Adeno-associated viral vector (AAV)-mediated gene delivery is a valuable tool for neurophysiology, but variability in transduction efficiency remains a bottleneck for experimental success. Repeated vector injections can help overcome this limitation but affect humoral immune state and transgene expression in ways that are poorly understood. We show that AAV vector injections into the primate central nervous system trigger long-lasting and serotype-specific immune responses, raising the possibility that switching serotypes may promote successful vector readministration.
Keywords: AAV vectors, nonhuman primate, optogenetics, neutralizing antibodies
Abstract
Gene delivery to the primate central nervous system via recombinant adeno-associated viral vectors (AAV) allows neurophysiologists to control and observe neural activity precisely. A current limitation of this approach is variability in vector transduction efficiency. Low levels of transduction can foil experimental manipulations, prompting vector readministration. The ability to make multiple vector injections into the same animal, even in cases where successful vector transduction has already been achieved, is also desirable. However, vector readministration has consequences for humoral immunity and gene delivery that depend on vector dosage and route of administration in complex ways. As part of optogenetic experiments in rhesus monkeys, we analyzed blood sera collected before and after AAV injections into the brain and quantified neutralizing antibodies to AAV using an in vitro assay. We found that injections of AAV1 and AAV9 vectors elevated neutralizing antibody titers consistently. These immune responses were specific to the serotype injected and were long lasting. These results demonstrate that optogenetic manipulations in monkeys trigger immune responses to AAV capsids, suggesting that vector readministration may have a higher likelihood of success by avoiding serotypes injected previously.
NEW & NOTEWORTHY Adeno-associated viral vector (AAV)-mediated gene delivery is a valuable tool for neurophysiology, but variability in transduction efficiency remains a bottleneck for experimental success. Repeated vector injections can help overcome this limitation but affect humoral immune state and transgene expression in ways that are poorly understood. We show that AAV vector injections into the primate central nervous system trigger long-lasting and serotype-specific immune responses, raising the possibility that switching serotypes may promote successful vector readministration.
genetic techniques for manipulating and monitoring signals in the primate central nervous system (CNS) require delivering engineered constructs to the brain, conventionally by viral vector injections. Viral vectors based on adeno-associated virus (AAV) are particularly useful for these applications because they transduce nondividing cells and are minimally pathogenic (Monahan and Samulski 2000; Diester et al. 2011). However, the transduction efficiency of seemingly identical AAV vector injections into the primate CNS is variable. This variability poses a significant barrier to progress because most primate studies use few animals, precluding remediation by averaging across animals. Low levels of transduction become clear only months after a vector injection has been made and can thwart experimental manipulations in ways that are difficult to correct. Increasing the reliability of AAV vector-mediated gene delivery is therefore an important goal for primate neurophysiology.
One factor that can influence the success of gene delivery is the immune status of the injected animal. Some animals harbor neutralizing antibodies (NAbs) in their blood that bind to AAV vectors and prevent transduction (Calcedo and Wilson 2013; Klasse 2014). This blockade is a major concern for gene therapy; a lack of preexisting immunity to AAV is a criterion for enrollment in some clinical trials (Jaski et al. 2009; Nathwani et al. 2011). Experiments in nonhuman primates show that low titers of NAbs to AAV can block transduction of some tissues (Hernandez et al. 1999; Jiang et al. 2006; Wang et al. 2011). The CNS is thought to be relatively protected against the effects of NAbs by the blood-brain barrier (Asokan et al. 2012; Freskgård and Urich 2016; Poduslo et al. 1994; Treleaven et al. 2012), but injections into the brain necessarily compromise this barrier, providing a route by which AAV vectors can interact with circulating antibodies and other components of the adaptive immune system. These interactions may accelerate the production of antibodies against AAV and limit the efficiency of AAV vector-mediated transduction.
Immune responses to AAV vectors have been investigated in the context of gene therapy, but differences in protocol between gene therapeutic and neurophysiological experiments complicate direct comparisons. Gene therapy studies typically involve the delivery of high vector doses via intravascular, intramuscular, or intrathecal routes, which affect the immune system in ways that differ qualitatively from the delivery of lower doses directly into the brain parenchyma, as is typical of neurophysiological studies. When gene therapy studies do make injections into the brain parenchyma, they typically use small openings in the cranium that are subsequently protected by host tissue, thereby limiting exposure to nonsterile environments. In contrast, craniotomies made in primate neurophysiological studies can be large, and foreign devices are inserted through them on a regular basis. These insertions compromise the blood-brain barrier and induce reactive changes in astrocytes, considerations that are particularly relevant given that AAV capsids can be detected in the brain weeks after an injection has been made (Samaranch et al. 2016) and that astrocytes are capable of antigen presentation (Fontana et al. 1984; Soos et al. 1998). Finally, gene therapy studies focus on AAV serotypes that have been approved for use in humans (e.g., AAV2) or those that have low prevalence of seropositivity in human populations (e.g., AAV.rh10) whereas AAV serotypes 1, 5, and 9, in addition to AAV2, are common in primate neurophysiological studies.
To assess humoral immune responses to AAV vector injections into the nonhuman primate brain, we quantified NAb titers in blood sera collected before and after injections. We found that injections of AAV1 and 9 into the cerebral and cerebellar cortices raised NAb titers consistently by at least threefold. These immune responses developed as early as 3 days after an injection, lasted for at least several months, and were specific to the serotype of AAV injected. Readministration of the same serotype continued to elevate NAb titer beyond that obtained after a single vector injection. Thus AAV vector injections typical of primate neurophysiological experiments have robust effects on humoral immune status.
MATERIALS AND METHODS
Experimental subjects.
Blood samples were obtained from three rhesus monkeys (Macaca mulatta): two females and one male (7–14 kg). Blood draws were performed by Washington National Primate Research Center veterinary staff as part of routine physical examinations after sedation (ketamine, 10 mg/kg). Approximately 2–4 ml of blood were drawn into collection tubes containing a clotting activator and gel for serum separation (BD Vacutainer, SST #367983). Following gentle agitation and a 30 min incubation period, collection tubes were centrifuged for 12 min at 1,200 rcf, and the separated serum was transferred to cryogenic vials and stored at –20°C. Blood draws were obtained before and after AAV vector injections into the brain (Fig. 1). Animal care conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee at the University of Washington. Animals were on a 12-h light-dark cycle and pair housed whenever possible. AAV injections were made in the laboratory during the day. This report was prepared in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
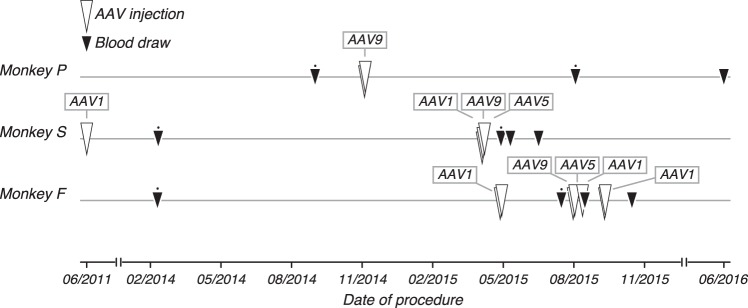
Fig. 1.
Blood draw and adeno-associated viral vector (AAV) injection timeline. Three rhesus monkeys received AAV vector injections (white triangles; also see Table 1) as part of optogenetic experiments. Blood samples were drawn (black triangles) before and after injections, and sera were collected for testing. Sera contributing to the data shown in Figs. 3–5 are highlighted (black dots).
AAV vector production.
AAV vectors were produced using a conventional three-plasmid transient transfection of human embryonic kidney cells (HEK 293T) with polyethylenimine (25 kDa, Polysciences). Cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 1% amphotericin B, penicillin (50 U/ml), and streptomycin (50 µg/ml) and incubated at 37°C with 5% CO2. Following 72 h of incubation, cells were harvested and pelleted by centrifugation. Vectors were released from the cells by repeated freeze-thaw cycles, purified by ultracentrifugation through an iodixanol gradient and exchanged into phosphate-buffered saline (PBS). Vector titers ranged from 1011 to 1013 genomic copies/ml (Table 1).
Table 1.
Details of viral vector injections made into the brain of nonhuman primates
| Monkey | Injection Date | Brain Area | AAV Vector Construct | Vector Titer, genomes/ml | Total Volume, μl |
|---|---|---|---|---|---|
| P | 11/10/2014 | V1 | AAV9–hSyn–oChIEF–citrine | 1.44 × 1013 | 16 |
| P | 11/11/2014 | V1 | AAV9–hSyn–oChIEF–citrine | 1.44 × 1013 | 16 |
| S | 06/08/2011 | V1 | AAV1–hSyn–ChR2–mCherry | 5.50 × 1011 | 12 |
| S | 03/31/2015 | Cerebellum | AAV1–L7–ChR2–mCherry | 8.45 × 1012 | 15 |
| S | 04/01/2015 | Cerebellum | AAV9– L7–ChR2–mCherry | 1.41 × 1013 | 15 |
| S | 04/03/2015 | Cerebellum | AAV5–hSyn–ArchT–eYFP | 8.08 × 1011 | 11 |
| F | 04/22/2015 | FEF | AAV1–hSyn–oChIEF–citrine | 2.2 × 1012 | 15 |
| F | 04/24/2015 | FEF | AAV1–hSyn–oChIEF–citrine | 2.2 × 1012 | 12 |
| F | 07/22/2015 | Cerebellum | AAV9– L7–ChR2–mCherry | 1.41 × 1013 | 17 |
| F | 07/23/2015 | Cerebellum | AAV5–hSyn–ArchT–eYFP | 8.08 × 1011 | 17 |
| F | 08/03/2015 | Cerebellum | AAV1–L7–ChR2–mCherry | 1.53 × 1013 | 17 |
| F | 09/01/2015 | Cerebellum | AAV1–L7–eNphR3.0–eYFP | 7.00 × 1012 | 17 |
AAV, adeno-associated viral vectors; FEF, frontal eye field; V1, primary visual cortex.
AAV vector injection methods.
Vectors were delivered to the brain via a cannula made of fused silica (Polymicro Technologies; ~150-μm inner diameter and ~360-μm outer diameter) that was connected to a Hamilton syringe (Hamilton, 100 μl) held in a manual pump (Stoelting with a Starrett micromanipulator no. 262M). The cannula and syringe were filled with silicone fluid (Octamethyltrisiloxane; Clearco Products) that was mixed with fluorescent leak detection dye (Dye-Lite; Tracerline) and filter-sterilized by passage through a Mixed Cellulose Esters membrane (Millex-GS 0.22 µm; SLGS033SB; EMD Millipore). The dyed silicone fluid served two purposes: 1) to occupy the full extent of the cannula, less the vector volume to be injected, and 2) to optimize visualization of the meniscus between the vector and silicone fluid under illumination with blue light during the injection procedure. Marks delineating 1-μl increments were placed along the cannula to facilitate tracking of the volume injected. The injection cannula was beveled at the tip to ease entry into the brain and was loaded into a custom dual-hydraulic Microdrive system (Narishige) alongside a tungsten recording electrode (FHC or Alpha Omega). The injection cannula and electrode were lowered into the brain through a common stainless steel guide tube and were independently moveable.
Before injections, we planned the placement of injection sites by mapping the extent of gray matter in the brain region of interest using standard extracellular neurophysiological techniques. Each injection started at the deepest point in a penetration, and the vector was delivered periodically during cannula retraction to fill the hole left by the cannula. We typically injected ~1 μl of vector every ~250–500 μm over 3–5 min while monitoring the position of the meniscus and waiting 3–5 min between injections. After the final vector injection, we waited 10–15 min before fully retracting the injection cannula to avoid the escape of vector along the injection track.
Assay for quantitating AAV NAbs.
To quantitate NAbs to AAV, we used an in vitro assay in which HEK 293T cells were treated with an AAV vector carrying the gene for green fluorescent protein (GFP) downstream of the cytomegalovirus promoter (CMV). Cells were seeded in 48-well plates (~12,500 cells/well), and after 24 h of incubation at 37°C with 5% CO2, the culture medium was replaced with new medium either 1) alone (i.e., “no virus” control), a condition that provided a lower bound on the fluorescence signal in the emission band of GFP; 2) containing AAV–CMV–GFP without serum (i.e., “no serum” control), a condition that provided an upper bound on the percentage of GFP-positive cells; or 3) containing a mixture of AAV–CMV–GFP and diluted blood serum.
Serum from each monkey was heat-inactivated at 56°C for 35 min to denature proteases and other heat-labile complement molecules that could affect cell health or inhibit transduction nonselectively (Bordet and Gengou 1901; Calcedo et al. 2009). Serial dilutions of serum in culture medium were prepared in ratios of 1:200, 1:400, 1:800, 1:1,600, and 1:3,200. To these diluted sera, we added AAV–CMV–GFP in an amount empirically determined to achieve GFP expression in ~25–35% of cells in the absence of serum (see Table 2). Three replicates of each condition were prepared, yielding a total of 21 samples for each serum tested (5 dilutions + no virus control + no serum control). All samples were incubated at 37°C for 1 h before application to cultured cells.
Table 2.
Number of genomic copies of AAV vector used in the assay
| AAV Serotype | Vector Titer genomes/ml | Vector Quantity genomes/sample |
|---|---|---|
| AAV1–CMV–GFP | 2.50 × 1012 | 1.97 × 108 |
| AAV2–CMV–GFP | 1.62 × 1012 | 3.44 × 106 |
| AAV5–CMV–GFP | 4.82 × 1012 | 2.54 × 109 |
| AAV9–CMV–GFP | 3.61 × 1013 | 6.27 × 109 |
GFP, green fluorescent protein; CMV, cytomegalovirus promoter.
After 48 h of additional incubation, cells were prepared for flow cytometry by detaching them from the culture plate (trypsin in 0.25% EDTA), and pelleting them by centrifugation (850 rcf for 6 min at 18°C). The cell pellet was then resuspended in 1× PBS, and analyzed immediately.
Quantifying GFP via flow cytometry.
Cells were counted using a BD LSR II flow cytometer (488 nm excitation; 530 nm emission). A total of 10,000 events were recorded for each sample tested. As per standard flow cytometry analysis methods, signal preprocessing included two stages of selection: 1) to exclude cellular debris and large aggregates of cells (Fig. 2A, gray polygon), and 2) to exclude doublets of cells (Fig. 2B, gray rectangle).
Fig. 2.
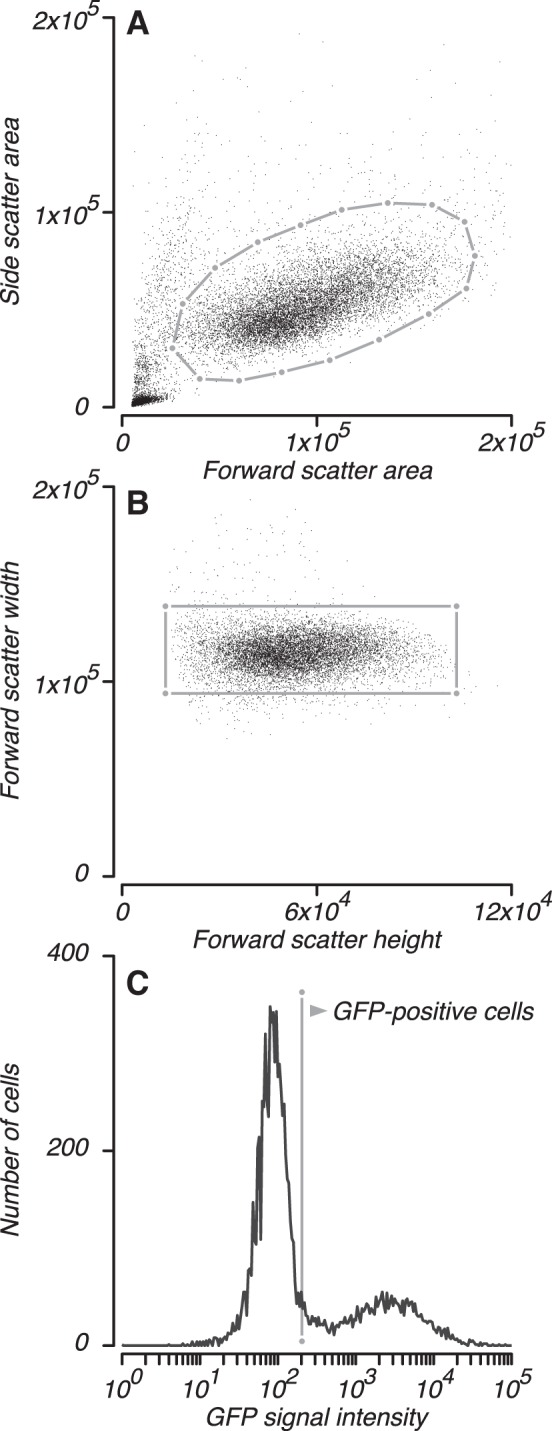
Flow cytometry methods. Cells were analyzed by fluorescence activated cell sorting. A: events were gated to exclude cellular debris and large aggregates of cells (gray polygon). B: events were also gated to exclude doublets of cells (gray rectangle). C: events that passed both gating procedures were analyzed for green fluorescent protein (GFP) signal; those that achieved a criterion level of GFP expression (gray line) were classified as GFP-positive.
We examined the distribution of GFP fluorescence intensity across cells that passed both gating procedures (Fig. 2C). The “no virus” control condition was used to select a criterion value of fluorescence intensity above which 1% of cells were classified as GFP positive (false alarms). For each of the remaining sample conditions (serum dilutions and “no serum” controls), we tallied the number of events that exceeded the mean criterion value across “no virus” replicates (Fig. 2C, gray line) and divided this count by the total number of events to obtain the percentage of GFP-positive cells.
We fit the data with a descriptive model based on a decaying exponential function:
where y is the percentage of GFP-positive cells, x is the reciprocal of blood serum dilution, and α and β are fitted parameters. The parameter α corresponds to the percentage of GFP-positive cells in “no serum” controls, less the lower bound of 1%. The parameter β corresponds to the efficacy with which the serum blocks AAV transduction. Both α and β were estimated using an optimization procedure (Matlab, fminsearch) that minimized the sum of squared differences between the observed and predicted percentage of GFP-positive cells. The NAb titer was defined as the reciprocal of the serum dilution corresponding to a 50% decrement in the percentage of GFP-positive cells from its maximal value (α + 1 in Eq. 1), referred to as D50. Differences in D50 between pre- and postinjection sera were tested for statistical significance by randomization tests (10,000 resamples) (Edgington and Onghena 2007). Statistical comparisons were made between serum samples that were processed on the same day using the same cell plating density and vector stocks.
RESULTS
To investigate immune responses to AAV vector injections into the primate brain, we analyzed blood serum samples from three rhesus macaques, drawn before and after injections (Fig. 1). AAV vectors carrying optogenetic constructs were injected into the primary visual cortex (V1), frontal eye fields (FEF), and cerebellum (Table 1). We used a standard in vitro immunological assay to test for the presence of NAbs to AAV in blood serum (Fig. 2; see materials and methods). For sera that do not contain NAbs to AAV, we expect comparable transduction efficiency across all samples, regardless of serum concentration. For sera that do contain NAbs to AAV, we expect that these antibodies will recognize and bind the AAV–CMV–GFP vector used in the assay, thereby reducing transduction efficiency and manifesting as an inverse relationship between serum concentration and the percentage of GFP-positive cells.
NAb titers increase after AAV injections into the brain.
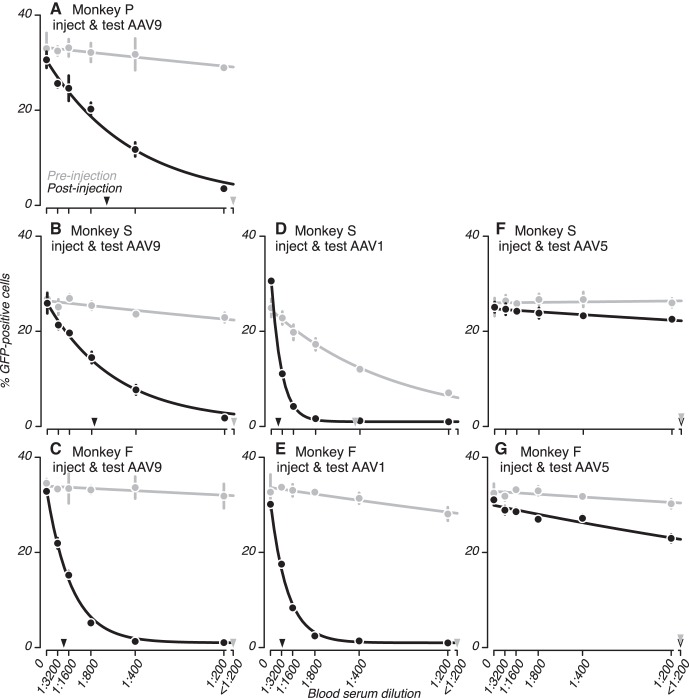
Serum samples collected before vector injections had little effect on transduction efficiency (Fig. 3, gray). Only one of these samples reduced transduction efficiency by >50%, an observation we return to shortly. In contrast, serum samples collected after AAV vector injections contained NAbs, as shown by the steeper, concentration-dependent reduction in the percentage of GFP-positive cultured cells (Fig. 3, black). Note that the serum sample from monkey S listed as “preinjection” (Fig. 3, B, D, and F; gray) was collected ~3 yr after this monkey was first injected with AAV1 (see Table 1) as part of an earlier study (Jazayeri et al. 2012). This monkey had not been previously injected with any other viral vector, and monkeys F and P had not been injected with any viral vector before their first blood draw.
Fig. 3.
Comparing neutralizing antibodies (NAbs) with AAV before and after vector injections. Sera collected before and after injections into the brain of 3 monkeys were analyzed for NAbs to the AAV serotype that was injected. A–G: preinjection sera (gray) tested negative for NAbs to AAV. The only exception was the preinjection serum for monkey S (in D), which was collected ~3 yr after an injection of AAV1. Postinjection sera (black) contained NAbs to AAV. We quantified NAbs in each serum using the serum dilution corresponding to a 50% decrement in the percentage of GFP-positive cells from its maximal value (D50) (triangles, see materials and methods). Error bars represent SD.
For each serum tested, we quantified NAbs to AAV as the reciprocal of the serum dilution corresponding to a 50% decrement in transduction efficiency from its maximal level (D50). Comparisons of D50 values from preinjection and postinjection sera demonstrate that injections of AAV vectors into the monkey brain made as part of optogenetic experiments can result in significant humoral immune responses to AAV (Fig. 3, triangles; also see Table 3). AAV1 and AAV9 injections raised NAb titers significantly (P < 0.01; Table 3), and AAV5 injections raised titers nearly significantly (P = 0.06 for monkey S and P = 0.03 for monkey F; compare Fig. 3, A–E with Fig. 3, F and G).
Table 3.
Quantification of anti-AAV antibodies before and after injections into the brain
| Monkey | AAV serotype | Preinjection D50 | Postinjection D50 | P Value |
|---|---|---|---|---|
| P | AAV9 | <1:200 | 1:589 ± 9 | P < 0.01 |
| S | AAV9 | <1:200 | 1:753 ± 35 | P < 0.01 |
| S | AAV1 | 1:423 ± 18* | >1:3,200 | P < 0.01 |
| S | AAV5 | <1:200 | <1:200 | P = 0.06 |
| F | AAV9 | <1:200 | 1:2,034 ± 161 | P < 0.01 |
| F | AAV1 | <1:200 | 1:3,000 ± 30 | P < 0.01 |
| F | AAV5 | <1:200 | <1:200 | P = 0.03 |
SE was estimated by bootstrapping (200 resamples). P values were estimated by randomization tests (10,000 resamples). D50, serum dilution corresponding to a 50% decrement in the percentage of GFP-positive cells from its maximal level.
Note: this animal had received an injection of AAV1 in 2011, ~3 yr before the collection of the serum sample tested for neutralizing antibodies to AAV1.
Serotype specificity of NAbs.
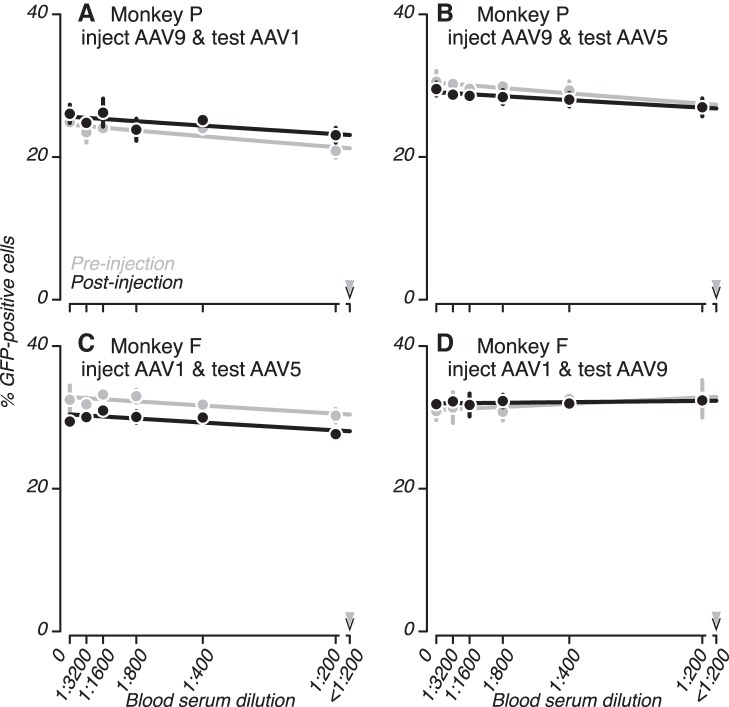
We considered the possibility that the reduction in transduction efficiency, which we interpret as an elevation of NAb titers to AAV, was caused by nonspecific factors in postinjection serum. If this were the case, we would expect that postinjection serum would block AAV-mediated transduction of cultured cells irrespective of which serotype was used in the assay. On the other hand, if the reduction in transduction efficiency was caused by antibodies that recognize specifically the AAV serotype injected into the brain, we expect to observe little or no blocking of transduction by postinjection serum when using other serotypes in the assay.
Sera from two of the three monkeys contributed to this experiment; sera from monkey S was excluded because we did not have pre- and postinjection samples flanking the injection of a single serotype. Serum from monkey P, who was injected with AAV9, was tested for NAbs to AAV1 and AAV5 (Fig. 4, A and B). Serum from monkey F, who was injected with AAV1, was tested for NAbs to AAV5 and AAV9 (Fig. 4, C and D). In none of these cases, did we find measurable differences in the transduction efficiency of vector that was incubated with pre- and postinjection serum (all D50 <1:200; P > 0.1), indicating that the factor in the serum that blocked transduction was specific to the serotype of AAV that had been injected into the brain.
Fig. 4.
Serotype specificity of NAbs. A–C: sera collected before and after injections into the brain of 2 monkeys were analyzed for NAbs to AAV serotypes that were not injected. Plotting conventions are as in Fig. 3.
As an additional test of serotype specificity, we measured NAbs titers to AAV2, a serotype we did not inject into any of the monkeys. Sera collected from monkey P and monkey F did not block transduction by AAV2 pre- or postinjection (Fig. 5, A and C; all D50 <1:200; P > 0.1). These data further attest to the serotype specificity of the humoral immune responses.
Fig. 5.
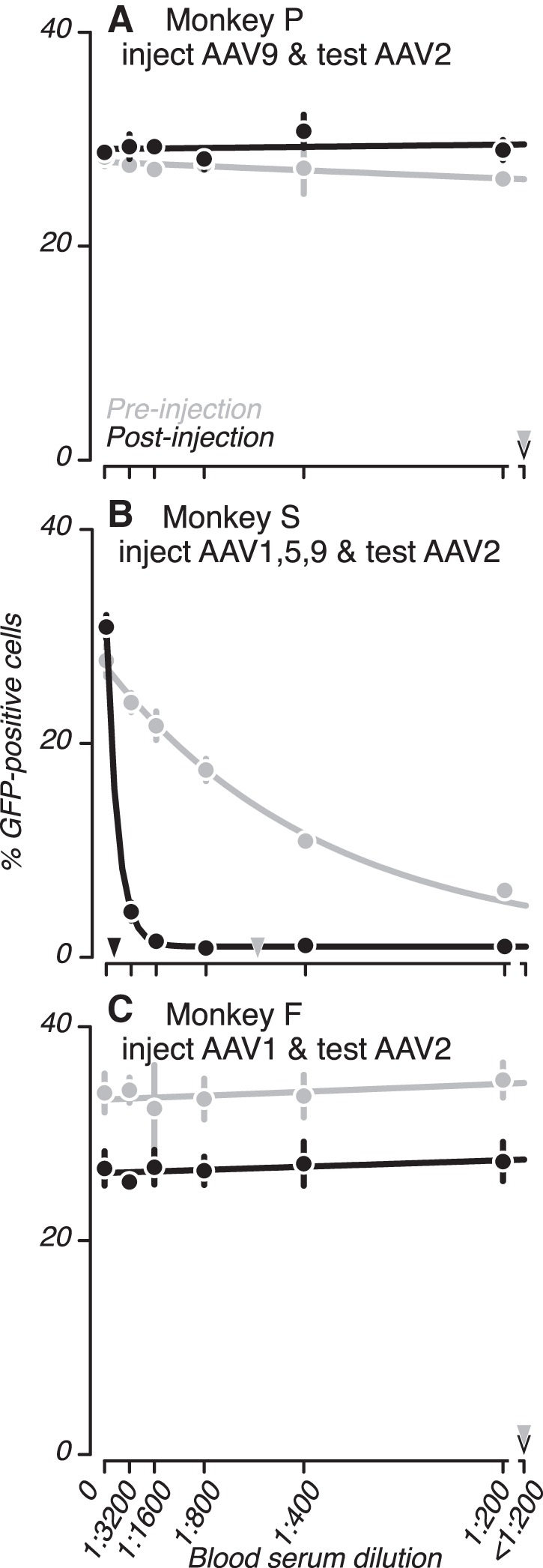
Testing for cross reactivity of NAbs to AAV2. A–C: sera collected before and after injections into the brain of 3 monkeys were analyzed for NAbs to AAV2, a serotype that was not injected. Plotting conventions are as in Fig. 3.
We did observe one case of apparent cross reactivity between serotypes. monkey S was injected with AAV1 into the brain ~3 yr before collection of the first serum sample, and this sample blocked transduction by AAV2 (Fig. 5B, gray) and by AAV1 (Fig. 3D, gray). Following additional injections of AAV1, AAV5, and AAV9 into monkey S, the NAb titer to AAV2 increased from a D50 of 1:527 ± 17 to >1:3200 (Fig. 5B, black; P < 0.01), suggesting that antibodies produced in response to AAV1 may have cross reacted with AAV2.
Time course of anti-AAV immune responses.
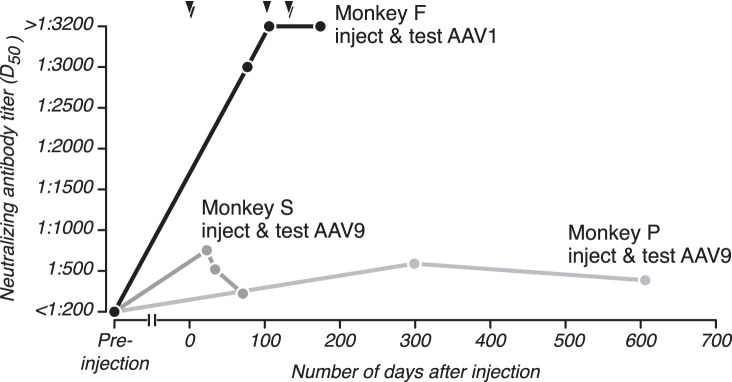
Monkey F received five AAV1 injections over the course of 4 mo (Table 1). To measure the effects of these repeated injections on immune status, we analyzed sera collected at various time points spanning vector injection dates and compared D50 values. Preinjection serum did not block transduction by AAV1 (Fig. 6, black; D50: <1:200), whereas serum collected after each injection (triangles) did (D50: 1:3,000 ± 30 and >1:3,200). Differences in the D50 values among the first three serum samples were significant (P < 0.01; comparing the first sample collected with the second sample collected, then the second to the third, etc.). The difference in D50 values for the last two sera was not significant (P = 0.66) because these sera had such high NAbs titers that even serum diluted at 1:3,200 was sufficient to block transduction by > 50%. These data demonstrate that repeated exposure to an AAV serotype can elevate AAV NAb titers beyond those obtained after a single injection.
Fig. 6.
Time course of AAV immune responses. Sera collected from 3 monkeys at different time points relative to a single vector injection, or repeated vector injections (triangles; only for monkey F), were analyzed for NAbs to the serotype that was injected.
To investigate the duration of NAb titer elevation without repeated injections, we tested sera from the other two monkeys at several time points after a single vector injection. For monkey S, we tested serum collected before an injection of AAV9, as well as sera collected 23, 34, and 71 days after the injection (Fig. 6, dark gray). Preinjection serum did not block transduction by AAV9 (D50: <1:200), whereas the first postinjection serum sample showed moderate blocking (D50: 1:753 ± 35). Sera collected 34 and 71 days postinjection showed progressively weaker blocking (D50: 1:518 ± 12 and 1:221 ± 18). For monkey P, we tested sera collected before an injection of AAV9, as well as 299 and 606 days after that injection was made (Fig. 6, light gray). Preinjection serum did not block transduction by AAV9 (D50 <1:200) whereas the first postinjection serum showed moderate blocking (D50: 1:589 ± 9), and serum collected 606 days after the injection showed weaker blocking (D50: 1:386 ± 15). For both monkeys, differences between each pair of sequential D50 values were significant (P < 0.01). These data demonstrate that humoral immune responses to AAV injections can last for many months but decrease over time (see also Mastakov et al. 2002; Petry et al. 2008).
DISCUSSION
Injections of AAV vectors into the brain, made as part of optogenetic experiments in rhesus macaques, elevated NAbs to AAV (Fig. 3). These immune responses were specific to the AAV serotype injected (Fig. 4) and were persistent (Fig. 6), in agreement with previous studies (Blacklow et al. 1968; Jiang et al. 2006; Nieto et al. 2012; Rivière et al. 2006; Zerah et al. 2015). Our single example of nonspecific blockade of transduction by AAV2 (Fig. 5B) is also consistent with another report of cross reactivity to AAV2 following injections of other serotypes (Kotterman et al. 2015). A new contribution of our study is the finding that small vector doses injected into the monkey CNS can produce high NAb titers. This result may be due to the chronic nature of the craniotomy through which we made injections or to repeated breach of the blood-brain barrier by electrodes or optical fibers preceding or following vector injections. Below, we discuss the strengths and limitations of the assay we used and how these affect our interpretation of the data. We then examine the implications of our results for neurophysiological studies that employ injections of AAV vectors into the primate brain.
Assay strengths and limitations.
The in vitro assay used in this study is the standard for quantifying NAbs to AAV (Calcedo and Wilson 2013) and is well suited for investigating the types of immune responses that are relevant to primate neurophysiology. In contrast, ELISA-based assays cannot discriminate neutralizing from nonneutralizing antibodies, and in vivo passive transfer assays provide NAb titers that correlate less well with transduction efficiency in macaques (Wang et al. 2011).
We quantified NAbs to AAV by estimating the blood serum dilution that produced a 50% decrement in transduction of cultured cells in the assay (D50) on the basis of a fitted function. Previous studies have not used fitted functions to interpolate antibody titers, but we found a mathematical function that described all of our data well, and the D50 values extracted from these fits were highly repeatable across replicates. Our use of flow cytometry to count a large number of cells and the short delay between sample preparation and cell counting may have contributed to the consistency across replicates and the quality of the fits.
Estimates of D50 depend on the percentage of GFP-positive cells in the absence of serum. This is expected: if each cell were transduced by many AAV particles, a high concentration of NAbs would be required to reduce transduction efficiency to half of the maximal value. To ensure that the proportion of GFP-positive cells in the absence of serum was similar across serotypes, we adjusted the concentration of AAV–CMV–GFP in the assay to compensate for variations in the tropism of each serotype for HEK 293T cells (Table 3). This adjustment, however, does not equate assay sensitivity across AAV serotypes (Calcedo and Wilson 2013; Wang et al. 2011). For serotypes requiring high vector concentrations, sensitivity is low because higher antibody concentrations are required to neutralize higher AAV concentrations. We used more AAV9 than AAV5 in the assay, and this is expected to reduce anti-AAV9 titers relative to anti-AAV5 titers. Nevertheless, anti-AAV9 titers exceeded anti-AAV5 titers in the sera we tested, an effect that cannot be explained by the concentrations of vector used in the assay.
The anti-AAV5 titers we measured may have been low because the AAV5 vector injected into the brain had the lowest concentration of the vectors we injected (Table 1) and therefore may have been insufficient to induce a strong humoral immune response. There is little evidence that AAV5 is less immunogenic than other serotypes: anti-AAV5 titers increase following intracerebral (Treleaven et al. 2012) or intranasal (Nieto et al. 2009) administration in mice, and intramuscular AAV5 injections prevent successful readminstration (Rivière et al. 2006). The anti-AAV9 titers we measured may have been higher because AAV9 crosses the blood-brain barrier more readily than AAV5 (Foust et al. 2009; Gray et al. 2011; Samaranch et al. 2012).
All of the preinjection sera we tested were negative for NAbs, except one (monkey S tested positive for anti-AAV2 antibodies). Had we tested higher serum concentrations, we might have measured NAb titers in other preinjection sera, but even if so, these titers would have necessarily been lower than the titers we did measure. We tested only serum concentrations <1:200 for three reasons. First, we were interested in large effects; low NAbs titers are less likely to be important for the reliability of AAV-mediated transduction of the brain than high titers. Second, nonspecific factors in serum affect transduction efficiency at high concentrations (van der Marel et al. 2011; Wang et al. 2010). Third, using low concentrations of serum allowed us to test for NAbs to a greater number of serotypes from each serum sample.
Implications for neurophysiological studies.
We found that injections of AAV1 and AAV9 increased NAb titers consistently. Readministration of these serotypes had mixed effects on the success of gene delivery in vivo. In one animal (monkey F), we successfully transduced multiple brain areas with multiple injections of a single serotype (AAV1) separated by many weeks. Nevertheless, it remains possible that the extent of transduction produced by the later injections would have been larger in an animal naïve to AAV1. In another animal (monkey S), readministraton of the same serotype (AAV1) failed to transduce cerebellar cortex after an injection into V1, whereas injection of a novel serotype (AAV9) into the cerebellar cortex was successful. This result suggests that switching serotypes may be an effective strategy for increasing the likelihood of successful readministation of AAV vectors to the primate CNS, consistent with studies performed in the primate airway (Nieto et al. 2012), rat striatum (Peden et al. 2004), and mouse skeletal muscle (Rivière et al. 2006).
Other strategies to overcome the anti-AAV immune response include immunosuppression and plasmapheresis (Lorain et al. 2008; Mingozzi and High 2013; Monteilhet et al. 2011), but these may have adverse consequences for monkeys prepared for chronic neurophysiological recordings. Whether such measures are warranted for neurophysiological studies is unclear. A practical guide to procedures that circumvent anti-AAV immune responses awaits a better understanding of the relationship between AAV transduction efficiency in vitro and in vivo. Clarifying this relationship requires experiments with multiple animals, sequential vector injections, blood draws, and quantitative histological analyses.
Knowing how high the NAb titer has to be to prevent CNS transduction would be useful but is challenging. NAb titers measured by different protocols are not directly comparable. Many additional factors contribute to transduction efficiency (e.g., vector dose, serotype, tissue target, route of administration, and time between injections), and disentangling the relative contributions of each factor is a daunting task. For example, pharmacological disruption of the blood-brain barrier in mice allows even modest NAb titers to block striatal transduction (Janelidze et al. 2014). Mechanical disruption of the blood-brain barrier, as is typical of optogenetic experiments in monkeys, may exert a similar effect.
Effects of preexisting immunity.
Different mammalian species have natural, preexisting immunity to different AAV serotypes. For example, monkeys tend to be seropositive for AAV7, AAV8, and AAV.rh10 but not AAV2, whereas the opposite is true for humans (Calcedo et al. 2009; Calcedo and Wilson 2013; Gao et al. 2003). Preexisting immunity limits AAV vector-mediated transduction of the liver and heart (Jaski et al. 2009; Jiang et al. 2006; Manno et al. 2006; Wang et al. 2010; Wang et al. 2011), but the CNS appears to be minimally affected (Jiang et al. 2006; Sanftner et al. 2004). Whether a lack of preexisting immunity should be a requirement for including a monkey in a neurophysiological study is an open question, but our results suggest that it is not critical. Naturally occurring NAb levels tend to be low (Calcedo et al. 2015; Rapti et al. 2012) and we were able to readminister AAV1 to monkey F successfully when this animal’s NAbs titer was well outside of this range.
Conclusion.
Delivery of optogenetic constructs to the primate CNS via injections of AAV vectors increased NAb titers to AAV capsid. The antibody titers we measured are easily in the range expected to limit the transduction of non-CNS tissues, but how high titers must be to block CNS transduction remains unclear. Significant effort has been directed toward documenting which serotypes of AAV best transduce which structures and cell types. Our results indicate that the humoral immune status of the animal used for experimentation may be an additional factor to consider when selecting a viral vector for gene delivery.
GRANTS
This work was supported by National Institutes of Health Grants EY-024362 and OD-010425.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D.M. and Y.E.-S. performed experiments; S.D.M. and Y.E.-S. analyzed data; S.D.M., Y.E.-S., and G.D.H. interpreted results of experiments; S.D.M. and Y.E.-S. prepared figures; S.D.M., Y.E.-S., and G.D.H. drafted manuscript; S.D.M., Y.E.-S., and G.D.H. edited and revised manuscript; S.D.M., Y.E.-S., and G.D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the veterinary staff at the Washington National Primate Center for assistance with blood serum collection and Donna Prunkard at the University of Washington Department of Pathology Flow Cytometry Core Facility for assistance with FACS analysis. We also thank Adam Kohn and Mehrdad Jazayeri for helpful comments on the manuscript.
REFERENCES
- Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 20: 699–708, 2012. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst 40: 319–327, 1968. [PubMed] [Google Scholar]
- Bordet J, Gengou O. Sur l’existence de substances sensibilisatrices dans la plupart des sérums antimicrobiens. Ann Inst Pasteur 15: 289–302, 1901. [Google Scholar]
- Calcedo R, Franco J, Qin Q, Richardson DW, Mason JB, Boyd S, Wilson JM. Preexisting neutralizing antibodies to adeno-associated virus capsids in large animals other than monkeys may confound in vivo gene therapy studies. Hum Gene Ther Methods 26: 103–105, 2015. doi: 10.1089/hgtb.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 199: 381–390, 2009. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 4: 341, 2013. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci 14: 387–397, 2011. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington ES, Onghena P. Randomization Tests. London: Chapman and Hall/CRC, 2007. [Google Scholar]
- Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307: 273–276, 1984. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27: 59–65, 2009. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freskgård PO, Urich E. Antibody therapies in CNS diseases. Neuropharmacology pii: S0028-3908(16)30086-7, 2016. doi: 10.1016/j.neuropharm.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, Calcedo R, Sanmiguel J, Abbas Z, Wilson JM. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA 100: 6081–6086, 2003. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19: 1058–1069, 2011. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez YJ, Wang J, Kearns WG, Loiler S, Poirier A, Flotte TR. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol 73: 8549–8558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Nordström U, Kügler S, Brundin P. Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson’s disease. J Gene Med 16: 300–308, 2014. doi: 10.1002/jgm.2779. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ, Investigators CT; Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators . Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 15: 171–181, 2009. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat Neurosci 15: 1368–1370, 2012. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, Mingozzi F, High KA, Pierce GF. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 108: 3321–3328, 2006. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse PJ. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol pii: 15789, 2014. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther 22: 116–126, 2015. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorain S, Gross DA, Goyenvalle A, Danos O, Davoust J, Garcia L. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther 16: 541–547, 2008. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12: 342–347, 2006. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mastakov MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol 76: 8446–8454, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122: 23–36, 2013. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today 6: 433–440, 2000. doi: 10.1016/S1357-4310(00)01810-4. [DOI] [PubMed] [Google Scholar]
- Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther 19: 2084–2091, 2011. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365: 2357–2365, 2011. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto K, Kern A, Leuchs B, Gissmann L, Müller M, Kleinschmidt JA. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antivir Ther 14: 1125–1137, 2009. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- Nieto K, Stahl-Hennig C, Leuchs B, Müller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum Gene Ther 23: 733–741, 2012. doi: 10.1089/hum.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol 78: 6344–6359, 2004. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry H, Brooks A, Orme A, Wang P, Liu P, Xie J, Kretschmer P, Qian HS, Hermiston TW, Harkins RN. Effects of viral dose on neutralizing antibody response and transgene expression after AAV1 re-administration in mice. Gene Therapy 15: 54–60, 2008. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci USA 91: 5705–5709, 1994. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, Hajjar RJ, Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther 20: 73–83, 2012. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther 13: 1300–1308, 2006. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Samaranch L, Hadaczek P, Kells AP, Bringas JR, Stockinger D, San Sebastian W, Macayan M, Samineni S, Pivirotto P, Forsayeth J, Bankiewicz KS. Slow AAV2 clearance from the brain of nonhuman primates and anti-capsid immune response. Gene Ther 23: 393–398, 2016. doi: 10.1038/gt.2015.87. [DOI] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 23: 382–389, 2012. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, Cunningham J. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther 9: 403–409, 2004. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol 161: 5959–5966, 1998. [PubMed] [Google Scholar]
- Treleaven CM, Tamsett TJ, Bu J, Fidler JA, Sardi SP, Hurlbut GD, Woodworth LA, Cheng SH, Passini MA, Shihabuddin LS, Dodge JC. Gene transfer to the CNS is efficacious in immune-primed mice harboring physiologically relevant titers of anti-AAV antibodies. Mol Ther 20: 1713–1723, 2012. doi: 10.1038/mt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H, Hommes DW, Ferreira V. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis 17: 2436–2442, 2011. doi: 10.1002/ibd.21673. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL, Wilson JM. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 22: 1389–1401, 2011. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, Sanmiguel J, Morizono H, Batshaw ML, Wilson JM. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther 18: 126–134, 2010. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerah M, Piguet F, Colle MA, Raoul S, Deschamps JY, Deniaud J, Gautier B, Toulgoat F, Bieche I, Laurendeau I, Sondhi D, Souweidane MM, Cartier-Lacave N, Moullier P, Crystal RG, Roujeau T, Sevin C, Aubourg P. Intracerebral gene therapy using AAVrh.10-hARSA recombinant vector to treat patients with early-onset forms of metachromatic leukodystrophy: preclinical feasibility and safety assessments in nonhuman primates. Hum Gene Ther Clin Dev 26: 113–124, 2015. doi: 10.1089/humc.2014.139. [DOI] [PubMed] [Google Scholar]