ABSTRACT
Family cars represent ∼74% of the yearly global output of motorized vehicles. With a life expectancy of ∼8 decades in many countries, the average person spends >100 min daily inside the confined and often shared space of the car, with exposure to a mix of potentially harmful microbes. Can commercial in-car microbial air decontamination devices mitigate the risk? Three such devices (designated devices 1 to 3) with HEPA filters were tested in the modified passenger cabin (3.25 m3) of a four-door sedan housed within a biosafety level 3 containment facility. Staphylococcus aureus (ATCC 6538) was suspended in a soil load to simulate the presence of body fluids and aerosolized into the car's cabin with a 6-jet Collison nebulizer. A muffin fan (80 mm by 80 mm, with an output of 0.17 m3/min) circulated the air inside. Plates (150 mm diameter) of Trypticase soy agar (TSA), placed inside a programmable slit-to-agar sampler, were held at 36 ± 1°C for 18 to 24 h and examined for CFU. The input dose of the test bacterium, its rate of biological decay, and the log10 reductions by the test devices were analyzed. The arbitrarily set performance criterion was the time in hours a device took for a 3-log10 reduction in the level of airborne challenge bacterium. On average, the level of S. aureus challenge in the air varied between 4.2 log10 CFU/m3 and 5.5 log10 CFU/m3, and its rate of biological decay was −0.0213 ± 0.0021 log10 CFU/m3/min. Devices 1 to 3 took 2.3, 1.5, and 9.7 h, respectively, to meet the performance criterion. While the experimental setup was tested using S. aureus as an archetypical airborne pathogen, it can be readily adapted to test other types of pathogens and technologies.
IMPORTANCE This study was designed to test the survival of airborne pathogens in the confined and shared space of a family automobile as well as to assess claims of devices marketed for in-car air decontamination. The basic experimental setup and the test protocols reported are versatile enough for work with all major types of airborne human pathogens and for testing a wide variety of air decontamination technologies. This study could also lay the foundation for a standardized test protocol for use by device makers as well as regulators for the registration of such devices.
KEYWORDS: airborne pathogens, automobiles, air decontamination, infection prevention, Staphylococcus aureus
INTRODUCTION
For safe driving, we are justifiably concerned with road conditions, weather, outdoor air quality, seatbelt use, and distracted and inebriated drivers as well as car and driver fitness. Should we also worry about the quality of air within the car? If yes, what risks does poor air quality pose to our health? These issues have come to the fore in recent years (1–3).
In general, the inside of an automobile is a confined and often shared space, and several reports in the past decade indicate that occupants thus face a higher risk of exposure to a variety of airborne infectious agents (1–3) and allergens (4), with possible harm to health. According to the 2015 report of the International Organization of Motor Vehicle Manufacturers (OICA; http://www.oica.net/), this is at a time when the global number of automobiles on the road is at an unprecedented level at the same time ongoing societal changes are increasing our exposure and vulnerability to infectious agents in general (5).
Cars for domestic or family use account for ∼74% of the world's yearly production of motorized vehicles (http://www.worldometers.info/cars/), with ∼80% of commuters using their own car and another 5.6% traveling as passengers. With the current life-expectancy of nearly 8 decades in many developed countries, the average time an individual spends driving a car is 4.3 years. This equates to nearly 100 min/day and a life-time driving distance of ∼1.3 million km inside this confined and often shared space with exposure to a mix of potentially harmful pathogens and other air pollutants in general. Can the increasing number and variety of commercial devices with claims for in-car air decontamination potentially reduce the risk from airborne pathogens? Robust and scientifically valid facilities and test protocols are currently unavailable to validate such claims; this study was initiated to address the gap.
RESULTS
RH and air temperature.
The relative humidity (RH) in the car chamber remained essentially in the midrange (50% ± 2%) and the air temperature stayed steady at 22 ± 1°C throughout a given test. The RH level changed from 49.5% to 50.5% immediately after the nebulization of the bacterial suspension due to the addition of moisture from spraying of the microbial suspension.
Testing microbial survival.
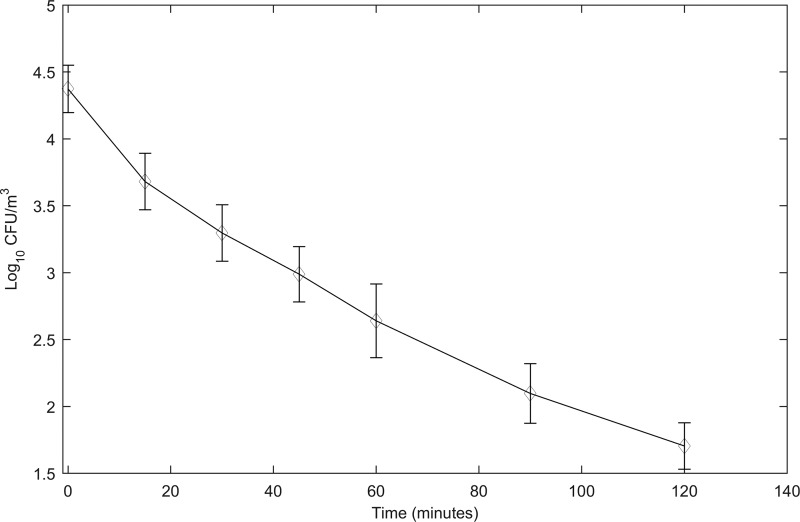
The rate of biological decay of S. aureus, based on three repeat experiments under the same experimental conditions in the car chamber, was found to be −0.0213 ± 0.0021 log10 CFU/m3 per min (Fig. 1). These data were used for comparing the air decontamination activity of the three test devices. In order to obtain the log10 reduction caused by a device at each time point, the log10 CFU/m3 of recovered bacteria in the efficacy test was subtracted from the log10 CFU/m3 of recovered bacteria in the stability-in-air test.
FIG 1.
Biological decay of Staphylococcus aureus in the air inside the car chamber. A suspension of S. aureus with a soil load was aerosolized into the car chamber using a Collison nebulizer. A muffin fan in the chamber helped distribute the aerosolized bacteria evenly throughout and also kept them resuspended in the air. A programmable slit-to-agar (STA) sampler was used to recover the airborne bacteria on plates of Trypticase soy agar (TSA). The plates were incubated at 36 ± 1°C for 18 to 24 h and CFU on them recorded to calculate the rate of biological decay. The RH inside the car chamber was 50% ± 5% and the air temperature 22 ± 1°C.
The activity of the air decontamination devices.
All three devices were tested twice each against S. aureus, as shown in Table 1, and the mean values used for statistical analysis. The devices differed in the rate with which they could recycle the in-car air; device 2 has the highest flow-rate.
TABLE 1.
Summary of log10 reductions in the CFU of airborne Staphylococcus aureus by the three in-car air decontamination devices
| Device no. | Log10 reduction in CFU/m3 | Time (min) | Estimated time in h to reach 3-log10 reduction | CFMa (m3/h) |
|---|---|---|---|---|
| 1 | 2.5 | 90 | 2.33 | 5.88 (10) |
| 2 | 3 | 90 | 1.5b | 7.06 (12) |
| 3 | 0.5 | 120 | 9.7 | 5.88 (10) |
CFM, cubic feet per minute.
Based on empirical data.
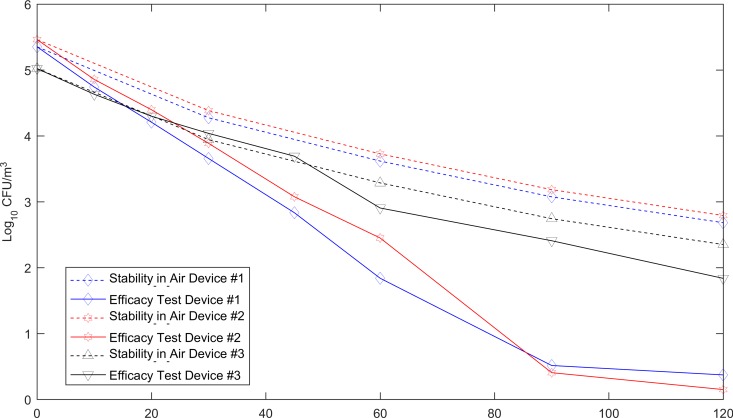
Figure 2 shows the performance of the three devices against S. aureus. The average input titers of S. aureus aerosolized into the chamber for the experiment with the three devices were 4.99, 5.288, and 4.858 log10 CFU/m3, respectively. Devices 1 and 2 could achieve a 3-log10 reduction in the viability of this microbe within 90 min (Fig. 2). Statistical analysis showed that the rates of reduction in the level of S. aureus by device 1 (P = 0.001) and device 2 (P = 0.0001) were significantly higher than the rate of natural biological decay. In contrast, there was no statistically significant difference between the performance of device 3 (P = 0.0655) and the rate of biological decay of S. aureus.
FIG 2.
Activity of the three commercial in-car air decontamination devices, using aerosolized S. aureus as the challenge. Before each test with an in-car air decontamination device the challenge bacterium was tested for stability in air; the data are shown in the top three lines (dotted) in the graph. Each one of the three in-car air decontamination devices was separately tested twice.
DISCUSSION
This work represents a systematic study on the survival of a representative airborne bacterial pathogen in the air inside family cars, which also assessed commercial in-car air decontamination devices. The basic setup and procedures described here have been adapted from our previous work in aerobiology (6). The setup described here is potentially suitable for work with other types of vegetative bacteria as well as other classes of human pathogens.
Pathogens become airborne either by direct ejection from infected/colonized individuals or by resuspension of already dried body fluids or other sources of contamination in the environment (7). Thus, pathogens in air are almost always embedded in droplet nuclei along with various levels and types of organic and inorganic materials—the soil load. Therefore, any air decontamination technologies assessed must be potent enough to reduce the pathogen load to the desired level while also coping with the soil load. In view of this, the liquid aerosolized using the Collison nebulizer consisted of the test microbial suspension and a soil load, together with antifoam to minimize frothing during nebulization (8). The antifoam and all components of the soil load were prescreened and found harmless to the test bacterium. The STA sampler was programmed for the duration of air sample collection. A filter at its exhaust prevented the release of any viable bacteria into the air.
While S. aureus and Klebsiella pneumoniae are both recommended by the U.S. EPA for testing indoor air decontamination, we found the latter less stable on nebulization and in air (data not shown). In preliminary tests, we also found Acinetobacter baumannii (7), a Gram-negative bacillus and an increasingly significant airborne pathogen (9), to be more robust and stable in indoor air than K. pneumoniae. Therefore, A. baumannii may prove to be a better surrogate than K. pneumoniae for Gram-negative bacteria in studies on the microbial quality of indoor air.
The 3-log10 reduction in the viability of the test bacterium was based on the recommendation of the U.S. Environmental Protection Agency (EPA), and also on practical limitations on the numbers of CFU that could be aerosolized and detected with consistency. A higher microbial titer would have confounded the results by overloading the test system while also increasing the challenge to levels generally not encountered in the field. Furthermore, air represents a dynamic milieu in contrast to, for example, environmental surfaces. Therefore, the concept of “contact time” for a specific log10 reduction cannot be readily applied to air decontamination. However, we have shown (7) that certain types of indoor air decontamination devices can successfully respond to continual fluctuations in microbial quality with reasonable speed.
Although all three devices tested were based on HEPA filtration, the protocol used could readily discern differences in their performance for regulatory purposes and for the information of prospective buyers. Further experimentation would be needed to identify the reasons for the differences in the performance of the three devices.
With regard to the microbial profile, human occupants of cars are the most common contributors of resident (e.g., Staphylococcus) as well as transient (e.g., influenza- and rhinovirus) microbiota. Pets such as dogs may also add to the complement of microbes with potential risks to humans (10).
Dust settled on carpets and upholstery may become resuspended, thus contaminating the air and/or other areas within the car (7). Sufficient levels of moisture from water/food spillage inside the car can also promote the replication of dust-carried microbes. Cargo in the passenger compartment may further contribute to the loading of dust-laden microbes, most of which are unlikely to be directly harmful to humans.
Opportunistic pathogens such as nontuberculous mycobacteria (NTM) and legionellae may enter cars via biofilms in car heaters/air conditioners (AC) (11, 12) and in windshield washer reservoirs (13), as well as other sources (14–16). Even though many types of NTM are increasingly recognized as human pathogens (17–19), there is virtually no information on their recovery from inside the family car. Any future studies on the microbiota in the family car should include assessment of NTM and their potential health impacts, especially in combination with other airborne pollutants in family cars (20).
Legionnaires' disease (LD) is a serious and potentially fatal pneumonia (21, 22), with Legionella pneumophila causing >90% of the cases. Legionellae are common in biofilms (21, 23, 24), and those individuals debilitated due to age, chronic smoking, and immunosuppression are at a higher risk. Legionellae have, in fact, been recovered from the condensates of car air conditioners and cabin air filters (25), with at least one case of legionellosis possibly linked to them (26).
Biofilms in windshield washer reservoirs may release legionellae (13, 27, 28), which may also enter cars from road dust and water in road puddles (14, 29). Although certain of the studies summarized above allude to the risk of LD for professional drivers and others have found components of an automobile's liquid- and air-handling systems to test positive for legionellae, the relevance of their findings to air quality in the family car remains unknown.
Li et al. (30) note the lack of data on risks associated with the exposure to microbial aerosols from automobile air conditioners (AC). They collected samples of dust from AC and engine filters from 30 automobiles in four coastal locations in China and analyzed them for bacteria, fungi, and endotoxins. Irrespective of the location of the tested vehicles, the dust from their AC filters revealed relatively high levels of bacteria (∼26,150 CFU/mg), fungi (∼1,287 CFU/mg), and endotoxins (∼5,527 endotoxin units/mg). More than 400 types of bacterial species were detected, including opportunistic pathogens such as Acinetobacter, Bacillus, Pseudomonas, and Stenotrophomonas spp. Some 18 types of allergenic fungal species were also found in abundance.
The influence of AC and heating systems on the levels of airborne bacteria and fungi inside automobiles has been assessed (1). Soon after the start of the AC systems, there was an increase in the levels of airborne microbes due to the purging of their pipes and also as a result of the resuspension of accumulated dust inside the cars. This was followed by significant drops in the aerosol levels in the next 5 to 35 min. In contrast, the heating systems did not show that initial increase in microbial aerosols, possibly because of microbial inactivation by the heating coils. The data in this study are based on five cars and the collection of 2-min air samples using a single-stage Andersen sampler. Such a sampler is much less accurate than an STA sampler, which is designed to show a time-related distribution of airborne particles.
The report by Knibbs et al. (31) is the only published one dealing with influenzaviruses and their possible airborne spread inside cars. They modeled virus spread in view of a suspected case of influenza spread during car travel in Australia (32). They noted wide variations in the efficiency of air circulation depending on the age and make of the car. Also, the estimated risk of influenza spread ranged from 59% to 99.9% for a 90-min trip when air was recirculated. These findings have implications for the design and operation of any in-car air decontamination device to deal with airborne viruses, including the enveloped ones.
Cars for domestic use entail certain unique factors to be considered when assessing the risks from exposure to infectious agents. The risk of exposure to a given infectious agent is directly related to the length of the commute and number of occupants, as well as with wide variation in their ages and immune statuses. The overall proportion of individuals with acquired (e.g., HIV), induced (e.g., organ transplantation and cancer therapy) and natural (aging) immunosuppression continues to change, with attendant impact on susceptibility to infectious agents in general. Those taking medication for common ailments (e.g., arthritis and diabetes) also suffer from depressed immune systems. In the U.S., for example, at least 3.6% of the general population is believed to be immunosuppressed at any given time (http://thebulletin.org/growing-number-immunocompromised). By its nature, driving can be a stressful activity, further exacerbated under conditions of heavy traffic and inclement weather. The possible impact of such stressors on rider susceptibility to infectious agents, including air pollutants in general, and their health outcomes (33) remains unexplored.
The relative concentrations as well as the variety of respirable particulates (e.g., PM2.5) on the road are likely to be higher than inside homes. Inhalation of such particulates, including those from tobacco smoke (33), and their retention in the respiratory system can predispose to many pathogens. In-car exposure to such particulates (34) and volatile organic compounds (VOCs) (35) may occur simultaneously, potentially leading to an additive negative impact on the health of the occupants (33–36).
According to OICA (2015), the global production and sales of motorized vehicles reached a record level of nearly 90 million units in 2014, a >34% increase since 2005. The world total of passenger cars has now surpassed the billion mark, with 174 vehicles/1,000 inhabitants, a >21% increase since 2005 (http://chartsbin.com/view/1113). In fact, the International Transport Forum (ITF) of the Organization for Economic Cooperation and Development predicts that the number of cars and light trucks globally will reach 2.5 billion by the year 2050 (http://www.ipsnews.net/2011/06/bike-vs-car-on-a-hot-planet/).
MATERIALS AND METHODS
Experimental setup.
To represent the inside of a family car, the passenger compartment of a discarded four-door 2005 Honda Civic (Fig. 3) was reassembled inside an aerobiology chamber (6) for additional biosafety. The inner walls of the compartment were lined with clear, locally purchased polyethylene sheeting (0.15 mm thickness) to create an airtight enclosure. Copper wiring was installed to dissipate any static electricity, thus avoiding the loss of bacteria by adsorption to the plastic. All other surfaces inside the cabin of the car were left uncovered. The total inside volume of the car chamber was ∼3.25 m3 (115 ft3). The nebulized bacteria entered the car via an inlet at the center of its roof and the air from inside was collected via a sealable port on one side. Before each experiment, the car chamber was filled with compressed air containing 10% hydrogen to check for leaks along all seams using a combustible gas leak probe and detector (Model BT-45; Quantum Instruments). Any leaks detected were patched using nylon tape.
FIG 3.
Diagram showing the general experimental set-up. Parts from a discarded four-door sedan were reassembled inside the biosafety chamber. The car chamber was placed inside an enclosure with plastic sheeting for additional biosafety. For a better view of the car chamber, the metallic support structures for the two proximal sides of the outer chamber are not shown in the diagram. The challenge bacterial aerosols were introduced into the car chamber using a Collison nebulizer. A muffin fan was used to keep the airborne bacteria evenly distributed. A programmable STA sampler was used to collect the airborne bacteria onto plates of TSA. The plates were held at 36 ± 1°C for 18 to 24 h before recording the CFU on them. Between experiments, the car chamber was purged with fresh air to remove any residual microbial contamination.
Test bacterium.
As recommended in the 2012 Guideline of the U.S. Environmental Protection Agency for air sanitizer performance (37), Staphylococcus aureus (ATCC 6538) was used as a representative Gram-positive airborne pathogen.
Trypticase soy broth (TSB; Oxoid) was used to prepare cultures of the test microbe and modified Letheen agar (MLA; Fisher Scientific) in 15- by 150-mm disposable plastic petri plates (Fisher) was used for determination of CFU in test stocks and for bacterial recovery from the air. All culture plates were incubated at 36 ± 1°C and observed after 18 ± 2 h of incubation and again after 4 days to detect CFU from any late-growing stressed or injured bacterial cells.
Consumables.
All items requiring sterilization prior to use were autoclaved at 121°C for 45 min. All used disposable labware was autoclaved and discarded as biomedical waste.
The soil load.
All bacterial suspensions to be nebulized contained a soil load (38) to simulate the presence of body fluids. The soil load consisted of a mixture of bovine serum albumin (BSA; Sigma-Aldrich), mucin from the bovine submaxillary gland (Sigma-Aldrich), and yeast extract powder (VWR). Stock solutions were prepared separately by dissolving 0.5 g, 0.04 g, and 0.5 g, respectively, in 10 ml of Dulbecco's phosphate-buffered saline (DPBS; pH 7.2 ± 0.2). The solutions were individually passed through a syringe-mounted polyethersulfone (PES; Sterlitech) membrane (0.2 μm in pore diameter), aliquoted in 1.5-ml volumes, and stored at −20 ± 2°C with a shelf-life of at least 1 year.
Air decontamination devices tested.
Three types of devices claiming in-car air decontamination were bought on the open market. All devices were free-standing and could readily be plugged into the car's power supply. Each one was separately placed inside the car chamber and tested at least twice. All devices were tested at their highest fan speeds. The car's air handling system was turned off during testing and a muffin fan (Mode Electronic 59-246-0) optimized with a 0.18-m3/min (6.45-ft3/min) output was used to circulate the air inside the compartment.
Methods.
Frozen stocks of the test bacterium were prepared by inoculating tubes with 9 ml of TSB and then incubating them at 36 ± 1°C for 18 ± 2 h. Each tube then received 1.0 ml of sterilized glycerol to yield a 10% by volume concentration of the cryopreservative. The suspension was aliquoted (0.5 ml volume) into cryovials for storage at −80 ± 2°C. To make a working suspension, 100 μl from a vial of the frozen stock was placed into 10 ml of TSB and the tube incubated for 18 ± 2 h. This represented the “refrigerated stock” for storage at 4 ± 2°C, used over 6 ± 1 days.
A bacterial suspension for nebulization was prepared by adding 100 μl of the refrigerated stock to 10 ml of sterile TSB which was incubated at 36 ± 1°C for 18 ± 2 h for a consistent yield of CFU. Fifty microliters of the culture was then added to 10.14 ml of DPBS along with 0.75 ml BSA, 1.05 ml yeast extract, 3.0 ml mucin, and 10 μl of Antifoam A concentrate (Sigma-Aldrich) for a total volume of 15.0 ml. To quantify the level of viable bacteria aerosolized into the chamber, the fluid in the nebulizer was assayed for CFU after spraying.
Aerosolization of test bacteria.
A 6-jet Collison nebulizer (CH Technologies, Westwood, NJ) was used to spray the test bacteria into the car chamber. Dry air from a compressed air cylinder was used at a pressure of ∼172 kPa (25 lb/in2) to operate the nebulizer. The length of nebulization varied depending on the type of experiment.
Sampling of air for viable bacteria.
The airborne survival of the test microbe and the activity of the air decontamination devices were determined by collecting the air from the chamber at the rate of 28.3 liter (1 ft3)/min using an externally placed slit-to-agar (STA) sampler (Particle Measuring Systems, Boulder, CO). The collection times for each sample and the total number of samples collected varied with the type of test. When the level of airborne bacteria in the car chamber was expected to be high, the air sample was collected for 2 min, the minimum time of operation for an STA sampler. The maximum time for air sampling was 20 min when the airborne bacterial load was expected to be low.
Testing microbial survival in air.
The natural rate of biological decay of airborne S. aureus in the car chamber was determined prior to testing the devices. To achieve this, a suspension of the test microbe was aerosolized into the compartment and a series of air samples collected. The plates from the STA sampler were incubated at 36 ± 1°C for 18 ± 2°C and CFU on them recorded. Digital camera pictures of the plates were taken. Plates with no visible CFU were reincubated for an additional 4 days and observed for any late-developing colonies before discarding the plates.
The use of a remote-sensing relative humidity (RH) and air temperature meter (Dickson WiZARD2; Dickson Co., Addison, IL) placed inside the car chamber allowed real-time monitoring and recording of these parameters; any changes could also be observed on a computer monitor placed in the vicinity of the larger aerobiology chamber.
Data analyses.
The rate of biological decay of the test bacterium was first transformed for comparison with the rate of loss in viability attained by a given test device. A one-way analysis of covariance (ANCOVA) was then carried out on the data using the “aoctool” function in the Matlab statistics toolbox (Mathworks, Natick, MA).
ACKNOWLEDGMENTS
Richard Kibbee assisted us in the establishment of the aerobiology facilities. Elizabeth Bruning of RB was most helpful in the administrative aspects of this study. André Bergeron (Manager, Biohazards Containment Facility) capably looked after all our technical needs.
The authors declare no conflict of interest.
REFERENCES
- 1.Jo WK, Lee JH. 2008. Airborne fungal and bacterial levels associated with the use of automobile air conditioners or heaters, room air conditioners, and humidifiers. Arch Environ Occup Health 63:101–107. doi: 10.3200/AEOH.63.3.101-107. [DOI] [PubMed] [Google Scholar]
- 2.Knibbs LD, Morawska L. 2012. Traffic-related fine and ultrafine particle exposures of professional drivers and illness: an opportunity to better link exposure science and epidemiology to address an occupational hazard? Environ Int 49:110–114. doi: 10.1016/j.envint.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson RE, Gutierrez D, Peters C, Nichols M, Boles BR. 2014. Elucidation of bacteria found in car interiors and strategies to reduce the presence of potential pathogens. Biofouling 30:337–346. doi: 10.1080/08927014.2013.873418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar P, Lopez M, Fan W, Cambre K, Elston RC. 1990. Mold contamination of automobile air conditioner systems. Ann Allergy 64:174–177. [PubMed] [Google Scholar]
- 5.Sattar SA, Tetro J, Springthorpe VS. 1999. Impact of changing societal trends on the spread of infections in American and Canadian homes. Am J Infect Control 27:S4–S21. doi: 10.1016/S0196-6553(99)70037-4. [DOI] [PubMed] [Google Scholar]
- 6.Sattar SA, Kibbee RJ, Zargar B, Wright KE, Rubino JR, Ijaz MK. 2016. Decontamination of indoor air to reduce the risk of airborne infections: studies on survival and inactivation of airborne pathogens using an aerobiology chamber. Am J Infect Control 44:e177–e182. doi: 10.1016/j.ajic.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Ijaz MK, Zargar B, Wright KE, Rubino JR, Sattar SA. 2016. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am J Infect Control 44: S109–120. doi: 10.1016/j.ajic.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijaz MK, Sattar SA, Johnson-Lussenburg CM, Springthorpe VS. 1985. Comparison of the airborne survival of calf rotavirus and poliovirus type 1 (Sabin) aerosolized as a mixture. Appl Environ Microbiol 49:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakupogullari Y, Otlu B, Ersoy Y, Kuzucu C, Bayindir Y, Kayabas U, Togal T, Kizilkaya C. 2016. Is airborne transmission of Acinetobacter baumannii possible: a prospective molecular epidemiologic study in a tertiary care hospital. Am J Infect Control 44:1595–1599. doi: 10.1016/j.ajic.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Esch KJ, Petersen CA. 2013. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev 26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diekmann N, Burghartz M, Remus L, Kaufholz AL, Nawrath T, Rohde M, Schulz S, Roselius L, Schaper J, Mamber O, Jahn D, Jahn M. 2013. Microbial communities related to volatile organic compound emission in automobile air conditioning units. Appl Microbiol Biotechnol 97:8777–8793. doi: 10.1007/s00253-012-4564-4. [DOI] [PubMed] [Google Scholar]
- 12.Simmons RB, Rose LJ, Crow SA, Ahearn DG. 1999. The occurrence and persistence of mixed biofilms in automobile air conditioning systems. Curr Microbiol 39:141–145. doi: 10.1007/s002849900435. [DOI] [PubMed] [Google Scholar]
- 13.Schwake DO, Alum A, Abbaszadegan M. 2015. Automobile windshield washer fluid: a potential source of transmission for Legionella. Sci Total Environ 526:271–277. doi: 10.1016/j.scitotenv.2015.03.122. [DOI] [PubMed] [Google Scholar]
- 14.van Heijnsbergen E, de Roda Husman AM, Lodder WJ, Bouwknegt M, Docters van Leeuwen AE, Bruin JP, Euser SM, den Boer JW, Schalk JA. 2014. Viable Legionella pneumophila bacteria in natural soil and rainwater puddles. J Appl Microbiol 117:882–890. doi: 10.1111/jam.12559. [DOI] [PubMed] [Google Scholar]
- 15.Falkinham JO., III 2015. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri A, Kneisel J, Kloster I, Kamal E, Lewin A. 2014. Abundance of Mycobacterium avium ssp. hominissuis in soil and dust in Germany—implications for the infection route. Lett Appl Microbiol 59:65–70. doi: 10.1111/lam.12243. [DOI] [PubMed] [Google Scholar]
- 17.Al-Anazi KA, Al-Jasser AM, Al-Anazi WK. 2014. Infections caused by non-tuberculous mycobacteria in recipients of hematopoietic stem cell transplantation. Front Oncol 4:311. doi: 10.3389/fonc.2014.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkle E, Winthrop KL. 2015. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med 36:91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guglielmetti L, Mougari F, Lopes A, Raskine L, Cambau E. 2015. Human infections due to nontuberculous mycobacteria: the infectious diseases and clinical microbiology specialists' point of view. Future Microbiol 10:1467–1483. doi: 10.2217/fmb.15.64. [DOI] [PubMed] [Google Scholar]
- 20.Sattar SA, Tetro JA, Springthrope VS. 2007. Effects of environmental chemicals and the host-pathogen relationship: Are there any negative consequences for human health?, p 2–30. In Zhu PC. (ed), New biocides development: the combined approach of chemistry and microbiology. ACS Symposium Series, vol. 967 Oxford University Press, Oxford, England. [Google Scholar]
- 21.Carratala J, Garcia-Vidal C. 2010. An update on Legionella. Curr Opin Infect Dis 23:152–157. doi: 10.1097/QCO.0b013e328336835b. [DOI] [PubMed] [Google Scholar]
- 22.Den Boer JW, Nijhof J, Friesema I. 2006. Risk factors for sporadic community-acquired Legionnaires' disease. A 3-year national case-control study. Public Health 120:566–571. doi: 10.1016/j.puhe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Ferranti G, Marchesi I, Favale M, Borella P, Bargellini A. 2014. Aetiology, source and prevention of waterborne healthcare-associated infections: a review. J Med Microbiol 63:1247–1259. doi: 10.1099/jmm.0.075713-0. [DOI] [PubMed] [Google Scholar]
- 24.Carratala J, Garcia-Vidal C. 2008. What is healthcare-associated pneumonia and how is it managed? Curr Opin Infect Dis 21:168–173. doi: 10.1097/QCO.0b013e3282f4f248. [DOI] [PubMed] [Google Scholar]
- 25.Alexandropoulou IG, Konstantinidis TG, Parasidis TA, Nikolaidis C, Panopoulou M, Constantinidis TC. 2013. First report of Legionella pneumophila in car cabin air filters. Are these a potential exposure pathway for professional drivers? Scand J Infect Dis 45:948–952. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto R, Ohno A, Nakahara T, Satomura K, Iwanaga S, Kouyama Y, Kura F, Noami M, Kusaka K, Funato T, Takeda M, Matsubayashi K, Okumiya K, Kato N, Yamaguchi K. 2009. Is driving a car a risk for Legionnaires' disease? Epidemiol Infect 137:1615–1622. doi: 10.1017/S0950268809002568. [DOI] [PubMed] [Google Scholar]
- 27.Palmer ME, Longmaid K, Lamph D, Willis C, Heaslip V, Khattab A. 2012. Legionella pneumophila found in windscreen washer fluid without added screenwash. Eur J Epidemiol 27:667. doi: 10.1007/s10654-012-9685-7. [DOI] [PubMed] [Google Scholar]
- 28.Wallensten A, Oliver I, Ricketts K, Kafatos G, Stuart JM, Joseph C. 2010. Windscreen wiper fluid without added screenwash in motor vehicles: a newly identified risk factor for Legionnaires' disease. Eur J Epidemiol 25:661–665. doi: 10.1007/s10654-010-9471-3. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto R, Ohno A, Nakahara T, Satomura K, Iwanaga S, Kouyama Y, Kura F, Kato N, Matsubayashi K, Okumiya K, Yamaguchi K. 2009. Legionella pneumophila in rainwater on roads. Emerg Infect Dis 15:1295–1297. doi: 10.3201/eid1508.090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Li M, Shen F, Zou Z, Yao M, Wu CY. 2013. Characterization of biological aerosol exposure risks from automobile air conditioning system. Environ Sci Technol 47:10660–10666. doi: 10.1021/es402848d. [DOI] [PubMed] [Google Scholar]
- 31.Knibbs LD, Morawska L, Bell SC. 2012. The risk of airborne influenza transmission in passenger cars. Epidemiol Infect 140:474–478. doi: 10.1017/S0950268811000835. [DOI] [PubMed] [Google Scholar]
- 32.Binns PL, Sheppeard V, Staff MP. 2010. Isolation and quarantine during pandemic (H1N1) 2009 influenza in NSW: the operational experience of public health units. N S W Public Health Bull 21:10–15. doi: 10.1071/NB09036. [DOI] [PubMed] [Google Scholar]
- 33.Chan ED, Kinney WH, Honda JR, Bishwakarma R, Gangavelli A, Mya J, Bai X, Ordway DJ. 2014. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis (Edinb) 94:544–550. doi: 10.1016/j.tube.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR, Zhu P, Tong J, Gong J, Zhu T, Zhang J. 2012. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med 186:1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose LJ, Simmons RB, Crow SA, Ahearn DG. 2000. Volatile organic compounds associated with microbial growth in automobile air conditioning systems. Curr Microbiol 41:206–209. doi: 10.1007/s002840010120. [DOI] [PubMed] [Google Scholar]
- 36.Sattar SA, Wright KE, Zargar B, Rubino JR, Ijaz MK. 2016. Airborne infectious agents and other pollutants in automobiles for domestic use: potential health impacts and approaches to risk mitigation. J Environ Public Health 2016:1548326. doi: 10.1155/2016/1548326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Environmental Protection Agency. 2012. Product Performance Test Guidelines OCSPP 810.2500: Air Sanitizers – Efficacy Data Recommendations. Docket ID EPA 730-C-11-003 U.S. Environmental Protection Agency, Washington, DC: https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0025. [Google Scholar]
- 38.Springthorpe VS, Sattar SA. 2007. Application of a quantitative carrier test to evaluate microbicides against mycobacteria. J AOAC Int 90:817–824. [PubMed] [Google Scholar]