1–4 hours without eating before the procedure. TE measurements were considered very reliable if the interquartile range divided by median (IQR/M) was <0.1, reliable if IQR/M was 0.1–0.3, and poorly reliable if IQR/M was >0.3 [20].
Follow-up visits for routine ART monitoring occurred at 2, 4, 8, and 12 weeks and every 3 months thereafter. At 1 year on ART, we repeated baseline measurements with the exception of HCV testing and, in addition, assessed ART adherence using the medication possession ratio (MPR) [21]. MPR was categorized as optimal (>95%), suboptimal (80%–95%), and poor (<80%). During follow-up, deaths were ascertained by report of the patient’s family/friend, the clinic staff, or a community health worker. Transfers to other clinics were documented when a patient informed the staff of their intent to establish HIV care at a distant site. We considered a patient lost to follow-up (LTFU) if there were no documented clinical or pharmacy visits for at least 6 months and no 1-year visit.
Study Outcomes
Our primary outcome was change in hepatic stiffness based on TE. We categorized each liver stiffness measurement (LSM) according to its equivalent Metavir liver fibrosis stage as no/minimal fibrosis (F0–F1), significant fibrosis (F2–F3), and cirrhosis (F4). This was done using thresholds that were previously established in published comparisons between TE and liver histology. For patients with only HIV, no/minimal fibrosis was LSM <7.1 kPa, significant fibrosis was 7.1–11.0 kPa, and cirrhosis was ≥11.1 kPa [22]. Among HIV–HBV patients, no/minimal fibrosis was <5.9 kPa, significant fibrosis was 5.9–9.3 kPa, and cirrhosis was ≥9.4 [23]. In HIV–HBV patients, we defined HBV virological suppression (VS) as a VL <20 IU/mL. In mid-2014, Zambian national treatment guidelines incorporated routine HIV VL monitoring; therefore, a subset of the cohort participants also had an HIV VL at 1 year on ART (Roche COBAS AmpliPrep/COBAS Taqman HIV-1 test, version 2.0, Pleasanton, California). HIV VS was defined as a VL <40 copies/mL.
Statistical Analyses
We excluded from analysis patients without TE at baseline. Among those with baseline TE, we further excluded those who died, transferred out, withdrew, became pregnant, or were LTFU, as well as those without a repeat TE at 1 year. We compared the demographic and clinical characteristics of participants analyzed at the primary outcome with those excluded using the Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical ones. In the analysis cohort, we compared baseline characteristics of patients with only HIV and those with HIV–HBV using the same tests. We graphed the distribution of fibrosis stages at baseline and 1 year and we analyzed the proportion who experienced a reduction in fibrosis using a McNemar test. In bivariable analyses, we considered the following potential correlates of liver fibrosis: age, sex, WHO stage, initial CD4+ count, body mass index, baseline ALT level, HBsAg positivity, HIV VS at 1 year, and hazardous alcohol consumption at 1 year. A stepwise logistic regression model was used to identify factors associated with significant fibrosis or cirrhosis (F2–F4) at 1 year with backward selection algorithm. The probability of removal was set at 0.2 using likelihood ratio test. As HIV VS was an important possible risk factor, we maintained that variable in the final model.
Among the subset with HIV–HBV coinfection, we described the efficacy of ART to suppress HBV replication as the median log10 change in HBV VL and the percentage of coinfected individuals with VS at 1 year. In bivariable analyses, we explored possible correlates of HBV VS including pretreatment HBV VL, HBeAg positivity, MPR, and HIV VS.
RESULTS
Cohort Characteristics
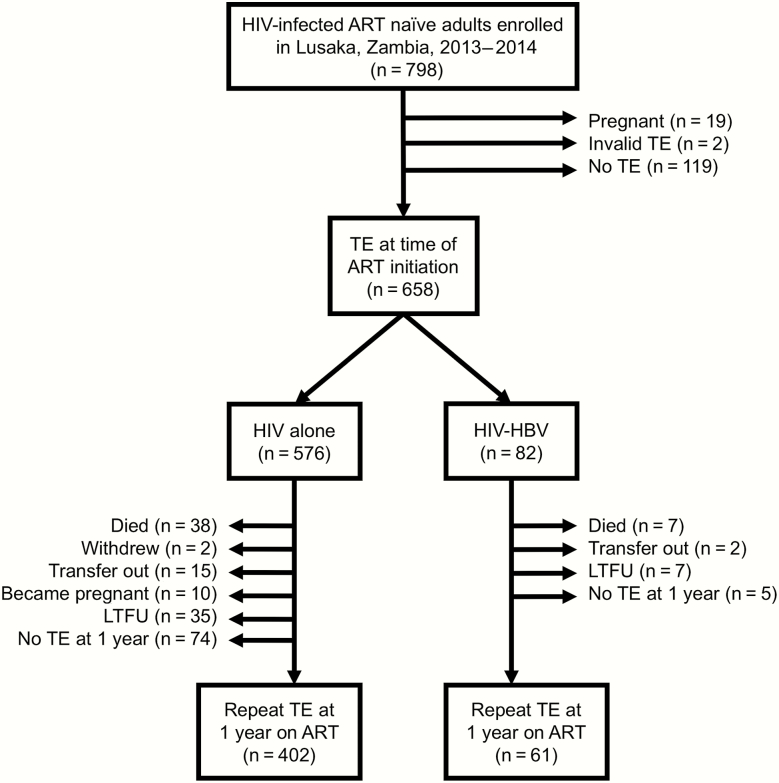
From October 2013 to August 2014, 798 eligible HIV-infected adults were enrolled in our cohort. Of these, 45 (5.6%) died, 19 (2.4%) withdrew or transferred to another facility, and 38 (4.8%) were LTFU during the first year on ART. In addition, 29 women were pregnant at either baseline or 1-year visits and could not undergo TE. Of the remaining 667 retained to the 1-year visit, 463 (69.4%) had valid TE at both time points and comprised the analysis cohort (Figure 1). Patients without TE (n = 204) were similar to those analyzed in terms of their median age (35 vs 35 years; P = .96) and percentage women (53.7% vs 53.8%; P = .98) but had slightly lower baseline CD4 counts (204 vs 240 cells/mm3; P = .03) and higher prevalence of WHO stage 3 or 4 (47.4% vs 36.6%; P = .01).
Figure 1.
Cohort flow diagram. Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; HIV, human immunodeficiency virus; LTFU, lost to follow-up; TE, transient elastography.
Within the analysis cohort (n = 463), 61 (13.2%) had HBV and none had active HCV coinfection (Table 1). HIV–HBV-coinfected individuals were more likely to have hazardous alcohol consumption and had higher baseline ALT levels and LSM compared to those with only HIV (Table 1). Nearly all patients (97.6%) received fixed-dose combination TDF, FTC, and EFV. MPR (ie, ART adherence) at 1 year was optimal for 404 (89.8%), suboptimal for 34 (7.6%), and poor for 12 (2.7%). CD4+ counts increased by a median of 106 cells/mm3 (IQR, 33–199) from baseline to 1 year. HIV VL was available at 1 year for 301 (65.0%) patients, and 253 (81.6%) were suppressed. HIV VS was observed in 84.1% of patients with optimal 1-year MPR, 66.7% with suboptimal, and 25.0% with poor adherence.
Table 1.
Baseline Characteristics of Human Immunodeficiency Virus–Infected Individuals Retained in Care for 1 Year on Antiretroviral Therapy in Lusaka, Zambia, by Hepatitis B Virus Coinfection Status
| Characteristic | HIV Alone (n = 402) |
HIV-HBV Coinfected (n = 61) |
P Value |
|---|---|---|---|
| Median age, y (IQR) | 35 (30–41) | 35 (29–39) | .57 |
| Female sex, n (%) | 221 (55.0) | 28 (45.9) | .18 |
| Body mass index, n (%) | |||
| <18.5 | 44 (11.1) | 9 (14.8) | .26 |
| 18.5–25 | 237 (59.6) | 40 (65.6) | |
| >25 | 117 (29.4) | 12 (19.7) | |
| World Health Organization clinical stage 3 or 4, n (%) | 191 (47.8) | 27 (45.0) | .69 |
| Tuberculosis, n (%) | 69 (17.2) | 9 (14.8) | .64 |
| Hazardous alcohol consumption, n (%) | 155 (39.0) | 33 (55.0) | .02 |
| Hepatitis C virus coinfection, n (%) | 0 | 0 | NA |
| Median CD4+ count (IQR) | 244 (130–338) | 235 (111–382) | .97 |
| Median ALT, U/L (IQR) | 18 (13–25) | 23 (13–40) | .02 |
| Elevated ALT, n (%) | 63 (19.6) | 18 (30.5) | .01 |
| Median liver stiffness measurement, kPa (IQR) | 5.0 (4.4–6.1) | 5.5 (4.4–6.8) | .04 |
| HBV viral load (IU/mL), n (%) | |||
| <20 | 12 (20.0) | ||
| 20–20000 | — | 21 (34.4) | — |
| >20000 | 27 (44.3) | ||
| HBV genotype, n (%) | — | — | |
| A (A1) | 17 (43.6) | ||
| E | 22 (56.4) | ||
| Hepatitis B e antigen reactive, n (%) | — | 18 (36.0) | — |
| Hepatitis delta virus antibody positive, n (%) | — | 2 (3.3) | — |
Abbreviations: ALT; alanine aminotransferase; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range.
Change in Liver Stiffness
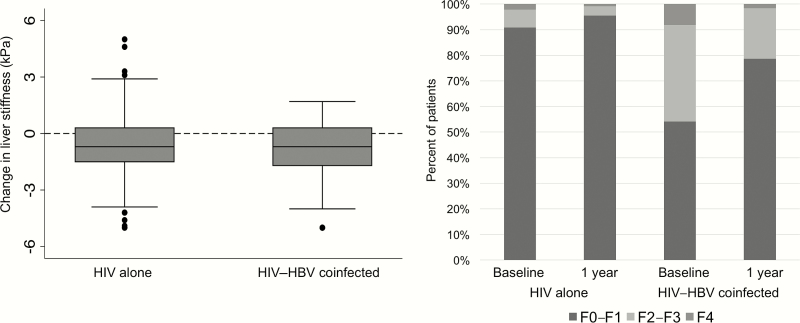
The median time between baseline and 1-year TE was 11.7 months (95% confidence interval, 11.1–12.6). Most TE measurements were either very reliable (n = 450; 48.6%) or reliable (n = 469; 50.6%) and very few were poorly reliable (n = 7; 0.8%). The median change in LSM was –0.70 kPa (IQR, –1.50 to +0.30) and was similar between patients with and without HBV coinfection (–0.7 vs –0.7 kPa; Figure 2). There was a significant reduction in the proportion with TE suggestive of significant fibrosis (14.0% vs 6.7%; P < .001) or cirrhosis (2.2% vs 1.1%; P = .01). Among patients with only HIV, the finding of significant fibrosis decreased from 9.2% to 4.5% (P = .002) while the finding of cirrhosis remained consistent (1.2% vs 1.0%; P = .10). For HIV–HBV-coinfected patients, a larger absolute reduction was observed for both significant fibrosis (45.9% vs 21.3%; P = <.001) and cirrhosis (8.2% vs 1.6%; P = .04). Among the 65 participants with F2–F4 at baseline, 46 (70.8%) experienced a reduction of liver fibrosis by 1 year on ART (Supplementary Table 1). Those with only HIV appeared more likely to experience a reduction compared to HIV–HBV-coinfected patients, but this association was not statistically significant (78.4% vs 60.7%; P = .12). Other patient factors including age, sex, CD4+ count, baseline ALT, hazardous drinking at 1 year, and HIV VS were not associated with a change in fibrosis (Supplementary Table 2), although the numbers in each group were small.
Figure 2.
Change in liver stiffness during the initial year of antiretroviral therapy among human immunodeficiency virus–infected adults in Zambia, by hepatitis B virus coinfection status. Abbreviations: HBV, hepatitis B virus; HIV, human immunodeficiency virus.
Correlates of Significant Fibrosis/Cirrhosis at 1 Year on ART
In multivariable analysis, men (adjusted odds ratio [AOR], 3.13; 95% CI, 1.10–8.93) and patients with HBV coinfection (AOR, 7.72; 95% CI, 2.89–20.57; Table 2) were more likely to have significant fibrosis/cirrhosis. With each 10-year increase in age there was an increased odds of the outcome (AOR, 1.81; 95% CI, 1.07–3.07). HIV VS by 1 year was not associated with reduced odds of significant fibrosis/cirrhosis (AOR, 0.89; 95% CI, 0.26–3.04). Neither HBV VL nor HBV genotype (A1 vs E) were associated with 1-year LSM among coinfected patients. During follow-up there were 12 patients (2.6%; 10 with only HIV alone and 2 with HIV–HBV) who experienced progression to a higher disease category.
Table 2.
Factors Associated With Significant Hepatic Fibrosis or Cirrhosis After 1 Year on Antiretroviral Therapy
| Factor | Crude OR (95% CI) |
Adjusted OR (95% CI) |
|---|---|---|
| Age, per 10-year increase | 1.34 (0.92–1.96) | 1.81 (1.07–3.07) |
| Sex | ||
| Female | Reference | Reference |
| Male | 3.61 (1.58–8.26) | 3.13 (1.10–8.93) |
| World Health Organization clinical stage | ||
| 1 or 2 | Reference | |
| 3 or 4 | 1.59 (0.76–3.33) | |
| Body mass index | ||
| <18.5 | Reference | |
| 18.5–25 | 2.10 (0.48–9.24) | |
| 25+ | 1.69 (0.34–8.22) | |
| Hepatitis B virus coinfection | 5.76 (2.66–12.50) | 7.72 (2.89–20.57) |
| Tuberculosis | 0.99 (0.39–2.49) | |
| Hazardous alcohol consumption at 1 year | 1.75 (0.83–3.68) | |
| CD4+ count at baseline, per 50 cells/mm3 increase | 1.03 (0.92–1.16) | |
| Human immunodeficiency virus viral load <40 copies/ mL at 1 year | 1.09 (0.35–3.37) | 0.89 (0.26–3.04) |
Abbreviations: CI, confidence interval; OR, odds ratio.
HBV Virological and Serological Outcomes
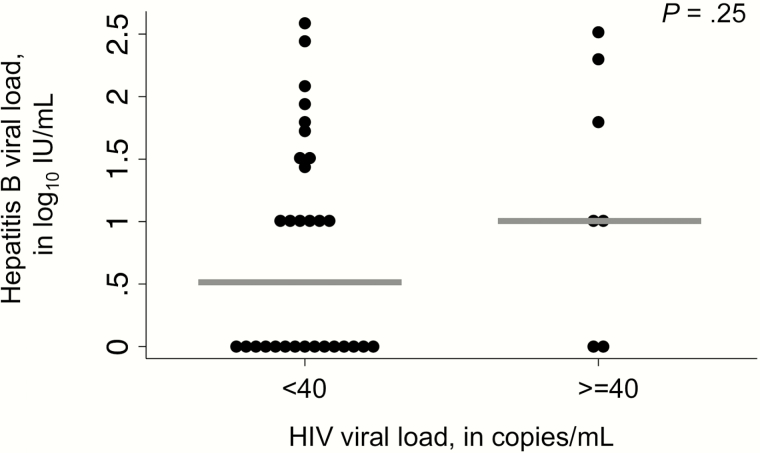
HIV–HBV patients also achieved significant virological control of HBV during the first year of ART. Among the 61 coinfected individuals, baseline HBV VL was available for all and 1-year VL was available for 51 (83.6%). Median baseline HBV VL was 3.7 log10 IU/mL and 27 (44.3%) had HBV VL >4.3 log10 IU/mL (20000 IU/mL; Table 1). HBV genotypes were A1 for 17 and E for 22 among the successfully sequenced samples. Nineteen were HBeAg positive, 34 were HBeAg negative, and the HBeAg status was not determined in 8 patients. Hepatitis delta antibodies were observed in 2 individuals. Following 1 year of ART, HIV–HBV-coinfected patients with baseline detectable HBV DNA experienced a median reduction in HBV VL of 4.8 log10 IU/mL and 33 (64.7%) achieved HBV VS (Supplementary Figure 1). HBV VS at 1 year was more common among patients with optimal adherence compared to those with suboptimal/poor adherence (76.5% vs 11.8%; P = .08). HBeAg-positive patients were less likely to have HBV VS (31.3% vs 79.3%; P = .001). There was a trend toward lower HBV VL in patients with HIV VS compared to those with detectable HIV RNA at 1 year (1.0 vs 0.5 log10 IU/mL; Figure 3), but this did not reach statistical significance (P = .25). Among those who were HBeAg positive, 1 of the 14 retested (7.1%) experienced an HBeAg loss. Of 58 patients with an available HBsAg at baseline and 1 year, 7 (12.1%) became HBsAg negative. Although the absolute numbers were small, patients with HBsAg loss tended to be female (71.4% vs 43.1%) and slightly younger (median age 28.4 vs 36.4 years) compared to those who remained HBsAg positive.
Figure 3.
Association between human immunodeficiency virus (HIV) and hepatitis B virus (HBV) viral suppression at 1 year on antiretroviral therapy among HIV–HBV-coinfected adults. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
During the first year of ART in Zambia, the percentage of HIV-infected Zambian adults with elevated liver stiffness suggestive of significant fibrosis/cirrhosis decreased following ART initiation—regardless of HBV status. This suggests that HIV infection plays a role in liver inflammation. HIV–HBV patients had a good early viral response to ART; however, this group continued to have higher levels of liver fibrosis markers at 1 year on therapy. These data support recommendations for HBsAg testing, early ART initiation, and close monitoring of liver disease in this at-risk group.
Our results provide further evidence of the benefits of effective ART on virological and clinical outcomes in HIV and HIV–HBV coinfection, especially regimens that contain potent HBV activity such as that provided by TDF. In line with previous reports from Europe and Africa, we observed that ART was associated with reductions in liver fibrosis markers in those with only HIV and HIV–HBV coinfection. Improvement in liver fibrosis was also reported in patients with CHB [8] and HIV–HBV coinfection [25] who received TDF. Among 348 CHB patients in Europe, 176 (51%) had histological regression of fibrosis over 5 years of TDF [8]. In Ghana, over 3 years of TDF-containing ART, the proportion of HIV monoinfected (30.8% to 21.8%) and HIV–HBV-coinfected individuals (56.4% to 38.0%) with elevated LSM reduced significantly [3].
At 1 year, we observed that increasing age, male sex, and HBV coinfection were associated with persistently elevated LSM suggestive of significant fibrosis/cirrhosis. This is consistent with other studies in the published literature. Increasing age and male sex have been shown to be potential cofactors for liver fibrosis progression [25, 26]. We did not find an association between hazardous drinking and TE at 1 year; however, quantifying alcohol consumption by self-report alone can be difficult and is subject to underreporting [27]. Similar to our findings, 44 of 76 (57.9%) HIV–HBV-coinfected Ghanaians had persistent elevations in LSM (>5.9 kPa) after switching from non-TDF to TDF-containing ART [24]. This may reflect that these patients have more severe liver disease before ART, incomplete HBV VS (as was observed in our study), or that TE measurements may be elevated by inflammation as well as fibrosis. We suspect that with additional follow-up, LSM will continue to improve in the setting of ART-treated HIV–HBV, as shown for CHB patients in China [9].
We also generated data on the short-term effectiveness of TDF-containing ART for HIV–HBV. Our finding that two-thirds of patients achieved HBV VS by 1 year is similar to findings from another study in the region and 1 in the United States [28, 29]. Longer follow-up of HBV VL may be needed to document full HBV VS. In a multicenter cohort of 397 HIV–HBV patients in the United States, for example, the median time to HBV VS was 28 months (IQR, 13–71) [30]. In the Netherlands, the proportion of HBV-infected patients suppressed on ART increased over time from 31% to 70% for those who were HBeAg positives and from 47% to 85% for those who were HBeAg negative [31]. In Zambia, we also demonstrated an association between drug adherence and HBV VS, which is important not only in HIV–HBV coinfection but also in CHB. Although it was not observed in our study, HIV VS, another marker of adherence, predicted HBV VS among 133 HIV–HBV patients on TDF-containing ART in the United States [29]. The rate of HBsAg loss (12.1%) we observed was higher than what has been listed in many reports from HIV–HBV-coinfected and CHB patients in high-income countries [32–34] but similar to the 17.6% reported by Hamers et al [29] and lower than the 36% observed by Anderson et al [35]. Our rate of HBsAg loss may be slightly overestimated due to our use of a rapid test on whole blood that has lower sensitivity than serum assays [36].
Our study is one of the first in Africa to longitudinally assess hepatic stiffness using TE in HIV-infected individuals receiving ART and supports the general value of ART for liver health. These data also provide evidence in support of WHO recommendations for HBV-active ART for HIV–HBV-coinfected patients. Our cohort had robust data on coinfections and comorbidities that may be liver disease cofactors. Because the study was nested within a public ART treatment program, we believe these data are likely to be representative of many HIV-infected and HIV–HBV-coinfected individuals in the region.
The main limitation of our study was incomplete serial TE data among approximately 30% of patients. Those with available data tended to be healthier and, as a consequence, we may have underestimated the prevalence of liver fibrosis in all patients starting ART. It is less likely that the analysis of risk factors for significant hepatic fibrosis or cirrhosis was affected by the missing data. Another limitation was our reliance on TE, which is a good but imperfect surrogate measure of liver fibrosis, and unlike newer versions, our TE device was not designed to assess steatosis. Time between TE measurements was only 1 year and, to some extent, observed changes may have reflected hepatic inflammation as well as fibrosis. Long-term follow-up is needed and will be pursued within our cohort. Additionally, more complete HIV VL data would have strengthened our ability to comment on HIV’s impact on liver inflammation and immune activation. We characterized a relatively small (n = 61) number of HIV-HBV patients, a key population at risk for cirrhosis and hepatocellular carcinoma (HCC) and acknowledge that more in-depth analysis of this group is warranted, including biannual screening for HCC. We also used a single positive HBsAg test at ART initiation to define HIV-HBV coinfection. Therefore, some patients with HBsAg loss at 1 year could have had acute HBV infection that did not progress to chronic infection. We think this is unlikely as only 1 of 10 patients with HBsAg loss had an elevated ALT at baseline consistent with acute infection.
In summary, initiation of TDF-containing ART was associated with reduced liver fibrosis/cirrhosis in patients with HIV alone and HIV-HBV coinfection. Although the majority experienced HBV VS and reduction in LSM, many HIV–HBV-coinfected patients had significant fibrosis/cirrhosis at 1 year on ART, suggesting the need for further follow-up and monitoring of this at-risk group.
Supplementary data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (U01 AI069924). In addition, M. J. V. was supported by the Fogarty International Center (K01 TW009998), B. H. C. was supported by NIAID (K24 AI120796), and G. W. was supported by an Ambizione-PROSPER fellowship from the Swiss National Science Foundation (PZ00P3_154730) to carry out this work.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Data Collection on Adverse Events of Anti-HIV Drugs Study Group. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med 2006; 166:1632. [DOI] [PubMed] [Google Scholar]
- 2. Redd AD, Wendel SK, Grabowski MK, et al. Liver stiffness is associated with monocyte activation in HIV-infected Ugandans without viral hepatitis. AIDS Res Hum Retroviruses 2013; 29:1026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grant J, Agbaji O, Muhammad M, et al. Long-term changes in liver fibrosis in HIV and HIV/HBV infected Nigerians on ART. Boston, MA: Conference on Retroviruses and Opportunistic Infections, IAS-USA, 2016. [Google Scholar]
- 4. Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010; 52:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HN, Nance R, Van Rompaey S, et al. Poorly controlled HIV infection: an independent risk factor for liver fibrosis. J Acquir Immune Defic Syndr 2016; 72:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO, 2013. [PubMed] [Google Scholar]
- 7. World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: WHO, 2015. [PubMed] [Google Scholar]
- 8. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381:468–75. [DOI] [PubMed] [Google Scholar]
- 9. Sun J, Xie Q, Tan D, et al. Five-year on-treatment systematically monitoring of dynamic changes of liver stiffness measurement with transient elastography compared with paired liver biopsies in chronic hepatitis B patients. Barcelona, Spain: International Liver Congress, EASL, 2016. [Google Scholar]
- 10. Egger M, Ekouevi DK, Williams C, et al. Cohort profile: the International Epidemiological Databases to Evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zambian Ministry of Health. Zambia consolidated guidelines for the treatment and prevention of HIV infection. Lusaka, Zambia:Zambian Ministry of Health; 2014. [Google Scholar]
- 12. Wandeler G, Mulenga L, Hobbins M, et al. Absence of active hepatitis C virus infection in human immunodeficiency virus clinics in Zambia and Mozambique. Open Forum Infect Dis 2016; 3:ofw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wandeler G, Musukuma K, Zürcher S, et al. ; IeDEA-Southern Africa Hepatitis B infection, viral load and resistance in HIV-infected patients in Mozambique and Zambia. PLoS One 2016; 11:e0152043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinikoor MJ, Zürcher S, Musukuma K, et al. Hepatitis B viral load in dried blood spots: a validation study in Zambia. J Clin Virol 2015; 72:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). alcohol use disorders identification test. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 16. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31:1208–17. [DOI] [PubMed] [Google Scholar]
- 17. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 18. Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–13. [DOI] [PubMed] [Google Scholar]
- 19. Vinikoor MJ, Mulenga L, Siyunda A, et al. ; International Epidemiologic Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) Association between hepatitis B co-infection and elevated liver stiffness among HIV-infected adults in Lusaka, Zambia. Trop Med Int Health 2016; 21:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boursier J, Zarski JP, de Ledinghen V, et al. ; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013; 57:1182–91. [DOI] [PubMed] [Google Scholar]
- 21. Vinikoor MJ, Schuttner L, Moyo C, et al. Short communication: late refills during the first year of antiretroviral therapy predict mortality and program failure among HIV-infected adults in urban Zambia. AIDS Res Hum Retroviruses 2014; 30:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morse CG, McLaughlin M, Proschan M, et al. Transient elastography for the detection of hepatic fibrosis in HIV-monoinfected adults with elevated aminotransferases on antiretroviral therapy. AIDS 2015; 29:2297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011; 18:61–9. [DOI] [PubMed] [Google Scholar]
- 24. Stockdale AJ, Phillips RO, Beloukas A, et al. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus (HBV) co-infection in Ghana. Clin Infect Dis 2015; 61:883–91. [DOI] [PubMed] [Google Scholar]
- 25. Poynard T, Mathurin P, Lai CL, et al. ; PANFIBROSIS Group A comparison of fibrosis progression in chronic liver diseases. J Hepatol 2003; 38:257–65. [DOI] [PubMed] [Google Scholar]
- 26. Bissell DM. Sex and hepatic fibrosis. Hepatology 1999; 29:988–9. [DOI] [PubMed] [Google Scholar]
- 27. Hahn JA, Fatch R, Wanyenze RK, et al. Decreases in self-reported alcohol consumption following HIV counseling and testing at Mulago Hospital, Kampala, Uganda. BMC Infect Dis 2014; 14:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamers RL, Zaaijer HL, Wallis CL, et al. ; PharmAccess African Studies to Evaluate Resistance HIV-HBV coinfection in southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. J Acquir Immune Defic Syndr 2013; 64:174–82. [DOI] [PubMed] [Google Scholar]
- 29. Hafkin JS, Osborn MK, Localio AR, et al. Incidence and risk factors for incomplete HBV DNA suppression in HIV/HBV-co-infected patients initiating tenofovir-based therapy. J Viral Hepat 2014; 21:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim HN, Rodriguez CV, Van Rompaey S, et al. ; Centers for AIDS Research Network of Integrated Clinical Systems Factors associated with delayed hepatitis B viral suppression on tenofovir among patients coinfected with HBV-HIV in the CNICS cohort. J Acquir Immune Defic Syndr 2014; 66:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology 2010; 139:1934–41. [DOI] [PubMed] [Google Scholar]
- 32. Boyd A, Maylin S, Gozlan J, et al. Use of hepatitis B surface and “e” antigen quantification during extensive treatment with tenofovir in patients co-infected with HIV-HBV. Liver Int 2015; 35:795–804. [DOI] [PubMed] [Google Scholar]
- 33. Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, et al. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J Infect Dis 2012; 206:974–80. [DOI] [PubMed] [Google Scholar]
- 34. Wursthorn K, Jung M, Riva A, et al. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology 2010; 52:1611–20. [DOI] [PubMed] [Google Scholar]
- 35. Anderson M, Gaseitsiwe S, Moyo S, et al. Slow CD4(+) T-cell recovery in human immunodeficiency virus/hepatitis B virus-coinfected patients initiating truvada-based combination antiretroviral therapy in Botswana. Open Forum Infect Dis 2016; 3:ofw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Njai HF, Shimakawa Y, Sanneh B, et al. Validation of rapid point-of-care (POC) tests for detection of hepatitis B surface antigen in field and laboratory settings in the Gambia, Western Africa. J Clin Microbiol 2015; 53:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.