One strategy by which animals adapt to their internal states and external environments is by adopting behavioral states. The roundworm Caenorhabditis elegans is an attractive model for investigating how behavioral states are genetically and neuronally controlled. Here we describe the hierarchical organization of behavioral states characterized by locomotory activity, feeding, and egg-laying. We show that decisions to engage in these behaviors are controlled by the nervous system through insulin-like signaling and the perception of food.
Keywords: behavioral state, insulin-like signaling, egg-laying, feeding, locomotion
Abstract
Animals optimize survival and reproduction in part through control of behavioral states, which depend on an organism’s internal and external environments. In the nematode Caenorhabditis elegans a variety of behavioral states have been described, including roaming, dwelling, quiescence, and episodic swimming. These states have been considered in isolation under varied experimental conditions, making it difficult to establish a unified picture of how they are regulated. Using long-term imaging, we examined C. elegans episodic behavioral states under varied mechanical and nutritional environments. We found that animals alternate between high-activity (active) and low-activity (sedentary) episodes in any mechanical environment, while the incidence of episodes and their behavioral composition depend on food levels. During active episodes, worms primarily roam, as characterized by continuous whole body movement. During sedentary episodes, animals exhibit dwelling (slower movements confined to the anterior half of the body) and quiescence (a complete lack of movement). Roaming, dwelling, and quiescent states are manifest not only through locomotory characteristics but also in pharyngeal pumping (feeding) and in egg-laying behaviors. Next, we analyzed the genetic basis of behavioral states. We found that modulation of behavioral states depends on neuropeptides and insulin-like signaling in the nervous system. Sensory neurons and the Foraging homolog EGL-4 regulate behavior through control of active/sedentary episodes. Optogenetic stimulation of dopaminergic and serotonergic neurons induced dwelling, implicating dopamine as a dwell-promoting neurotransmitter. Our findings provide a more unified description of behavioral states and suggest that perception of nutrition is a conserved mechanism for regulating animal behavior.
NEW & NOTEWORTHY One strategy by which animals adapt to their internal states and external environments is by adopting behavioral states. The roundworm Caenorhabditis elegans is an attractive model for investigating how behavioral states are genetically and neuronally controlled. Here we describe the hierarchical organization of behavioral states characterized by locomotory activity, feeding, and egg-laying. We show that decisions to engage in these behaviors are controlled by the nervous system through insulin-like signaling and the perception of food.
animals make decisions regarding behavior by integrating information about their internal states and external environments (McFarland 1977). A nearly universal strategy is to assume one or more behavioral states, defined as sets of behavioral outputs that tend to be coincidental and occur for a discrete period of time. A common example is sleep, a behavioral state occurring in many animals.
Environmental stimuli can modulate behavioral states in different ways. In mammals, the circadian regulation of sleep episodes is largely internal and is driven by an evolutionarily conserved cellular-molecular clock (Stanewsky 2003), although it may be entrained by external light-dark cycles (Roenneberg and Merrow 2007). By contrast, mammalian hibernation is a behavioral state primarily driven by external factors (e.g., low temperature, short length of day, and food shortages) rather than internal ones (Carey et al. 2003).
Behavioral states can occur exclusive of or coincident with other behavioral states. During an episode of sleep, for example, an animal may exhibit multiple distinct behavioral substates such as slow-wave sleep and random eye movement sleep (Aserinsky and Kleitman 1953). Multiple behavioral states are manifest during hibernation as well. While the primary state during hibernation is torpor, characterized by low body temperature, reduced metabolism, and the absence of locomotory activity, animals also rhythmically rouse from torpor (Michener 1977; Pengelley and Fisher 1961). The mechanisms by which these episodic behavioral states interact with one another or remain distinct are largely unclear.
The nematode Caenorhabditis elegans has become an important model for investigating the neural and molecular bases of behavior and behavioral states. Advantages of this roundworm include its genetic tractability, optical transparency, well-mapped nervous system, and a well-described behavioral repertoire including diverse locomotory (Fujiwara et al. 2002; Ghosh and Emmons 2008), egg-laying (Tong Zhou et al. 1998), and sleeplike (Sulston et al. 1983; Trojanowski and Raizen 2016) behavioral states.
Studies of C. elegans locomotory behavioral states have largely focused on behavior on standard laboratory agar plates containing food bacteria. These investigations have revealed two locomotory behavioral states, roaming and dwelling (Fujiwara et al. 2002). Roaming is characterized by rapid locomotion and infrequent turns, while dwelling is characterized by slow locomotion and frequent turns. The relative proportions of dwelling and roaming a worm exhibits are responsive to the presence of food in the environment, with food encouraging dwelling (Ben Arous et al. 2009). A third state, quiescence, is characterized by very little or no movement. Distinct forms of quiescence are observed during developmental transitions called lethargus, after cellular stress (Hill et al. 2014; Nelson et al. 2013, 2014; Raizen et al. 2008), and after feeding on rich food sources (You et al. 2008).
These three locomotory states—dwelling, roaming, and quiescence—are regulated in response to food, development, and environmental stressors by biogenic amine and neuropeptide signaling (Choi et al. 2013; Flavell et al. 2013; Hill et al. 2014; Nagy et al. 2014b; Nelson et al. 2013; Turek et al. 2013; You et al. 2008). For example, nlp-22 (Nelson et al. 2013), flp-13 (Nelson et al. 2014), and daf-7 (You et al. 2008) encode neuropeptides that promote quiescent states, pdf-1 and pdf-2 encode neuropeptides that promote wakefulness or roaming (Choi et al. 2013; Flavell et al. 2013), and serotonin-gated chloride channels encoded by mod-1 promote dwelling (Flavell et al. 2013).
In addition to displaying locomotory states as described above, C. elegans also changes its locomotory patterns according to the mechanical environment. On moist, solid surfaces worms generally move via short-wavelength, low-frequency dorsoventral undulations, a mode termed crawling. In low-viscosity liquid media, worms move with long-wavelength, high-frequency undulations, a mode called swimming (Pierce-Shimomura et al. 2008). Continuous variation in mechanical load yields a continuum of locomotory patterns, showing that locomotion is modulated continuously by mechanical loading (Berri et al. 2009; Fang-Yen et al. 2010; Lebois et al. 2012).
A behavior called episodic swimming has been described for worms immersed in liquid without food. Under such conditions, worms switch between swimming and quiescence (Ghosh and Emmons 2008, 2010). However, it is unclear why worms should display a unique behavioral state only in liquids, and it is unclear to what extent if any swimming and quiescence in liquid are comparable to other states such as roaming and dwelling on solid surfaces.
This interplay between food and the mechanical environment in determining these two pairs of behavioral states is therefore puzzling: roaming/dwelling and swimming/quiescence both involve alternation between high-activity and low-activity states. However, on solid media food seems to be required for episodic behavior, while in liquid media food is not required. On solid media the low-activity state (dwelling) consists of a relatively low but nonzero level of activity, while in liquid media the low-activity state (quiescence) is nearly motionless.
We sought to unify our understanding of episodic behavioral states and their modulation by food and the mechanical environment. We also aimed to broaden our description of these states by examining them with respect to other behaviors and by exploring their neural and genetic bases.
METHODS
Strains and worm culture.
Strains used in the study are listed in Table 1. We grew all worms on nematode growth medium (NGM) plates (Brenner 1974) seeded with Escherichia coli OP50 bacteria at either 15°C or 20°C. Experiments were performed with first-day adult hermaphrodites. For experiments with temperature-sensitive strains, worms were grown at the permissive temperature, 15°C, picked as L4 larvae, and grown at the restrictive temperature of 25°C overnight. We noted that the egg-laying defective (egl) phenotypes of egl-21(n476) worms are more severe at 25°C than at 20°C. Animals observed to be bagged (larvae hatched within the body) were discarded from activity analyses.
Table 1.
List of strains
| Strain | Genotype |
|---|---|
| N2 | Wild type |
| PR881 | osm-6(p881) |
| MT1074 | egl-4(n479) |
| NQ20 | egl-4(n479); qnEx21 [tax-4::egl-4a; unc-119::GFP] |
| CB1033 | che-2(e1033) |
| CB1124 | che-3(e1124) |
| KP2018 | egl-21(n476) |
| DA521 | egl-4(ad450gf) |
| VC461 | egl-3(gk238) |
| KP1873 | egl-3(nu349) |
| DA509 | unc-31(e928) |
| GR1337 | daf-2(e1370); njEx38[Punc-54::daf-2cDNA::Punc-54 3′UTR; Punc-54::GFP] |
| GR1336 | daf-2(e1370); njEx32[Pges-1::daf-2; Pges-1::GFP] |
| GR1339 | daf-2(e1370); mgEx376[Punc-14::daf-2;rol-6] |
| GR1340 | daf-2(e1370); mgEx373[unc-119 promoter::daf-2 cDNA::unc-54 3′UTR)] |
| GR2107 | daf-2(e1370); mgEx371[dpy-30 promoter::daf-2 cDNA::unc-54 3′UTR)] |
| YX82 | daf-2(e1370); egl-21(n476) strain 1 |
| YX84 | daf-2(e1370); egl-21(n476) strain 2 |
| CF2093 | daf-16(mu86) I; daf-2(e1370) III; muIs131 [unc-119p::GFP::daf-16 + rol-6(su1006)] |
| CF2005 | daf-16(mu86) I; daf-2(e1370) III; muIs120 [ges-1p::GFP::daf-16 + rol-6(su1006)] |
| CF2102 | daf-16(mu86) I; daf-2(e1370) III; muIs126 [myo-3p::GFP::daf-16 + rol-6(su1006)] |
| CF1724 | daf-16(mu86) I; daf-2(e1370) III; muIs105 [daf-16p::GFP::daf-16 + rol-6] |
| NQ731 | qnEx397[Punc-17:gCAMP6//SL2//dsRED + rol-6 + ladder] (roller control) |
| AQ2028 | lite-1(ce314) X; ljIs100 [Pdat-1::ChR2::YFP; unc-122::GFP] |
| AQ2050 | lite-1(ce314) X; ljIs102[tph-1::ChR2::YFP; unc-122::GFP] |
| YX121 | lite-1(ce314) X; tph-1(n4622) II; ljIs100 [Pdat-1::ChR2::YFP; unc-122::GFP] strain 1 |
| YX122 | lite-1(ce314) X; tph-1(n4622) II; ljIs100 [Pdat-1::ChR2::YFP; unc-122::GFP] strain 2 |
| ZM5016 | lin-15(n765ts) X; hpIs178[Punc17::NpHR::ECFP; lin-15(+)] |
WorMotel behavioral assays and imaging.
We conducted longitudinal behavioral assays in 3-mm-diameter concave wells of a WorMotel device made of polydimethylsiloxane molded from a three-dimensional-printed master (Churgin and Fang-Yen 2015). Worms were immersed in NGM buffer (NGMB) solutions alone or NGMB with agarose, food, or dextran as indicated. NGMB consists of the same constituents as NGM agar but without peptone, cholesterol, or agar.
For assays performed with food, a 50-ml lysogeny broth medium culture was inoculated with OP50 and grown 24 h at 20°C. An aliquot of this culture was centrifuged and resuspended, unless otherwise indicated, at a concentration 8 times that of the original liquid culture (Stiernagle 2006). Approximate optical densities (OD600) corresponding to 0.125×, 0.25×, 0.5×, 1×, 4×, and 8× were 0.11, 0.14, 0.17, 0.59, 1.1, 1.8, and 4.1, respectively. Unless indicated otherwise, all experiments marked “+food” have a concentration of 8× food.
Image sequences of worms in the WorMotel were collected with Imaging Source cameras [DMK23GP031 (2,592 × 1,944 pixels) or DMK31BU03 (1,024 × 768 pixels)] mounted to lenses (Fujinon HF12.5SA-1, 12.5-mm focal length), using darkfield red LED illumination, as previously described (Churgin and Fang-Yen 2015). Images were taken every 1 s with exposure time 1/30 s. We positioned the camera pointing upward and the wells facing downward so that when worms and bacteria settled in liquid they moved toward the camera. This orientation minimized occlusion of worms by bacteria. We conducted experiments inside a small water-cooled incubator to control the temperature.
Activity analysis.
After recording, activity calculations were performed with an image subtraction algorithm similar to that previously described (Churgin and Fang-Yen 2015). In brief, we measured worm locomotion indirectly by examining the sum of the absolute value of pixel differences between images. For each experiment we calculated the activity between each successive frame (1 s apart) as well as a second activity value for frames acquired 10 s apart (see Table 2).
Table 2.
Settings for automated assessment of behavioral states
| Fasting Conditions | Food Conditions | |
|---|---|---|
| Quiescent threshold | 1% | 0.10% |
| Active threshold | 30% | 40% |
| Time below quiescent threshold to flag Q bout | 30 s | 30 s |
| Time above active threshold to flag being of S bout | 180 s | 180 s |
| Time near beginning of assay used for scaling | 3,000 s | 10,000 s |
| Activity calculation interval | 1 s | 10 s |
| Quiescence by algorithm compared with manual | 27% underestimate | 21% overestimate |
We present most examples of individual worm behavior as actigraphs, plots of activity over time. In WorMotel imaging there is sometimes well-to-well variability in brightness, volume, or worm size [for example, egl-4(gof) mutants are small]. To compare worms to one another we calculated the mean activity for the top 5% of all activity values observed during a certain time frame (see Table 2) and normalized the data by this value. As a result of changing optical conditions during the experiment, such as the settling of bacteria, normalized activity values sometimes exceeded 1. In <0.5% of frames the camera recorded an image shifted in one direction by several hundred pixels. Either these frames were removed or spurious activity values were replaced with an average of the prior and subsequent frames.
We found that 1-s activity intervals were useful in analyzing data for fasting worms and worms immersed in low concentrations of food (Table 2), whereas for higher food concentrations analysis was performed with a 10-s interval. A 10-s time lapse improves the ability to detect head movements that occur during dwelling that might otherwise be missed in 1-s intervals.
To assess latency until the first quiescent bout, an actigraph of scaled activity was generated for each worm and the experimenter subjectively picked the first quiescent bout and recorded the time the bout began. To assess sedentary/active bout durations, the period (time between arousal and a swim/roam state) was calculated by an algorithm designed to 1) recognize and record the time at which initiation of a low-activity episode occurs by finding instances where activity falls below a low-activity threshold for at least 30 s and 2) recognize and record the arousal time point, which was defined as the time at which the average activity was above the low-activity threshold for the subsequent 180 s. The threshold value was set to the same level for every worm analyzed, and usually the low-activity threshold was 0.3 for the analysis of active and sedentary episodes. During the analysis of bout durations we ignored the first active episode and the first quiescent bout.
To approximate the amount of time spent in each of three different locomotory states, we calculated activity and used an algorithm to process data in the following steps: 1) We flagged the initiation of quiescent bouts when the average activity for the following 30-s period was below a quiescent threshold. 2) After detection of a quiescent bout worms were presumed to be in a sedentary state where entry into quiescence might be common, so all subsequent activity was scored as quiescent (below the quiescent threshold), dwelling (above the quiescent threshold), or swimming above a second threshold called the active threshold. 3) During the sedentary state the algorithm searches for arousal from the sedentary to the active episode by finding instances where the activity during the next 180 s is, on average, above the active threshold. After arousal is detected, all activity is scored as either swimming (above the active threshold) or dwelling (below the active threshold). 4) If activity falls below the quiescent threshold again for 30 s a new quiescent bout is initiated, and the worm continues to be treated as if in a sedentary episode.
Through repeating these steps in the algorithm we approximated the amount of swimming, dwelling, and quiescence without information about centroid position, curvature, or velocity that other studies have used. To validate the algorithm we manually assessed locomotory behavior in three WorMotels and observed each occupied well for three sessions of 100 s each. We then allowed the algorithm to score the same exact session that the manual observer scored. We corrected the values in Fig. 8 by a percent error that we calculated for each food concentration tested. The standard errors (SEs) shown for the three experiments in Fig. 8 were calculated as the SE of three experiments as scored by the algorithm, but then the SE values were increased by the absolute value of the percent error calculated by the experiment performed manually. Therefore the error bars shown (Fig. 8, C–E) show a conservative error range that reflects both experimental error and error compared with manually validated controls. Our manual validation process also only samples a small portion (<3% of all of the data in the experiment), and this could lead to undersampling artifacts. We did not impose a manual validation correction to data other than that shown in Fig. 8, C and D.
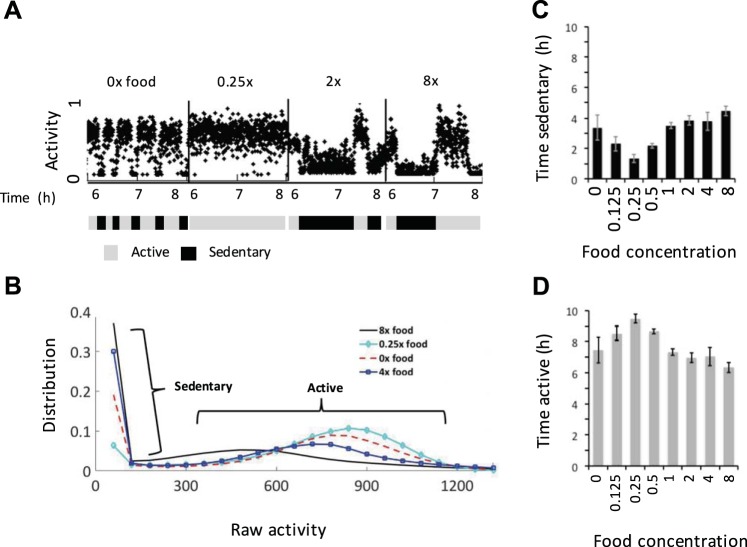
Fig. 8.
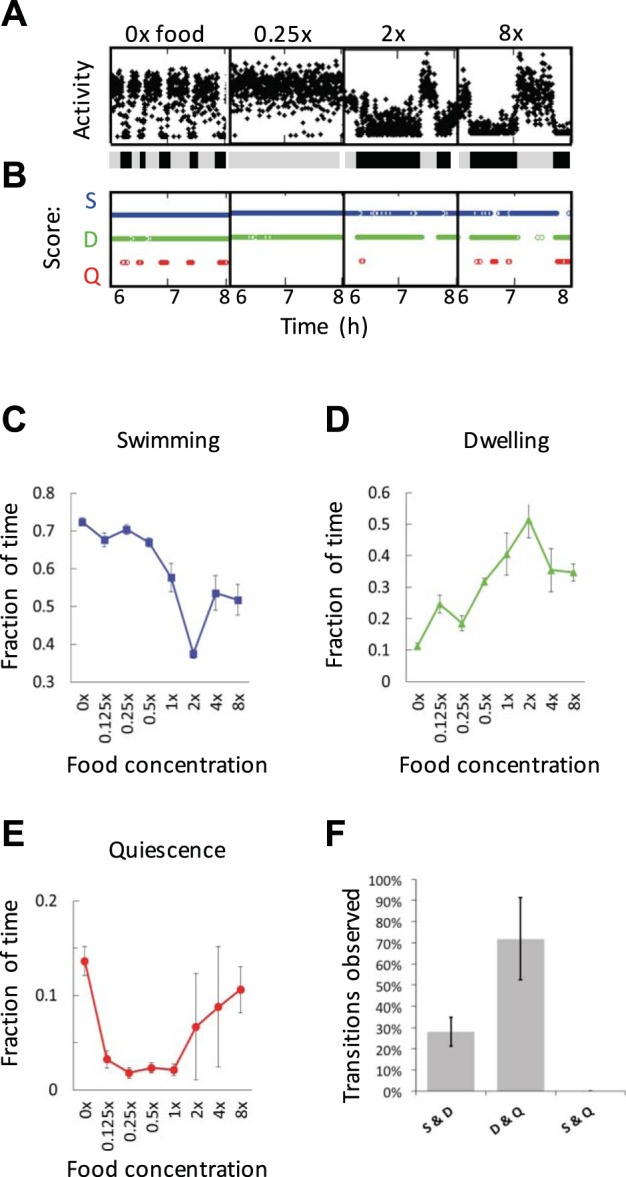
Food regulates the incidence of dwelling, roaming, and quiescence. A: same as Fig. 7A. B: automated scoring of swimming (S), dwelling (D), and quiescence (Q) based on activity corresponding to the data shown in A. A shows 2 h of activity from individual worms immersed in different concentrations of food, and B shows the same data points after being rescored as S, D, or Q. C–E: proportion of time spent in a given behavioral state for each food concentration tested. Worms in 2×, 4×, and 8× food were analyzed with high-food algorithm conditions and the rest with low-food algorithm conditions (see methods and Table 2). The values and error markers show means and SE of 5 experiments totaling ~24 worms for each condition, and values were adjusted by a % of error calculated by comparing algorithm scores to manual scoring. F: relative % of behavioral transitions observed. Direction of transitions was not recorded. Error bars represent the SE of 3 experiments containing a total of 403 sessions from 3 experiments.
To manually assess locomotory state we examined each worm in three sessions of 100 s 1 h apart and used certain criteria to score behavioral state. 1) Moving worms were scored as either swimming or dwelling, excluding only movements of the tip of the nose or rare twitches. 2) For posture, partial paralysis or lack of curvature in the posterior of the body during movement was scored as dwelling whereas full body movement was scored as swimming. 3) Nonmoving worms were quiescent if they remained nonmoving for 10 s. Movement such as a single twitch that was not succeeded by arousal was ignored, and movement of worms floating passively through liquid without postural changes was also ignored and considered as quiescence.
Asterisks and bars in figures generally represent comparison of two groups with an unpaired Student’s t-test. Groups compared were comprised of the means of experimental repeats of at least two experiments, and bars representing the standard error of the mean (SE) show the SE of experiments. All other types of comparisons are specifically noted. For example, SE bars and statistical comparisons were performed on n worms such as in Fig. 1, D and E.
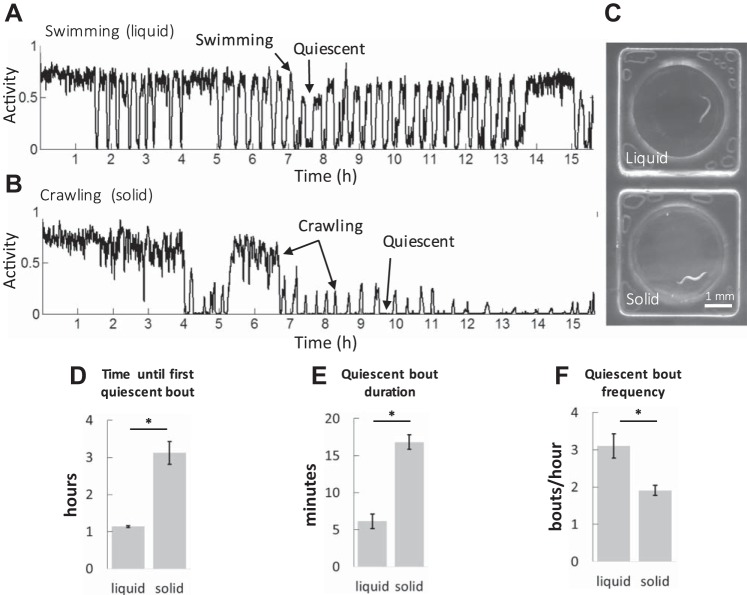
Fig. 1.
Fasting worms enter quiescence in different mechanical environments. “Activity” here and elsewhere in this study is given in arbitrary units scaled to relative body size (see methods). Actigraphs showing a single line plot (A and B) represent activity averaged each minute. A: example activity in NGMB. B: example activity in NGMB with 2% agarose. For each locomotory state indicated an arrow points to an example of corresponding activity. C: darkfield images of worms in a single WorMotel well in the liquid or solid condition. The images show representative postures for moving worms characteristic for each mechanical environment. D: latency until the onset of the first quiescent bout. n > 30 worms for each condition. Error bars show the SE of 2 experimental means, P = 0.02. E and F: bars show the error of the mean of n = 7 worms with 79 quiescent bouts and n = 20 worms with 115 quiescent bouts in liquid or solid, respectively. The time frame examined was hours 6–10 of the experiment. *P < 0.001.
Nontargeting analysis of curvature and undulation frequency analysis.
To assess worm curvature we picked worms into the appropriate NGMB solution and sandwiched worms between two microscope slides separated by 80- to 100-µm-thick plastic shims. In this setup worms’ locomotion was largely restricted to two dimensions. NGMB contained food (see Fig. 4C, dwelling, and Fig. 9) or no food (see Fig. 4C, swimming and quiescence). Locomotion was then recorded at 30 frames per second (fps) with a DMK22BUC03 camera (Imaging Source). Curvature analysis was performed with custom MATLAB scripts as previously described (Fang-Yen et al. 2010). To assess undulation frequency, worms were prepared as described above with food present and allowed to incubate for 2 h in a humidified chamber before video recording. Worms’ behavioral state was subjectively assessed based on posture and movement. Time between head undulations was counted manually from image sequences. For optogenetic analysis of curvature we illuminated worms with a fluorescence stereo microscope for 10 s, using green fluorescent protein (GFP; for blue light) or DsRed (for green light) filters. Irradiance was 0.1 mW/mm2 for blue light and 0.3 mW/mm2 for green light. For optogenetic analysis of undulation frequency we illuminated worms for 15 s. To test whether optogenetic stimulation could induce a sedentary bout we illuminated worms for 5 s at 0.57 mW/mm2. Ptph-1::ChR2 worms showed no response to optogenetic manipulation immediately after being picked to liquid, so they were allowed to acclimate for 15 min before experimentation.
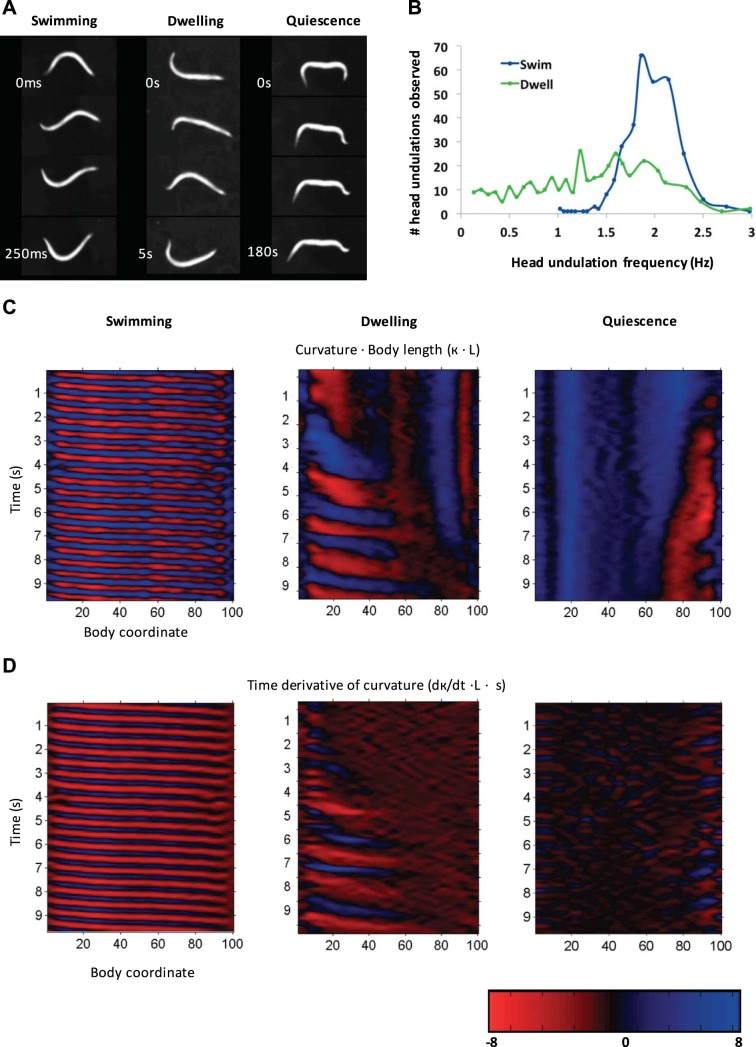
Fig. 4.
Locomotion and head undulation frequency differ by behavioral state. A: representative images of worms in the indicated behavioral state. Anterior is to left. Swimming worms’ curvature varies from anterior to posterior as their whole body bends, but dwelling worms lack bending in the posterior. Quiescent worms have almost no movement, but their body changes posture through relaxation and occasional twitching. B: head undulation frequency of worms in either the swimming or dwelling state suggests that dwelling worms have a broader range of head undulation speeds including some speeds similar to crawling worms even though these worms are in liquid. The histogram represents data from n = 16/11 (swimming/dwelling) worms and n = 376/312 undulations. C and D: curvature analysis of the same worms from A over 9 s across the whole body. Red and blue color map indicates curvature (C) or time derivative of curvature (D) in the dorsal and ventral directions, respectively.
Fig. 9.
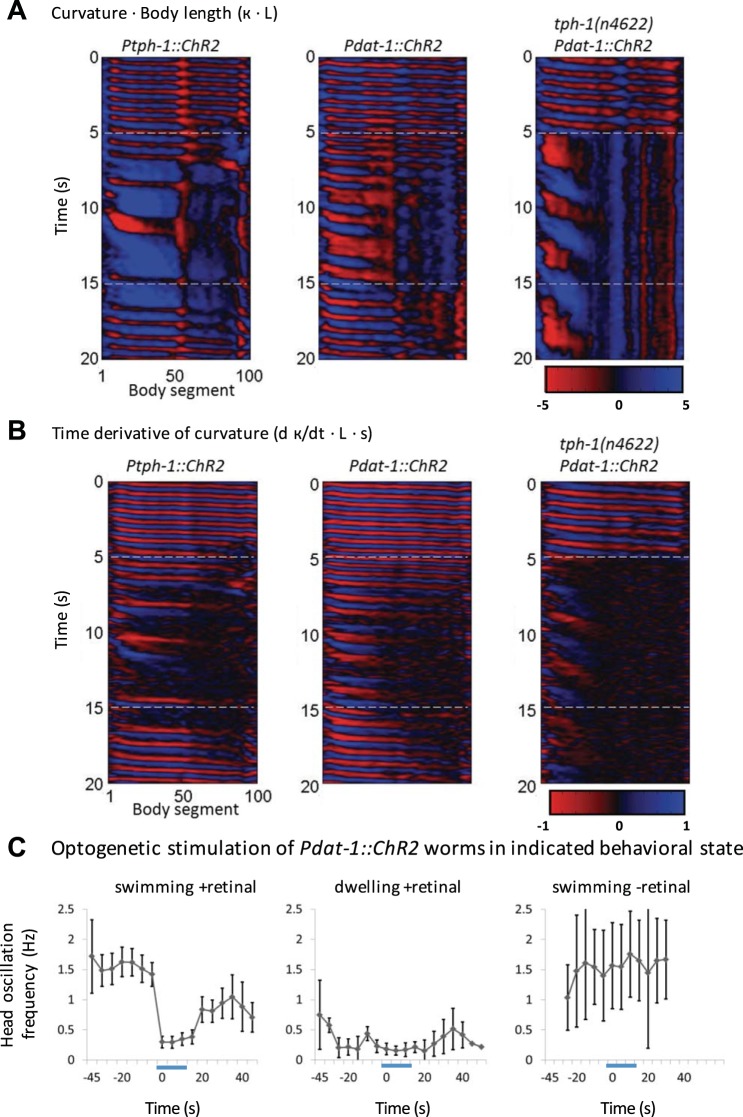
Stimulation of dopaminergic and serotonergic neurons produces dwelling locomotory characteristics. A: curvature of a representative worm of the indicated genotype. B: time derivative of curvature for the same worm. 17/20, 11/11, and 11/11 worms of the indicated genotypes showed slowing in response to blue light for the respective genotypes shown. C: head movement frequency was assessed in worms expressing ChR2 in dopaminergic neurons before, during, and after optogenetic stimulation. Worms were stimulated after they were assessed to be either swimming or dwelling. n = 20, 9, and 3 worms, respectively.
Targeted optogenetic illumination.
To illuminate specific neurons in freely moving Pdat-1::ChR2 or Ptph-1::ChR2 animals, we used a system for illuminating sections of the body in freely moving worms similar to that previously described (Leifer et al. 2011). Blue light was provided by a diode-pumped solid-state laser (Shanghai Laser & Optics Century BL473T3, 473-nm wavelength). For each region-specific experiment, ~20 animals were added to a chamber composed of two microscope slides separated by a 80- to 100-µm-thick plastic spacer and containing 20% by mass dextran in NGMB solution lacking food. Worms were selected for illumination if they were swimming forward and illuminated once for 8 s along the indicated fraction of the body. After 20 illuminations (~20 worms), the chamber was discarded. A minimum of eight trials were conducted for each illumination condition.
Curvature maps of the worm center line were computed for each worm in real time and saved to disk with each video frame. The amplitude and frequency of undulation during illuminations (between 1 and 3.5 s after the 8-s illumination started) were computed with a Fourier analysis of the time derivative of the length-normalized curvature data.
Pump rate analysis.
To measure pump rates in liquid we manually followed the movement of worms magnified ×100, using a motorized stage with joystick control while simultaneously recording the worm head and pharynx at 100 fps with a Phantom v9.1 camera (Vision Research). We counted pumps manually by reviewing videos at 5 fps.
Egg-laying assays.
For liquid media, worms were recorded in darkfield in upside-down WorMotels containing NGMB and food at a magnification of 7 µm/pixel. This magnification was adequate for detecting eggs. Videos were then manually reviewed to record the time of every egg-laying event. For solid media, we recorded single adult worms in brightfield overnight on ~60-mm-diameter bacterial lawns at a magnification of 6.3 µm/pixel and calculated relative activity for the entire assay. We also calculated the instantaneous velocity every 10 s based on centroid position within a 3-h window. The time of egg-laying events was scored manually by watching recorded videos, and worm velocity was calculated by calculating the change in centroid position after each 10-s interval. To assess the activity and/or velocity surrounding individual egg-laying events we registered activity or velocity values to the frame in which an egg-laying event occurred.
RESULTS
Animals become quiescent during fasting in any mechanical environment.
Worms initiate short bouts of quiescence when immersed in liquid environments, in which swimming is the primary mode of active movement. However, similar bouts were not observed on solid media (Ghosh and Emmons 2008). It has therefore been proposed that this behavioral pattern could be called episodic swimming because animals alternate between swimming and quiescent behavioral states. However, it is unclear why worms in a liquid environment should display unique behavioral states since C. elegans can grow and survive in liquid culture.
To better understand the interaction between mechanical environment and episodic swimming/quiescent behavior, we monitored worms in liquid or solid media lacking food longitudinally for up to 16 h. Previous investigations used similar conditions but only monitored worms for 4 h (Ghosh and Emmons 2010).
In both solid and liquid media, fasting worms exhibited an initial period of active movement followed by alternation between high activity and quiescence (Fig. 1, A and B; Supplemental Videos S1 and S2). [Supplemental Material for this article is available online at the Journal website.] The onset of the first quiescent episode was delayed when worms were on solid compared with liquid media (Fig. 1D).
We calculated the average quiescent episode duration and frequency during hours 6–10 of the assay. We found that in liquid media worms have, on average, ~5-min-long quiescent episodes, occurring approximately three times per hour, consistent with previous reports (Ghosh and Emmons 2008). Worms on solid media displayed ~16-min-long quiescent bouts occurring approximately two times per hour (Fig. 1, E and F).
We observed that many quiescent episodes greatly increased in duration after 10 h. We quantified this effect for worms in the liquid environment lacking food (Fig. 2A). We found that quiescent bout durations increased approximately fourfold by the end of a 16-h assay (Fig. 2B) whereas swimming bout durations changed relatively little until after 14 h (Fig. 2C).
Fig. 2.
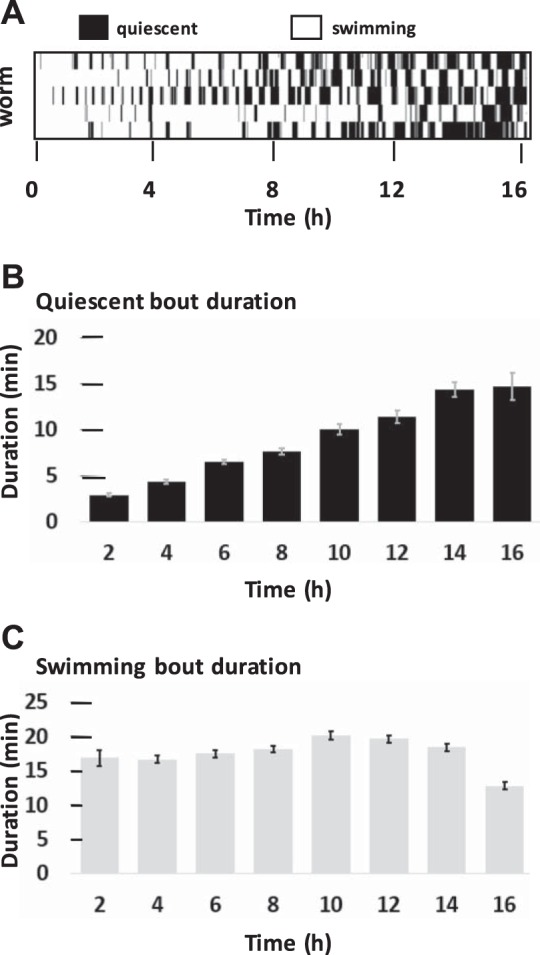
Quiescent bouts increase in duration with continued fasting. A: example binary activity plot showing swimming in white and quiescence in black generated by simple thresholding of activity data. Each row represents 1 worm. B: average quiescent episode duration during 2 h before the number shown on the x-axis (from hours 0–2, 2–4, etc.). For each bar, n = 59–223 episodes from 20 worms. C: swimming episode durations represented as in B. Swimming episodes were calculated by an algorithm imposing requirements for both time and activity level relative to a threshold (see methods).
We also performed experiments with worms in viscous dextran solutions (Fang-Yen et al. 2010) representing an intermediate point between solid and liquid assays. We found that, as in liquid or solid locomotion, worms in viscous solutions exhibited episodic alternation between movement and quiescence (data not shown).
We hypothesized that locomotory quiescence might be driven by a lack of food as opposed to the mechanical environment. To test this idea, we examined worms in the presence of food in liquid and found that this factor greatly changed behavior (Fig. 3; described further below). However, bacteria-conditioned culture media did not alter behavior (data not shown). These data show that food absence enables the specific pattern of alternation between swimming and quiescence.
Fig. 3.
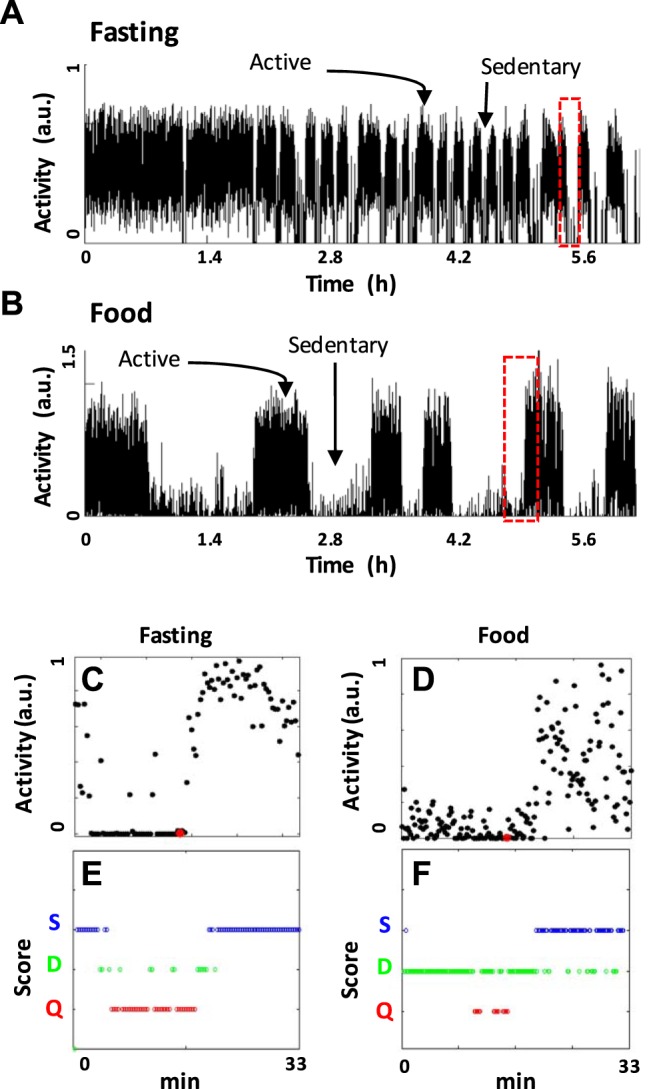
Example data and algorithm outputs used to calculate metrics about sedentary/active episodes and to quantify quiescent, dwelling, and swimming behavior. A and B: actigraphs of activity [in arbitrary units (a.u.)] scaled to the top 5% of activity displayed in the first 2.7 h of the assay with no temporal averaging. The graph shows black lines that connect the position between subsequently plotted data points. Data were plotted every 1 s. A: a representative fasting worm. B: a representative worm with 8× food present. The red highlighted regions in A and B are expanded in C–F. C and D: scaled activity as black dots plotted every 10 s. A red dot marks the algorithmically determined end of a sedentary episode and the beginning of an active episode. E and F: algorithmic scoring locomotory state. Plots show small open circular dots that each represent a unique score for the time point observed. Colored circles correspond to quiescence (red), dwelling (green), or swimming (blue). In these plots dots and circles are oversized and appear to overlap in the plot, but each time point is given only 1 score.
These results show that episodic alternation between movement and quiescence is not unique to liquid environments but occurs in arbitrary mechanical environments. The mechanical environment does affect the duration, frequency, and initial latency of quiescent episodes. Therefore the mechanical environment influences episodicity but is not a deterministic factor required to induce the quiescent state. Since this behavioral pattern is suppressed by the addition of food, we suggest that bouts of quiescence induced by fasting should more properly be called fasting-induced quiescence.
Worms display episodic alternation between active and sedentary states.
We have shown that fasting worms alternate between relatively active and inactive behavioral states. However, this behavioral pattern may not be unique to fasting, since it has been observed that worms grown with food switch between roaming and dwelling (Fujiwara et al. 2002). We asked whether worms in liquid containing food also display episodic behavior.
First, we examined worm behavior in liquid media containing food (E. coli OP50) at concentrations similar to those used for liquid growth media (Stiernagle 2006). Like fasting worms (Fig. 3A), fed worms swam continuously during an initial period but eventually exhibited episodes of low activity (Fig. 3B). However, unlike fasting worms, which became nearly motionless (Fig. 3, C and E), fed animals maintained a low level of activity (Fig. 3, D and F), primarily in the form of continued movement of the anterior of the body while the posterior was quiescent (Fig. 4; Supplemental Videos S3 and S4). Episode alternation was continuous during our assays, but we noted that the duration of low-activity episodes increased over time (data not shown). Activity during low-activity episodes was typically between 1% and 40% of swimming activity (Fig. 3D), compared with below<1% for quiescent worms. Overall, low-activity behavior resembles dwelling (Fujiwara et al. 2002), a behavior characterized by slow locomotion or movements restricted to the anterior of the animal and that increases in the presence of food (Ben Arous et al. 2009; Shtonda and Avery 2006).
Our results show that in either the presence or absence of food animals alternate between high-activity and low-activity episodes. Moreover, our analyses so far show that episodes may contain multiple locomotory behaviors. Specifically, during a low-activity episode a worm exhibits both locomotory quiescence and dwelling (Fig. 3F), while during a high-activity episode a worm exhibits swimming and some dwelling. For clarity, we call high-activity and low-activity periods active and sedentary episodes, respectively, independent of the food conditions.
Roaming, dwelling, and quiescent worms are distinguished by their locomotory characteristics.
To gain more insight into roaming, dwelling, and quiescent behavioral states in liquid media, we sought to examine their characteristics in more detail.
Our first observation was that during active episodes worms are predominantly swimming, suggesting that swimming may represent a locomotory mode equivalent to roaming in liquid (Fig. 4A; Supplemental Video S5). As previously shown, swimming worms exhibited relatively low-curvature postures (Fang-Yen et al. 2010; Pierce-Shimomura et al. 2008) in both the presence and absence of food (Fig. 4, C and D), with anterior-to-posterior undulatory waves that propagated through the entire body, with frequency ranging over 1.5–2.5 Hz (Fig. 4B). Henceforth, we refer to this type of locomotion in liquid interchangeably as either swimming or roaming.
Next we examined the locomotory characteristics of dwelling worms. We reasoned that during sedentary episodes if worms are not quiescent they might be dwelling. Previously dwelling on solid media has been characterized by low speed, high turning rate, and the animal remaining within a restricted area (Fujiwara et al. 2002). In our experiments, we observed that worms in sedentary episodes remained in a restricted area often with a hockey stick-like posture in which the body is nearly straight except for bending in the head (Fig. 4A). Undulatory waves propagated only about halfway through the body so that the posterior appeared paralyzed (Fig. 4, C and D; Supplemental Video S6), and the frequency of head undulations is variable, ranging from 0.1 to 2.5 Hz (Fig. 4B). Overall, our observations of moving worms in sedentary episodes suggests that they are dwelling. Therefore we chose to define the liquid dwelling locomotory characteristics as reduced locomotion, continued head movement, and lack of curvature propagation in the posterior (Fig. 4C). These characteristics are consistent with observations of dwelling in “artificial dirt” devices composed of microfabricated hexagonal post arrays filled with liquid (Lockery et al. 2008; Nagy et al. 2014a).
During fasting-induced quiescence, worms were completely immobile. Frequently both the head and tail or only the head remained bent during the quiescent episode, and the body slowly straightened (Fig. 4A), often ending in a characteristic hockey stick-like posture (see Supplemental Video S1 at the 7 min 42 s mark) (Ghosh and Emmons 2008; Tramm et al. 2014).
These findings show that swimming, dwelling, and quiescent worms display distinct postural and locomotory patterns. Our observation that dwelling worms have a straightened posterior posture in liquid is useful since a similar posture is not often observed on solid media. This difference may reflect the capillary forces exerted on the worm from the liquid/air interface on moist solid media, which does not exist in liquid media.
Feeding characteristics differ between quiescent and nonquiescent animals.
Since food status is important for determining behavioral state, we reasoned that these behavioral states might differ not only in locomotion but also in feeding behavior. C. elegans feeds through rhythmic contractions and relaxations (pumping) of the pharynx, a double-bulbed neuromuscular tube connecting the buccal cavity and intestine (Avery and Shtonda 2003; Fang-Yen et al. 2009).
Measuring feeding behavior in liquid is challenging because of the animals’ rapid movement during swimming. There are reports that worms suppress feeding while swimming (Vidal-Gadea et al. 2012). However, worms are routinely maintained in liquid culture in which they can both feed and reproduce (Avery and Horvitz 1990; Stiernagle 2006).
To explore how swimming affects feeding behavior, we immersed worms in NGMB containing bacteria (see methods) and imaged worms at 100 fps for 10 s. We found that immediately after transfer from plates to liquid worms cease pharyngeal pumping (Fig. 5A). However, pumping rates return to normal over time (Supplemental Videos S7–S10). Resumption of feeding occurred after 15 min in some animals. All worms resumed pumping within 2 h. These results show that the feeding is temporarily inhibited when a worm enters a liquid environment.
Fig. 5.
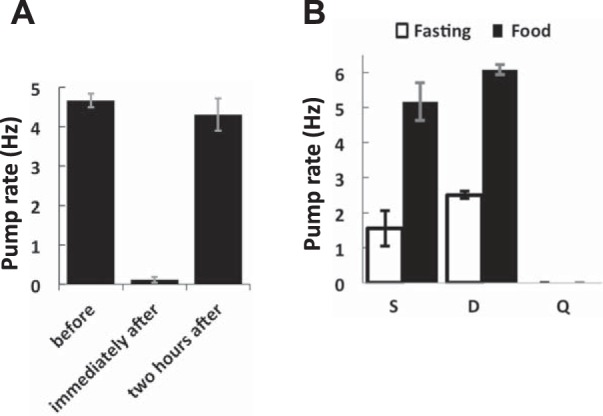
Feeding characteristics differ by behavioral state. A: pump rate was assessed before, immediately after, or 2 h after being picked from standard solid media with food into NGMB containing food. n = 5 worms. B: worms were immersed in NGMB with or without food and mounted on slides, where they incubated for 2 h. After 2 h worms on a given slide could be identified as swimming (S), dwelling (D), or quiescent (Q) based on locomotion. After finding and centering on worms their behavior was immediately recorded for 10 s at 100 Hz, and movies were reviewed at a later time to assess the pump rate. Number of worms observed in the indicated behavioral state for each condition from left to right: n = 9, 10, 2, 13, 13, and 3. n(fed) = 26; n(fasted) = 24. Error bars show SE.
Next, we assessed pharyngeal pumping rates during roaming, dwelling, and fasting-induced quiescence. Worms were immersed in liquid either with or without food for 2 h. We observed worms on a microscope to assay their locomotory behavioral state, and recorded videos for 10 s. Fed roaming worms in liquid displayed pharyngeal pumping rates similar to those on solid media (5.2 ± 0.5 Hz; Fig. 5B). Fasting roaming worms pumped at a lower rate (1.6 ± 0.5 Hz). Fed dwelling worms pumped at a rate not significantly different from that of roaming worms (6.1 ± 0.1 Hz; Fig. 5B), suggesting that pumping is not differentially regulated when worms alternate between these two states. In a few rare instances (2/24 observations of fasting worms) fasting worms’ locomotion was equivalent to dwelling. We observed that the pump rate for such fasting dwellers was 2.5 ± 0.1 Hz. Quiescent worms exhibited no pharyngeal pumping (n = 13). In a few (3/26) cases we also observed quiescent worms in the presence of food, and these worms also had no pumping. It is possible that these worms may have been in bouts of satiety quiescence (You et al. 2008).
These results show that pharyngeal pumping proceeds at similar rates in roaming and dwelling worms but ceases during locomotory quiescence. Therefore the presence or absence of pharyngeal pumping, in addition to locomotory characteristics, is a reflection of a worm’s behavioral state.
Egg-laying occurs preferentially during roaming of active episodes.
Next, we asked whether C. elegans egg-laying behavior, like feeding, is also modulated by behavioral state. Previous work has shown that three states characterize egg-laying (Tong Zhou et al. 1998). In the inactive state (I) worms do not lay eggs. A second state is the active state (A), which seems to be a permissive state for egg-laying. While in state A worms can enter the third state (E), which corresponds to the moment eggs are laid (Tong Zhou et al. 1998). The inactive and active states are separated by relatively long time intervals (20 min), and the duration of the states can be genetically manipulated (Hardaker et al. 2001; Tong Zhou et al. 1998; Waggoner et al. 1998). Given that active and sedentary episodes last for comparable durations when food is present (active: 25–30 min, sedentary: 11–29 min), we hypothesized that active and sedentary episodes correspond to egg-laying states A and I, respectively.
To test this idea, we recorded videos of individual worms in liquid, where active and sedentary episodes are easily discerned while recording egg-laying events (Supplemental Video S11), and registered egg-laying events to activity (Fig. 6A). We observed that egg-laying was associated with active episodes: 93% (125 of 135) egg-laying events occurred during swimming episodes, and the remaining 7% occurred within 1 min of the beginning or end of active episodes. Moreover, all egg-laying events occurred when worms were swimming/roaming, not dwelling. Worms were sedentary about one-third of the time during these assays, so if egg-laying were randomly timed, only about two-thirds of egg-laying events would occur during the active episode.
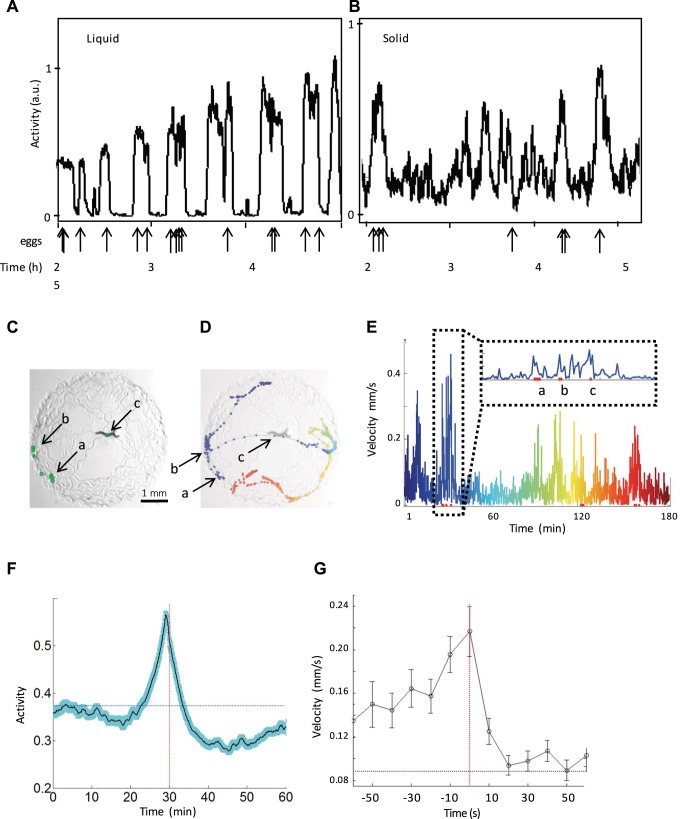
Fig. 6.
Egg-laying preferentially occurs during active episodes. A and B: actigraph showing activity for young adult worms in either liquid (in WorMotel; A) or solid (a standard NGM plate with a small lawn; B) media. Arrows below the x-axes point to manually scored egg-laying events. Ticks below these 2 graphs mark hours 2, 3, 4, and 5 of the assay, which is shown below the arrows. B: worm velocity was calculated every 10 s. C–E: an example of egg-laying events that are spread out spatially but occur during the same roaming episode. C: adult worm roaming. Black arrows labeled a, b, and c point to eggs laid (green ovals) within the same active episode; a, b, and c are also labeled in D and E. D: worm tracks marked by colored dots that begin at blue and end at red, overlaid on the image from C. The velocity observed at each colored point in time corresponds to the plot displayed in E. E: worm velocity of the worm shown in B–D calculated by tracking the centroid position of the worm every 10 s. Red dots at the base of the graph mark egg-laying events that correspond to the arrows shown in B. An expanded section shows a single active episode during which multiple egg-laying events occur. A chronologically coordinated color scale, ranging from blue to red, matches the colored dots that mark position of the worm on the lawn shown in D. F: activity alignment for the 30 min before and after egg-laying events; data from n = 361 events from 10 worms. The black line shows average activity, and the shaded cyan region shows standard error of the mean of aligned events. The horizontal blue dotted line shows the average activity observed for all activity values included in the experiment, and the vertical red dotted line marks the point to which all egg-laying events were aligned. G: velocity immediately before and after egg-laying in a 1-min window. The red line marks egg-lay timing, and the blue line marks the average velocity observed throughout the experiment including data outside the 1-min window shown.
Our results show that egg-laying events occur during the roaming period of active episodes in liquid, but we also noted that dwelling worms have little bending near the vulva during sedentary episodes because of a characteristic lack of posterior bending. One possibility is that a bent body posture increases egg-laying rate. If this is true, we reasoned that the correlation between active episodes and egg-laying might only be present in liquid.
To address this concern, we asked whether this result also holds true on solid media, where egg-laying active and egg-laying inactive behavioral states were originally described (Tong Zhou et al. 1998). For this assay we calculated both activity and centroid velocity. Velocity has been previously used as a correlate of roaming behavior (Flavell et al. 2013; Fujiwara et al. 2002; Gallagher et al. 2013a). We observed that egg-laying events often coincide with activity peaks as well as with high-velocity peaks (Fig. 6, B and E). An example worm is shown in Fig. 6 that, during course of several minutes of high-velocity movement, lays eggs in three locations on the plate within a short time (Fig. 6, C–E), suggesting that the worm is both in an active episode and also roaming. An aggregate analysis of all egg-laying and activity data (Fig. 6F) shows that worms’ activity is higher than average for up to 7 min before egg-laying and that afterwards activity is higher than average for ~3 more minutes. This result shows that on solid media, as in liquid media, worms lay eggs during active episodes.
We note that animals lay eggs near peaks in activity, suggesting that egg-laying occurs near the middle of active episodes as opposed to during transitions between behavioral states. However, we also calculated velocity relative to an egg-laying event in a shorter 1-min window surrounding egg-laying events and noted that animals immediately slow after laying an egg (Fig. 6G). This suggests that while animals are in an active episode and probably roaming immediately before laying an egg, they may change their behavior to reduce velocity immediately after laying an egg while maintaining a high activity level. Although we did not quantify worm locomotion immediately after egg-laying, the observation that activity remains high after egg-laying but velocity immediately declines suggests that animals begin to turn more frequently or switch between forward and reverse locomotion after laying eggs.
Together our results show that C. elegans egg-laying occurs preferentially during roaming in active episodes. Our data strongly suggest that the active and sedentary episodes we describe here correspond to the previously defined states A and I (Tong Zhou et al. 1998; Waggoner et al. 1998).
Architecture of behavioral states is regulated by food levels.
Increase in food levels or food quality promotes dwelling in C. elegans (Ben Arous et al. 2009; Shtonda and Avery 2006). Previous studies examined overall dwelling and did not monitor the incidence of sedentary episodes, during which worms are often dwelling. Furthermore, prior work has treated food presence as a binary quantity and has not quantified how behavior depends on food availability in a quantitative manner. To address these limitations, we sought to determine the incidence of locomotory states as a function of food concentration.
We recorded worm behavior in food concentrations ranging from 0× to 8×, relative to a standard liquid culture concentration (see methods), and then calculated activity and automatically scored the episode state and the locomotory state for individual animals as previously shown (Fig. 2). For a subset of the data, we also performed manual scoring of behavioral state (see methods) to validate the automated assessment.
We found that increasing food concentrations generally increases sedentary behavior (Fig. 7). However, there is one exception: sedentary behavior decreases when food concentration goes from zero to a small but nonzero value (Fig. 7, B–D). This result suggests that sedentary behavior is promoted both by food and by fasting.
Fig. 7.
Food regulates the incidence of dwelling, roaming, and quiescence. A: activity in several food concentrations. Bottom: bars mark active (gray) and sedentary (black) episodes. B: histograms showing a distribution of unscaled activity (x) for observations (y) every 1 s for 11 h. C and D: average time spent either sedentary or active during a 10.3-h period. Means and SE of 3 experiments.
We found that the amount of quiescence also has a nonmonotonic dependence on food concentration (Fig. 8, B and E). Quiescence is highest for worms on zero or high concentrations of food and lowest for intermediate concentrations. The peak of quiescence at zero food is consistent with the previous findings that fasting induces quiescence. The finding that quiescence increases at high food concentration suggests that worms are experiencing satiety quiescence.
Consistent with past observations that food edibility influences roaming and dwelling amounts (Ben Arous et al. 2009), we found that lower food increases the occurrence of roaming/swimming and that increased food increases dwelling (Fig. 8, C and D).
Transitions between behaviors can yield insights into how they are regulated (Roberts et al. 2016). We sought to determine how worms transition between roaming, dwelling, and quiescence. Using a manual analysis of locomotory state (403 samples of 100 s each), we found that worms show a bias when transitioning between locomotory states (Fig. 8F). We often observed that worms’ transitions were between dwelling and quiescence (n = 68) and between dwelling and swimming (n = 27) (Fig. 8F). However, we did not observe any direct transitions between swimming and quiescence. In six samples we observed all three locomotory states in the same 100-s video. In each of these six cases animals transitioned into dwelling for an average of 40 ± 10 s before entering the next behavioral state. These results suggest that dwelling is an intermediate locomotory state through which worms must pass when moving between roaming and quiescence.
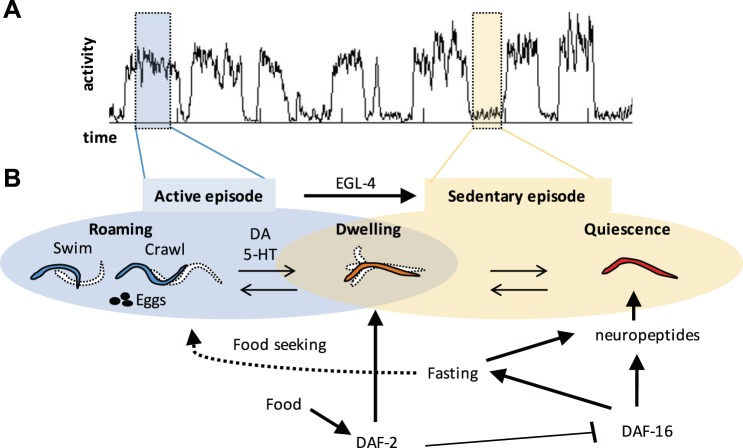
We observed dwelling during sedentary episodes and during transitions between active and sedentary episodes but next wondered whether dwelling occurred during active episodes, since our automatic scoring finds dwelling during active episodes. Dwelling during active episodes would be consistent with previous results showing that worms can abruptly switch between active wakefulness and quiet wakefulness (Nagy et al. 2013). To investigate, we examined WorMotel video recordings at food concentrations at which worms were predominantly active (from 0.5× to 0.125× food) and looked for dwelling. We found that in 49/144 (34%) of sessions observed at these food concentrations worms were dwelling for the entire 1.6-min session. This amount of dwelling is higher than the expected amount of sedentary behavior, an average of 18.9% across these concentrations (Fig. 7C). We also observed worms switching in brightfield videos (see Supplemental Video S4, worm arriving from the top). These results confirm that dwelling type locomotion occurs during active episodes but can have high activity values, which we can refer to as “active dwelling” (Table 3). A summary of the relationships observed between active/sedentary episodes and corresponding locomotion, feeding, and egg-laying behaviors is given in Table 3 and Fig. 16. Overall our results show that roaming, dwelling, and quiescent locomotory states are nested within active and sedentary episodic states.
Table 3.
Characteristics of active and sedentary episodes
| Active Episode |
Sedentary Episode |
|||
|---|---|---|---|---|
| Roaming | Active dwelling | Sedentary dwelling | Quiescence | |
| Activity | High | High/intermediate | Low | Low/none |
| Locomotion | High | High/intermediate | Intermediate | Low/none |
| ΔCurvature | High | Anterior (high), posterior (low) | Anterior (high), posterior (low) | Low/none |
| Pumping (food present) | High | High* | High | Low/none |
| Pumping (food absent) | Low | Low* | Low | Low/none |
| Egg-laying | High | High | Low/none | Low/none |
Predicted result; no actual observation was made.
Fig. 16.
A: actigraph of wild-type worms from (also shown in Fig. 7) in the presence of 8× food. An active episode (highlighted in blue on left) and a sedentary episode (highlighted in yellow on right) are indicated. B: expected and/or stereotyped locomotory states expected to be observed in an active episode (blue highlighted bubble) or a sedentary episode (yellow highlighted bubble). Black arrows pointing between locomotory behavioral states show possible transitions. Other black lines with arrow (promoting) or bar (inhibiting) network together different factors influencing decisions over behavioral state.
Stimulation of specific dopaminergic and serotonergic neurons induces dwelling locomotion without inducing sedentary episodes.
Of the three locomotory states, dwelling is the least well understood. Our results show that dwelling worms display distinct locomotory characteristics in liquid (Fig. 4). We sought to use these characteristics to better understand the circuits and cells that promote dwelling. To this end, we examined the behavior of worms during Channelrhodopsin-2 (ChR2)-mediated optogenetic stimulation of single or multiple neuron types.
Serotonergic neurons have been shown to promote transitions to dwelling states (Flavell et al. 2013). Moreover, acute optogenetic stimulation of serotonergic neurons causes slowing (Ezcurra et al. 2011; Iwanir et al. 2016). We sought to examine dwelling induction in a liquid medium context and to identify other pathways that promote dwelling.
We first examined locomotion in liquid during and after optogenetic activation of all serotonergic neurons (Ezcurra et al. 2011) in liquid. We found that stimulation of serotonergic neurons caused a transition to dwellinglike locomotory behavior in which head undulation frequency is reduced and curvature propagation toward the posterior is inhibited. Seventeen of twenty worms displayed slowing. Locomotion of worms lacking all-trans-retinal, a cofactor required for ChR2 function, showed no change upon illumination (data not shown). These results confirm that optogenetic activation of dwell-promoting circuits, previously shown to induce dwelling on solid media (Flavell et al. 2013), also promotes dwelling locomotory characteristics in liquid.
Like the serotonergic neurons, the dopaminergic neurons have been described as causing slowing. Dopamine has been reported to act as a food signal to modulate chemosensory behaviors (Ezcurra et al. 2011; Sawin et al. 2000). We asked whether dopamine, like serotonin, might also induce a transition from swimming to dwelling.
When we optogenetically stimulated dopaminergic neurons in swimming worms, we found that stimulation of dopaminergic neurons induced dwellinglike behavior, in the form of inhibited curvature propagation in the posterior of the animal (Fig. 9, A and B). Although multiple neurotransmitters may be involved, this result suggests that release of dopamine, like serotonin, promotes dwelling.
We sought to determine whether the dwellinglike behavior induced by stimulation of serotonergic or dopaminergic neurons can be distinguished from slowed but nondwelling locomotion. In particular, we asked whether slowing in liquid is necessarily accompanied by loss of curvature propagation in the worm’s posterior.
We compared optogenetic stimulation of dopamine release with optogenetic inhibition of cholinergic neurons under otherwise identical experimental conditions (see methods). Dopaminergic neuron stimulation produced a typical dwell-like slowing including cessation of posterior wave propagation in 10 of 10 worms. By contrast, seven of seven worms expressing Halorhodopsin in cholinergic neurons (Punc-17::NpHR) (Wen et al. 2012) exhibited slowing but maintained posterior wave propagation (3 worms reversed, and slowing was not assessed) (data not shown). However, since cholinergic neuron inhibition slowed worms’ head undulation frequency to a lesser degree than dopaminergic neuron stimulation, we also cannot rule out a model in which the magnitude of slowing has an effect on wave propagation in the animal posterior. These observations support the finding that stimulation of dopaminergic neurons induces dwelling in C. elegans.
It has been reported that serotonin-dependent slowing behavior occurs independently of dopamine production (Iwanir et al. 2016; Sawin et al. 2000), suggesting that serotonin-dependent dwelling (Flavell et al. 2013) is also likely to be independent of dopamine. We asked the complementary question of whether dopaminergic neuron stimulation could induce dwelling in mutants deficient for serotonin production. We examined worms expressing dopaminergic ChR2 that were homozygous for the presumptive null mutation tph-1(n4622) in tryptophan hydroxylase, required for serotonin production (Sze et al. 2000). We found that stimulation of dopaminergic neurons induces slowing even in tph-1 mutants (Fig. 9, A and B).
Overall, these observations confirm that serotonergic neuron activity induces dwelling and show that dopaminergic neurons also induce dwelling in a manner independent of serotonin.
It was previously shown that the inhibition of sensory neurons expressing NpHR under the mod-1 promoter can extend the duration of dwelling (Flavell et al. 2013). This result suggests that optogenetic manipulations can affect dwelling behavior. We asked whether optogenetic stimulation of dwell-promoting neurons could induce a sedentary episode.
To test whether serotonin or dopamine release was sufficient to produce long-lasting sedentary episodes, we acutely activated neurons in roaming worms and monitored the duration of the resulting dwelling behavior. Worms were tested in an NGMB-food suspension in which naturally occurring sedentary episodes would last an average of ~10 min. After stimulation of serotonergic neurons, dwelling persisted for only 28 ± 5 s (n = 24 worms). Stimulation of dopaminergic neurons produced dwelling for 26 ± 5 s (n = 12 worms). These data show that acute optogenetic activation of dwell-promoting neurons is insufficient to trigger a sedentary episode that lasts as long as a natural episode. It is possible that our manipulations shortened the duration of the active episode. These results support a model in which the timing and duration of sedentary episodes require upstream regulation such as persistent serotonergic activation or the persistent inhibition of sensory input to produce episodic behavior.
Next, we sought to address observations that serotonin from NSM can cause both increases (Fig. 6E in Vidal-Gadea et al. 2011) and decreases (Flavell et al. 2013; Ranganathan et al. 2001; Sawin et al. 2000) in characteristics associated with locomotory speed in C. elegans. These experiments measured different locomotory characteristics and were obtained with different experimental protocols. For example, Vidal-Gadea et al. (2011) examined worms moving from solid to liquid and showed that NSM promotes a crawling to swimming transition during which animals increase their body bending frequency. In contrast, results obtained for worms on solid media (Flavell et al. 2013; Ranganathan et al. 2001; Sawin et al. 2000) show that the NSM neurons mediate slowing and promote dwelling that are characterized by decreases in speed and body bending. These experiments to test the role of NSM in locomotion utilized genetic methods and neuron ablations.
To clarify the roles for NSM in regulating locomotory states, we tested behavioral effects of selective optogenetic stimulation of NSM or other serotonergic neurons. First we illuminated the entire head where the NSM- and ADF-type neurons are located in freely moving worms by a method similar to that described previously (Leifer et al. 2011). Worms exhibited bending amplitude reductions upon illumination (Fig. 10, A and D). Illumination of the entire body posterior to the head, which includes the region containing the HSNs, had no effect (Fig. 10, B and D). We noted, however, that expression of the ChR2::YFP fusion protein in HSNs was dimmer than the expression of ChR2::YFP in the NSMs and ADFs (data not shown), which could result in only weak stimulation of HSN. These results show that the ADFs and/or NSMs can mediate dwelling locomotion.
Fig. 10.
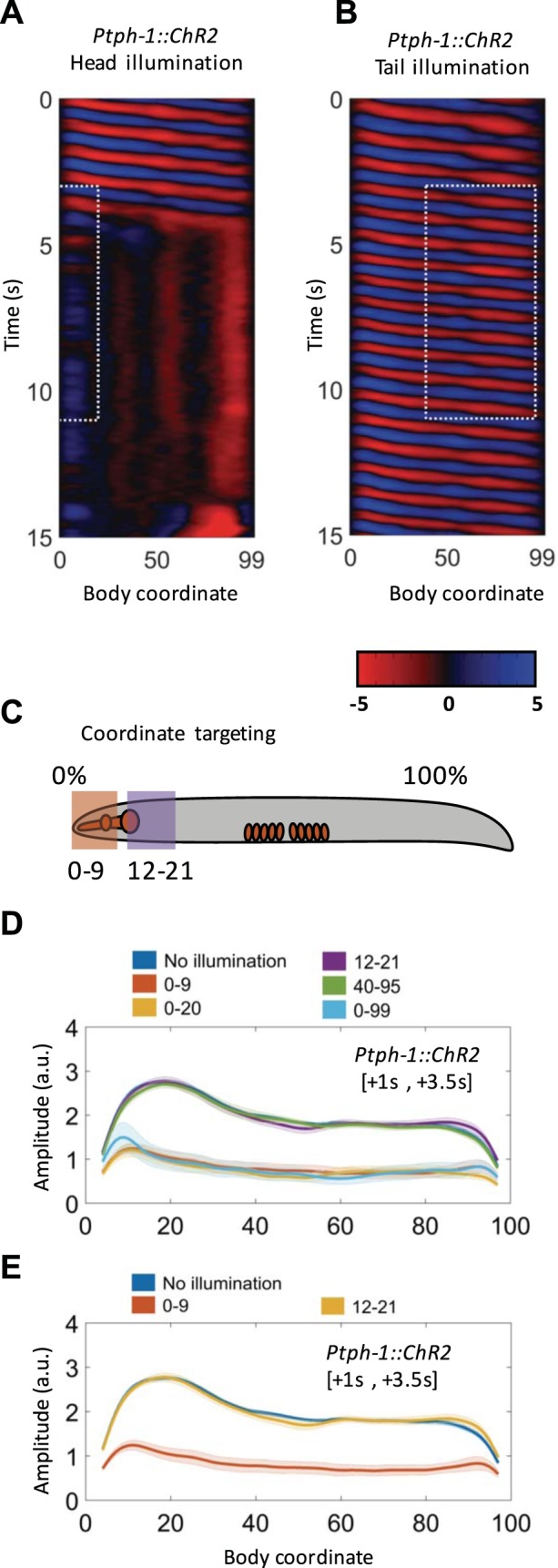
The NSM neurons mediate locomotory slowing. A: optogenetic stimulation of the head serotonergic neurons (ADF and NSM) causes tail paralysis and reduced head activity. B: illumination of the posterior of the worm (HSN) fails to produce slowing. C: schematic indicating the body coordinate, with two regions provided as examples. D: amplitude of bending (mean and SE) along the worm body while portions of the body are illuminated by blue light. E: illumination of small subregions of the head, which likely stimulate the cell bodies of NSM (body coordinate 0–9%) and ADF (body coordinate 12–21%).
We next illuminated more restricted regions of the head. First we illuminated a region corresponding to a body coordinate from 0% to 9% (where 0% is the tip of the nose and 100% is the end of the tail). This corresponds to the nose and includes the proximal bulb of the pharynx, but not the terminal bulb, and therefore illuminates NSM and the ADF dendrites that extend toward the nose. When this region was illuminated the same amount of amplitude reduction was observed as when the entire head (0–20%) was illuminated (Fig. 10E). These results show that activation of the NSM cell body and the ADF dendrites is sufficient to mediate all amplitude reduction that is caused by serotonergic head neurons. We next illuminated from 12% to 21%, which is a region starting approximately at the terminal bulb and extending posteriorly to include part of the gut. In this illumination the ADF cell bodies should be illuminated, and possibly a portion of NSM axons that extend through the pharyngeal isthmus toward the terminal bulb. We observed no amplitude reduction in response to this illumination (Fig. 10E). Together these results suggest that the NSM neurons mediate the slowing we observe in our assays.
Previously, neuron ablation studies showed that three types of dopaminergic neurons (the 4 CEP, 2 ADE, and 2 PDE cells) respond to the mechanical environment to mediate locomotory slowing (Sawin et al. 2000). However, in a different study investigators showed that animals have less body bending and modestly slower speeds after transitioning from a liquid to a solid environment if ADE or PDE neurons are ablated (Fig. 3, F and G, in Vidal-Gadea et al. 2011), suggesting that ADE and PDE increase locomotion on land. We asked which dopaminergic neurons mediate the dwell-promoting effects we observed in our experiments in liquid and tested them by selective optogenetic activation.
First we selectively illuminated a head region ranging approximately from the metacorpus to the terminal bulb (5–15%) of worms expressing Pdat-1::ChR2::YFP. This stimulation should illuminate CEP as well as ADE and/or ADE processes. From this manipulation we observed slowing and amplitude reduction identical to when the whole body was illuminated (Fig. 11, A and C). In contrast, when only the body and tail (35–99%) were illuminated no initial amplitude reduction was observed, but we did note that worms eventually slowed a small amount after a 5- to 8-s delay (Fig. 11, B and D), showing that illumination of PDE alone contributes weakly to dwellinglike bending characteristics.
Fig. 11.
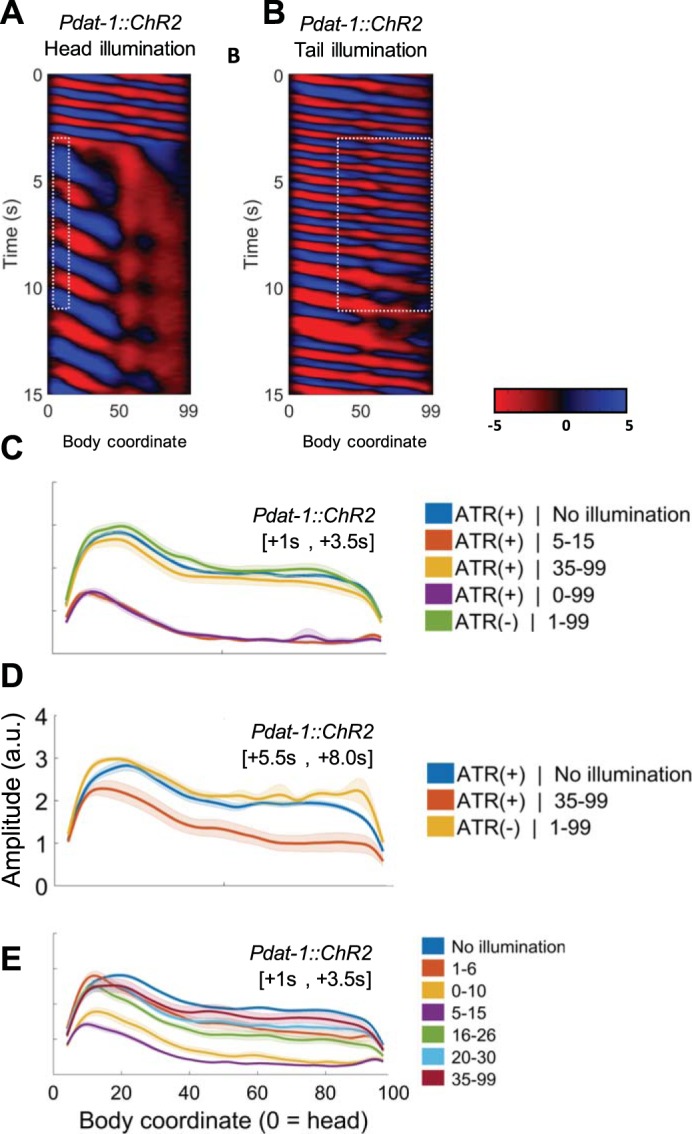
Stimulation of head dopaminergic neurons (ADE, CEP, and PDE) causes dwellinglike behavior. A and B: representative curvature kymograms of worms in which the head (A) or posterior 2/3 (B) has been illuminated by blue light. White dashed boxes indicate the spatial and temporal extents of the illumination. Note that marked head slowing and tail paralysis occurs immediately during head illumination but not during tail illumination. However, a milder version of this phenotype occurs after 5–6 s of posterior (PDE) illumination. C–E: mean and SE amplitude as a function of body coordinate for worms in each illumination condition. All numeric illumination conditions are % of worm length, from the head. In C and E, amplitude is measured between 1.0 and 3.5 s after the start of illumination. In D, it is measured between 5.5 and 8.0 s after the start of illumination.
To further assess which dopaminergic head neurons mediate slowing we illuminated only the tip of the nose (1–6%), since this region includes dendritic extensions of each of the four CEP neurons. This produced mild slowing, which we observed as a reduction of amplitude (Fig. 11E) in the posterior as well as a reduction in the head oscillation frequency (data not shown).
Next we attempted to selectively illuminate ADE. Before the experiment we examined the fluorescence in Pdat-1::ChR2::YFP worms. We noted that ADE neurons often appeared to be positioned posterior to the terminal bulb and in some cases overlapped with fluorescent granules of gut cells (data not shown). Therefore to illuminate ADE we targeted region 16–26%, which approximately begins near the posterior of the terminal bulb and extends posteriorly toward the gut. This illumination region would be predicted to miss ADE sometimes based on typical ADE position, which has been reported to be adjacent to the terminal bulb (WormAtlas). Illumination of this region also produced mild amplitude reduction (Fig. 11E) that was similar to the illumination of CEP dendrites. We then performed a control in which we illuminated from 20% to 30% of the worm body. This illumination begins after the beginning of the gut, and we believed it would not include ADE cell bodies but would include PDE axons. This illumination also produced some amplitude reduction (Fig. 11E), suggesting that either the anteriormost axon segment of PDE or off-target illumination of ADEs produced slowing. In contrast, illumination of 35–95%, a region that includes the posterior portion of PDE axons and the PDE cell body, produced no significant slowing (Fig. 11E). Overall these results show that dwelling can be driven by short illumination of CEP dendrites, long illumination of PDE, and short illuminations of a region containing ADE and the anteriormost portion of the PDE axon. We consider it unlikely that short illumination of the anterior segment of the PDE axon should result in dwelling when short illumination of the cell body does not. This means it is likely that ADE can also mediate slowing. These results show that all eight dopaminergic neurons mediate slowing.
Our results show that dopamine, like serotonin, induces dwellinglike behavior. Our results confirm by selective neuron illumination that specific neurons promote dwelling: NSM, ADE, CEP, and PDE. Also, we note that our optogenetic illuminations were performed either in the presence (Fig. 9) or absence (Fig. 10 and Fig. 11) of food. In both of these situations dwelling behavior was inducible. Our observation that optogenetic activation of dwell-promoting neurons does not produce long-lasting sedentary episodes shows that the regulation of sedentary episode induction or stability is regulated by other factors, possibly located upstream of dwell-promoting neurons.
Normal fasting behavior requires neuropeptide signaling.
When worms are removed from food and placed in fasting conditions, they initially swim/roam and then become progressively more quiescent over time (Fig. 1 and Fig. 2). We sought to gain a better understanding of the molecular control of this response to environmental change by examining a role for neuropeptides in controlling behavior.
Previously, it has been noted that specific neuropeptides and processing of neuropeptides are required for various forms of quiescence, including satiety quiescence (You et al. 2008), lethargus (Nagy et al. 2014b; Nelson et al. 2013; Turek et al. 2013), and stress-induced quiescence (Hill et al. 2014; Nelson et al. 2014). There are also exist arousal-promoting peptides (Choi et al. 2013; Flavell et al. 2013). We hypothesized that the fasting response is a neurally regulated process that requires neuropeptide signaling.
To test this idea, we examined egl-3, egl-21, and unc-31 mutants under fasting conditions. These genes (egl-3, egl-21) are required to processes neuropeptides (Kass et al. 2001; Jacob and Kaplan 2003; Husson et al. 2007) or to secrete neuropeptides (unc-31) (Charlie et al. 2006). First, we found that egl-21 mutants displayed an ~80% reduction in the total amount of time spent in quiescence during fasting (Fig. 12, A and B). We also observed that egl-21 animals display unique locomotory phenotypes: during swimming egl-21 mutants exhibit drops in activity during which the mutants continuously coil and uncoil (Supplemental Video S12). Coiling in egl-21 mutants has been reported previously (Trent et al. 1983), but these data suggest that coiling may occur in bouts.
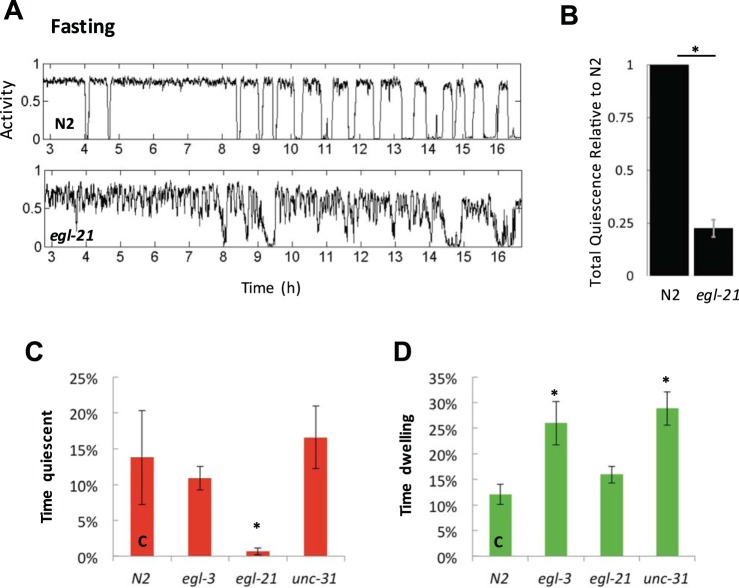
Fig. 12.
The neuropeptide processing protein EGL-21 is required for fasting-induced quiescence. A: actigraphs showing either N2 or egl-21(n476) mutants in liquid fasting conditions. B: comparison of total quiescence for N2 compared with egl-21 mutants. Error bar shows the SE of 2 experiments. P = 0.015. C and D: % of time quiescent or dwelling over the course of a 13.3-h fasting assay for the indicated genotypes. Error bars represent the SE of 2 experiments, but asterisks mark significance (P < 0.001) by comparing groups of pooled worms from both experiments. n = 22 or more worms in each condition. Experiments were performed with both egl-3(gk238) and egl-3(nu349), and the data were pooled.
We then examined egl-3, egl-21, and unc-31 mutants together and monitored levels of quiescence, dwelling, and swimming/roaming. As in our previous experiment, egl-21 mutants showed reduced quiescence compared with N2, but egl-3 and unc-31 mutants did not have altered quiescence levels (Fig. 12C). However, egl-3 and unc-31 mutants dwell more than N2 controls (Fig. 12D) and swim less (data not shown). These data show that mutations affecting neuropeptide function have complex effects on the fasting behavioral response. Loss of egl-21 results in reduced quiescence, suggesting a loss of quiescence-promoting neuropeptides. In contrast, loss of egl-3 and unc-31 results in more dwelling and less roaming, suggesting that these mutations may also affect arousal peptides, as previously suggested (Nagy et al. 2014b).
Overall, these results show that neuropeptides are required for a normal fasting quiescence response.
Sensory neurons and Foraging homolog EGL-4 regulate behavior through control of active/sedentary episodes.
Mutations that impair normal sensory neuron function have been found to cause changes to the overall proportion of dwelling and of roaming (Fujiwara et al. 2002). For example, a mutation in osm-6 that encodes an intraflagellar transport component results in high dwelling (Fujiwara et al. 2002). In contrast, loss-of-function mutations in the cGMP-sensitive protein kinase egl-4 result in higher roaming and reduced levels of quiescence (Fujiwara et al. 2002; Raizen et al. 2008). We predicted that mutations impairing sensory neuron function might alter the overall levels of these behaviors by altering proportions of active and sedentary episodes, which has not previously been investigated.
To test this idea, we examined worms harboring mutations compromising sensory neuron functions that have known effects on behavioral state. We found that loss-of-function mutants for genes che-2, che-3, and osm-6, genes contributing to ciliary neuron structure or intracellular transport, show a decrease in overall active behavior and an increase in sedentary behavior (Fig. 13, A and B). This is consistent with past findings that these mutations increase dwelling (Fujiwara et al. 2002).
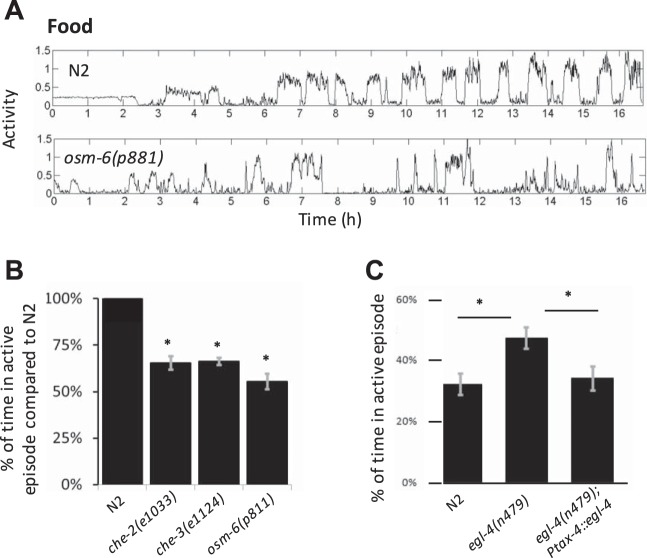
Fig. 13.
Sensory neuron mutants swim less and dwell more than wild-type worms in the presence of food. A: representative activity of an N2 mutant and an osm-6 mutant, which is a known hyperdweller. B: average time swimming as assessed by thresholding for various hyperdwelling mutants compared with N2. Error bars show the SE of the mean of at least 2 experiments. *P < 0.001. C: percent of time swimming for egl-4 mutants, which are typical hyperroamers, compared with either wild type or a rescue of egl-4 in sensory neurons. Error bars show the SE of the mean of 3 experiments. *P < 0.03.
Since active and sedentary episodes are regulated in a food-responsive manner, conserved food-responsive genes might regulate episodes. One such gene proposed to be conserved in food responsiveness is the Drosophila gene Foraging (Fitzpatrick and Sokolowski 2004; Kaun and Sokolowski 2009), which regulates diverse physiological processes including foraging strategy and energy homeostasis. EGL-4 is the Foraging homolog in C. elegans. Indeed, a loss-of-function mutation in egl-4 (a cGMP-sensitive protein kinase) causes increased roaming and decreased dwelling (Fujiwara et al. 2002), so we investigated egl-4 further.
We found that egl-4 mutation resulted in more time spent in active episodes (Fig. 13C). Restoring egl-4 function in sensory neurons restored the proportions of active/sedentary states to normal levels (Fig. 13C), consistent with previous findings (Fujiwara et al. 2002; Raizen et al. 2008). Overall these results reaffirm previous findings that sensory neuron function regulates behavioral states and show that this occurs in part through the regulation of active/sedentary episodes.
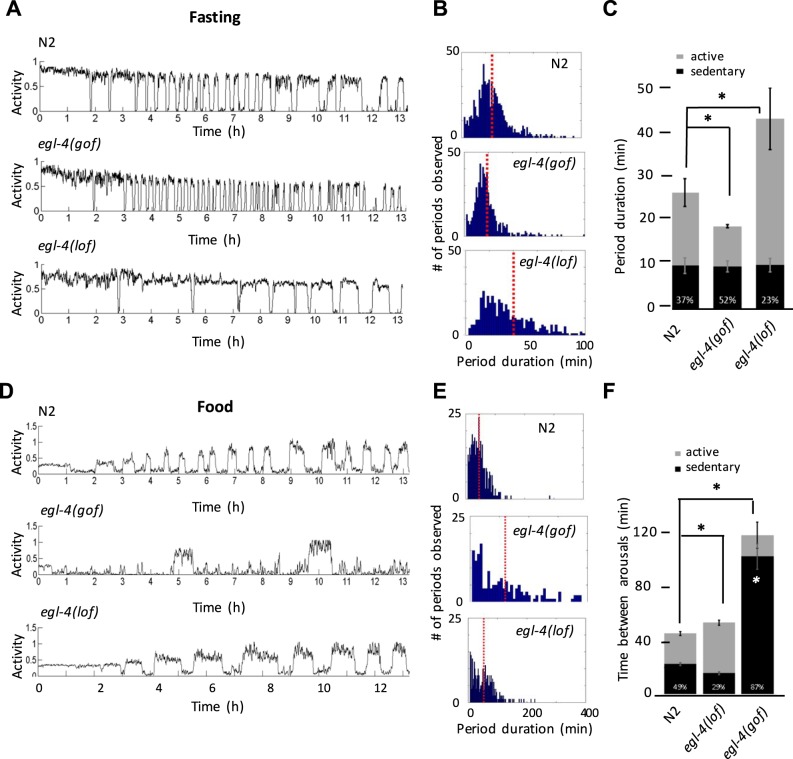
Next we examined worms in liquid either with or without food present and assessed the duration of active and sedentary bouts by quantifying periodicity (the time between arousals to an active episode). We examined wild-type (WT), egl-4(n479) loss-of-function (lof), and egl-4(ad450) gain-of-function (gof) alleles that have been reported to be hyperactive and hypoactive, respectively (Raizen et al. 2006).
For fasting worms, we found that egl-4(lof) mutants displayed longer periods between arousals compared with WT while egl-4(gof) mutants had shorter periods than WT (Fig. 14, A–C). Differences in period appear to be predominantly due to differences in the duration of the active episode, since the duration of the sedentary episode was not different (Fig. 14C). Furthermore, when we examined the distribution of periods in WT vs. egl-4 mutants we observed what appears to be a normal distribution for WT, suggesting that the time between arousals is indeed periodic, but in egl-4 mutants these periods are shifted to be shorter or longer. These results show that in fasting conditions EGL-4 primarily acts to shorten the duration of active episodes, suggesting that EGL-4 may be part of a pacemaker system that regulates the timing of active and sedentary episodes.
Fig. 14.
EGL-4 promotes transitions to low-activity behavioral states. A: actigraphs of fasting worms comparing N2 to egl-4 loss-of-function (lof) or gain-of-function (gof) mutants. Representative traces show swimming and quiescent bouts, and it appears that egl-4 mutants have altered bout frequencies. B: histograms showing the number of periods observed (blue bars) and the average period duration (red dotted line). egl-4(gof) mutants have slightly shorter periods, whereas egl-4(lof) mutants have longer periods. C: changes to period duration in fasting conditions can be attributed mostly to the changes in duration of swimming bouts. Bar heights correspond to the period (duration of the swimming episode + subsequent quiescent episode). The average duration of quiescent episodes alone is shown by the height of the black bar. A percentage at the base of the bar shows the duration of quiescent bouts/period duration. D–F show results of an experiment performed the same way as A–C except in the presence of food. In F, the white asterisk denotes that egl-4(gof) sedentary bout duration is longer than both N2 and egl-2(lof) sedentary bouts. Error bars and comparisons are of pooled worms from 2 experiments. n = 32 for each condition. *P < 0.05 in each marked comparison.
When we performed the same experiment in the presence of food, we found that egl-4(lof) exhibited a largely unchanged period, but with a slightly greater proportion of time spent roaming resulting in an overall decrease in the amount of time spent dwelling compared with WT. egl-4(gof) mutants had elongated periods that were mostly due to an increase in the duration of sedentary episodes as well as a decrease in the duration of active episodes (Fig. 14, D–F). Therefore when food is present EGL-4 still acts to shorten active episodes but also acts to extend sedentary episode duration. Taken together, these results suggest that EGL-4 activity promotes transitions to and maintains sedentary behavioral episodes.
Insulin-like signaling in nervous system promotes dwelling by antagonizing daf-16-dependent quiescence during sedentary state.
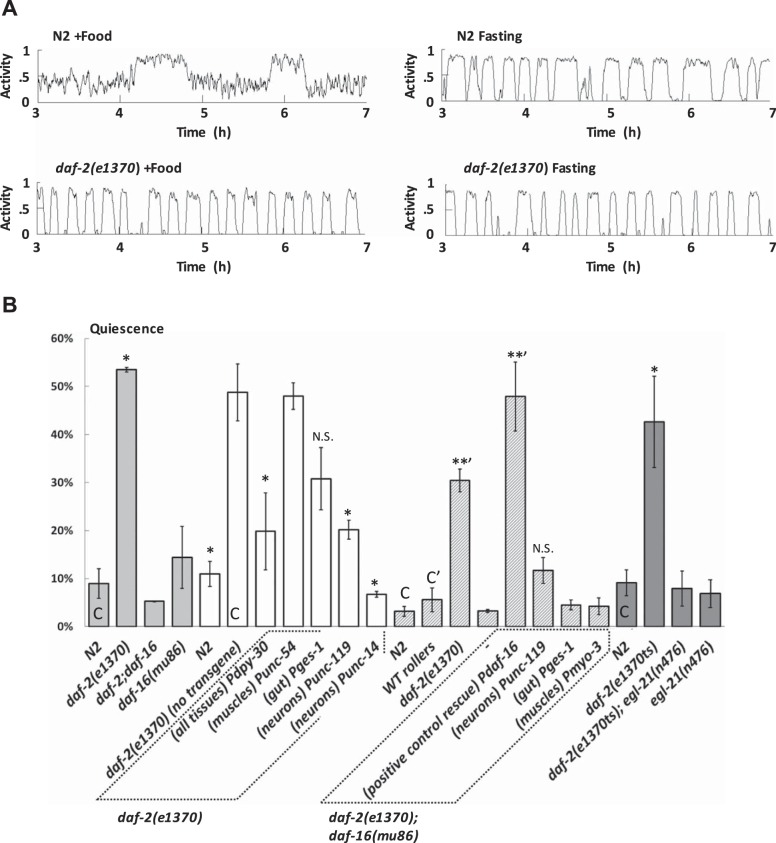
Insulin and insulin-like signaling have conserved roles in sensing and responding to nutritional status in animals (reviewed in Avery 2010). C. elegans has only one known insulin-like receptor, DAF-2, which is expressed in many tissues, including the nervous system (Kimura et al. 2011). Since reductions in DAF-2 function have been described as changing roaming (Ben Arous et al. 2009) and quiescent (Gaglia and Kenyon 2009; Gems et al. 1998) behavior, we asked whether DAF-2 is also involved in the selection of behavioral states.
We chose to work primarily with a temperature-sensitive mutant, daf-2(e1370). To assess null-like effects we performed experiments on worms shifted from 15°C to the restrictive temperature 25°C at the late L4 stage.
For fed conditions, we found that daf-2 mutants have decreased dwelling, increased quiescence, and an increased alternation frequency between active and sedentary episodes (Fig. 15B; Supplemental Video S13).
Fig. 15.
Insulin-like signaling in the nervous system prevents a fasting-like behavioral response in daf-2 mutants. A: actigraphs showing activity for worms in NGMB either fasting or with 8× food during hours 3–5 of the experiment for the indicated genotypes. B: quantification of the total % of time worms spend in quiescence. Worms of the indicated genotypes immersed in NGMB with 8× food. During recording WorMotels were maintained at the restrictive temperature for daf-2 mutants (see methods). Four sets of experiments are indicated by the light gray, white, hatched, and dark gray bars. Asterisks mark statistical significance in comparison to a control. C marks the control genotype to which any bar marked with * or N.S. was compared for that experimental group. Similarly C′ were compared with bars marked with *′. Strains are indicated beneath the bar. Genotypes surrounded by a dotted box also have the mutations labeled underneath the box. All experiments were performed at the restrictive temperature, 25°C.
During fasting, daf-2(e1370) worms resembled WT fasting worms, except that daf-2 worms spent more total time in quiescence and switched more frequently between episodes than WT worms (Fig. 15A). For fed worms, daf-2(e1370) were quiescent 50% of the time compared with 10% in WT. This reduction in movement was due not to a loss of swimming but to a loss of dwelling (data not shown). We examined several other daf-2 alleles to confirm our result and found that, while daf-2(e1391) displays an increase in quiescence similar to daf-2(e1370), daf-2(m596) and daf-2(e1368) do not show similar increases (data not shown).
The two daf-2 mutations showing an increase in quiescence are both in the kinase domain; the other mutations affect ligand binding (Kimura et al. 1997; Scott et al. 2002). However, it is not clear whether allele strength or the molecular nature of the mutation was responsible for phenotypic differences.
These results suggest that insulin-like signaling is required for the decision to dwell or become quiescent during sedentary behavioral episodes or alternatively that the loss of insulin-like signaling induces a behavioral repertoire similar to long-term fasting.
Many phenotypes caused by the mutation of daf-2, such as life span extension and dauerlike quiescent behavior, can be suppressed by mutation of daf-16, the conserved forkhead box O (FOXO)-like transcription factor that is negatively regulated by insulin-like signaling in C. elegans (Gaglia and Kenyon 2009; Lin et al. 1997). Therefore, we tested whether daf-16 mutation could also suppress quiescence in our assays. Indeed, we found that daf-16 mutation suppressed the high levels of quiescence in daf-2(e1370) mutants (Fig. 15B; Supplemental Video S14).
In fasting conditions daf-16 single mutants have quiescence levels identical to WT worms, suggesting that fasting induces quiescence independently of DAF-16. In light of these results it is clear that DAF-16 is not required for a fasting response. However, since daf-2 mutants behave like fasting worms, we hypothesized that DAF-16 might regulate quiescence by producing a fasting state in C. elegans. Indeed, it has been shown that daf-2 mutants have reduced feeding (Chow et al. 2006; Dillon et al. 2016; Dwyer and Aamodt 2013; Gems et al. 1998).
We next asked whether reductions in feeding rate correlated with quiescent phenotypes. In liquid containing food, daf-2(e1370) is highly quiescent, whereas daf-2(m596) is active. Similarly, daf-2(e1370) worms had low pump rates in these conditions, while daf-2(m596) had normal pump rates (data not shown). In a second experiment, on solid media, we examined the feeding behavior of daf-2(e1370) compared with other strains. Worms were grown at the permissive temperature (15°C) and then shifted at L4 to the semipermissive temperature (20°C). The following day pump rates were assessed by manually counting pumps for 30 s [N2: 3.4 Hz, daf-2(e1370): 2.4 Hz, daf-16(mu86): 3.4 Hz, daf-2;daf-16: 3.4 Hz, egl-21: 3.0 Hz, daf-2;egl-21: 1.76 Hz]. Then, adults were shifted to the restrictive temperature, 25°C, for 24 h and rates were assessed again [N2: 3.85 Hz, daf-2(e1370): 0.3 Hz, daf-2;daf-16: 3.3 Hz, egl-21: 3.2 Hz, daf-2;egl-21: 0.2 Hz]. The number of worms was n = 10, 9, 11, 4, 8, and 6, respectively. These data show that, at the restrictive temperature, daf-2(1370) has a feeding rate reduced by 92%.
These results suggest that reductions of insulin signaling lead to feeding cessation phenotypes that depend on DAF-16. These feeding cessation phenotypes might have a cumulative effect of inducing a fastinglike state in worms that leads to quiescence.
We hypothesized that daf-2 quiescence was induced by similar mechanisms as fasting-induced quiescence. If so, then phenotypes should be suppressed by egl-21 mutation. To test this idea we examined daf-2;egl-21 double mutants. We found that egl-21 mutation suppresses the level of quiescence back to WT levels (Fig. 15B). However, this suppressive effect may only be temporary, since daf-2;egl-21 mutants do not have large reductions in quiescence throughout their life span compared with daf-2 alone (Churgin and Fang-Yen, unpublished data). The result also suggests that EGL-21 may act downstream of DAF-16 to generate these phenotypes.
We next asked whether DAF-2 and DAF-16 were acting from within the nervous system to mediate behavioral effects. To test this idea, we measured the total time spent in given behavioral states in daf-2(e1370) mutants expressing DAF-2 under tissue-specific promoters (Wolkow et al. 2000). We found that expression of DAF-2 under promoters dpy-30 (all tissues), unc-119 (neuronal), and unc-14 (neuronal) was capable of suppressing quiescent phenotypes in daf-2(e1370ts) worms (Fig. 15B). By contrast, DAF-2 expression under unc-54 (body wall muscle) or ges-1 (gut) promoters did not significantly rescue quiescence (Fig. 15B). DAF-16 is also expressed in the nervous system (Liang et al. 2006), so we tested whether transgenic tissue-specific expression of DAF-16 in the nervous system (Gaglia and Kenyon 2009; Libina et al. 2003) could restore quiescence in daf-2;daf-16 mutants. We found that DAF-16 expression under its own promoter was able to rescue; however, expression of DAF-16 in neurons alone did not restore quiescence. Together, these data suggest that DAF-2 is required in the nervous system to prevent DAF-16-dependent induction of quiescence.
DISCUSSION
Behavioral episodes in C. elegans.
Our work helps to unify the findings of different studies under a common framework for behavior regulation (Fig. 16; Table 3). We show that episodic locomotion/quiescence alternation, previously described for swimming worms, occurs under arbitrary mechanical environments. During active episodes, worms primarily roam, display some dwelling, and lay eggs. During sedentary episodes, worms rarely roam but are often dwelling, do not lay eggs, and may enter quiescence, during which time they do not move or feed. When worms are fasting, the proportion of locomotory behaviors during active and sedentary bouts changes so that sedentary bouts mostly contain quiescence. These findings show that certain behaviors in C. elegans either are organized hierarchically within other states or are often coincident. Similar correlations of certain behaviors within behavioral states [compound behavioral states (Nagy et al. 2013)] have been previously described, such as dwelling- and roaming-specific combinations of reversals and turns (Flavell et al. 2013) and quiet wakefulness during otherwise active states (Nagy et al. 2013).
Our results clarify previous reports that animals become quiescent in liquid either with or without food (Ghosh and Emmons 2008, 2010). These studies considered a worm quiescent if it displayed <2 body bends per 5 s, a definition that partly includes what we call dwelling behavior. We believe this definition of quiescence is misleading since we show that inactive (sedentary) episodes when food is present are comprised of quiescent and dwelling locomotion as well as active or quiescent feeding behaviors. Going forward, we propose that behavioral episodes simply be called active or sedentary when the type of locomotion contained within the behavioral episode is not discerned in detail.
Episodic regulation of C. elegans behavior is complex because episode incidence and timing are regulated by the interplay between multiple circuit systems. Prior research shows that quiescent episodes (sedentary bouts when animals are fasting) are induced in part by ACh and that quiescence duration is shortened when command interneurons are ablated and extended when command interneurons are activated (Ghosh and Emmons 2008). However, switching between swimming and quiescence still occurred even after the complete ablation of command interneurons, suggesting that induction occurs at the motor neuron level or by some other mechanism. In our experiments we find that episode regulation requires neuropeptide processing and responsiveness to food levels. Also, we show that episode timing also depends on PKG function, which builds on results showing that PKG and calcineurin act in sensory neurons to antagonistically to regulate episodes (Ghosh and Emmons 2010). Overall these studies on episodic behavior show that multiple systems coordinate to regulate episode organization.
Food regulation of quiescence.
By examining locomotory behavior under varied food concentrations, we show that the incidence of locomotory states depends on food in a nonmonotonic manner. We found that quiescence was promoted both by fasting (absence of food) and by high concentrations of food (8× food). The concept that continued fasting might produce inactivity in worms has been reported previously. Previous observations show that C. elegans (Boyd et al. 2003), and other nematodes such as Prionchulus punctatus (Maertens 1975; Van Gundy 1965), become inactive when starved. Our data are the first effort to quantify and observe fasting-induced quiescence in nematodes and to suppress the induction of that quiescent behavior by feeding worms.
Quiescence is also observed in conditions in which animals are given high levels of food. This quiescence might be equivalent to satiety quiescence (You et al. 2008), which is induced by high-quality food. Moreover, fasting and satiety quiescence might be related because it has been shown that satiety quiescence is enhanced by prior fasting for up to 12 h (You et al. 2008). One interpretation of this result is that fasting simply enhances satiety quiescence through a single pathway. Alternatively, enhanced satiety quiescence might be a hybrid quiescent state induced by two pathways: satiety and fasting-induced quiescence. Future experiments will be needed to resolve the relationship between these two behaviors.
This part of the discussion highlights the importance of quantitative assays capable of discerning effects that would be lost if food were treated as a binary variable (i.e., either present or absent).
Food regulates behavioral state architecture.
Our results show that food regulates behavioral architecture of both episodes and behavior within episodes. First, food affects the alternation rate and duration of active and sedentary episodes. Second, active and sedentary episodes can vary, since the states appear to compound behavioral states because they contain a mixture of different behaviors. For example, at 8× food high levels of quiescence are observed, possibly suggesting satiety quiescence. In contrast, at 2× food sedentary episodes are still common, but quiescence is much lower.
Other environmental factors also influence episodic behavior. We showed that mechanical environment is one of these factors since it alters the timing of episodes. Another likely example is that oxygen may affect episodes. This is because it is an environmental gas that, when reduced, promotes dwelling behavior (Cheung et al. 2005). However, low-oxygen-induced dwelling depends on the presence of food (Cheung et al. 2005), showing that behavioral state decisions are multifactorial.
Overall our findings show, in a detailed way, how food is an environmental parameter that influences behavioral state architecture in liquid. Secondarily, we also show that other factors such as internal perturbations by genetics also affect episodes. Continued studies of behavioral states in C. elegans will show how behavioral states are regulated in complex ways by multiple environmental and internal influences.
Feeding and egg-laying manifestations of behavioral states.
We have shown that episodic behavioral states are not restricted to locomotion but also influence feeding and egg-laying behaviors. Locomotory quiescence during fasting is accompanied by feeding quiescence, and this feeding quiescence is not due to being immersed in liquid except for a brief period.
We found that the previously described egg-laying active behavioral states (Tong Zhou et al. 1998) correspond to active episodes in C. elegans. An inference we can make from this observation is that active and sedentary episodes may be states equivalent to the previously described egg-laying active and inactive states. These observations have interesting implications for future studies in C. elegans since many mutants have egl phenotypes (Trent et al. 1983). In addition to those we have examined (e.g., egl-4, egl-21), it is likely that other egl mutations also affect the regulation of behavioral episodes.
Overall, our results show that episodes in C. elegans may reflect the likelihood of other types of behaviors like feeding, egg-laying, and possibly others not studied here. These results suggest that the observed behavioral states are the outward manifestation of organism-wide modulations.
Dopaminergic and serotonergic circuitry drive dwelling locomotion.
Previous investigations have described that both dopamine and serotonin, whether released by neurons or administered exogenously, can produce slowing and/or paralytic effects in C. elegans (Ezcurra et al. 2011; Horvitz et al. 1982; Iwanir et al. 2016; Schafer and Kenyon 1995). Moreover, both of these biogenic amines have been described as mediating slowing in response to food and/or the mechanical sensation of food (Sawin et al. 2000). Our experiments add to the evidence that serotonergic neurons mediate dwelling and extend findings to show that dopaminergic neurons also mediate dwelling. We also show that slowing effects can be attributed to NSM, ADE, CEP, and PDE neurons. However, ADF neurons have also been reported to produce slowing (Iwanir et al. 2016), and we can only surmise that ADF does not produce dwelling in our assay.
Regulation of behavioral states by PKG signaling and insulin-like signaling in nervous system.
Our results show that conserved food-responsive signaling pathways (Avery 2010) regulate episodic behavioral states in C. elegans. We found that EGL-4, a homolog of the food-behavior related gene Foraging in D. melanogaster, regulates active and sedentary episodes as a mechanism to regulate roaming and dwelling, consistent with previous findings that EGL-4 promotes low-activity behaviors (Fujiwara et al. 2002; Gallagher et al. 2013b; Ghosh and Emmons 2008, 2010; Raizen et al. 2008; You et al. 2008;). Overall our results extend the understanding of how EGL-4 functions to include active and sedentary episodes.
Insulin signaling is a conserved pathway for detecting food (Kimura et al. 2011). Reductions in insulin signaling in daf-2 mutants have been shown to induce behavioral quiescence that depends on the FOXO-like transcription factor DAF-16 (Gaglia and Kenyon 2009). We set out to test how mutation of the daf-2 gene affected behavioral states and found that without DAF-2 animals behave as if fasting by frequently switching between active and sedentary episodes. Moreover, sedentary episodes are comprised primarily of quiescence, which occurs in WT fasting worms. Expression of DAF-2 in the nervous system is sufficient to rescue these phenotypes.
How does loss of DAF-2 in the nervous system lead to quiescent behavior? Our hypothesis is that reduction of DAF-2 function at the restrictive temperature leads to an increase in DAF-16, which induces chronic feeding reduction, which leads to fasting-induced quiescence. Indeed, feeding reductions in daf-2 mutants have been reported (Chow et al. 2006; Dillon et al. 2016; Dwyer and Aamodt 2013; Gems et al. 1998). The reduction in feeding rates we observed was higher than in some reports, likely because we perform our experiments at the restrictive temperature, 25°C, at which DAF-2 becomes undetectable in the nervous system (Kimura et al. 2011).
Overall, the observation that insulin-like signaling in the nervous system regulates feeding and locomotory behavior in C. elegans could be important if similar mechanisms regulate behavior in other animals. For example, the human disease diabetes mellitus has been proposed to be caused, in part, by a reduction of insulin-signaling sensitivity correlated with obesity. Therefore, it is possible that insulin insensitivity in the brain (Baskin et al. 1987) might reinforce food-seeking behavior that exacerbates weight gain.
Episodic regulation of behavior in C. elegans is an adaptive behavioral strategy.
Our work provides insights into episodic behavior as an adaptive strategy for survival. We show that active and sedentary episodes, as well as roaming, dwelling, and quiescence, are sensitive to both external (food) and internal (insulin-like signaling, neuropeptides, biogenic amines) signals. However, the question remains: Why do behavioral episodes exist, and what survival advantage do they provide?
We hypothesize that episodes in C. elegans represent the outward manifestation of switches modulating multiple neural systems. Such switches could have evolved for at least two reasons. First, we note that episodes likely have adaptive value in responding to the local environment. For example, when faced with low levels of food animals suppress sedentary episodes, resulting in increased roaming. In the continued absence of food worms become quiescent, representing a strategy to save energy. When food levels are high, sedentary episodes and dwelling are common. This strategy promotes resource exploitation and reduces the probability that worms will leave the food cache.
Second, coordination of compatible behaviors likely provides a survival advantage. For example, why do worms switch back to being active if food levels are optimal? Our finding that egg-laying occurs almost exclusively during the active episode suggests that the reproductive system is more active during roaming. This idea is consistent with the finding that PDF controls both roaming and egg-laying in C. elegans (Flavell et al. 2013; Meelkop et al. 2012). Such a coordination of roaming and egg-laying could represent a strategy for egg dispersal. Another possible coincident behavior is that males may preferentially mate with sedentary hermaphrodites, as suggested by previous studies (Bahrami and Zhang 2013). We show that osm-6 mutation results in high levels of sedentary behavior, while others have shown that osm-6 mutation results in hermaphrodites being more receptive to mating with males (Bahrami and Zhang 2013). Mating during sedentary episodes ensures a lower likelihood of the hermaphrodite crawling away and also ensures that mating occurs at times exclusive from egg-laying. Overall, these ideas support the notion that behavioral episodes facilitate behavioral diversity by creating distinct states that are compatible with multiple behavioral outputs.
GRANTS
R. J. McCloskey was supported by NIH Grant T32 HL-007712-23. A. D. Fouad and M. A. Churgin were supported by NIH Grant R01 NS-84835-03. C. Fang-Yen was supported by the NIH, the Ellison Medical Foundation, and the Alfred P. Sloan Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.J.M., M.A.C., and C.F.-Y. conceived and designed research; R.J.M. and A.D.F. performed experiments; R.J.M., A.D.F., and M.A.C. analyzed data; R.J.M., A.D.F., M.A.C., and C.F.-Y. interpreted results of experiments; R.J.M., A.D.F., and C.F.-Y. prepared figures; R.J.M., A.D.F., and C.F.-Y. drafted manuscript; R.J.M., A.D.F., M.A.C., and C.F.-Y. edited and revised manuscript; R.J.M., A.D.F., M.A.C., and C.F.-Y. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Hu for helpful discussion and technical assistance and Gary Ruvkun and William Schafer for providing worm strains. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD-010440).
REFERENCES
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118: 273–274, 1953. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Avery L. Caenorhabditis elegans behavioral genetics: where are the knobs? BMC Biol 8: 69, 2010. doi: 10.1186/1741-7007-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool 253: 263–270, 1990. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol 206: 2441–2457, 2003. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami AK, Zhang Y. When females produce sperm: genetics of C. elegans hermaphrodite reproductive choice. G3 (Bethesda) 3: 1851–1859, 2013. doi: 10.1534/g3.113.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Figlewicz DP, Woods SC, Porte D Jr, Dorsa DM. Insulin in the brain. Annu Rev Physiol 49: 335–347, 1987. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- Ben Arous J, Laffont S, Chatenay D. Molecular and sensory basis of a food related two-state behavior in C. elegans. PLoS One 4: e7584, 2009. doi: 10.1371/journal.pone.0007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berri S, Boyle JH, Tassieri M, Hope IA, Cohen N. Forward locomotion of the nematode C. elegans is achieved through modulation of a single gait. HFSP J 3: 186–193, 2009. doi: 10.2976/1.3082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Cole RD, Anderson GL, Williams PL. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem 22: 3049–3055, 2003. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Charlie NK, Schade MA, Thomure AM, Miller KG. Presynaptic UNC-31 (CAPS) is required to activate the Galphas pathway of the Caenorhabditis elegans synaptic signaling network. Genetics 172: 943–961, 2006. doi: 10.1534/genetics.105.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol 15: 905–917, 2005. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM. Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron 78: 869–880, 2013. doi: 10.1016/j.neuron.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DK, Glenn CF, Johnston JL, Goldberg IG, Wolkow CA. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp Gerontol 41: 252–260, 2006. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churgin MA, Fang-Yen C. An imaging system for C. elegans behavior. Methods Mol Biol 1327: 199–207, 2015. doi: 10.1007/978-1-4939-2842-2_14. [DOI] [PubMed] [Google Scholar]
- Dillon J, Holden-Dye L, O’Connor V, Hopper NA. Context-dependent regulation of feeding behaviour by the insulin receptor, DAF-2, in Caenorhabditis elegans. Invert Neurosci 16: 4, 2016. doi: 10.1007/s10158-016-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Aamodt EJ. Insulin/IGF-1 signaling, including class II/III PI3Ks, β-arrestin and SGK-1, is required in C. elegans to maintain pharyngeal muscle performance during starvation. PLoS One 8: e63851, 2013. doi: 10.1371/journal.pone.0063851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J 30: 1110–1122, 2011. doi: 10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang-Yen C, Avery L, Samuel AD. Two size-selective mechanisms specifically trap bacteria-sized food particles in Caenorhabditis elegans. Proc Natl Acad Sci USA 106: 20093–20096, 2009. doi: 10.1073/pnas.0904036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang-Yen C, Wyart M, Xie J, Kawai R, Kodger T, Chen S, Wen Q, Samuel ADT. Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 107: 20323–20328, 2010. doi: 10.1073/pnas.1003016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick MJ, Sokolowski MB. In search of food: exploring the evolutionary link between cGMP-dependent protein kinase (PKG) and behaviour. Integr Comp Biol 44: 28–36, 2004. doi: 10.1093/icb/44.1.28. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154: 1023–1035, 2013. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron 36: 1091–1102, 2002. doi: 10.1016/S0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Gaglia MM, Kenyon C. Stimulation of movement in a quiescent, hibernation-like form of Caenorhabditis elegans by dopamine signaling. J Neurosci 29: 7302–7314, 2009. doi: 10.1523/JNEUROSCI.3429-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T, Bjorness T, Greene R, You YJ, Avery L. The geometry of locomotive behavioral states in C. elegans. PLoS One 8: e59865, 2013a. doi: 10.1371/journal.pone.0059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T, Kim J, Oldenbroek M, Kerr R, You YJ. ASI regulates satiety quiescence in C. elegans. J Neurosci 33: 9716–9724, 2013b. doi: 10.1523/JNEUROSCI.4493-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Emmons SW. Episodic swimming behavior in the nematode C. elegans. J Exp Biol 211: 3703–3711, 2008. doi: 10.1242/jeb.023606. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Emmons SW. Calcineurin and protein kinase G regulate C. elegans behavioral quiescence during locomotion in liquid. BMC Genet 11: 7, 2010. doi: 10.1186/1471-2156-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol 49: 303–313, 2001. doi: 10.1002/neu.10014. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Mansfield R, Lopez JM, Raizen DM, Van Buskirk C. Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol 24: 2399–2405, 2014. doi: 10.1016/j.cub.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014, 1982. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Janssen T, Baggerman G, Bogert B, Kahn-Kirby AH, Ashrafi K, Schoofs L. Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J Neurochem 102: 246–260, 2007. doi: 10.1111/j.1471-4159.2007.04474.x. [DOI] [PubMed] [Google Scholar]
- Iwanir S, Brown AS, Nagy S, Najjar D, Kazakov A, Lee KS, Zaslaver A, Levine E, Biron D. Serotonin promotes exploitation in complex environments by accelerating decision-making. BMC Biol 14: 9, 2016. doi: 10.1186/s12915-016-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci 23: 2122–2130, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci 21: 9265–9272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Sokolowski MB. cGMP-dependent protein kinase: linking foraging to energy homeostasis. Genome 52: 1–7, 2009. doi: 10.1139/G08-090. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Riddle DL, Ruvkun G. The C. elegans DAF-2 insulin-like receptor is abundantly expressed in the nervous system and regulated by nutritional status. Cold Spring Harb Symp Quant Biol 76: 113–120, 2011. doi: 10.1101/sqb.2011.76.010660. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946, 1997. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Lebois F, Sauvage P, Py C, Cardoso O, Ladoux B, Hersen P, Di Meglio JM. Locomotion control of Caenorhabditis elegans through confinement. Biophys J 102: 2791–2798, 2012. doi: 10.1016/j.bpj.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel AD. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods 8: 147–152, 2011. doi: 10.1038/nmeth.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Moussaif M, Kuan CJ, Gargus JJ, Sze JY. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab 4: 429–440, 2006. doi: 10.1016/j.cmet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115: 489–502, 2003. doi: 10.1016/S0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322, 1997. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lockery SR, Lawton KJ, Doll JC, Faumont S, Coulthard SM, Thiele TR, Chronis N, McCormick KE, Goodman MB, Pruitt BL. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol 99: 3136–3143, 2008. doi: 10.1152/jn.91327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens D. Observations on the life cycle of Prionchulus punctatus (Cobb, 1917) (Nematoda) and some culture conditions. Biol Jb Dodonaea 43: 197–218, 1975. [Google Scholar]
- McFarland DJ. Decision making in animals. Nature 269: 15–21, 1977. doi: 10.1038/269015a0. [DOI] [Google Scholar]
- Meelkop E, Temmerman L, Janssen T, Suetens N, Beets I, Van Rompay L, Shanmugam N, Husson SJ, Schoofs L. PDF receptor signaling in Caenorhabditis elegans modulates locomotion and egg-laying. Mol Cell Endocrinol 361: 232–240, 2012. doi: 10.1016/j.mce.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Michener GR. Effect of climatic conditions on the annual activity and hibernation cycle of Richardson’s ground squirrels and Columbian ground squirrels. Can J Zool 55: 693–703, 1977. doi: 10.1139/z77-091. [DOI] [Google Scholar]
- Nagy S, Raizen DM, Biron D. Measurements of behavioral quiescence in Caenorhabditis elegans. Methods 68: 500–507, 2014a. doi: 10.1016/j.ymeth.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S, Tramm N, Sanders J, Iwanir S, Shirley IA, Levine E, Biron D. Homeostasis in C. elegans sleep is characterized by two behaviorally and genetically distinct mechanisms. eLife 3: e04380, 2014b. doi: 10.7554/eLife.04380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S, Wright C, Tramm N, Labello N, Burov S, Biron D. A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Gαs signaling. eLife 2: e00782, 2013. doi: 10.7554/eLife.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Lee KH, Churgin MA, Hill AJ, Van Buskirk C, Fang-Yen C, Raizen DM. FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr Biol 24: 2406–2410, 2014. doi: 10.1016/j.cub.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Trojanowski NF, George-Raizen JB, Smith CJ, Yu CC, Fang-Yen C, Raizen DM. The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans. Nat Commun 4: 2846, 2013. doi: 10.1038/ncomms3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelley ET, Fisher KC. Rhythmical arousal from hibernation in the golden-mantled ground squirrel, Citellus lateralis tescorum. Can J Zool 39: 105–120, 1961. doi: 10.1139/z61-013. [DOI] [Google Scholar]
- Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci USA 105: 20982–20987, 2008. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Cullison KM, Pack AI, Sundaram MV. A novel gain-of-function mutant of the cyclic GMP-dependent protein kinase egl-4 affects multiple physiological processes in Caenorhabditis elegans. Genetics 173: 177–187, 2006. doi: 10.1534/genetics.106.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 451: 569–572, 2008. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci 21: 5871–5884, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Augustine SB, Lawton KJ, Lindsay TH, Thiele TR, Izquierdo EJ, Faumont S, Lindsay RA, Britton MC, Pokala N, Bargmann CI, Lockery SR. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. eLife 5: 1–41, 2016. doi: 10.7554/eLife.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol 72: 293–299, 2007. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631, 2000. doi: 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375: 73–78, 1995. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296: 2388–2391, 2002. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol 209: 89–102, 2006. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. J Neurobiol 54: 111–147, 2003. doi: 10.1002/neu.10164. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: WormBook Bethesda, MD: The C. elegans Research Community, 2006. doi: 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119, 1983. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403: 560–564, 2000. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Tong Zhou G, Schafer WR, Schafer RW. A three-state biological point process model and its parameter estimation. IEEE Trans Signal Process 46: 2698–2707, 1998. doi: 10.1109/78.720372. [DOI] [Google Scholar]
- Tramm N, Oppenheimer N, Nagy S, Efrati E, Biron D. Why do sleeping nematodes adopt a hockey-stick-like posture? PLoS One 9: e101162, 2014. doi: 10.1371/journal.pone.0101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104: 619–647, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski NF, Raizen DM. Call it worm sleep. Trends Neurosci 39: 54–62, 2016. 10.1016/j.tins.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek M, Lewandrowski I, Bringmann H. An AP2 transcription factor is required for a sleep-active neuron to induce sleep-like quiescence in C. elegans. Curr Biol 23: 2215–2223, 2013. doi: 10.1016/j.cub.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Van Gundy SD. Factors in survival of nematodes. Annu Rev Phytopathol 3: 43–68, 1965. doi: 10.1146/annurev.py.03.090165.000355. [DOI] [Google Scholar]
- Vidal-Gadea A, Topper S, Young L, Crisp A, Kressin L, Elbel E, Maples T, Brauner M, Erbguth K, Axelrod A, Gottschalk A, Siegel D, Pierce-Shimomura JT. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci USA 108: 17504–17509, 2011. doi: 10.1073/pnas.1108673108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea AG, Davis S, Becker L, Pierce-Shimomura JT. Coordination of behavioral hierarchies during environmental transitions in Caenorhabditis elegans. Worm 1: 5–11, 2012. doi: 10.4161/worm.19148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Zhou GT, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21: 203–214, 1998. doi: 10.1016/S0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Wen Q, Po MD, Hulme E, Chen S, Liu X, Kwok SW, Gershow M, Leifer AM, Butler V, Fang-Yen C, Kawano T, Schafer WR, Whitesides G, Wyart M, Chklovskii DB, Zhen M, Samuel AD. Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron 76: 750–761, 2012. doi: 10.1016/j.neuron.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290: 147–150, 2000. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- You YJ, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab 7: 249–257, 2008. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.