Abstract
The present study investigated the use of waste non-edible oil cakes (Jatropha, Karanja, Neem, and Mahua) as a substrate for the growth of Paecilomyces variotii and dipicolinic acid (DPA) production. Previous researches proved the efficacy of DPA in suppressing certain pathogens that are deleterious to the plants in the rhizosphere. DPA production was statistical optimized by amending non-edible oil cakes in growing media as nitrogen and sugars (Dextrose, Glucose, and Lactose) as carbon source. Plackett-Burman design (PBD), indicated that Jatropha cake, Karanja cake, and Dextrose were the most significant components (p < 0.05) of the media and were further optimized using response surface methodology (RSM). Jatropha cake, Karanja cake, and Dextrose at the concentration of 12.5, 4.5, and 10 g/l, respectively, yielded 250 mg/l of DPA, which was 2.5 fold more than that obtained from basal medium. HPLC analysis of the optimized medium (peak at retention time of 30 min) confirmed the enhanced DPA production by P. variotii. The scanning electron microscopy (SEM) images showed that optimized medium impose a stress like condition (due to less C:N ratio) for the fungus and generated more spores as compared to the basal medium in which carbon source is easily available for the mycelial growth. The antimicrobial activity of the fungal extract was tested and found to be effective even at 10−2 dilution after 72 h against two plant pathogens, Fusarium oxysporum and Verticillium dahlia. Statistical experimental design of this study and the use of non-edible oil cakes as a substrate offer an efficient and viable approach for DPA production by P. variotii.
Keywords: jatropha cake, karanja cake, dipicolinic Acid (DPA), Paecilomyces variotii, medium optimization, biocontrol
Introduction
Previous researches proved the efficacy and adaptability of Paecilomyces in effectively controlling different pathogens under various environmental conditions (Dunlap et al., 2007; Anastasiadis et al., 2008; Sharma et al., 2012; Barakat and Saleh, 2016). Paecilomyces is morphologically characterized by having flask-shaped phialides, which is differentiated into swollen base and a distinct neck and generate conidia that are arranged end to end in a chain. Paecilomyces species is known to utilize a variety of substrates due to its ability to consume high ammonia and nitrogen rich substrates (Liu et al., 2016). Furthermore, produces bioactive substances such as dipicolinic acid (DPA), which has been reported to suppress certain pathogens that are deleterious to the plants in the rhizosphere (Asaff et al., 2005, 2006). Use of microbial inoculants as biocontrol agents for controlling disease and pests of crop plants is continuously gaining attention as an alternative tool against the use of chemical pesticides in developing countries (Dale et al., 2007). Very few experimental studies have been reported on the DPA production from Paecilomyces strain and its use as an agent for control of plant pathogens.
Reported by several workers that media constituents simply affect the virulence of biocontrol agents against plant pathogens (Cliquet and Jackson, 2005; Nisha and Kalaiselvi, 2016) and its composition plays a significant role in growing fungal mycelia with increased sporulation (Sun and Liu, 2006; Gao et al., 2007). Liu and Chen (2002) reported that fungi can use wide range of substrates as carbon and nitrogen sources. However, low cost and simple media will be a better choice for their mass-production and also for field application. For successful use of a mycopesticide in biocontrol, it is essential to produce high yields of propagules which are pathogenic toward target pathogen. The quality as well as quantity of propagules is affected by medium composition and culture conditions (Gupta et al., 2016). Carbon-Nitrogen ratio as well as type of sugar in media plays an important role in determining the pathogenicity of the fungus (Vidal et al., 1998; Gao et al., 2007; Zhao et al., 2013; Nisha and Kalaiselvi, 2016). Therefore, nitrogen and carbon sources were screened for the production of bioactive molecule because they may influence the activity of phytopathogens, and enhances antibiotics production in biocontrol strains and contribute to the variability of biocontrol in different soils and also on host crops (Milner et al., 1995; Thomashow and Weller, 1996; Cliquet and Jackson, 2005; Moorthi et al., 2015).
Previously, media optimization studies reported the use of one factor at a time for various biomolecules production which is laborious and time consuming process, it also often misleads the understanding of the system behavior, generates confusion, and variation in prediction (Singh and Chhatpar, 2010; Saharan et al., 2011; Nisha and Kalaiselvi, 2016). Plackett and Burman's design (PBD), when applied to evaluate medium components, will screen the insignificant factors out of a large number of possible factors at fairly early stages of the experiment. Box Behnken Design (BBD) is useful in determining the effect of key factors, by minimum number of experiments for further optimization (Singh and Chhatpar, 2010; Zhao et al., 2013). No published reports to the best of our knowledge are available for increased production of the bioactive DPA from P. variotii using response surface methodology (RSM). The findings of this study strongly support the hypothesis that better yields can be achieved by optimization of C and N sources using RSM technique.
Materials and methods
Microorganism and culture condition
Paecilomyces variotii strain employed in this study was procured from the culture collection of International Centre for Agriculturally Important Microbes (ICAIM), PUSA, New Delhi, India. The potato dextrose agar (PDA) was used to grow the fungus on plates at 28°C for 7 days and it stimulated the production of conidia. These conidia were sub-cultured under submerged fermentation (SmF) in Erlenmeyer flask (500 ml) containing 100 ml of the medium containing (per liter): Glucose (30 g), yeast extract (3 g), KH2PO4 (0.39 g), Na2HPO4.12H2O (1.42 g), MgSO4.7H2O (0.60 g), NH4NO3 (0.70 g), and KCl (1.00 g) (Fargues et al., 1992). The medium pH was adjusted to 5.6 before sterilization and inoculated with 4 ml of 107 conidia/ml from conidial suspension. The flasks were then incubated at 27°C for 7 days in an incubator (Scigenics Biotech, India) and the inoculum thus prepared was used for harvesting conidial spores. The spores were collected in sterile solution (0.05% Tween 80) and 50% glycerol stocks were maintained from Tween 80 conidial suspension culture and stored at −20°C. This spore suspension was utilized as inoculums in all further experiments. For using oil cakes as media, the non-edible oil cakes i.e., Jatropha (Jatropha curcas), Karanja (Pongamia pinnata), Neem (Azadirachta indica), and Mahua (Madhuca indica) were oven dried at 55°C for 24 h, grinded, and sieved to obtain 0.8- and 2.0-mm particle size. C:N ratio in the cakes was analyzed. Oil cake was soaked in water overnight, then heated for 2 h and final volume was adjusted by adding water to prepare cake broth.
Analytical methods
Biomass estimation
Gravimetric analysis was used to determine biomass in submerged fermentation as described by Soundrapandian and Chandra (2007). The liquid from flask was filtered through pre-weighed Whatman filter paper Grade 1 (pore size, 11 μm) and dried at 70°C under vacuum (0.6 atm) until reaching the constant weight, by determining the weight difference of the filter paper. Spore count was done with a Neubeaur's hemocytometer.
Chemical analysis of the samples
The culture filtrate was centrifuged at 10,000 rpm for 10 min and supernatant was filtered through nylon filter (pore size 0.45 μm) and divided into two parts for analysis. One part was kept for spectrophotometric analysis of DPA and second part (50-ml aliquots) was freeze-dried and then extracted using sonication with 50 ml methanol acetate at 25°C for 10 min.
Qualitative analysis
The extracts with methanol and ethyl acetate were used for Thin Layer Chromatography (TLC). Silica gel GF254 plate (E. Merck, Dramstadt, Germany) was used with 1-butanol/water/acetic acid (12:5:3, by vol.) solvents. The plates were exposed to UV light (254 nm) to visualize the bands and confirmation was done by spraying a 0.051 M cerium ammonium sulfate solution and heating on a hot plate for color development. A DPA standard (Sigma) (Rf 0.90) was used for determining the presence of DPA (Asaff et al., 2005).
High-Performance Liquid Chromatography (HPLC) analysis of the collected samples were performed using a Thermo-separation (Constametric 4100) chromatograph using following conditions: UV detector and a Phenomenex OOH-0138-KO column, temp 30°C, mobile phase 5 mM H2SO4, flow rate of 0.6 ml/min and 210 nm wavelength for compound detection (Asaff et al., 2005).
DPA estimation
DPA content was quantified using a UV-visible spectrophotometer (Asaff et al., 2006). Sample (0.8 ml) to be quantified was mixed with 0.2 mL of ferrous ammonium sulfate (1 g) and ascorbic acid (1 g) and dissolved in 100 ml of 0.5 M acetate buffer and with a final pH of 5.5. Absorbance was measured at 440 nm wavelength in UV-visible spectrophotometer (PerkinElmer model Lambda 25 UV/VIS, Massachusetts, USA).
Experiment design
Culture medium was optimized using non-statistical as well as statistical experimental designs. Medium components were selected by using one-factor-at-a-time approach. The preliminary experiments revealed the optimal range of carbon (Glucose, Dextrose, and Lactose) and nitrogen sources (Jatropha oil cake, Karanja oil cake, Neem oil cake, and Mahua oil cake) and its effect on sporulation and DPA production by the fungus (Table 1). This data was utilized for designing further experiments using PBD.
Table 1.
Growth and bioactive substance (DPA) production by P. variotii on non-edible oil cakes used as solid medium and broths (after 10 days).
| Non-edible oil cake | Treatment | Solid medium | Broth | |||
|---|---|---|---|---|---|---|
| Growth/day (cm/day) | Spore count (spores/g of substrate) | Dry mycelial weight (g/100 ml) | Spore count (spores/ml) | DPA production (mg/l) | ||
| C1 | Alone | 0.42 ± 0.01c | 6.7 × 109 | 0.29 ± 0.04d | 2.7 × 109 | 102 ± 3.5 |
| + PDA (1:1) | 0.35c | 9.0 × 109 | 0.15 ± 0.016a | 7.9 × 108 | 110 ± 1.2 | |
| + dextrose | 0.36d | 5.1 × 1010 | 0.31 ± 0.02d | 3.4 × 109 | 135 ± 2.3 | |
| C2 | Alone | 0.44d | 5.1 × 109 | 0.271 ± 0.02d | 2.0 × 109 | 70 ± 1.2 |
| + PDA (1:1) | 0.32 ± 0.02d | 6.2 × 109 | 0.135 ± 0.005a | 3.1 × 108 | 78 ± 3.5 | |
| + dextrose | 0.23 ± 0.01c | 2.9 × 1010 | 0.28 ± 0.04d | 1.9 × 109 | 83 ± 1.3 | |
| C3 | Alone | 0.25 ± 0.02d | 4.5 × 109 | 0.275 ± 0.02d | 2.1 × 109 | 108 ± 2.1 |
| + PDA (1:1) | 0.31 ± 0.02d | 1.0 × 109 | 0.13 ± 0.01a | 3.0 × 108 | 116 ± 1.1 | |
| + dextrose) | 0.35 ± 0.01d | 2.1 × 1010 | 0.28 ± 0.04d | 1.8 × 109 | 136 ± 2.5 | |
| C4 | Alone | 0.18 ± 0.015b | 7.1 × 108 | 0.20 ± 0.03b | 10 × 107 | 60 ± 3.4 |
| + PDA (1:1) | 0.18 ± 0.01c | 8.3 × 107 | 0.12 ± 0.04a | 8.7 × 107 | 52 ± 1.8 | |
| + dextrose) | 0.20c | 7.7 × 107 | 0.18 ± 0.01b | 6.5 × 107 | 45 ± 3.4 | |
| Control | PDA (Potato Dextrose Agar) | 0.26 ± 0.01c | 2.5 × 109 | 0.249 ± 0.01c | 1.5 × 109 | 91 ± 1.0 |
C1, Jatropha oil cake; C2, Neem oil cake; C3, Karanja oil cake; C4, Mahua oil cake. Same letter used in each column indicates insignificant difference among the treatments (p < 0.05) according to DMRT. Value are means of n = 4.
All experiments were performed in triplicate in 500 ml Erlenmeyer flasks with 100 ml working volume. The average crude DPA concentration and spore count were taken as the dependent variables or response (Y). A correlation was also determined between production of DPA and spore of the fungus.
PBD for identification of the significant variables
To increase DPA production, carbon and nitrogen sources were optimized using PBD. PBD screen “n” variables in just a minimum of “n + 1” number of experiments (Plackett and Burman, 1946). It was used to identify the relatively important medium constituents i.e., C and N sources. PBD design evaluates the impact of main factors, as well as their interaction in production of DPA and produce optimal or near optimal responses. A total of seven factors were studied as described in Table 2 including their low (−1) and high levels (+1). The PBD was based on the first order model:
Table 2.
Levels of various Carbon and Nitrogen sources tested in Plackett-Burman Design (PBD).
| Variables code | Independent Variables | Levels (g/l) | |
|---|---|---|---|
| Low level −1 | High level +1 | ||
| x1 | Glucose | 15 | 60 |
| x2 | Dextrose | 15 | 60 |
| x3 | Lactose | 15 | 60 |
| x4 | Karanja | 10 | 40 |
| x5 | Jatropha | 12 | 48 |
| x6 | Neem | 12 | 48 |
| x7 | Mahua | 17 | 56 |
| (1) |
Where Y is the response (DPA production), β0 is the model intercept, and βi is the variable estimates. The factor level with confidence level above 95% is considered the most significant factor that affects the DPA production. A 25 PBD leading to 30 sets of experiments were performed in triplicate to verify the most significant factors affecting the DPA production, as shown in Table 3.
Table 3.
PBD and results of the fractional factorial design.
| Run | x1 | x2 | x3 | x4 | x5 | x6 | x7 | DPA (mg/l) | |
|---|---|---|---|---|---|---|---|---|---|
| Predicted | Observed | ||||||||
| 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 89.4 | 88.2 |
| 2 | −1 | 1 | −1 | −1 | 1 | −1 | −1 | 90.1 | 94.3 |
| 3 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 79.7 | 78.2 |
| 4 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | 89.6 | 90.2 |
| 5 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 90.9 | 91.2 |
| 6 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | 88.6 | 88.6 |
| 7 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | 96.2 | 94.6 |
| 8 | 1 | −1 | 1 | 1 | 1 | −1 | 1 | 93.1 | 94.2 |
| 9 | 1 | −1 | −1 | 1 | 1 | −1 | −1 | 140.1 | 138 |
| 10 | −1 | −1 | 1 | −1 | −1 | 1 | −1 | 96.2 | 98.4 |
| 11 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 99.4 | 100.6 |
| 12 | −1 | −1 | 1 | 1 | 1 | −1 | −1 | 104 | 105.4 |
| 13 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 96.8 | 96.8 |
| 14 | −1 | 1 | −1 | 1 | 1 | 1 | −1 | 152 | 155 |
| 15 | 1 | 1 | 1 | 1 | 1 | −1 | −1 | 94 | 91.2 |
| 16 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | 95.2 | 94.2 |
| 17 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 97.3 | 98.8 |
| 18 | −1 | 1 | 1 | 1 | −1 | −1 | 1 | 130.2 | 132 |
| 19 | −1 | 1 | 1 | −1 | 1 | −1 | 1 | 93.3 | 94.4 |
| 20 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 91 | 89.8 |
| 21 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | 98.2 | 97.8 |
| 22 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 99.7 | 100.4 |
| 23 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 93 | 92.2 |
| 24 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 93 | 92.3 |
| 25 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | 100 | 99.6 |
| 26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 68.7 | 70.3 |
| 27 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | 88.3 | 88.6 |
| 28 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | 92.4 | 93.2 |
| 29 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 92 | 91.2 |
| 30 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | 92.4 | 93.6 |
Optimization of the selected trace concentration by response surface methodology (RSM)
RSM is an efficient method to answer multivariate problems and optimizing several responses by minimizing the number of experiments (Kumar et al., 2016). Box Benkhen design (BBD) and PBD are part of RSM. In this experiment PBD was used initially to select the components affecting DPA production and BBD was used to optimize the major variables designated as x1, x2, and x3. Seventeen experimental runs were generated by the software comprising of different combinations of three factors. A second-order polynomial function was fitted to correlate the relationship between independent variables and response for predicting the optimal point. For three factors this equation is
| (2) |
Where, Ypred is the predicted response, xi and xj are the input variables which influence the response Y. βi is the ith linear coefficient, βij is the ijth interaction coefficient and βo is the constant. A quadratic polynomial equation was used to fit the data obtained.
Software for experimental design
The “Design-Expert” software version 7.0 (Stat-Ease, Inc., Minneapolis, USA) was used for the construction and evaluation of the statistical experimental design. The RSM contour plot analysis provides the optimal C and N values for DPA production.
Preparation of P. variotii samples for morphological study
The P. variotii cells were harvested by centrifugation from the run showing a maximum yield of DPA and control after the completion of BBD. The harvested cells were prefixed with 2.5% glutaraldehyde solution overnight at 4°C. The cells were centrifuged and washed thrice with 0.1 M sodium phosphate buffer (pH 7.2) and serial dehydration of the cells was done using different gradient of ethanol. Later, the cells were dried at “critical point” (Tyagi and Malik, 2010).
For SEM standard protocol was used to study the morphology of the P. variotii cells under SEM (ZEISS EVO 50, Germany). The SEM images were clicked using following parameters: EHT = 20.00 kV, WD = 12.0 mm, Signal A = SE1.
Biocontrol activity in vitro
The antagonistic activity of the P. variotii against two phytopathogenic fungi Fusarium oxysporium and Verticillium dahilae was studied by dual culture test by following the method of Rahman et al. (2009). P. variotii was inoculated on both the sides of Potato dextrose Agar (PDA) plate which contained 6 mm disc of pre-grown phytopathogenic fungi at the center. The biocontrol activity of P. variotii against the fungal pathogens was observed in terms of measuring the diameter of zone of inhibition. Phase Contrast Microscope (Leica Model, DME, Germany) at 10 and 40X magnification after 2–5 days of incubation at 28°C was used for identification of fungal mycelium along the edges of the inhibited colonies facing P. variotii.
Results and discussion
Carbon and nitrogen are known to be most essential nutrients required by fungi for their growth. C:N ratio in the medium is important since it significantly affects the spore yield of the bioherbicidal fungi (Engelkes et al., 1997; Gao et al., 2007). Media having 15:1 C:N ratio promote more conidial production along with biocontrol efficacy. Results showed that C:N ratio is more important for the sporulation of P. variotii. The cakes of Jatropha, Karanja, Neem, and Mahua were found to have C:N ratios of 2.76, 2.29, 3.28, and 6.3 respectively.
PBD for the screening of important factors for DPA production
Seven medium elements which have the possibility to influence the production of active substance, namely Glucose, Dextrose, Lactose (carbon sources), and Jatropha oil cake, Karanja oil cake, Neem oil cake, and Mahua oil cake were selected for a 2-level PBD. The predicted regression equation for the DPA (Y) production as a function of the coded values of critical variables is given in the following equation:
| (3) |
From Equation (3) it can be observed that Karanja oil cake (x4), due to its higher coefficient effect was the most significant factor. Although, the results have also given the picture that the Jatropha oil cake and dextrose are also necessary in certain amounts to switch on the maximum production of DPA. These factors contain some important elements that may influence the activity of some enzymes (Deqing, 2002; Saharan et al., 2011) and some enzymes included in non-ribosomal peptide synthetase (NRPSs). The biochemical mechanism related to the yield of DPA by non-edible oil cakes needs further research. The results of F-test showed significant difference (p < 0.01) for variance between the average of observation of two-level experiment and center point. The result revealed that the optimum point was in accordance with the design used in the experiment.
Optimization of selected C & N sources by RSM using BBD
Screening of the factors helped in the detection of gross curvature in the design space. The BBD was applied to estimate quadratic effects, pure process variability and reassess gross curvature, with active substances produced as response (Wang and Liu, 2008). Based on the variable identified by the PBD, a 3-level BBD was developed for variables significantly affecting DPA production (Table 4). The BBD of the three screened variables in coded format, along with DPA concentration as responses is depicted in Table 5. The maximum yield of DPA production, experimentally observed, was 250 mg/l in run 12. In order to get the optimum values of the variables which correspond toward maximum DPA production, a second-degree polynomial model was proposed. By applying a second-degree polynomial equation (Equation 4) in multiple regression analysis, explains the function of each variable and their interactions in the production of active substance:
Table 4.
Coded and real values for Box Behnken design (concentration g/l).
| Variables | Level of variables | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Dextrose | 5 | 10 | 15 |
| Jatropha | 1 | 2 | 3 |
| Karanja | 2 | 4 | 8 |
Table 5.
Response from Box Behnken design experiment (conc. g/l).
| Run | x1 | x2 | x3 | DPA (mg/l) |
|---|---|---|---|---|
| 1 | −1.000 | 0.000 | −1.000 | 172.5 |
| 2 | 0.000 | 0.000 | 0.000 | 251.2 |
| 3 | 0.000 | 0.000 | 0.000 | 249.8 |
| 4 | 1.000 | 0.000 | 1.000 | 107 |
| 5 | 0.000 | 0.000 | 0.000 | 250.4 |
| 6 | −1.000 | 0.000 | 1.000 | 138 |
| 7 | −1.000 | 1.000 | 0.000 | 155 |
| 8 | 0.000 | −1.000 | 1.000 | 130 |
| 9 | 1.000 | 0.000 | −1.000 | 99.2 |
| 10 | 0.000 | −1.000 | −1.000 | 140 |
| 11 | 1.000 | 1.000 | 0.000 | 115 |
| 12 | 0.000 | 0.000 | 0.000 | 248.2 |
| 13 | 0.000 | 0.000 | 0.000 | 253 |
| 14 | −1.000 | −1.000 | 0.000 | 140 |
| 15 | 0.000 | 1.000 | 1.000 | 128 |
| 16 | 0.000 | 1.000 | −1.000 | 175 |
| 17 | 1.000 | −1.000 | 0.000 | 115 |
| (4) |
Where Y is the predicted active substance (DPA) yield, x1 is Dextrose, x2 is Karanja oil cake, and x3 is Jatropha oil cake.
In first order, main effect of Jatropha oil cake (p = 0.0501) and Karanja oil cake (p = 0.0045) are less significant than their quadratic main effect (p < 0.001) indicating the effect of Jatropha and Karanja oil cake in DPA production (Table 6). Similarly, the interaction of Dextrose—Jatropha (p < 0.0001) and Dextrose—Karanja (p < 0.0001) and the quadratic are also quite significant among all interactions. Results of second order response surface model using ANOVA (analysis of variance) are shown in Table 7. The low probability value obtained from Fisher's F-test [(P model > F) < 0.0001] showed the high significance of the model. The coefficient (R2 = 0.9833) described the fitness of the model, which ascertained that > 98% sample variation was ascribed to the variables and only <2% of the total variance could not be explained by the model (Table 7). The model significance was also justified by the adjusted determination coefficient (Adj R2 = 0.9847) and predicted determination coefficient (0.8962). The statistically insignificant lack of fit was also shown by model [(P model > F) = 0.22], making the model adequate for prediction within the range of variables employed. A coefficient variation having low value (CV = 4.26%) proposed the preciseness and reliability of experiments. The linear pattern in the normal plot of residuals, demonstrated normality in the error term, i.e., there were no indications of any problem in the data (Draper and Smith, 1981). The correlation coefficient of 0.94 reveals the strong positive correlation between the both spore formation and DPA production. Increase in the number of spore's leads to more production of DPA by the fungus.
Table 6.
Significance of quadratic model coefficients for DPA production.
| Factor | Coefficient values | F-values | p-value |
|---|---|---|---|
| Intercept | 341.2 | 115.35 | <0.0001 |
| x1 | 47.0 | 69.50 | <0.0001 |
| x2 | 119.6 | 5.59 | 0.0501 |
| x3 | 225.5 | 16.99 | 0.0045 |
| −2.66 | 1.09 | 0.3309 | |
| −13.1 | 8.68 | 0.0215 | |
| −97.1 | 6.64 | 0.0367 | |
| x1x2 | 0.37 | 363.08 | <0.0001 |
| x1x3 | 2.83 | 225.96 | <0.0001 |
| x2x3 | −6.16 | 244.14 | <0.0001 |
Table 7.
ANOVA for response surface quadratic model obtained for DPA production.
| Source | Sum of Squares | Mean Square | DF | F-value | P > F |
|---|---|---|---|---|---|
| Model | 53514.61 | 5946.07 | 9 | 115.35 | <0.0001 |
| Residual | 360.85 | 51.55 | 10 | ||
| Lack of Fit | 295.32 | 49.22 | 6 | 3.004 | 0.22 |
| Pure Error | 65.53 | 16.382 | 4 | ||
| Total | 53875.46 | 19 |
CV % = 4.26; R2 = 0.9833; Adj R2 = 0.9847; Predicted R2 = 0.8962.
Value of “p >F” less than 0.05 indicate model terms are significant.
The CV value >4 indicates adequate precision in the model.
Table 7 inferred the significance of each coefficient which was determined by F-test and P-value. The greater the magnitude of F-value the lesser the p-value, the more significant is the corresponding coefficient. The generated response surfaces of three variables tested expressed a linear effect on the response (p < 0.001). Dextrose, Karanja, and Jatropha showed a positive effect on the production of bioactive substance.
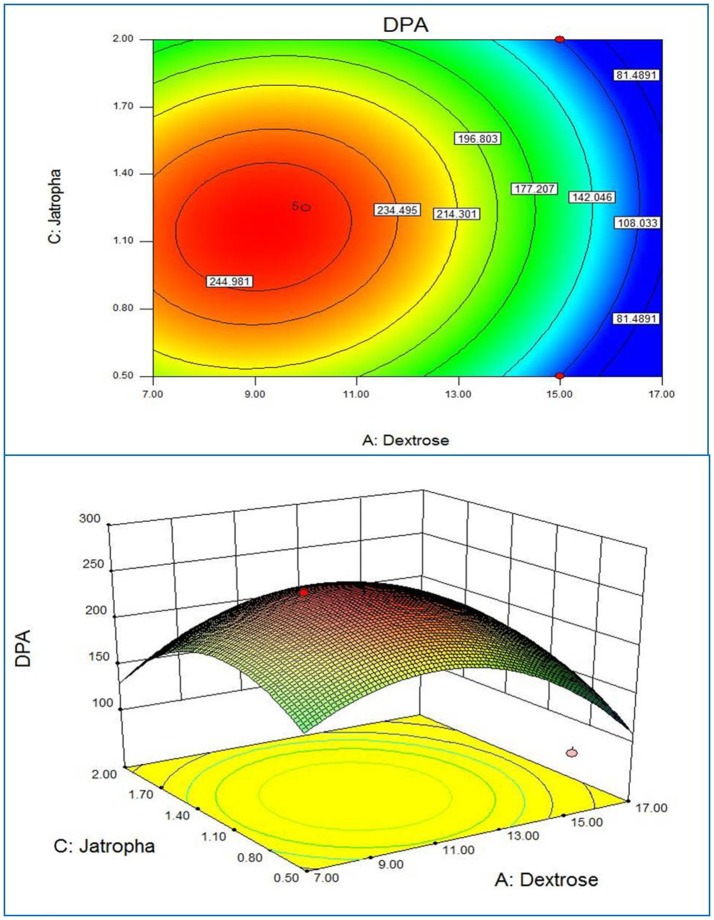
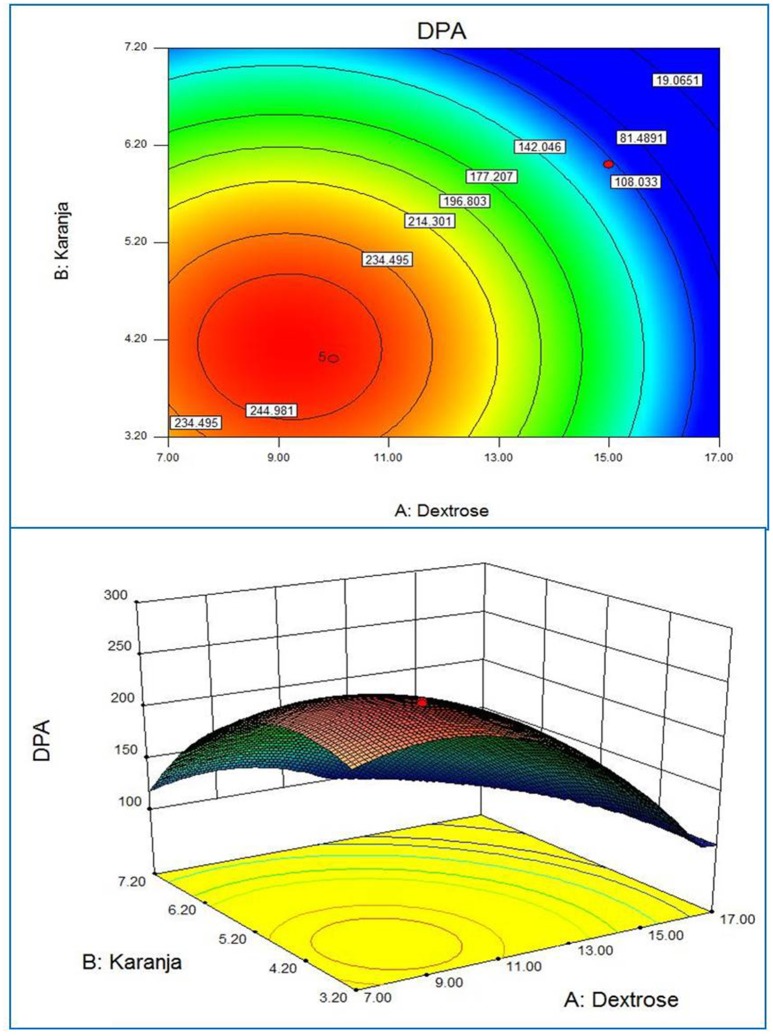
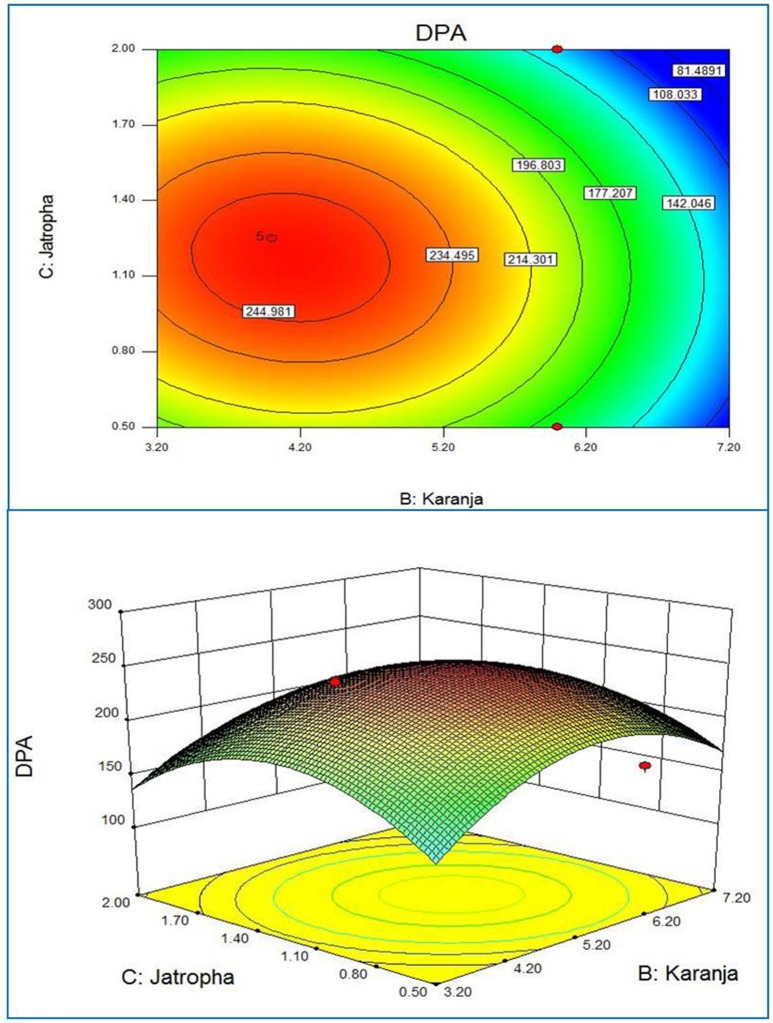
Response surface plots of the model generated by the software gave the visualization of the role of the independent variables on the dependent one (Xu et al., 2008). The RSM plot presents a system to foresee the yield of active substances for various values at different test variables and the contours of the plot generated are useful in recognition of the kind of interaction lies in test variables. The contour plots generated by using the fitted quadratic polynomial equation obtained from regression analysis are shown in Figures 1–3. Each figure shows the effect of two independent variables on the active substances production, while the other variable was held at zero level. The circular contours of the plot surfaces implicate the negligible interaction between the corresponding variables whereas an elliptical or saddle contours indicates the significant interactions between the related variables (Murthy et al., 2000). The effect of Jatropha oil cake and dextrose on the DPA production at the fixed Karanja oil cake level is shown in Figure 1. Similarly, the Figures 2, 3 correspond to the effect of two variables on the production of DPA, holding the 3rd variable at zero level. All three cases, revealed a clear optimal convergence. The optimal levels provided three independent variables on DPA production. A significant interaction was noted between the carbon source dextrose and the nitrogen source Karanja and Jatropha oil cakes. The C:N ratio directly influenced the growth of the fungus and accumulation of the metabolites (Ramachandran et al., 2007; Abd EL-Aziz et al., 2015). Although the actual situation may be more complex than explained, an attempt for the medium optimization has been made by RSM.
Figure 1.
2D contour plot and 3D response surface curve of Jatropha oil cake and Dextrose predicted by the full quadratic model.
Figure 2.
2D contour plot and 3D response surface curve of Karanja and Dextrose predicted by the full quadratic model.
Figure 3.
2D contour plot and 3D response surface curve of Karanja and Jatropha predicted by the full quadratic model.
Validation of the predicted concentration in the optimal medium
Based on medium optimization, the maximum production of DPA was 250 mg/l predicted by quadratic model when the dextrose, Karanja oil cake, and Jatropha were at 10, 40, and 12.5 g/l, respectively. Validation experiment was performed in a 5 l fermentor and compared with the predicted data from the model to verify the predicted results. Fermentor experiment was done only for validation of the results obtained from the batch fermentation and was carried out in exactly same conditions. The volume was increased from 100 ml to 5 l. Scale up experiment with 5l fermentor was done at 27°C and 150 rpm (Scigenics Biotech, India) for 7 days. In the 5 l fermentor, the average concentration of DPA in the broth was 373.5 mg/l, showing 50% increase in DPA yield, suggesting that the accuracy of the model is over 95%. Where as, the predicted value for DPA by the model was 251 mg/l which translates into a deviation of only 4.64%. The level of DPA production obtained using optimized carbon and nitrogen sources amendment was 2.5 folds higher than the non-optimized basal medium for fungus. This is the first report on DPA production optimization using RSM designs in synthetic medium.
Morphological alteration of P. variotii
SEM images show the morphological changes in P. variotii spores grown in basal medium and in the oil cake (Figure 4). Spores in the best-selected concentration of oil cakes (Jatropha, Karanja) underwent morphological alterations considerably in comparison to the control when observed under SEM (Figure 4). Control spores are spotted round and spherical and less in number, also there is more mycelial content (Figure 4A) while the oil cakes treated spores are numerous and oval shaped having ornamented rough surface (Figure 4B).
Figure 4.
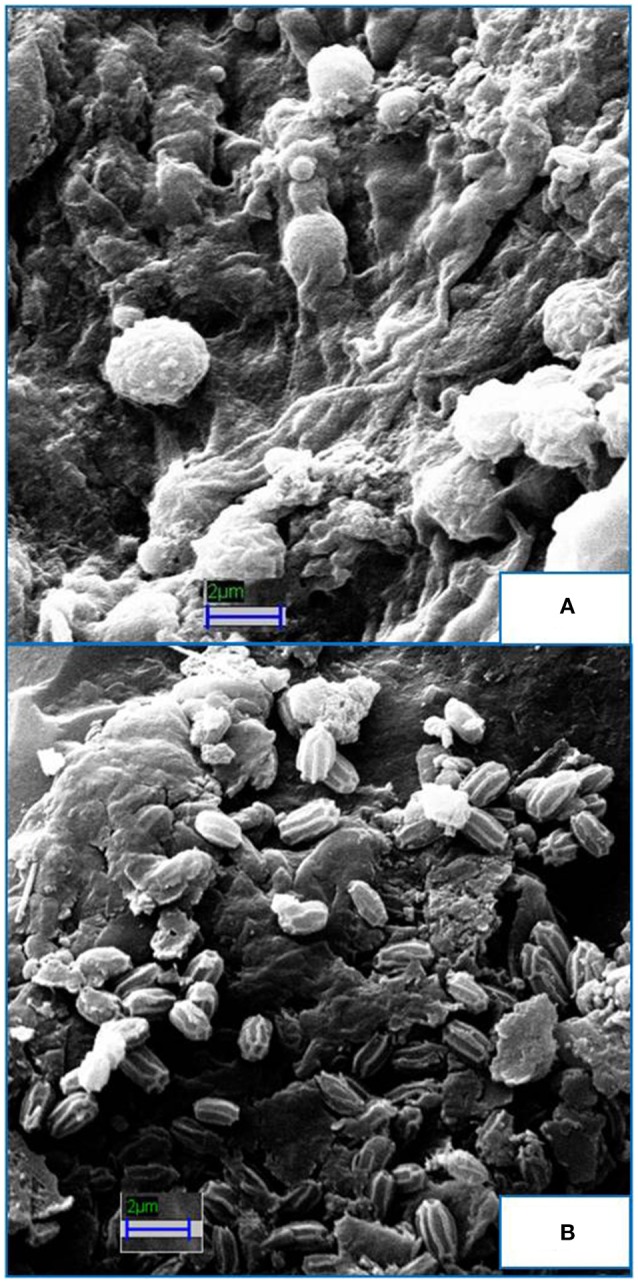
Scanning Electron Micrographs of P.variotii spores grown in basal medium and optimized non-edible oil cake medium. (A) Spores in normal PDB are less in number, spherical, and with smooth surface. (B) Spores in optimized non-edible oil cake medium are numerous, oval shaped, and ornamented surface.
Biocontrol activity
Production of bioactive substance establishes that P. variotii confirmed strong inhibition in dual culture after 2–5 days of incubation against F. oxysporium and V. dahilae. On further incubation, the pathogenic fungal mycelia (F. oxysporium and V. dahilae) growing toward the interacting zone inhibited and continuously lost vigor due to degradation. P. variotii mycelium was grew faster and covered the most part of the petriplate. It showed strong inhibition activities in vitro against above mentioned phytopathogenic fungi tested.
The fungistatic and fungicidal concentrations of P. variotii extract contains DPA as observed by spectrophotometric assay are presented in Figure 5. Fungal filtrate exhibited the strong antimicrobial effect even at very low concentrations. The Minimum Inhibitory Concentrations (MICs) against both the pathogens (F. oxysporum and V. dahilae) was identified at the concentration C3 i.e., 1E-2 dilution of the filtrate (Figures 5A,B).
Figure 5.
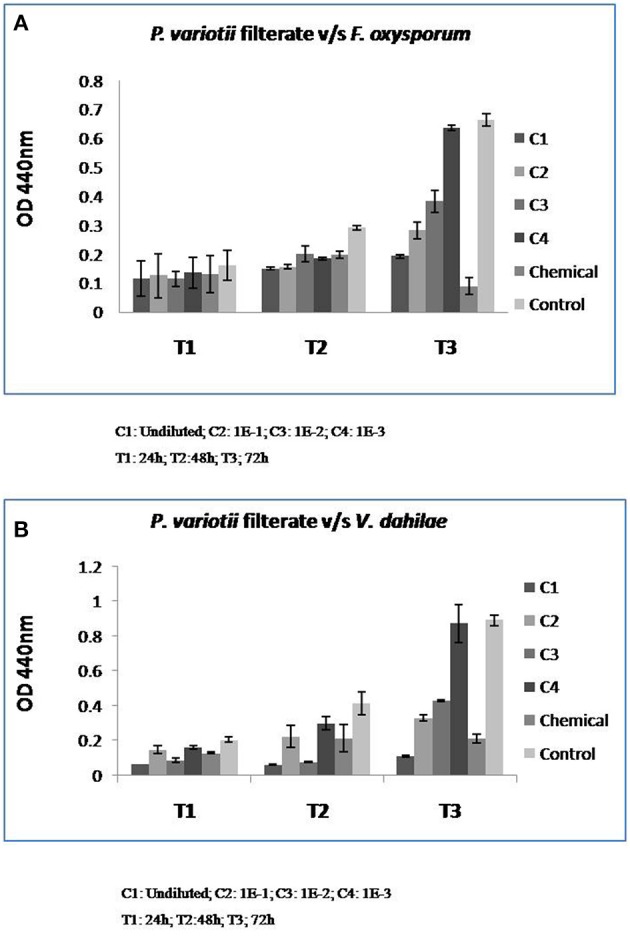
Minimum Inhibitory Concentration (MIC) analysis of P. variotii filtrate against F. oxysporum (A) and V. dahilae (B).
This study is novel in terms of optimization of media for DPA production by P. variotii, a low cost raw material such as non-edible oil cakes and in studying biocontrol of plant pathogens by the fungal extract. Studies and field trials proved that P. variotii is potential in controlling Fusarium and Verticillium wilt of tomato was aided by production of various bioactive compounds such as DPA, oxalic acid, protease, etc. The plants that were applied with DPA and fungal extract of P. variotii were tolerant to the severe attack of Fusarium and Verticillium wilt pathogens. Biopesticidal applications involve high amount of bioactive components which can be attained by medium components optimization for exploiting a low cost material such as non-edible oil cakes (NEOCs) (Abd EL-Aziz et al., 2015). Use of NEOCs in biodiesel sector for the production of DPA could be a solution for oil cakes disposal, since these cakes are toxic and cannot be used as animal feed like other edible oil cakes. Several factors predominantly medium constituents are known to influence production of DPA. Particularly C and N are significant for the production of these bioactive compounds. Ping (2000) and Ramachandran et al. (2007) have reported, various industrial applications of oil cakes in biotechnological and fermentation processes and their value addition by implementation in feed and energy source as well. Sharma et al. (2012) in their study used the non-edible oil cakes as a potential substrate for Paecilomyces lilacinus and its application as biopesticidal agent against termites. PBD was useful in recognizing Jatropha oil cake, Karanja oil cake, and Dextrose as vital factors affecting DPA production by P. variotii. No earlier studies are available on the effect of NEOCs on DPA production by P. variotii. Furthermore, the interaction effect among Jatropha oil cake, Karanja oil cake, and Dextrose was highly significant in enhancing the DPA production by P. variotii. The scaling up in a 5 l fermentor in an optimized medium promote enhanced DPA production by 50%. Like other metabolites maximum DPA production was also attained in the stationary phase. Our results are in agreement with Azeredo et al. (2004) and Singh and Chhatpar (2010), who observed that maximum enzyme production, obtained at the stationary phase of growth.
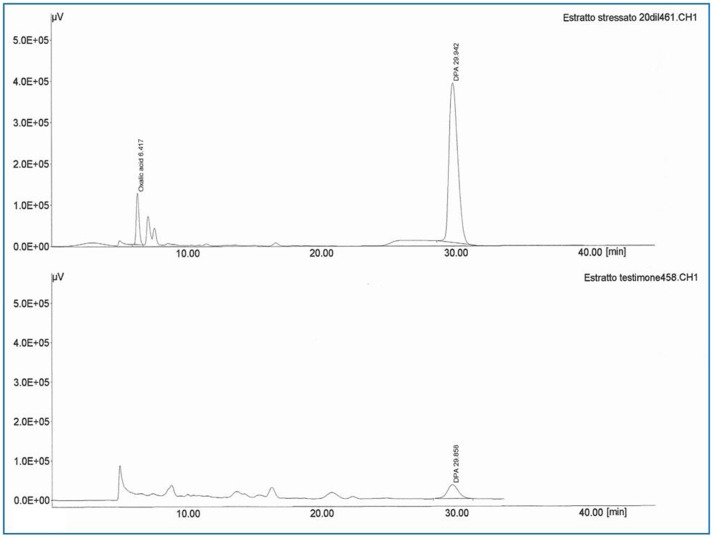
The HPLC analysis of the optimized medium also confirmed the enhanced DPA production by P. variotii (Figure 6). The SEM analysis showed that oil cakes create a stress like condition (due to less C:N ratio) for the fungus and it generated more spores as compared to basal medium in which carbon source is easily available for the mycelial growth. The dramatic change in the morphology of the fungal cells has been observed. These results are supported by our previous observations of the DPA production during sporulation (unpublished data). During the biocontrol experiment at laboratory level the fungal extract (containing DPA) was found to be effective against F. oxysporum and V. dahilae even at low concentrations. This might be due to the Pyridine and amide moieties present in DPA. The antimicrobial activity of DPA might be due to the presence of the amide linkage groups and nitrogen heterocyclic rings, which enhances their activity (Al-Salahi et al., 2010). Moreover, the plate studies revealed the absence of any direct contact between P. variotii and the pathogenic fungi. This suggested that the inhibition of phytopathogenic fungi was due to exocellular, bioactive substance that diffuse through the agar medium, which substantiates the production of DPA and other bioactive substances that might inhibit the pathogen growth (Mohammadi et al., 2016). Visual observation under a phase contrast microscope, showed that the pathogenic fungal mycelia growth toward the P. variotii were thin and that the cytoplasmic contents of the cell became collate. However, on the control petriplate, pathogenic fungal mycelia showed regular radial growth. The results of the current work correlates with the studies conducted by Wang and Liu (2008), which optimized the medium for antifungal substance from Paenibacillus sp.
Figure 6.
High-Performance Liquid Chromatography (HPLC) for DPA production of control and best NEOCs (Karanja oil cake + Jatropha oil cake + Dextrose) treatment (at Retention Time 30 min).
This study indicated that statistical experimental design recommends an efficient and realistic methodology for optimizing the requirements for DPA production by P. variotii. The maximum DPA production (251 mg/l), was 2.5 folds more when compared to the basal medium, achieved by optimizing carbon and nitrogen sources. This study will also contribute toward improving the DPA production by other microorganisms and the usage of trace elements. Integrated into a broader study of carbon and nitrogen sources on the production of DPA as bioactive substance, this work should help to build more rational control strategy, possibly involving scale-up production of DPA.
Author contributions
KA performed the experiments, applied RSM, and prepared the manuscript. SS designed the experiments and reviewed the manuscript. AK, SK, and JA edited and revised the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Ms. Krishna Saharan and Prof. Vikram Sahai, Fermentation Lab, DBEB, Indian Institute of Technology, Hauz Khas, New Delhi for RSM modeling and providing Design Expert software for RSM analysis. This project has been implemented with financial contributions from the Indian Institute of Technology, New Delhi, India. SK is thankful to DUT, Durban for Research Associateship.
References
- Abd EL-Aziz A. B. E.-D., Awad A. A. E.-N., Zaki G. H. (2015). Reduction of olive oil mill waste water phenolic compounds and COD using Paecilomyces variotii. Trends Ind. Biotechnol. Res. 1, 1–9. [Google Scholar]
- Al-Salahi R. A., Al-Omar M. A., Amr A-G. (2010). Synthesis of chiral macrocyclic or linear pyridine carboxamides from pyridine-2,6-dicarbonyl dichloride as antimicrobial agents. Molecules 15, 6588–6597. 10.3390/molecules15096588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis I. A., Giannakou I. O., Prophetou-Athanasiadou D. A., Gowen S. R. (2008). The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot. 27, 352–361. 10.1016/j.cropro.2007.06.008 [DOI] [Google Scholar]
- Asaff A., Cerda-García-Rojas C. M., Gonzalez G. V., Torre M. (2006). Carbon distribution and redirection of metabolism in Paecilomyces fumosoroseus during solid state and liquid fermentations. Process Biochem. 41, 1303–1310. 10.1016/j.procbio.2006.01.001 [DOI] [Google Scholar]
- Asaff A., Cerda-García-Rojas C. M., Torre M. (2005). Isolation of dipcolinic acid as an insecticidal toxin from Paecilomyces fumosoroseus. Appl. Microbial. Biotechnol. 68, 542–547. 10.1007/s00253-005-1909-2 [DOI] [PubMed] [Google Scholar]
- Azeredo L. A. I., Freire D. M. G., Soares R. M. A., Leite S. G. F., Coelho R. R. R. (2004). Production and partial characterization of thermophilic proteases from Streptomyces sp. isolated from Brazilian cerrado soil. Enzyme Microb. Technol. 34, 354–358. 10.1016/j.enzmictec.2003.11.015 [DOI] [Google Scholar]
- Barakat K., Saleh M. (2016). Bioactive betulin produced by marine Paecilomyces WE3-F. J. Appl. Pharm. Sci. 6, 34–40. 10.7324/JAPS.2016.60306 [DOI] [Google Scholar]
- Cliquet S., Jackson M. A. (2005). Impact of carbon and nitrogen nutrition on the quality, yield and composition of blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. J. Ind. Microbiol. Biotechnol. 32, 204–210. 10.1007/s10295-005-0232-3 [DOI] [PubMed] [Google Scholar]
- Dale B. M., Walter J. G., Wayne L. T., Michael J. M. (2007). Global and local optimization using radial basis function response surface models. Appl. Math. Model. 31, 2095–2110. 10.1016/j.apm.2006.08.008 [DOI] [Google Scholar]
- Deqing Z. (2002). Tutorial of Microbiology, 2nd Edn. Beijing: Higher Education Press. [Google Scholar]
- Draper N. R., Smith H. (1981). Applied Regression Analysis, 2nd Edn. Beijing: Higher Education Press. [Google Scholar]
- Dunlap C. A., Jackson M. A., Wright M. S. (2007). A foam formulation of Paecilomyces fumosoroseus, an entomopathogenic biocontrol agent. Biocont. Sci. Technol. 17, 513–523. 10.1080/09583150701311614 [DOI] [Google Scholar]
- Engelkes C. A., Nuclo R. L., Fravel D. R. (1997). Effect of carbon, nitrogen, and C:N ratio on growth, sporulation, and biocontrol efficacy of talaromyces flavus. Phytopathology 87, 500–505. 10.1094/PHYTO.1997.87.5.500 [DOI] [PubMed] [Google Scholar]
- Fargues J., Maniania N., Delmas J., Smits N. (1992). Influence of the temperature on the in vitro growth of entomopathogenic hypomycetes. Agronomie 12, 556–557. 10.1051/agro:19920708 [DOI] [Google Scholar]
- Gao L., Sum M. H., Liu X. Z., Che Y. S. (2007). Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol. Res. 111, 87–92. 10.1016/j.mycres.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Gupta A., Sharma S., Kumar A., Alam P., Ahmad P. (2016). Enhancing nutritional contents of Lentinus sajor-caju using residual biogas slurry waste of detoxified mahua cake mixed with wheat straw. Front. Microbiol. 7:1529. 10.3389/fmicb.2016.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Chanderman A., Makolomakwa M., Perumal K., Singh S. (2016). Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit. Rev. Environ. Sci. Technol. 46, 556–591. 10.1080/10643389.2015.1131562 [DOI] [Google Scholar]
- Liu X. Z., Chen S. Y. (2002). Nutritional requirements of the nematophagous fungus Hirsutella rhossiliensis. Biocontrol Sci. Technol. 12, 381–393. 10.1080/09583150220128167 [DOI] [Google Scholar]
- Liu Z., Liu G., Cai H., Shi P., Chang W., Zhang S. (2016). Paecilomyces variotii: a fungus capable of removing ammonia nitrogen and inhibiting ammonia emission from manure. PLoS ONE 11:e0158089. 10.1371/journal.pone.0158089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J. L., Raffel S. J., Lethbridge B. J., Handelsman J. (1995). Culture conditions that influence accumulation of zwittermicin A by Bacillus cereus UW85. Appl. Microbiol. Biotechnol. 43, 685–691. 10.1007/BF00164774 [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Soltani J., Khosro P. (2016). Soilborne and invertebrate pathogenic Paecilomyces species show activity against pathogenic fungi and bacteria. J. Crop Prot. 5, 377–387. [Google Scholar]
- Moorthi P. V., Balasubramanian C., Ramar M., Murugan K. (2015). Biocontrol potential of entomopathogenic fungi against Spodoptera litura. Sci Agric. 12, 23–27. [Google Scholar]
- Murthy M., Swaminathan T., Rakshit S. K., Kosugi Y. (2000). Statistical optimization of lipase catalyzed hydrolysis of methyloleate by response surface methodology. Bioprocess Eng. 22, 35–39. 10.1007/PL00009097 [DOI] [Google Scholar]
- Nisha M. K., Kalaiselvi M. (2016). Effect of carbon and nitrogen sources on pectinase by Paecilomyces variotii. Int. J. Curr. Adv. Res. 5, 874–877. [Google Scholar]
- Ping S. (2000). Microbiology, 1st Edn. Beijing: Higher Education Press. [Google Scholar]
- Plackett R. L., Burman J. P. (1946). The design of optimum multi factorial experiments. Biometrika 33, 305–325. 10.1093/biomet/33.4.305 [DOI] [Google Scholar]
- Rahman M. A., Begum M. F., Alam M. F. (2009). Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Mycobiology 37, 277–285. 10.4489/MYCO.2009.37.4.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S., Singh S. K., Larroche C., Soccol C. R., Pandey A. (2007). Oil cakes and their biotechnological applications – A review. Bioresour. Technol. 98, 2000–2009. 10.1016/j.biortech.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Saharan K., Sarma M. V. R. K., Prakash A., Johri B. N., Bisaria V. S., Sahai V. (2011). Shelf-life enhancement of bio inoculant formulation by optimizing the trace metals ions in the culture medium for production of DAPG using fluorescent pseudomonad R62. Enzyme Microb. Technol. 48, 33–38. 10.1016/j.enzmictec.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Sharma S., Verma M., Sharma A. (2012). Utilization of non edible oil seed cakes as substrate for growth of Paecilomyces lilacinus and as biopesticide against termites. Waste Biomass Valor. 4, 325–330. 10.1007/s12649-012-9134-6 [DOI] [Google Scholar]
- Singh A. K., Chhatpar H. S. (2010). Optimization of protease production by Streptomyces sp. A6 using statistical approach for reclamation of shellfish waste. World J. Microbiol. Biotechnol. 26, 1631–1639. 10.1007/s11274-010-0339-1 [DOI] [Google Scholar]
- Soundrapandian P., Chandra R. (2007). Mass production of entomopathogenic fungus Metarhizium anisopliae (Deutromycota: Hyphomycetes) in the laboratory. Res. J. Microbiol. 2, 690–695. 10.3923/jm.2007.690.695 [DOI] [Google Scholar]
- Sun M. H., Liu X. Z. (2006). Carbon requirements of some nematophagous, entomopathogenic and mycoparasitic Hyphomycetes as fungal biocontrol agents. Mycopathologia 161, 295–305. 10.1007/s11046-006-0249-9 [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. (1996). Current concepts in the use of introduced bacteria for biological disease control mechanisms and antifungal metabolites, in Plant-microbe Interactions, eds Stacey G., Keen N. T. (New York, NY: Chapman and Hall; ), 187–235. [Google Scholar]
- Tyagi A. K., Malik A. (2010). Liquid and vapour phase antifungal activities of selected essential oils against Candida albicans: microscopic observations and chemical characterizations of Cymbopogon citrates. BMC Complement. Altern. Med. 10:65. 10.1186/1472-6882-10-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal C., Fargues J., Lacey L. A., Jackson M. A. (1998). Effect of various liquid culture media on morphology, growth, propagule production and pathogenic activity to Bemisia argentifolii of the entomopathogenic Hypomycete, Paecilomyces fumosoroseus. Mycopathologia 143, 33–46. 10.1023/A:1006962808712 [DOI] [Google Scholar]
- Wang Z. W., Liu X. L. (2008). Medium optimization for antifungal active substances production from a newly isolated Paenibacillus sp. using response surface methodology. Bioresour. Technol. 99, 8245–8251. 10.1016/j.biortech.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Xu H., Sun L. P., Shi Y. Z., Wu Y. H., Zhang B., Zhao D. Q. (2008). Optimization of cultivation conditions for extracellular polysaccharide and mycelium biomass by Morchella esculenta AS51620. Biochem. Eng. J. 39, 66–73. 10.1016/j.bej.2007.08.013 [DOI] [Google Scholar]
- Zhao P., Quan C., Jin L., Wang L., Wang J., Fan S. (2013). Effects of critical medium components on the production of antifungal lipopeptides from Bacillus amyloliquefaciens Q-426 exhibiting excellent biosurfactant properties. World J. Microbiol. Biotechnol. 29, 401–409. 10.1007/s11274-012-1180-5 [DOI] [PubMed] [Google Scholar]