ABSTRACT
A clinical isolate of Salmonella enterica serovar Senftenberg, isolated from an outbreak linked to the herb Ocimum basilicum L. (basil), has been shown to be resistant to basil oil and to the terpene alcohol linalool. To better understand how human pathogens might develop resistance to linalool and to investigate the association of this resistance with resistance to different antimicrobial agents, selective pressure was applied to the wild-type strain by sequential exposure to increasing concentrations of linalool. The results demonstrated that S. Senftenberg adapted to linalool with a MIC increment of at least 8-fold, which also resulted in better resistance to basil oil and better survival on harvested basil leaves. Adaptation to linalool was shown to confer cross protection against the antibiotics trimethoprim, sulfamethoxazole, piperacillin, chloramphenicol, and tetracycline, increasing their MICs by 2- to 32-fold. The improved resistance was shown to correlate with multiple phenotypes that included changes in membrane fatty acid composition, induced efflux, reduced influx, controlled motility, and the ability to form larger aggregates in the presence of linalool. The adaptation to linalool obtained in vitro did not affect survival on the basil phyllosphere in planta and even diminished survival in soil, suggesting that development of extreme resistance to linalool may be accompanied by a loss of fitness. Altogether, this report notes the concern regarding the ability of human pathogens to develop resistance to commercial essential oils, a resistance that is also associated with cross-resistance to antibiotics and may endanger public health.
IMPORTANCE Greater consumer awareness and concern regarding synthetic chemical additives have led producers to control microbial spoilage and hazards by the use of natural preservatives, such as plant essential oils with antimicrobial activity. This report establishes, however, that these compounds may provoke the emergence of resistant human pathogens. Herein, we demonstrate the acquisition of resistance to basil oil by Salmonella Senftenberg. Exposure to linalool, a component of basil oil, resulted in adaptation to the basil oil mixture, as well as cross protection against several antibiotics and better survival on harvested basil leaves. Collectively, this work highlights the hazard to public health while using plant essential oils without sufficient knowledge about their influence on pathogens at subinhibitory concentrations.
KEYWORDS: essential oils, antibiotic resistance, foodborne pathogens, host-pathogen interactions
INTRODUCTION
It has long been recognized that volatile plant metabolites, known as essential oils (EOs), have antibacterial, antiviral, antifungal, and insecticidal properties (1, 2). Nowadays, EOs are commercially extracted from different parts of plants (leaves, flowers, bulbs, seeds, etc.) for flavors, fragrances, and preservation purposes (2–4). One such extract (basil oil) is obtained from Ocimum basilicum L., which has widely been reviewed for its antimicrobial properties. Basil oil and each of its principal constituents—linalool, estragole, and eugenol (2, 5–10)—have largely been used in the food, sanitary, cosmetic, and perfume industries, with an estimated annual global level of production of more than 100 tons (7, 11–15). The industrial usage of basil oil is likely to grow steadily in the future due to its potential applications in antimicrobial technologies and the global green consumerism trend that has resulted in increasing demand for natural compounds (3, 9).
The antibacterial mode of action of the terpene alcohol linalool, one of the potent antimicrobial substances in basil oil, was recently investigated. It was shown that linalool perforates the bacterial membrane of Gram-negative bacteria, resulting in the increased permeability of both membranes and the leakage of vital molecules (16). Linalool also directly or indirectly inhibits cell motility and causes bacterial aggregation in Salmonella enterica serovar Senftenberg by an unknown mechanism (16, 17). The MIC of linalool against pathogens like Escherichia coli, Salmonella enterica, Staphylococcus spp., and Streptococcus spp. typically ranges from 0.6 to 125 mM (17, 18). However, much of the commercial use of linalool involves preparations with unspecified concentrations of linalool which may fall into its sublethal dosage range and, hence, provide considerable opportunity for bacterial adaptation to linalool (higher MIC values) when other barriers to bacteria applied in the product are not sufficient to eliminate the pathogens (16). For example, commercial fruit juices contain 0.02 to 90 mM linalool (19), and pet feed may contain up to 0.6 mM linalool (20).
Many researchers have suggested that spontaneous resistance to plant extracts is unlikely to arise, since the multicomponent nature of EOs incorporates various mechanisms of action against several targets and functions with respect to microbial membranes (1, 2, 21, 22). For example, Hammer et al. showed that single and multiple exposures to tea tree oil have little impact on the development of bacterial resistance (21). On the contrary, few studies have indicated that exposure of various bacteria to EOs, such as thyme oil, pine oil, oregano oil, and tea tree oil, has contributed to the development of resistance not only to the plant-derived extracts but also to antibiotics. In these cases, the resistance mechanisms allowed adaptation to various types of stresses (23–28). These contradictions, as well as the inadequate research addressing adaptation to pure single dominant compounds and cross-resistance, signify the importance of additional investigation in this field.
In 2007, a foodborne outbreak that was traced to contaminated fresh basil was reported in Europe. Salmonella enterica serovar Senftenberg was identified to be the cause of this outbreak (29). Investigation of a clinical isolate from this outbreak revealed that S. Senftenberg possessed an increased resistance to basil oil and each of its major compounds, which correlated with better survival on basil plants pre- and postharvest (30). The inhibition zone of linalool against S. Senftenberg, for instance, was 12.5 mm, whereas it was 21.0 mm against S. Typhimurium (30). The naturally reduced susceptibility of S. Senftenberg to EO components highlights the great health risks posed by the emergence of resistant pathogens adapted to plant-derived antimicrobial agents. Specifically, the resistance of S. Senftenberg to linalool is attributed to concurrent mechanisms, including chemotaxis-controlled motility, the selective permeability of the cell envelope, and regulated efflux/influx (16). The ability to form larger aggregates in the presence of linalool was also described, but its association with resistance is still unclear (16). It was shown that exposure to linalool enhances the transcription of mcpL, an STM1657-like gene, in S. Senftenberg. This gene encodes a putative methyl-accepting chemotaxis protein (MCP) with serine sensor receptors (16) which is possibly involved in the detection of chemotactic signals (31). Deletion of this gene in S. Senftenberg increases its sensitivity to linalool (16). In addition, linalool is a potential substrate of the AcrAB efflux pump. AcrAB/TolC efflux pumps are often overexpressed by Salmonella strains with increased resistance to various classes of antibiotics, such as quinolones, tetracycline, ampicillin, and chloramphenicol, as well as dyes, detergents, and disinfectants like triclosan (32–34). The expression of acrAB as a response to different stresses is known to be regulated by AcrR, MarA, Rob, SoxS, and RamA, whereas each regulatory factor is induced by different stress signals (35, 36). In fact, these activators jointly control the expression of both AcrAB and MicF, an antisense RNA which downregulates the porin OmpF. Since porins allow the passive diffusion of small molecules through the outer membrane, inhibition of OmpF expression by MicF decreases the influx of such molecules (37, 38). So far, however, there has been little discussion about the expression of these integrated sets in linalool-resistant bacteria.
To gather better insight about bacterial resistance to EOs, we investigated whether resistance to high concentrations of linalool or basil oil may develop following a multistep exposure to sublethal concentrations of linalool. We then intensively explored the adaptation mechanisms in S. Senftenberg and determined the effect of adaptation on bacterial sensitivity to clinically used antibiotics and on bacterial survival on basil plants and harvested basil leaves.
RESULTS
Selection of linalool-resistant strains.
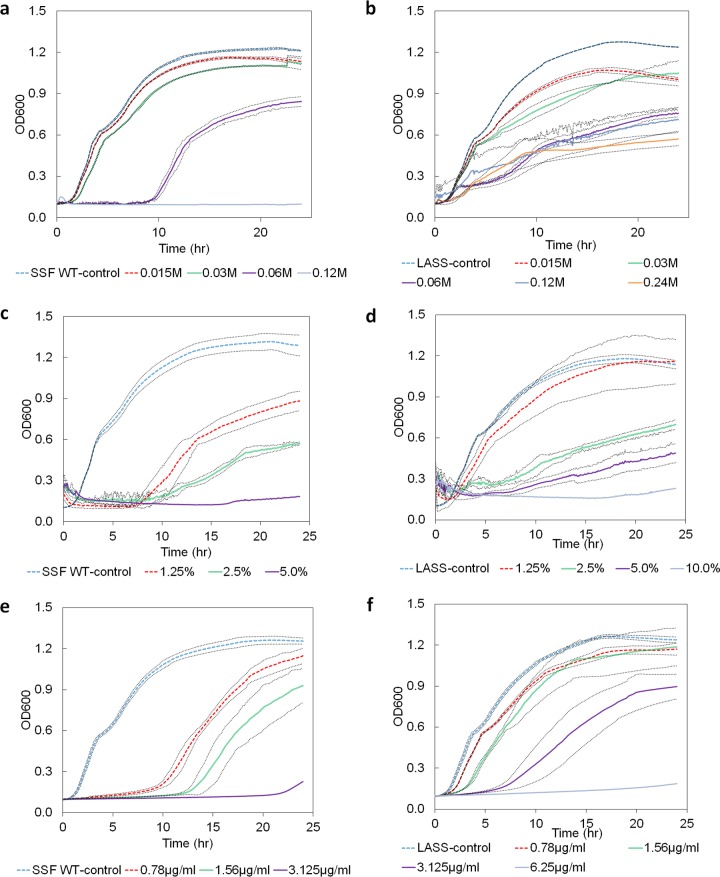
Following a selective pressure procedure that included seven serial transfers of S. Senftenberg in increasing concentrations of linalool, the bacteria were able to increase their resistance to linalool by 8-fold, reaching a final MIC of 0.96 M (16%, vol/vol) under the current experimental conditions. The induced resistance was proved to be stable: bacteria stored at −80°C, recultured in Luria-Bertani (LB) agar in the absence of linalool, and subsequently exposed to linalool retained their tolerance both in LB broth and on LB agar plates. One colony of the linalool-adapted strains was named linalool-adapted S. Senftenberg (LASS) and was chosen for further analysis. The 90% minimal bactericidal concentrations (MBC90s) for the LASS strain and the S. Senftenberg wild type (WT) were determined to be twice the value of the MIC for each strain. LASS was able to grow on agar plates and in broth medium containing up to 0.48 M linalool, although at linalool concentrations higher than 0.06 M, a significant 1.6-fold decrease in maximal growth (absorbance at the maximal optical density of 600 nm [OD600]) was observed (Fig. 1a and b).
FIG 1.
Growth curves of the S. Senftenberg wild type (SSF WT) and the LASS strain exposed to linalool, tetracycline, and basil oil. Bacterial growth in LB broth supplemented with linalool, tetracycline, or basil oil was determined by measuring the OD600 during 24 h of incubation at 37°C. Bacterial growth in pure LB broth served as a control. (a) Growth curves of the S. Senftenberg WT exposed to linalool; (b) growth curves of the LASS strain exposed to linalool; (c) growth curves of the S. Senftenberg WT exposed to basil oil; (d) growth curves of the LASS strain exposed to basil oil; (e) growth curves of the S. Senftenberg WT exposed to tetracycline; (f) growth curves of the LASS strain exposed to tetracycline. Each point represents the mean from two independent experiments performed in duplicate. Black dotted curves, 95% confidence bands.
Resistance of LASS to basil oil and to antibiotics.
The susceptibility of LASS to linalool, basil oil (a commercial mixture of components containing approximately 15% linalool), pure estragole, pure eugenol, and 14 antibiotics (from different classes with various mechanisms of action) was compared to the susceptibility of the wild type using a disk diffusion assay (Table 1) and a broth microdilution assay (Table 2). LASS demonstrated a lower inhibition diameter not only with pure linalool but also with the basil oil mixture (P < 0.05), with a large effect size being detected (Cohen's d statistic ([d] = 4.95, 95% confidence interval [CI] = 1.72, 8.18). Moreover, although LASS demonstrated only a 2-fold increment of the basil oil MIC, its higher maximal absorbance and lower lag duration compared to those for the wild type grown in broth indicated bacterial adaptation (Fig. 1c and d). No significant differences in resistance to pure eugenol or estragole were seen (Table 1).
TABLE 1.
Antibacterial activities of basil oil, its constituents, and several antibiotics against the S. Senftenberg WT and the LASS strain
| Antibacterial agent (amt per disk) | Inhibition diam (mm)a |
|
|---|---|---|
| S. Senftenberg wild type | LASS strain | |
| Saline | NDb | ND |
| Linalool (8.7 mg) | 11.3 ± 1.2 | 7.2 ± 0.3* |
| Basil oilc (9.56 mg) | 9.0 ± 0 | 6.5 ± 0* |
| Estragole (9.65 mg) | 11.0 ± 1.0 | 12.7 ± 1.2 |
| Eugenol (10.6 mg) | 13.5 ± 0.5 | 12.7 ± 0.6 |
| Polymyxin B (300 units) | 12 ± 1.4 | 12.3 ± 0.4 |
| Streptomycin (10 μg) | 13.7 ± 1.2 | 12.5 ± 0.7 |
| Trimethoprim (25 μg) | 20.7 ± 0.6 | ND* |
| Trimethoprim-sulfamethoxazole (25 μg) | 19.7 ± 1.2 | ND* |
| Chloramphenicol (30 μg) | 26.3 ± 1.5 | 16.2 ± 1.6* |
| Gentamicin (10 μg) | 19.0 ± 1.0 | 20.3 ± 1.5 |
| Colistin (10 μg) | 10.3 ± 1.2 | 10.2 ± 0.8 |
| Tetracycline (30 μg) | 15.0 ± 1.7 | 10.0 ± 1.0* |
| Carbenicillin (100 μg) | 21.7 ± 1.2 | 17.7 ± 1.5 |
| Norfloxacin (10 μg) | 26.0 ± 1.0 | 22.0 ± 1.4 |
| Amikacin (30 μg) | 17.0 ± 1.0 | 18.7 ± 0.6* |
| Cefepime (30 μg) | 24.0 ± 1.0 | 22.7 ± 1.2 |
| Ceftriaxone (30 μg) | 25.0 ± 1.0 | 23.7 ± 1.2 |
| Piperacillin (100 μg) | 18.3 ± 0.6 | 12.5 ± 0.5* |
The mean diameter of the paper disk (6 mm) ± standard deviation is included. *, significant differences (P < 0.05) between the S. Senftenberg wild-type and LASS strains.
ND, no inhibition was detected.
The basil oil was from Av-On, Israel.
TABLE 2.
Comparison of MICs of various antimicrobial compounds and antibiotics for the S. Senftenberg WT and the LASS strain
| Genotype | Linalool MIC (M) | Basil oil MIC (% [vol/vol]) | MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|---|
| Tetracycline | Chloramphenicol | Trimethoprim | Trimethoprim-sulfamethoxazole | Piperacillin | |||
| S. Senftenberg WT | 0.12 | 5 | 6.25 | 25 | 3.13 | 6.25 | 3.13 |
| LASS strain | 0.96 (8)a | 10 (2) | 12.5 (2) | 50 (2) | 100 (32) | 50 (8) | 12.5 (4) |
Values in parentheses are the fold increase in the MIC.
While the tolerance of LASS to amikacin was significantly lower than the tolerance of the wild type (P < 0.05), its tolerance to trimethoprim, trimethoprim-sulfamethoxazole, chloramphenicol, tetracycline, and piperacillin was significantly higher (P < 0.05, minimal effect size [d] = 3.54, 95% CI = 0.97, 6.10); no significant differences in resistance to the other investigated antibiotics were observed between the two strains. The antibiotics that the two strains resisted differently target diverse systems and function by different mechanisms. Amikacin is an aminoglycoside that blocks the 30S ribosomal subunit and hampers protein production. Trimethoprim and sulfamethoxazole inhibit different pathways in bacterial DNA synthesis. Chloramphenicol and tetracycline inhibit translation by binding to the 50S and 30S ribosomal subunits, respectively, and piperacillin is a broad-spectrum β-lactam antibiotic that inhibits peptidoglycan synthesis. Further MIC analysis showed that the highest MIC increment was for trimethoprim (32-fold), whereas the MICs of trimethoprim-sulfamethoxazole and piperacillin showed increases of 8-fold and 4-fold, respectively. Chloramphenicol and tetracycline showed the least increment of the MIC (2-fold). The adaptation to tetracycline was also shown by a higher value of maximal absorbance and a shorter lag duration in comparison with those for the wild type (Fig. 1e and f).
Survival on basil plants.
Comparison of the survival of both strains on harvested basil leaves during storage at 4°C showed that the numbers of S. Senftenberg wild-type bacteria were reduced (0.6 log reduction; P < 0.05) with a large effect size (d = 4.48, 95% CI = 2.36, 6.60), while the numbers of LASS bacteria remained stable. To determine whether the increased resistance of LASS to linalool and basil oil is also beneficial for fitness on basil plants, the survival of the LASS strain on the basil phyllosphere and rhizosphere was compared to that of the wild type 2 days after spray irrigation. The basil phyllosphere harbored the highest levels of both bacterial strains tested (7.4 log CFU/g), with no significant difference between the levels of the two strains being detected. A lower level of LASS was observed on the roots, but the difference was not significant (P > 0.05), while in the soil, significantly lower survival levels (1 log reduction; P < 0.05) with a large effect size (d = 3.76, 95% CI = 1.88, 5.64) were observed for LASS.
Similar membrane perforation by linalool in the S. Senftenberg wild type and LASS.
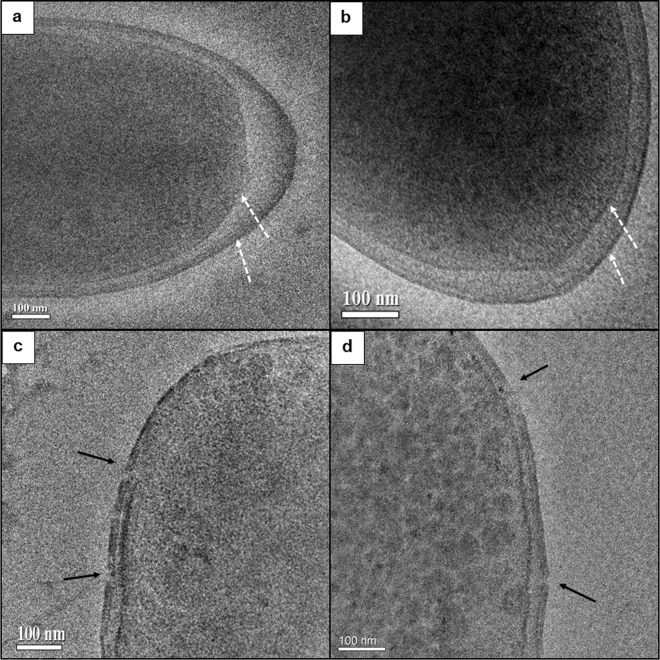
Cryogenic transmission electron microscopy (cryo-TEM) images showed that the morphology of the unaffected membranes of the S. Senftenberg wild type and LASS seemed smooth and intact (Fig. 2a and b). Incubation of these bacteria with subinhibitory concentrations of linalool (6 mM) for 1 h resulted in small pores in their outer membranes (Fig. 2c and d). The pore size ranges of the wild-type and LASS strains were of the same order of magnitude and were determined to be 10 to 200 nm and 22 to 177 nm, respectively. The damage on the inner membrane was less apparent in both strains. These results indicate that linalool similarly affects the membrane integrity of both strains.
FIG 2.
Typical cryogenic transmission electron micrographs of untreated cells of the S. Senftenberg wild type (a) and the LASS strain (b) and linalool-treated cells of the S. Senftenberg wild type (c) and LASS strain (d). Treated cells were incubated with 6 mM linalool for 1 h at 37°C. Dashed white arrows point to the outer and inner membranes. Black arrows point to membrane pores or tears.
Reduced membrane permeabilization of LASS and its contribution to linalool resistance.
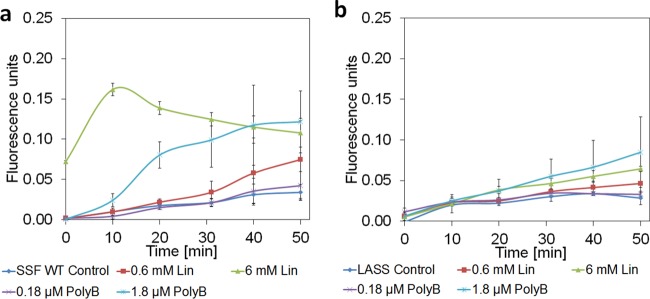
A membrane permeability assay was implemented by measuring the uptake of the fluorescent dye ethidium bromide (EtBr) by the S. Senftenberg wild type and LASS. The membranes of both strains showed very low permeability without any antimicrobial compound (Fig. 3) and during incubation with 0.6 mM linalool. An increase in the linalool concentration to 6 mM impacted the permeability of both strains significantly (P < 0.05) but appeared to do so with a greater effect on the wild-type strain (d = 2.04, 95% CI = 0.71, 3.43).
FIG 3.
Time-related ethidium bromide fluorescence emission by the S. Senftenberg wild type (SSF WT) and the LASS strain. (a) The S. Senftenberg WT incubated with polymyxin B (PolyB) or linalool (Lin); (b) the LASS strain incubated with polymyxin B or linalool. Cells not incubated with polymyxin B or linalool served as controls. Fluorescence levels were determined during 50 min of incubation at 37°C. The results shown are the averages from 2 independent repeats performed in triplicate. Higher fluorescence values correlate with increased membrane permeability. Data for the wild type were published previously (16) and are provided here for the convenience of the reader.
To determine if LASS also resists the activities of other compounds that damage the membrane, the bacteria were exposed to polymyxin B, a cationic polypeptide, which primarily damages the membrane of Gram-negative bacteria (39). LASS showed significantly lower permeability with 1.8 μM polymyxin B than the wild type with a large effect size (P < 0.05, d = 2.28, 95% CI, 0.83, 3.74), whereas with 0.18 μM polymyxin B, only minimal permeability was noticed (Fig. 3). These results demonstrate that the LASS strain somehow resists an increase in permeability, even though the pores in the membrane appear to form similarly to those in the wild-type strain.
Fatty acid composition of the membranes.
The membrane fatty acid compositions of the untreated S. Senftenberg wild type and the LASS strain were analyzed, since the membrane fatty acid composition affects the accessibility of the membranes to hydrophobic compounds like linalool and the ability to overcome membrane damage (40). Palmitic acid (C16:0) was the most abundant fatty acid in the membranes of both strains, accounting for approximately 40% of all fatty acids (Table 3). Two more abundant fatty acids were cyclopropaneoctanoic acid 2-hexyl and oleic acid (C18:1), which were present in estimated proportions of 12 to 21%. Comparison of the fatty acid profiles of the S. Senftenberg wild type and LASS revealed significant differences (P < 0.05), with moderate to large effect sizes in the relative concentrations of palmitoleic acid (C16:1; d = 0.71, 95% CI = −0.57, 1.99), cyclopropaneoctanoic acid 2-hexyl (C18:0; d = 1.69, 95% CI = 0.25, 3.14), and the fatty acid methyldihydrosterculate (d = 1.26, 95% CI = −0.10, 2.61) being seen. A notable increase (P < 0.05) was observed for the palmitoleic acid of LASS, probably on account of the other two fatty acids, cyclopropaneoctanoic acid 2-hexyl and methyldihydrosterculate. Although in both strains total saturated fatty acids (SFAs) were present in larger amounts (more than 50%) than unsaturated fatty acids (UFAs), elevated levels (P < 0.05) of SFAs (an approximately 7% increase with a large effect size [d = 1.45, 95% CI = 0.06, 2.85]) were detected in the LASS strain (Table 3). In accordance with this finding, a significant reduction (P < 0.05) in the UFA-to-SFA ratio was observed in LASS: the ratio was approximately 0.72 in LASS, whereas it was 0.94 in the wild type (d = 1.31, 95% CI = −0.06, 2.67). While the relative proportions of the short fatty acids (C14 to C16) and the long fatty acids (C18) in the wild type were nearly the same, the relative proportion of short fatty acids in LASS was ca. 50% higher (d = 3.21, 95% CI = 1.33, 5.08]) than the relative proportion of long fatty acids (Table 3). After 1 h of exposure to linalool, the relative concentrations of the fatty acids did not significantly change (data not shown).
TABLE 3.
Percentage of total fatty acids identified in S. Senftenberg WT and LASS strain membranes
| Common name | Type | No. of carbon atoms | Relative proportiona (%) |
|
|---|---|---|---|---|
| S. Senftenberg WT | LASS | |||
| Palmitic acid | Saturated | 16 | 39.5 ± 2.4 | 43.6 ± 5.1 |
| Cyclopropaneoctanoic acid 2-hexyl | Alicyclic | 18 | 20.9 ± 5.2 | 12.8 ± 4.3* |
| Oleic acid | Unsaturated | 18:1 | 13.4 ± 6.9 | 15.1 ± 3.4 |
| Methyldihydrosterculate | Unsaturated | 20 | 6.8 ± 3.4 | 3.3 ± 2.1* |
| Plamitoleic acid | Unsaturated | 16:1 | 6.2 ± 4.7 | 9.9 ± 5.8* |
| Stearic acid | Saturated | 18 | 6.3 ± 0.7 | 7.0 ± 1.0 |
| Myristic acid | Saturated | 14 | 3.7 ± 0.8 | 4.9 ± 0.8 |
| Pentadecanoic acid | Saturated | 15 | 1.6 ± 0.3 | 1.6 ± 0.6 |
| Linoleic acid | Unsaturated | 18:2 | 1.1 ± 0.6 | 0.6 ± 0.5 |
| Margaric acid | Saturated | 17 | 0.9 ± 0.6 | 0.9 ± 0.6 |
| SFAb | 51.7 ± 3.0 | 58.6 ± 6.0* | ||
| UFAc | 47.3 ± 3.0 | 39.6 ± 6.0* | ||
| UFA/SFA ratio | 93.9 ± 12.8 | 72.3 ± 19.5* | ||
| Short fatty acids (C14–C16) | 51.0 ± 2.4 | 60.1 ± 2.7* | ||
| Long fatty acids (C18) | 48.6 ± 3.2 | 38.8 ± 3.2* | ||
| Long fatty acid/short fatty acid ratio | 95.7 ± 10.5 | 65.1 ± 10.3* | ||
The averaged calculated proportion (on the basis of the results of 5 experiments) of each fatty acid out of the total fatty acids extracted ± standard deviation. *, significant differences (P < 0.05) between the S. Senftenberg wild type and the LASS strain.
SFA, total saturated fatty acids, including C14:0, C15:0, C16:0, C17:0, and C18:0 fatty acids.
UFA, total unsaturated fatty acids, including C16:1, C18:1, and C18:2 fatty acids, methyldihydrosterculate, and cyclopropane octanoic acid, 2-hexyl.
Transcription of acrAB, micF, marRAB, soxS, rob, and ramA.
As shown in Table 2, LASS is less susceptible than the wild type to the antibiotics trimethoprim, trimethoprim-sulfamethoxazole, chloramphenicol, tetracycline, and piperacillin. These antibiotics belong to different classes but share a feature: all are excluded by efflux pumps, and S. enterica strains with increased resistance to these antibiotics often overexpress the AcrAB/TolC efflux pump and downregulate the porin OmpF through overexpression of MicF (32–34). Therefore, we analyzed the S. Senftenberg wild type and LASS for the transcription of acrAB, micF, and their major regulatory elements, MarA, Rob, SoxS, and RamA (35, 36). Using green fluorescent protein (GFP) reporting systems in which the promoter of each investigated gene was fused to the gfp gene, LASS expressed higher basal levels of GFP than the S. Senftenberg wild type under the control of all tested promoters at a ratio of 1.6- to 7.2-fold (d = 0.72, 95% CI = −0.36, 1.80), except for pramA, which showed a 2-fold decrease (Table 4). As the high basal level of expression in the LASS strain could be the consequence of point mutations in the relevant promoters, we sequenced S. Senftenberg WT promoters and compared their sequences to those of their counterparts in the LASS strain. However, comparison of the promoter sequences showed that they were identical.
TABLE 4.
Activities of the promoters pmarRAB, pacrAB, pmicF, prob, psoxS, pramA, and pmcpL in the S. Senftenberg WT and the LASS strain (basal levels) and in response to linalool or decanoatea
| Promoter | Ratio of expression by LASS/wild type with no inducerb | Fold induction compared with the basal level of expression withc: |
Ratio of expression by LASS/wild type with induction with 6 mM linaloold | |||
|---|---|---|---|---|---|---|
| 5 mM decanoate |
6 mM linalool |
|||||
| S. Senftenberg WT | LASS | S. Senftenberg WT | LASS | |||
| marRAB | 5.2 ± 1.7* | 13.9 ± 3.2 | 5.6 ± 1.1* | 1.5 ± 0.5 | 1.2 ± 0.2* | 0.8 ± 0.3* |
| acrAB | 1.7 ± 0.6* | 1.1 ± 0.8 | 1.8 ± 0.7* | 0.9 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.7 |
| micF | 7.2 ± 1.5* | 18.6 ± 4.0 | 4.1 ± 0.7* | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.1 ± 0.2 |
| rob | 1.6 ± 0.3* | 0.5 ± 0.2 | 1.2 ± 0.2* | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.9 ± 0.3 |
| soxS | 2.9 ± 0.8* | 1.6 ± 1.2 | 3.1 ± 0.9* | 1.1 ± 0.6 | 1.6 ± 0.5* | 1.4 ± 0.9* |
| ramA | 0.5 ± 0.2* | 2.1 ± 0.7 | 3.5 ± 1.3* | 2.1 ± 0.7 | 1.9 ± 0.8 | 0.9 ± 0.5 |
| mcpL | 43.5 ± 8.6* | 8.5 ± 4.8 | 1.5 ± 0.2* | 2.5 ± 1.3 | 1.0 ± 0.1* | 0.4 ± 0.2* |
Data were calculated from at least four experiments performed in triplicate. *, significant difference (P < 0.05) in promoter induction levels between the LASS strain and the S. Senftenberg wild type.
The numbers represent the average ratio of the fluorescence of the LASS mutant without an inducer normalized to the fluorescence of the same promoter without induction for the wild type. The basal promoter activities of the S. Senftenberg wild type represented by the normalized fluorescence are 725 for marRAB, 1,810 for acrAB, 5,938 for micF, 4,031 for rob, 388 for soxS, 2,467 for ramA, and 124 for mcpL.
The numbers represent the average ratio of the fluorescence of the promoter with induction normalized to the fluorescence of the basal level of the same promoter.
The numbers represent the average ratio of the fluorescence of the LASS mutant with induction with 6 mM linalool normalized to the fluorescence of the same promoter with induction with 6 mM linalool of the wild type.
Cultures were also monitored after exposure to 6 mM linalool to determine the effect of linalool on the expression of the investigated genes. Decanoate served as a positive control. Decanoate is a salt of decanoic acid, a medium-chain bile acid that is known to upregulate the expression of the activators evaluated as well as AcrAB (37, 38). All promoters, with the exception of pramA, showed significantly higher activity levels in favor of the LASS strain after exposure to linalool or decanoate (d for decanoate = 0.92, 95% CI = 0.14, 1.70). Incubation of the S. Senftenberg wild type with linalool caused a low but significant induction only in the case of pramA (2.1 ± 0.7) (Table 4). Compared to the induction of pramA achieved with linalool, a similar induction of pramA was observed with decanoate in the wild-type strain, whereas a pronounced induction of pmarRAB (13.9 ± 3.2) and pmicF (18.6 ± 4.0) was measured. prob was downregulated in the wild-type strain (0.5 ± 0.2), and the regulation of pacrAB was unaffected (Table 4). Incubation of LASS with linalool resulted in a low induction profile similar to that of the wild type for most promoters; the exceptions were psoxS, which showed a slightly higher but significant level of induction compared with that in the wild type, and pmarRAB, which showed a significantly lower level of induction compared with that in the wild type (P < 0.05). Due to the higher basal level of expression under the control of pmarRAB in LASS (compared with that in the wild type), the florescence values were significantly higher with linalool, too, despite the lower level of induction (Table 4). In the presence of decanoate, some promoters, like pmarRAB, showed a significantly (d = 3.48, 95% CI = 2.31, 4.66) lower level of induction in LASS (5.6 ± 1.1) than in the wild type, while promoters like psoxS showed a higher level of induction (d = 1.48, 95% CI = 0.37, 2.59). For pacrAB, a low but significant level of induction (1.8 ± 0.7) was detected only in LASS following exposure to decanoate.
To summarize, the basal transcription levels of the promoters acrAB, marRAB, micF, rob, and soxS were higher in LASS than in the wild type. Whereas linalool induced some of these promoters (marRAB, micF, rob, and soxS) in the wild type, the effect size was minimal, and their total expression was higher in LASS even after exposure to linalool or decanoate.
Linalool-induced aggregation.
We further determined how adaptation to linalool affects linalool-induced aggregation. The particle size distribution of LASS and wild-type cells grown without linalool displayed a mean particle size of 1.1 μm (equivalent sphere), corresponding to that of planktonic bacteria (Fig. 4). After incubation of both cultures with 0.6 mM linalool, only 7% of LASS cells accumulated in small clusters of 3 to 20 μm. Incubation of the cultures with 3 mM linalool led to a shift in the mean particle size to 20 μm and 30 μm for the S. Senftenberg wild type and LASS, respectively (Fig. 4), indicating bacterial aggregation. LASS revealed a higher percentage (d = 0.74, 95% CI = −1.11, 2.59) of accumulating cells (23%) with a cluster size of 3 to 100 μm than the wild type (19%), whereas 13% of the LASS particles and only 5% of the wild-type particles were larger than 20 μm (P < 0.05). An increase in the linalool concentration to 6 mM affected only the LASS strain (d = 4.99, 95% CI = 1.74, 8.23); the proportion of aggregates with sizes of 3 to 100 μm increased to 40%. An additional increment of the linalool concentration to 12 mM (Fig. 4) led to the accumulation of the majority of the LASS cells present in the culture (50% had a particle size range of 3 to 20 μm, and 29% had a particle size range of 20 to 100 μm) (P < 0.05). The wild type was unable to grow in the presence of linalool at this concentration.
FIG 4.
Particle size distribution of linalool-untreated and -treated cultures of the S. Senftenberg wild type (SSF WT) and the LASS strain. The particle size distributions of S. Senftenberg WT (left) and LASS strain (right) cells after 22 h of incubation in the absence and presence of 0.6 mM, 3 mM, 6 mM, and 12 mM linalool are shown. Data for the wild type were already published (16) and are provided here for the convenience of the reader.
Impact of linalool on chemotaxis and motility.
The activity of the mcpL promoter in LASS was monitored by measurement of the fluorescence of GFP fused to the mcpL promoter with and without linalool. The results revealed that LASS has a basal level of expression of this gene 2 orders of magnitude higher than that of the wild type (d = 7.96, 95% CI = 5.34, 10.58) (Table 4). In the wild type, exposure to linalool induced the transcription of mcpL 2.5-fold compared to the basal level of transcription, while no induction was observed in LASS (Table 4), yet with linalool the level of transcription of mcpL by LASS was also much higher than that by the wild type due to the significant basal level of expression in LASS.
The motility of LASS was compared to that of the wild type. In the absence of linalool, LASS demonstrated reduced motility (d = 15.64, 95% CI = 6.65, 24.63) in comparison to the wild type (Fig. 5). Exposure to linalool at a concentration of 0.6 mM resulted in inhibition of the motility of both strains (d for the wild-type strain = 7.14 [95% CI = 2.79, 11.49], d for LASS = 9.45 [95% CI = 3.87, 15.03]) compared to that of their control untreated counterparts, but LASS demonstrated the most intense reduction (47%). An increase in the concentration of linalool to 6 mM further affected the motility only of the wild type (d = 38.74, 95% CI = 16.76, 60.71), resulting in similar motility in both strains (Fig. 5). The motility of LASS was also compared to the motility of a mcpL null mutant (ΔmcpL) (described in reference 16). While LASS demonstrated lower motility than the ΔmcpL mutant at low concentrations of linalool or without linalool, the ΔmcpL mutant treated with 6 mM linalool showed the most significant reduction in motility (d = 5.83, 95% CI = 2.17, 9.50) (Fig. 5).
FIG 5.
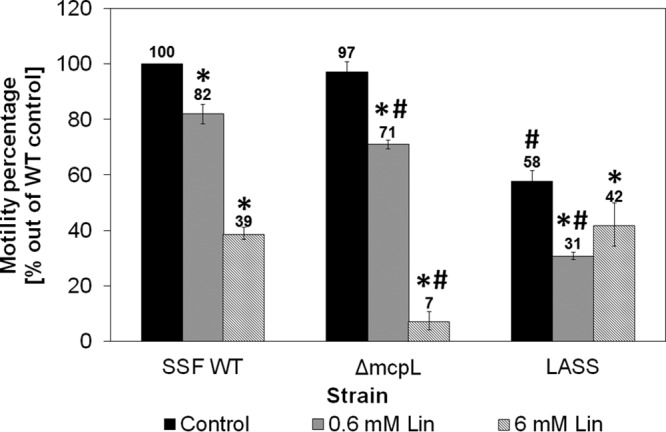
Motility of the S. Senftenberg wild type (SSF WT), the ΔmcpL mutant, and the LASS strain in the absence and presence of 0.6 and 6 mM linalool. The diameters of the areas of bacterial motility of the S. Senftenberg WT, ΔmcpL mutant, and LASS strain were measured after 6 h of incubation at 37°C. The ratio of the diameter of the areas of motility in the presence and absence of linalool in relation to the diameter of the area of motility of the S. Senftenberg WT without linalool (33 mm) was calculated as a reference. *, statistical significance of the motility of each strain in the presence of linalool compared to that of their counterparts without linalool (marked as controls); #, statistical significance of motility in comparison to that of the S. Senftenberg WT. Each column represents the mean ± SEM from three independent experiments performed in duplicate. Data for the wild type and the ΔmcpL mutant were already published (16) and are provided here for the convenience of the reader.
DISCUSSION
In the last decade, the European Commission (EC) and the U.S. Food and Drug Administration (FDA) have approved the use of several EOs, including basil oil, in food products (2, 41). Since then, linalool has become a dominant substance used in the fragrance, food, and cosmetic industries, and its worldwide production is about to expand in the near future (15, 42). Our results demonstrated that a multistep exposure of a wild-type S. Senftenberg strain to sublethal concentrations of linalool is adequate to increase the resistance of the strain not only to linalool itself but also to the whole oily extract of basil oil and even to several antibiotics used in clinical and veterinary medicine, suggesting that the commercial use of an unspecified sublethal concentration of linalool may selectively reduce bacterial susceptibility to this compound and even to antibiotics. The resistance that had been developed in vitro was stable, indicating that the resistance resulted from a genetic shift rather than epigenetic changes. The current findings contradict the general thought that bacteria would rarely develop resistance to EOs (21, 23, 43) and resemble the results of Nelson (28) and Becerril et al. (23), in which repeated exposure to tea tree oil and oregano oil concluded with the development of tolerance to these plant-derived extracts. The acquisition of resistance to antibiotics following the selective pressure of plant-derived antimicrobial agents has been reported for other bacterial species (23–25). However, the distinct feature of our study is that it was conducted with pure linalool; most of these studies examined resistance to the plant extracts and not to their pure constituents.
We have previously shown that the wild-type S. Senftenberg strain survives on soil, as does S. Typhimurium, but survives better on basil plants pre- and postharvest and is less sensitive to basil oil and to linalool than S. Typhimurium (30). Here, the selective pressure of linalool on the same S. Senftenberg strain further increased the linalool resistance of the resultant LASS strain and did not significantly affect its growth in broth or on agar medium, nor did it affect its persistence on the phyllosphere of growing basil plants; furthermore, it improved its survival on harvested basil during refrigerated storage. This in vitro adaptation of LASS, however, had a significant fitness cost in the environment, as reflected from its compromised survival in soil. Altogether, it might be suggested that during survival on basil plants, S. Senftenberg had already adapted to basil by developing natural resistance to its antimicrobials and reached a good balance between resistance and survival on basil and its environment. The addition of resistance to linalool, obtained by exposure to increasing concentrations of linalool, was not beneficial for survival in the environment. The fact that further acquisition of resistance may carry a fitness cost in vitro or in the host has already been described (44) but has rarely been investigated in the environment. For example, Paulander et al. showed that the development of intrinsic resistance to streptomycin in S. Typhimurium leads to impaired growth on rich medium (45). While the fitness cost is also known to be affected by environmental conditions (46), the relatively low rate of survival of the LASS strain in soil probably indicates that the acquisition of excessive linalool resistance has its own fitness cost and makes a poor contribution to survival on basil plants in nature. Further study should be conducted in order to investigate the potential effects of adaptation to linalool on long-term survival in soil and on plants.
LASS developed resistance to linalool and to several antibiotics but remained sensitive to eugenol, estragole, and the other antibiotics investigated and even became more sensitive to aminoglycosides, suggesting that the specific intrinsic mechanisms of resistance and not general mechanisms, such as the efficient production of a biofilm, which are expected to improve resistance to most investigated antimicrobial agents, contribute to resistance. We have previously identified the mechanisms of adaptation of the S. Senftenberg wild type to linalool by a different approach, in which a transposon library of S. Senftenberg was constructed and screened for linalool-sensitive strains (16). In the second approach, described here, we characterized a resistant strain obtained by exposure to increasing concentrations of linalool. Interestingly, these two approaches suggested that the same mechanisms are involved in resistance to linalool: changes in the envelope that affect cell permeability, improved efflux, reduced influx, and different motilities with and without linalool. This indicates that several mechanisms that work simultaneously contribute to resistance.
Adaptation to linalool resulted in better resistance to tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, and piperacillin, antibiotics that have different structures and targets. Tetracycline and chloramphenicol, for example, target ribosomes, while piperacillin targets cell walls. Despite these differences, all these antibiotics are known substrates of the AcrAB/TolC efflux pump in Escherichia coli and Salmonella (24, 27, 32–34, 47–49). A dominant mechanism known to confer resistance to these antibiotics involves overexpression of the AcrAB efflux pumps and inhibition of porin production (38). The same mechanism also contributes to the adaptation to detergents and antimicrobials that target membranes, probably because damage to the inner membrane has severe consequences on cell viability and may be prevented by the activity of efflux pumps that expel the compounds from the periplasmic space (50). Here we show that overexpression of AcrAB efflux pump and MicF is also correlated with increased resistance to linalool, since the LASS strain expressed higher levels of AcrAB and MicF under basal conditions. This finding is in accord with our earlier observations, which showed that the deletion of AcrA or AcrB sensitized the bacteria to linalool (16). Furthermore, linalool induced the transcription of micF, ramA, and marA, indicating that linalool is not only a substrate of the efflux pump but also a direct or indirect inducer of the mar regulon. Altogether, these observations suggest that induced efflux and reduced influx are associated with linalool tolerance acquisition. The results of this study place linalool as a potential substrate for the AcrAB efflux pump and highlight the broad specificity of this pump, which can handle diverse classes of compounds, including plant-derived oils. The increased resistance and adaptation of E. coli to pine and thyme oils were also associated with the overexpression of micF, marA, and acrAB and reduced expression of the outer membrane porins OmpF and OmpC (24, 27). However, the fact that marA is induced in Salmonella serovars during survival on basil plants (G. Kisluk and S. Yaron, submitted for publication), together with our finding that AcrA has no contribution to bacterial persistence on basil plants (16), may suggest that the bacteria use other efflux pumps as well in order to expel toxic plant-derived compounds.
The second mechanism associated with linalool tolerance and identified by both screening approaches includes changes in the envelope structure that affect membrane permeability. Although linalool caused similar pores in the membranes of LASS and the wild type, the LASS membrane was less permeable to EtBr in the presence of linalool or polymyxin B. This could be the consequence of increased efflux and decreased influx, as described above, or a result of the changes in membrane structure and fluidity via phospholipid configuration alteration (40, 51). Characterization of the fatty acid profile in the LASS strain indicated an extensive incorporation of short SFAs coupled with a relative decrease in the amounts of UFAs or long-chain fatty acids. Incorporation of the short SFAs, which can be efficiently packed together into the membrane, may decrease membrane permeability and recompense the membrane pores formed in the presence of linalool (52). An increase in the amount of SFAs or short-chain fatty acids is a known bacterial response that induces membrane fluidity (53–55) and was shown with a variety of bacteria that were exposed to EOs (56). On the contrary, other bacteria adapt to EOs by decreasing their fluidity. For example, adaptation to carvacrol by Bacillus cereus is involved in a decrease in the level of membrane fluidity (40). This inconsistency therefore implies that changes in fatty acid composition depend on the compound in question and the bacterial strain (55).
The third mechanism identified to be linked to linalool resistance is associated with the chemoreceptor-like protein McpL. McpL is a putative transmembrane protein with an unknown function. Its sequence is homologous to that of methyl-accepting chemotaxis proteins (MCPs) with serine sensor receptors. The MCPs mediate chemotaxis through the detection of unknown chemotactic signals (31). The signals are transduced through a cascade of phosphorylated regulators and cause switching in the direction of rotation of the rotary flagellar motor, thus enabling cells to move toward nutrients or to avoid toxic compounds (57, 58). Here we show that linalool induced the transcription of mcpL, while the LASS strain expressed significantly higher levels of McpL than the wild type with and without linalool. These observations may indicate that McpL is active in the presence of linalool and most likely contributes to the survival of the bacteria. This is also in accord with the findings of our previous research, which showed that McpL null mutants of both S. Senftenberg and S. Typhimurium were much more sensitive to linalool than the wild type in growth medium and were limited in their survival on basil leaves at 7 days postinoculation (16).
Contrary to expectations, although the LASS strain expressed McpL in significantly larger amounts than the wild type, it was less motile than the wild-type strain in the absence of linalool, and linalool had relatively little influence on its motility. This result presents a somewhat confusing picture, since an mcpL null mutant also demonstrated reduced motility in the presence of linalool (16). All these results may be explained by the fact that S. enterica has several known chemoreceptor genes: tar (which senses aspartate and maltose), tsr (which senses serine), trg (which senses ribose, galactose, and glucose), tcp (which senses citrate and phenol), aer (which senses oxygen), tip, mcpA, and mcpB (which sense l-cystine), and mcpC (which senses l-cystine) (59). The deletion of mcpL alone apparently does not eliminate the chemotaxis of the null mutant in linalool-free medium. Though the function of McpL remains to be determined, its upregulation following exposure to linalool in both the S. Senftenberg wild type and the LASS strain, together with its elevated basal levels seen in the latter, suggest that it is important for adaptation and coping with linalool, which might be mediated through changes in motility behavior. A further study with a greater focus on the association between linalool and McpL and the other MCPs is therefore suggested.
LASS also formed more and larger aggregates than the wild type in the presence of linalool. An aggregation of bacterial cells was previously shown with other compounds that originated from plants, such as the flavonoids galangin and catechin (60). An aggregation of Salmonella on leaves contributes not only to the persistence of the pathogen on the plant but also to its resistance to disinfection approaches (61). Further research should be done to investigate the causes of linalool-induced aggregation, its role in resistance to antimicrobials, and the mechanism by which resistance develops.
To conclude, resistance to linalool and basil oil can be acquired and thereby confer protection against a range of clinically significant antibiotics. Nonetheless, this process may also affect additional properties and pathways in the bacterium, as it has some fitness cost (i.e., a cost on fitness for survival in soil). The developed resistance involves the incorporation of several phenotypes, including induced efflux, reduced influx, changes in membrane composition, controlled motility, and the formation of aggregates. Taken together, these findings signify the concern that the extensive usage of products containing EOs at subinhibitory concentrations can provoke the emergence of resistant human pathogens, providing cross protection against antibiotics, hence enabling the pathogens to colonize new niches that select for such resistance and pose an increased risk to public health.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Salmonella enterica serovar Senftenberg ATCC 07885, a clinical isolate from the 2007 outbreak linked to basil, was used in this study (30, 62). S. Senftenberg was grown at 37°C in Luria-Bertani (LB) broth and agar (Difco), which was supplemented with 50 μg/ml kanamycin (Kan) when necessary. Electrocompetent S. Senftenberg cells were transformed by electroporation, using a MicroPulser electroporator (Bio-Rad Laboratories), with a set of pCS21a plasmids containing the gfp gene fused to one of the following promoters: marRAB, acrAB, micF, robA, soxS, ramA, and mcpL (16, 38).
Antimicrobial agents.
Pure linalool and estragole (methyl chavicol) were purchased from Sigma-Aldrich, Rehovot, Israel. Eugenol was obtained from Domca SA, Spain. The principal components of commercial basil oil (purchased from Av-On, Israel), determined by gas chromatography, were as follows: 82.0% estragole, 14.7% linalool, 1.0% humulene, 0.6% eucalyptol, and 0.6% α-bergamotene.
The antibiotics used in this study were streptomycin, norfloxacin, amikacin, trimethoprim, trimethoprim-sulfamethoxazole, colistin, and cefepime (all of which were purchased from Oxoid Microbiology Products, Hampshire, England) and chloramphenicol, gentamicin, tetracycline, carbenicillin, polymyxin B, ceftriaxone, and piperacillin (all of which were purchased from HiMedia Laboratories, Mumbai, India). The concentration for each antibiotic applied is listed in Tables 1 and 2.
Isolation of strains resistant to linalool by use of selective pressure.
The S. Senftenberg wild type was grown in a 96-well plate containing 2-fold serial dilutions of linalool in LB medium ranging from 240 mM (4%, vol/vol) to 0.5 mM (0.0078%, vol/vol). The cultures were incubated at 37°C for 24 h with agitation. Cells growing on the highest concentration of linalool were diluted (1:10) in fresh LB medium without added linalool or with the bacterium's subinhibitory concentration of linalool (1/2 the MIC) and grown overnight. Overnight cultures were then diluted 1:100 in fresh LB medium, and portions of 100 μl of the diluted bacteria were added into a 96-well plate containing the different concentrations of linalool. The plate was incubated again at 37°C for 24 h with agitation. This entire process was repeated with increasing concentrations of linalool (up to 0.96 M linalool) until highly resistant colonies of S. Senftenberg were isolated. Further transfers were not applied due to the low solubility of linalool in water or in growth medium (LB medium) without surfactant addition. The use of other solvents was avoided due to possible solvent-derived effects. Linalool-resistant colonies were stored in 20% glycerol at −80°C. One of the resistant colonies, named linalool-adapted S. Senftenberg (LASS), was picked for further characterization.
Essential oils and antibiotic susceptibility assays.
The antibacterial activities of basil oil, its major components (eugenol, linalool, and estragole), and different antibiotics against the S. Senftenberg wild type and the LASS strain were measured by a paper disk diffusion assay (30). Briefly, overnight cultures were diluted 1:100 in fresh LB medium and then grown to an optical density at 600 nm (OD600) of 0.4 (approximately 107 CFU/ml). One hundred-microliter portions of each culture were plated on an agar plate. Sterilized paper disks (diameter, 0.6 cm) soaked either with 10 μl of each essential oil or with the appropriate amount of each antibiotic (listed in Table 1) were placed on the plates, and the diameters of the inhibition zone were measured after 24 h of incubation at 37°C. Paper disks soaked with saline served as controls. The LASS strain was also tested for reduced susceptibility to linalool, basil oil, and antibiotics using a broth microdilution assay. For MIC measurement, 2-fold serial dilutions of basil oil and linalool ranging from 20% to 0.04% and from 240 mM to 0.5 mM, respectively, were prepared in 96-well plates. Wells with LB medium without basil oil or linalool served as controls. After 24 h, 100 μl of the culture from each well was spread on an agar plate, and the concentration at which no growth was observed was determined to be the minimum bactericidal concentrations (MBC). The experiment was performed in duplicate and repeated at least twice. Antibiotics were also 2-fold diluted, with the tetracycline concentration ranging from 50 μg/ml to 0.0975 μg/ml and the concentrations of the remaining antibiotics ranging from 100 μg/ml to 0.195 μg/ml. Bacterial growth was determined by measuring the OD600 every 5 min during a 24-h incubation with shaking at 37°C (SynergyHT; Bio-Tek Instruments Inc., Winooski, VT, USA). The disk diffusion assay was repeated three times in triplicate, while the microdilution assay was repeated at least twice in duplicate.
Cryo-TEM.
Overnight broth cultures were harvested by centrifugation (5 min, 14,000 × g) and washed twice with sterile saline (0.85% NaCl). The cultures were resuspended in sterile saline and diluted 1:4 to prepare bacterial suspensions of approximately 108 CFU/ml. The cells were incubated with 6 mM linalool for 1 h at 37°C. Cells incubated without linalool served as controls. Subsequently, 1 ml from each tube was harvested by centrifugation (5 min, 14,100 × g), washed twice with sterile saline, and finally, resuspended with 250 μl phosphate-buffered saline (pH 7.4). Bacterial samples for cryogenic transmission electron microscopy (cryo-TEM) analysis were prepared as described previously (16, 63, 64). Images of dozens of cells were recorded digitally on a Gatan MultiScan 791 (CM120) cooled charge-coupled-device camera in the low-dose imaging mode to minimize beam exposure and electron-beam radiation damage, and the images were analyzed using the DigitalMicrograph software package.
EtBr permeation assay.
To determine cell permeability, a fluorescence-based assay was implemented (65). Briefly, cultures of the S. Senftenberg wild type and the LASS strain were grown overnight in LB broth at 37°C under shaking. Bacteria were diluted 1:100 in fresh LB medium and grown to an OD600 of 1.0. The cultures were washed twice in saline (0.85% NaCl) and resuspended in saline containing 0.5% (wt/vol) glucose. Afterwards, the cells were incubated at 37°C for 10 min. One hundred microliters of saline containing 0.005% (vol/vol) ethidium bromide (EtBr), 0.5% (wt/vol) glucose, and polymyxin B (0.18 or 1.8 μM) or linalool (0.6 mM or 6 mM) was added to each well of a flat-bottomed 96-well plate. Subsequently, 100 μl of LB medium containing the bacteria (approximately 108 CFU/ml) was added to each well to a final volume of 200 μl. The EtBr fluorescence in each well was monitored every 1 min for 50 min (excitation, 530 nm; emission, 590 nm; Wallac 1420 Victor2 multilabel counter; PerkinElmer). Each experiment was performed twice in triplicate.
Analysis of transcription levels using GFP as a reporter.
The transcription levels of the genes marRAB, acrAB, micF, rob, soxS, ramA, and mcpL were determined using the GFP reporter protein (33, 38). A set of pCS21a plasmids, each of which contained the gfp gene fused to one of the investigated promoters (16, 38, 66, 67), was transformed into cells of the S. Senftenberg wild type and the LASS strain. Cultures of the S. Senftenberg wild type and the LASS strain harboring each of the GFP plasmids were grown at 37°C in LB broth containing kanamycin. Overnight cultures were diluted 1:200 in fresh LB broth, LB broth containing 6 mM linalool, or LB broth containing 5 mM sodium decanoate. Aliquots of 200 μl were distributed into a flat-bottomed 96-well plate. LB broth without inducer was used as a control. The plates were covered with a sterile breathable adhesive pad (Diversified Biotech) and incubated for 22 h with continuous shaking at 37°C in a multilabel counter (Victor2) with continuous measurement of the absorbance (OD600) and fluorescence (excitation, 535 nm; emission, 485 nm). The experiments were repeated at least 3 times in triplicate for each inducer. The fluorescence intensity and OD600 measurements of triplicate wells at each time point were averaged, and the appropriate standard deviations were calculated. The fluorescence intensity (Flu) was calculated by the equation Flut = FLt(test) − FLt(control) as described previously (68), where Flut is the fluorescence intensity at time t, FLt(test) is the fluorescence value at time t of culture with a plasmid harboring promoter, and FLt(control) is the fluorescence value at time t of the same culture grown with a pCS21a plasmid without an insert (promoter). The normalized fluorescence intensity (NFlu) was calculated by the equation NFlut = Flut/[ODt(test) − OD0(test)], where ODt(test) and OD0(test) are the absorbance (600 nm) at time t and time zero of culture with a plasmid harboring a promoter, respectively.
Motility assay.
The motility of the investigated strains was evaluated on motility agar medium containing 0.3% agar with or without 0.6 mM or 6 mM linalool (69). Once the agar got solidified, 2 μl of overnight culture of the S. Senftenberg wild-type, mcpL null mutant, or LASS strain was added to the center of the plates. The mcpL null mutant was previously identified to be linalool susceptible during screening of a transposon library for strains with an altered response to linalool (16). The plates were incubated for 6 h at 37°C. The average swarming diameter of every variant was calculated from three sets of measurements in triplicate.
Aggregate size measurement.
Cultures of the S. Senftenberg wild type and the LASS strain were grown in LB medium for 24 h at 37°C under shaking, diluted 1:50 in fresh medium, and grown for 22 h at 37°C in the absence and presence of linalool at a concentration of 0.6 mM, 3 mM, 6 mM, or 12 mM. Particle size experiments were carried using a Mastersizer 2000 instrument (Malvern) equipped with a Hydro 2000s dispersing unit (Malvern) (70) with the following modifications: measurements were taken in the range of 0.02 to 2,000 μm under conditions of a particle refractive index of 1.6 (defined as lipids), a particle obscuration coefficient of 10 to 15%, and a water refractive index of 1.33 and by use of the general calculation model for irregular particles. Three measurement cycles of 10 s each (background measurements of 20 s) were taken, and the data obtained were averaged with the appropriate software (Malvern Mastersizer 2000, version 5.60). Each experiment was repeated at least twice in triplicate.
Membrane fatty acid composition.
Bacteria were grown overnight in 50 ml LB medium at 37°C. The total lipid was extracted using the method of Evans et al. (71) with modifications. Briefly, bacteria were washed and resuspended in 4 ml of sterile distilled water. Fifteen milliliters of methanol-chloroform (2:1, vol/vol) was added, and the mixture was shaken. After 2 h, the mixture was centrifuged at 800 × g for 15 min. The supernatant (indicated as SN1) was decanted and retained. The pellet was resuspended in 19 ml of water-methanol-chloroform (0.8:2:1, vol/vol/vol) and the process was repeated once again: the mixture was shaken and left for a minimum of 2 h and then was centrifuged at 800 × g (2,500 rpm) for 15 min for removal of the supernatant (indicated as SN2). The two supernatants were pooled and supplemented with [(SN1 + SN2)/3.8] ml of chloroform followed by the same volume of water. After phase separation occurred, the lower chloroform fraction, which contained the lipids, was removed and placed into a tapered Erlenmeyer flask for further chemical transmethylation, as follows. Twenty milliliters of 2.5% (vol/vol) sulfuric acid dissolved in methanol solution was added, and the mixture was heated under stirring. Methanol vapors were condensed in a reflux system. Once the mixture was boiled, a constant temperature was set. The system was cooled down after 2 h, and 5 ml of a petroleum ether-diethyl ether solution (1:1, vol/vol) was added. Distilled water was added until the organic phase was clearly separated. The solution was mixed vigorously and left for 20 min until the phases completely separated. The upper organic ether phase was removed, placed into a glass vial, and completely evaporated under a stream of nitrogen gas, while the residue was resuspended in a small volume (500 μl) of ethyl acetate (EtAc). The identification of the fatty acids was conducted by gas chromatography-mass spectrometry (GC-MS) using a 6890N GC-MS instrument (Agilent Technologies, CA, USA) equipped with a capillary HP-5 column (30 m by 250 μm by 0.25 μm; Agilent Technologies), a capillary DB-23 column (60 m by 250 μm by 0.25 μm; Agilent Technologies), and a flame ionization detector (FID). Samples of 1 μl were injected in a split mode (1/10). The initial HP-5 column temperature was 150°C, and the column was kept at this temperature for 1 min; the temperature was then raised to 200°C at 5°C min−1 and maintained at this temperature for 10 min. Then, the temperature was raised to 270°C at a rate of 20°C min−1 and maintained at this temperature for 5 min. The temperature of the injector was set at 300°C. The initial DB-23 column temperature was 150°C, and the column was kept at this temperature for 1 min; the temperature was then raised to 240°C at 5°C min−1 and maintained at this temperature for 10 min. The temperatures of the injector and the detector were set at 270°C and 320°C, respectively. Nitrogen was used as a carrier gas at a column flow rate of 2.2 ml min−1. The fatty acid methyl esters (FAMEs) were identified on the basis of the retention times of FAME analytical standards (GLC-10 FAME mix; Sigma-Aldrich, Rehovot, Israel) and quantified as percentages on the basis of the peak area ratio between each FAME and the total FAMEs identified. The relative surfaces of the peaks were analyzed with Enhanced ChemStation D software (version 2.00.275) by Agilent Technologies.
Survival in planta and on basil leaves during storage at 4°C.
Plants of sweet basil (Ocimum basilicum L.) were grown in a greenhouse and contaminated as described previously (72). Briefly, GFP-expressing Salmonella strains were grown overnight at 37°C with aeration in LB broth supplemented with Kan (50 μg/ml). Subsequently, the cultures were diluted 1:100 in fresh LB medium and grown overnight to reach stationary phase. Bacteria were harvested by centrifugation, washed, and resuspended in sterile saline (0.85% NaCl) to prepare saline suspensions holding approximately 108 CFU ml−1 bacteria. These solutions were used for spray irrigation of basil plants that reached an average height of 20 to 40 cm (when they were 7 to 12 weeks old) (72). At 48 h after irrigation, leaves, stalks, roots, and soil samples (20 g each) were collected and placed in sterile stomacher bags for processing. Stalks, roots, and soil samples were collected in triplicate. In addition, 3 additional samples of leaves were stored overnight at 4°C immediately after collection. Samples were processed immediately upon collection by pummeling with a stomacher and plating on LB agar plates supplemented with Kan for Salmonella recovery (72), whereas the leaves stored at 4°C were processed after overnight storage.
Statistical analysis.
At least two independent repetitions were performed, unless specifically mentioned otherwise. The number of colonies per plate was converted to the number of CFU per gram (fresh weight) and log transformed for statistical analysis. To test for differences between LASS and the wild type in all experiments listed above, a t test analysis (paired t test for two samples for means) was conducted using Microsoft Excel software (version 2010). Effect sizes were calculated using Cohen's d statistics with 95% confidence intervals. Maximal bacterial growth rates were calculated at the exponential phase using Microsoft Excel software. Differences were considered significant when P was ≤0.05.
ACKNOWLEDGMENTS
This work was supported in part by the Technion Russell Berrie Nanotechnology Institute (RBNI).
We thank Ayelet Fishman and Sabina Ifraimov for their help in running samples in the gas chromatograph-mass spectrometer and the Mastersizer 2000 instruments. We express our full gratitude to Yeshayahu Talmon and Ellina Kesselman for their help with the cryo-TEM analysis. Lastly, we thank Judy Henn for her English editing of the manuscript.
We declare no conflict of interest.
REFERENCES
- 1.Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari BK, Valdramidis VP, O'Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. 2009. Application of natural antimicrobials for food preservation. J Agric Food Chem 57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 4.Van de Braak SAAJ, Leijten GCJJ. 1999. Essential oils and oleoresins: a survey in the Netherlands and other major markets in the European Union, p 116 CBI, Centre for the Promotion of Imports from Developing Countries, Rotterdam, Netherlands. [Google Scholar]
- 5.Elgayyar M, Draughon FA, Golden DA, Mount JR. 2001. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot 64:1019–1024. doi: 10.4315/0362-028X-64.7.1019. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Marshall MR, Wei C. 1995. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem 43:2839–2845. doi: 10.1021/jf00059a013. [DOI] [Google Scholar]
- 7.Lachowicz KJ, Jones GP, Briggs DR, Bienvenu FE, Wan J, Wilcock A, Coventry MJ. 1998. The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Lett Appl Microbiol 26:209–214. doi: 10.1046/j.1472-765X.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Sienkiewicz M, Lysakowska M, Pastuszka M, Bienias W, Kowalczyk E. 2013. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules 18:9334–9351. doi: 10.3390/molecules18089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suppakul P, Miltz J, Sonneveld K, Bigger SW. 2003. Antimicrobial properties of basil and its possible application in food packaging. J Agric Food Chem 51:3197–3207. doi: 10.1021/jf021038t. [DOI] [PubMed] [Google Scholar]
- 10.Wan J, Wilcock A, Coventry MJ. 1998. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. J Appl Microbiol 84:152–158. doi: 10.1046/j.1365-2672.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC. 2005. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 12.Javanmardi J, Khalighi A, Kashi A, Bais HP, Vivanco JM. 2002. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem 50:5878–5883. doi: 10.1021/jf020487q. [DOI] [PubMed] [Google Scholar]
- 13.Liber Z, Carovic-Stanko K, Politeo O, Strikic F, Kolak I, Milos M, Satovic Z. 2011. Chemical characterization and genetic relationships among Ocimum basilicum L. cultivars. Chem Biodivers 8:1978–1989. doi: 10.1002/cbdv.201100039. [DOI] [PubMed] [Google Scholar]
- 14.Paton A, Harley RM, Harley MM. 1999. Ocimum: an overview of classification and relationships, p 1–38. In Hiltunen R, Holm Y (ed), Basil: the genus Ocimum. Harwood Academic Publishers, Basingstoke, United Kingdom. [Google Scholar]
- 15.Radulovic NS, Blagojevic PD, Miltojevic AB. 2013. Alpha-linalool—a marker compound of forged/synthetic sweet basil (Ocimum basilicum L.) essential oils. J Sci Food Agric 93:3292–3303. doi: 10.1002/jsfa.6175. [DOI] [PubMed] [Google Scholar]
- 16.Kalily E, Hollander A, Korin B, Cymerman I, Yaron S. 2016. Mechanisms of resistance to linalool in Salmonella Senftenberg and their role in survival on basil. Environ Microbiol 18:3673–3688. doi: 10.1111/1462-2920.13268. [DOI] [PubMed] [Google Scholar]
- 17.Zengin H, Baysal AH. 2014. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SN, Lim YK, Freire MO, Cho E, Jin D, Kook JK. 2012. Antimicrobial effect of linalool and alpha-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 18:369–372. doi: 10.1016/j.anaerobe.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Bazemore R, Rouseff R, Naim M. 2003. Linalool in orange juice: origin and thermal stability. J Agric Food Chem 51:196–199. doi: 10.1021/jf0257291. [DOI] [PubMed] [Google Scholar]
- 20.Fuller ML. October 1981. Dog food compositions of improved palatability to dogs. US patent US4294857 A.
- 21.Hammer KA, Carson CF, Riley TV. 2012. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother 56:909–915. doi: 10.1128/AAC.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becerril R, Nerin C, Gomez-Lus R. 2012. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathog Dis 9:699–705. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- 24.Fadli M, Chevalier J, Hassani L, Mezrioui NE, Pages JM. 2014. Natural extracts stimulate membrane-associated mechanisms of resistance in Gram-negative bacteria. Lett Appl Microbiol 58:472–477. doi: 10.1111/lam.12216. [DOI] [PubMed] [Google Scholar]
- 25.McMahon MA, Blair IS, Moore JE, McDowell DA. 2007. Habituation to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia) is associated with reduced susceptibility to antibiotics in human pathogens. J Antimicrob Chemother 59:125–127. [DOI] [PubMed] [Google Scholar]
- 26.McMahon MA, Tunney MM, Moore JE, Blair IS, Gilpin DF, McDowell DA. 2008. Changes in antibiotic susceptibility in staphylococci habituated to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia). Lett Appl Microbiol 47:263–268. doi: 10.1111/j.1472-765X.2008.02420.x. [DOI] [PubMed] [Google Scholar]
- 27.Moken MC, McMurry LM, Levy SB. 1997. Selection of multiple-antibiotic-resistant (mar) mutants of Escherichia coli by using the disinfectant pine oil: roles of the mar and acrAB loci. Antimicrob Agents Chemother 41:2770–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson RR. 2000. Selection of resistance to the essential oil of Melaleuca alternifolia in Staphylococcus aureus. J Antimicrob Chemother 45:549–550. doi: 10.1093/jac/45.4.549. [DOI] [PubMed] [Google Scholar]
- 29.Pezzoli L, Elson R, Little CL, Yip H, Fisher I, Yishai R, Anis E, Valinsky L, Biggerstaff M, Patel N, Mather H, Brown DJ, Coia JE, van Pelt W, Nielsen EM, Ethelberg S, de Pinna E, Hampton MD, Peters T, Threlfall J. 2008. Packed with Salmonella—investigation of an international outbreak of Salmonella Senftenberg infection linked to contamination of prepacked basil in 2007. Foodborne Pathog Dis 5:661–668. doi: 10.1089/fpd.2008.0103. [DOI] [PubMed] [Google Scholar]
- 30.Kisluk G, Kalily E, Yaron S. 2013. Resistance to essential oils affects survival of Salmonella enterica serovars in growing and harvested basil. Environ Microbiol 15:2787–2798. doi: 10.1111/1462-2920.12139. [DOI] [PubMed] [Google Scholar]
- 31.Falke JJ, Hazelbauer GL. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem Sci 26:257–265. doi: 10.1016/S0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy SB. 2002. Active efflux, a common mechanism for biocide and antibiotic resistance. Symp Ser Soc Appl Microbiol 2002:65S–71S. [PubMed] [Google Scholar]
- 33.Solnik-Isaac H, Weinberger M, Tabak M, Ben-David A, Shachar D, Yaron S. 2007. Quinolone resistance of Salmonella enterica serovar Virchow isolates from humans and poultry in Israel: evidence for clonal expansion. J Clin Microbiol 45:2575–2579. doi: 10.1128/JCM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaron S, White DG, Matthews KR. 2003. Characterization of an Escherichia coli O157:H7 marR mutant. Int J Food Microbiol 85:281–291. doi: 10.1016/S0168-1605(02)00547-0. [DOI] [PubMed] [Google Scholar]
- 35.Li XZ, Zhang L, Poole K. 1998. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol 180:2987–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez A, Poza M, Aranda J, Latasa C, Medrano FJ, Tomas M, Romero A, Lasa I, Bou G. 2012. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother 56:6256–6266. doi: 10.1128/AAC.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol 16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 38.Hartog E, Ben-Shalom L, Shachar D, Matthews KR, Yaron S. 2008. Regulation of marA, soxS, rob, acrAB and micF in Salmonella enterica serovar Typhimurium. Microbiol Immunol 52:565–574. doi: 10.1111/j.1348-0421.2008.00075.x. [DOI] [PubMed] [Google Scholar]
- 39.Hancock RE. 1997. Peptide antibiotics. Lancet 349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 40.Ultee A, Kets EP, Alberda M, Hoekstra FA, Smid EJ. 2000. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch Microbiol 174:233–238. doi: 10.1007/s002030000199. [DOI] [PubMed] [Google Scholar]
- 41.Hyldgaard M, Mygind T, Meyer RL. 2012. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UNEP. 2002. Linalool. OECD SIDS initial assessment report for SIAM 14, March 2002. UNEP, Paris, France. [Google Scholar]
- 43.Hammer KA, Carson CF, Riley TV. 2008. Frequencies of resistance to Melaleuca alternifolia (tea tree) oil and rifampicin in Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis. Int J Antimicrob Agents 32:170–173. doi: 10.1016/j.ijantimicag.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Melnyk AH, Wong A, Kassen R. 2015. The fitness costs of antibiotic resistance mutations. Evol Appl 8:273–283. doi: 10.1111/eva.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulander W, Maisnier-Patin S, Andersson DI. 2009. The fitness cost of streptomycin resistance depends on rpsL mutation, carbon source and RpoS (sigmaS). Genetics 183:539–546, 1SI–2SI. doi: 10.1534/genetics.109.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol 8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 47.Mazzariol A, Cornaglia G, Nikaido H. 2000. Contributions of the AmpC beta-lactamase and the AcrAB multidrug efflux system in intrinsic resistance of Escherichia coli K-12 to beta-lactams. Antimicrob Agents Chemother 44:1387–1390. doi: 10.1128/AAC.44.5.1387-1390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visalli MA, Murphy E, Projan SJ, Bradford PA. 2003. AcrAB multidrug efflux pump is associated with reduced levels of susceptibility to tigecycline (GAR-936) in Proteus mirabilis. Antimicrob Agents Chemother 47:665–669. doi: 10.1128/AAC.47.2.665-669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaknoon F, Goldberg K, Sarig H, Epand RF, Epand RM, Mor A. 2012. Antibacterial properties of an oligo-acyl-lysyl hexamer targeting Gram-negative species. Antimicrob Agents Chemother 56:4827–4832. doi: 10.1128/AAC.00511-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lande MB, Donovan JM, Zeidel ML. 1995. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J Gen Physiol 106:67–84. doi: 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y-M, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 53.Ingram LO. 1976. Adaptation of membrane lipids to alcohols. J Bacteriol 125:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingram LO. 1982. Regulation of fatty acid composition in Escherichia coli: a proposed common mechanism for changes induced by ethanol, chaotropic agents, and a reduction of growth temperature. J Bacteriol 149:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rozes N, Peres C. 1998. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl Microbiol Biotechnol 49:108–111. doi: 10.1007/s002530051145. [DOI] [Google Scholar]
- 56.Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. 2007. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem 55:4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- 57.Lux R, Jahreis K, Bettenbrock K, Parkinson JS, Lengeler JW. 1995. Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc Natl Acad Sci U S A 92:11583–11587. doi: 10.1073/pnas.92.25.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 59.Lazova MD, Butler MT, Shimizu TS, Harshey RM. 2012. Salmonella chemoreceptors McpB and McpC mediate a repellent response to l-cystine: a potential mechanism to avoid oxidative conditions. Mol Microbiol 84:697–711. doi: 10.1111/j.1365-2958.2012.08051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cushnie TP, Hamilton VE, Chapman DG, Taylor PW, Lamb AJ. 2007. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J Appl Microbiol 103:1562–1567. doi: 10.1111/j.1365-2672.2007.03393.x. [DOI] [PubMed] [Google Scholar]
- 61.Brandl MT, Huynh S. 2014. Effect of the surfactant Tween 80 on the detachment and dispersal of Salmonella enterica serovar Thompson single cells and aggregates from cilantro leaves as revealed by image analysis. Appl Environ Microbiol 80:5037–5042. doi: 10.1128/AEM.00795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger CN, Shaw RK, Brown DJ, Mather H, Clare S, Dougan G, Pallen MJ, Frankel G. 2009. Interaction of Salmonella enterica with basil and other salad leaves. ISME J 3:261–265. doi: 10.1038/ismej.2008.95. [DOI] [PubMed] [Google Scholar]
- 63.Danino D, Bernheim-Groswasser A, Talmon Y. 2001. Digital cryogenic transmission electron microscopy: an advanced tool for direct imaging of complex fluids. Colloids Surf A 183–185:113–122. [Google Scholar]
- 64.Devi KP, Nisha SA, Sakthivel R, Pandian SK. 2010. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella Typhi by disrupting the cellular membrane. J Ethnopharmacol 130:107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg K, Sarig H, Zaknoon F, Epand RF, Epand RM, Mor A. 2013. Sensitization of gram-negative bacteria by targeting the membrane potential. FASEB J 27:3818–3826. doi: 10.1096/fj.13-227942. [DOI] [PubMed] [Google Scholar]
- 66.Schlisselberg DB, Kler E, Kisluk G, Shachar D, Yaron S. 2015. Biofilm formation ability of Salmonella enterica serovar Typhimurium acrAB mutants. Int J Antimicrob Agents 46:456–459. doi: 10.1016/j.ijantimicag.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Zaslaver A, Bren A, Ronen M, Itzkovitz S, Kikoin I, Shavit S, Liebermeister W, Surette MG, Alon U. 2006. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat Methods 3:623–628. doi: 10.1038/nmeth895. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Barak Z, Streckel W, Yaron S, Cohen S, Prager R, Tschape H. 2006. The expression of the virulence-associated effector protein gene avrA is dependent on a Salmonella enterica-specific regulatory function. Int J Med Microbiol 296:25–38. [DOI] [PubMed] [Google Scholar]
- 69.Malapaka RR, Adebayo LO, Tripp BC. 2007. A deletion variant study of the functional role of the Salmonella flagellin hypervariable domain region in motility. J Mol Biol 365:1102–1116. doi: 10.1016/j.jmb.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 70.Sadowski Z, Maliszewska IH, Grochowalska B, Polowczyk I, Kozlecki T. 2008. Synthesis of silver nanoparticles using microorganisms. Materials Sci Poland 26:419–424. [Google Scholar]
- 71.Evans RI, McClure PJ, Gould GW, Russell NJ. 1998. The effect of growth temperature on the phospholipid and fatty acyl compositions of non-proteolytic Clostridium botulinum. Int J Food Microbiol 40:159–167. doi: 10.1016/S0168-1605(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 72.Kisluk G, Yaron S. 2012. Presence and persistence of Salmonella enterica serotype Typhimurium in the phyllosphere and rhizosphere of spray-irrigated parsley. Appl Environ Microbiol 78:4030–4036. doi: 10.1128/AEM.00087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]