ABSTRACT
Here, we describe a novel clade within Ensifer meliloti and consider how geographic and ecological isolation contributed to the limited distribution of this group. Members of the genus Ensifer are best known for their ability to form nitrogen-fixing symbioses with forage legumes of three related genera, Medicago L., Melilotus Mill., and Trigonella L., which are members of the tribe Trifolieae. These legumes have a natural distribution extending from the Mediterranean Basin through western Asia, where there is an unsurpassed number of species belonging to these genera. Trigonella suavissima L. is unusual in that it is the only species in the tribe Trifolieae that is native to Australia. We compared the genetic diversity and taxonomic placement of rhizobia nodulating T. suavissima with those of members of an Ensifer reference collection. Our goal was to determine if the T. suavissima rhizobial strains, like their plant host, are naturally limited to the Australian continent. We used multilocus sequence analysis to estimate the genetic relatedness of 56 T. suavissima symbionts to 28 Ensifer reference strains. Sequence data were partitioned according to the replicons in which the loci are located. The results were used to construct replicon-specific phylogenetic trees. In both the chromosomal and chromid trees, the Australian strains formed a distinct clade within E. meliloti. The strains also shared few alleles with Ensifer reference strains from other continents. Carbon source utilization assays revealed that the strains are also unusual in their ability to utilize 2-oxoglutarate as a sole carbon source. A strategy was outlined for locating similar strains elsewhere.
IMPORTANCE In this study, we employed a biogeographical approach to investigate the origins of a symbiotic relationship between an Australian legume and its nitrogen-fixing rhizobia. The question of the ancestral origins of these symbionts is based on the observation that the legume host is not closely related to other native Australian legumes. Previous research has shown that the legume host Trigonella suavissima is instead closely related to legumes native to the Mediterranean Basin and western Asia, suggesting that it may have been introduced in Australia from those regions. This led to the question of whether its rhizobia may have been introduced as well. In this study, we were unable to find persuasive evidence supporting this hypothesis. Instead, our results suggest either that the T. suavissima rhizobia are native to Australia or that our methods for locating their close relatives elsewhere are inadequate. A strategy to investigate the latter alternative is proposed.
KEYWORDS: Ensifer, rhizobium, Sinorhizobium meliloti, Trigonella, biogeography, clade, symbiotic nitrogen fixation
INTRODUCTION
In this study, we describe a novel clade of Ensifer meliloti and consider how geographic and ecological isolation may have contributed to the evolution of this group of bacteria. Members of the genus Ensifer (formerly Sinorhizobium) are best known for their ability to form nitrogen-fixing symbioses with forage legumes of three related genera, Medicago L., Melilotus Mill., and Trigonella L. These legumes, which are members of the tribe Trifolieae, have a natural distribution that extends from the Mediterranean Basin through western Asia (1, 2). This region contains an unsurpassed number of legume species belonging to these genera, and it also contains a rich diversity of microsymbionts that nodulate these legumes. In the study in which this extensive rhizobial diversity was first identified, a strongly divergent genotype within the species E. meliloti was noted (3). The strain possessing this genotype was USDA6670 (syn., CC2013), a strain symbiotically effective with the native Australian legume Trigonella suavissima Lindl. (4). In subsequent studies, four other T. suavissima symbionts were also described as having multilocus genotypes similar to that of USDA6670 (5, 6).
Trigonella suavissima is a herbaceous annual legume that is widely distributed in the arid interior of Australia and is well adapted to low-lying areas of flood plains, particularly those in the Channel Country of South West Queensland (7). The presence of T. suavissima on that continent was first documented during a collection expedition there in 1835 (8). In that report, the similarity of T. suavissima to other legume species of southern Europe was noted. Unlike its widely cultivated relative Medicago sativa L. (alfalfa or lucerne), which is native to western Asia and which can be nodulated by at least two Ensifer species, T. suavissima is more specific in its requirements for nitrogen-fixing microsymbionts (4, 9). Trigonella suavissima is also the only member of the tribe Trifolieae that is endemic to Australia (7). This observation and the observation that most other species of Trigonella are native to the Mediterranean region and western Asia (2, 10) suggest that T. suavissima may have been introduced into Australia from those regions. Although the rhizobial populations in Australia that nodulate T. suavissima have been characterized with regard to their abundance, symbiotic effectiveness, and host range (7), there is little information on the range and extent of their genetic diversity or their phylogenetic relatedness to Ensifer strains from other continents.
The objective of the current study was to assess the genetic diversity within a collection of 61 rhizobia isolated from 29 localities in southeastern Australia. Forty-seven of the strains were isolated from T. suavissima and 14 of the strains were isolated from M. sativa. Although both hosts are members of the same cross-inoculation group, the symbiotic effectiveness of a strain may differ between the host species. An additional 32 Ensifer sp. reference strains from other continents were included for genotype and phenotype comparisons. These comparisons were intended to aid in the taxonomic placement of the Australian T. suavissima rhizobial strains and to provide evidence as to whether they might be closely related to native rhizobial lineages found elsewhere. For the purposes of this study, a T. suavissima strain is defined as a rhizobial strain that either was isolated from a T. suavissima nodule or can fix nitrogen with T. suavissima but was isolated from a related legume species, such as M. sativa. In our comparative studies, we included representatives of three other Ensifer species (E. medicae, E. arboris, and E. numidicus) that were previously shown to be closely related to E. meliloti (11–13).
Currently, the two species of Ensifer having the greatest agricultural significance are E. meliloti and E. medicae (14), which are capable of symbiotic nitrogen fixation with a broad range of perennial and annual forage legumes, respectively (13). Their genomes consist primarily of a single circular chromosome of approximately 3.65 Mb and two large accessory replicons (or megaplasmids) (14, 15). Ensifer spp. may also contain smaller auxiliary plasmids, the number and identity of which vary widely among strains (16) and the functional importance of which is largely unknown. Of the two megaplasmids, one is referred to as a symbiotic megaplasmid and is approximately 1.3 Mb in size (pSymA and pSMED02 in E. meliloti and E. medicae, respectively). It carries genes important in the development and function of the nitrogen-fixing symbiosis. The other megaplasmid has been referred to as a chromid (17) and is approximately 1.6 Mb in size (pSymB and pSMED01 in E. meliloti and E. medicae, respectively). This replicon carries genes that are important for other accessory functions, including the import of small molecules and polysaccharide biosynthesis (15). For taxonomic placements, we relied primarily on comparisons of the chromosomal genotypes across the strains. However, we were also interested in the evolutionary histories of their large extrachromosomal replicons because of their agricultural and ecological significance.
In the first part of the study, chromosomal genotypic profiles of 56 T. suavissima strains were compared to those of related species through the use of a genomic indexing method called multilocus sequence typing (MLST) (18, 19). Previous whole-genome sequence analyses of diverse E. meliloti and E. medicae strains have shown that chromosomal MLST analyses can provide a robust description of the taxonomic boundaries between Ensifer species (20). In the second part of this study, we used a similar MLST approach to examine the range of megaplasmid genotypes in a subsample of the T. suavissima strains used for chromosomal profiling. In addition to the genotypic analyses, we also compared phenotypic characteristics of the strains to explore possible genotype-by-phenotype correlations.
RESULTS
Taxonomic placement of the Australian Trigonella strain USDA6670 (syn., CC2013).
To obtain a preliminary taxonomic placement for the Australian isolates, we compared the previously published nucleotide sequences of two evolutionarily conserved loci (the 16S rRNA gene and atpD) in T. suavissima USDA6670 with the homologous sequences in six Ensifer type strains (Table 1). The sequence comparisons revealed that the 16S and atpD alleles of USDA6670 are most similar to their respective homologs in E. meliloti USDA1002T and that the USDA6670 alleles are less similar to their respective homologs in E. numidicus and E. medicae. The corresponding alleles in the other species, particularly E. psoraleae and E. fredii, share the smallest number of nucleotides with their corresponding alleles in USDA6670.
TABLE 1.
Distance matrix showing the number of pairwise nucleotide sequence differences in atpD and 16S rRNA alleles between the Australian Trigonella suavissima strain USDA6670 (CC2013) and Ensifer (Sinorhizobium) type strains
| Ensifer species and strain | Strain designation | No. of sequence differences with strain designationa: |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| E. meliloti USDA6670b | 1 | 12 | 38 | 39 | 47 | 44 | 47 | |
| E. meliloti USDA1002T | 2 | 1 | 32 | 33 | 43 | 36 | 41 | |
| E. numidicus ORS1407T | 3 | 4 | 4 | 32 | 40 | 35 | 42 | |
| E. medicae A321T | 4 | 5 | 4 | 6 | 42 | 42 | 41 | |
| E. arboris HAMBI 1552T | 5 | 5 | 6 | 7 | 6 | 45 | 42 | |
| E. psoraleae CCBAU 65732T | 6 | 10 | 9 | 11 | 9 | 11 | 42 | |
| E. fredii ATCC 35243T | 7 | 15 | 10 | 17 | 18 | 18 | 23 | |
Differences are based on a 420-bp segment of atpD (shown in boldface type) and a 1,404-bp segment of the 16S rRNA gene (shown in lightface type). The accession numbers for the atpD/16S rRNA sequences for the type strains are AM418760/X67222 (E. meliloti), AM946551/AY500254 (E. numidicus), AM418754/L39882 (E. medicae), AM418767/Z78204 (E. arboris), EU617988/EU618039 (E. psoraleae), and AM418761/D14516 (E. fredii).
Homologous atpD/16S rRNA sequences were obtained from the GenBank draft genome sequence, accession number NZ_ATWE00000000.
Two criteria that are commonly used to determine species affiliations are average genomic nucleotide identity (ANI) and 16S rRNA gene sequence similarity. It has been estimated that most bacterial species encompass organisms that have an ANI of greater than 95% (21). This threshold has been shown to correspond to a level of 16S rRNA gene sequence similarity of approximately 98.7% (22). The percentage of sequence similarity between the 16S gene of USDA6670 and that of the E. meliloti type strain USDA1002T is greater than 99.9% (Table 1). A two-way ANI comparison was calculated for the draft genome sequence of USDA6670 and the chromosomal sequence of the E. meliloti genome reference strain USDA1021 (23). The comparison revealed a shared ANI of 95.2%. Thus, both the 16S rRNA gene sequence comparisons in Table 1 and the shared ANI estimate indicate that based solely on genotype, the Australian T. suavissima strain USDA6670 is either at or within the generally accepted species limits for E. meliloti.
Chromosomal genetic divergence and allelic diversity among the T. suavissima strains and related species.
The nucleotide sequences of 10 chromosomal loci in the T. suavissima strains and corresponding loci in the four related Ensifer species were screened for polymorphic nucleotide sequence positions. The results revealed that each locus was represented by multiple alleles. A comparison of the multilocus allelic profiles for each of the 84 strains revealed 61 distinct chromosomal sequence types, or CSTs (see Table S1 in the supplemental material). Table 2 lists the 61 CSTs, the 84 strains associated with each of the individual CSTs, the geographical origins of the strains, and the respective strain phenotypes. Fifty-six of the 61 CSTs were represented by only one or two strains, while the other five CSTs were represented by multiple strains of differing geographic origins. For example, two CSTs (CST 2 and CST 46) were represented both by a strain from Israel and by two strains from another country. In the case of CST 2, the two additional strains were from Australia, while in the case of CST 46, the two additional strains were from the United States.
TABLE 2.
CSTs, strain origins, and phenotypes of the 84 strains in the MLST analysis
| CSTa | Strain | Origin |
Symbiotic effectiveness |
2-oxo growthf | Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Countryb | State | Locality | Year | Trap hostc | Tsd | Mse | ||||
| 1 | CC2137 | Au | NSW | Bootingeeg | 1960 | Ts | NDh | I | + | |
| 2 | CC2283c | Is | NAi | Tel Sharuhen | 1965 | Ta | I, E | I | +/− | 9 |
| CC5033 | Au | NSW | North Bourke | 2004 | Ts | I/E | I/E | + | ||
| CC5043 | Au | Qld | Jackson | 2004 | Ts | I/E | I | + | ||
| 3 | CC2155b | Au | Qld | Malagargag | 1963 | Ts | E | I | + | 6, 7 |
| 4 | CC2129 | Au | Qld | Wyandra | 1960 | Ts | ND | I | + | 6 |
| 5 | CC2341 | Au | Qld | Currawillag | ND | Ts | E | I | ND | |
| 6 | CC5015 | Au | SA | Etadunnag | 1999 | Ts | ND | ND | + | |
| CC5022 | Au | NSW | Cuttabura Creek | 2000 | Ts | ND | ND | + | ||
| CC5035 | Au | Qld | Birdsville | 2004 | Ts | ND | ND | + | ||
| CC5036 | Au | Qld | Birdsville | 2004 | Ts | ND | ND | + | ||
| 7 | CC5019 | Au | NSW | Louth | 2000 | Ts | ND | ND | + | |
| 8 | CC5021 | Au | NSW | Cuttaburra Creek | 2000 | Ts | ND | ND | + | |
| 9 | CC5038 | Au | Qld | Innamincka stationg | 2004 | Ts | ND | ND | + | |
| CC5049 | Au | Qld | Birdsville | 1999 | Ts | ND | ND | 0 | ||
| 10 | CC5037 | Au | Qld | Birdsville | 2004 | Ts | E | I/E | + | |
| CC5040 | Au | Qld | Innamincka stationg | 2004 | Ts | ND | ND | + | ||
| 11 | CC5039 | Au | Qld | Innamincka stationg | 2004 | Ts | ND | ND | ND | |
| 12 | CC5044 | Au | Qld | Jackson | 2004 | Ts | ND | ND | + | |
| 13 | CC5053 | Au | NSW | Menindee | 2000 | Ts | ND | ND | + | |
| 14 | CC5031 | Au | NSW | North Bourke | 2004 | Ts | ND | ND | + | |
| 15 | CC5032 | Au | NSW | North Bourke | 2004 | Ts | ND | ND | + | |
| 16 | CC5028 | Au | NSW | Menindee | 2000 | Ts | ND | ND | + | |
| 17 | CC5034 | Au | Qld | Glengyleg | 2004 | Ts | ND | ND | 0 | |
| 18 | CC2325 | Au | NSW | Fords Bridge | 1995 | Ts | I/E | I/E | +/− | |
| CC5020 | Au | NSW | Louth | 2000 | Ts | ND | ND | + | ||
| CC5027 | Au | SA | Marree | 2000 | Ts | ND | ND | + | ||
| CC5029 | Au | NSW | Bourke | 2004 | Ts | ND | ND | + | ||
| CC5030 | Au | NSW | Bourke | 2004 | Ts | ND | ND | +/− | ||
| 19 | CC5045 | Au | NSW | Wilcannia | 2004 | Ts | ND | ND | + | |
| 20 | CC5024 | Au | SA | Margaret Creek | 2000 | Ts | ND | ND | + | |
| 21 | CC2324 | Au | NSW | North Bourke | 1995 | Ts | ND | I | + | |
| 22 | CC2160 | Au | NSW | Birriegoolpa Bore | 1967 | Ts | E | I | + | 5, 6, 7 |
| 23 | CC5023 | Au | SA | Strzelecki Creek | 2000 | Ts | ND | ND | + | |
| 24 | CC5026 | Au | SA | Marree | 2000 | Ts | ND | ND | + | |
| 25 | CC2017 | Au | Qld | Roma | 1958 | Ms | E | E | + | 4, 7, 9 |
| 26 | CC5016 | Au | Qld | Marion Downsg | 1999 | Ts | ND | ND | 0 | |
| CC5017 | Au | Qld | Marion Downsg | 1999 | Ts | ND | ND | 0 | ||
| 27 | CC2154 | Au | Qld | Malagargag | 1960 | Ts | E | I/E | + | 7, 9 |
| 28 | CC5006 | Au | NSW | Menindee | 2006 | Ts | E | I/E | + | 7 |
| 29 | CC2153 | Au | Qld | Malagargag | 1960 | Ts | E | I | + | 5, 7 |
| CC2156b | Au | Qld | Malagargag | 1963 | Ts | E | I | + | 7 | |
| 30 | USDA6670 (CC2013) | Au | NSW | Moonie River | 1966 | Ms | E | E | + | 4, 5 |
| 31 | CC2157 | Au | NSW | Teurikag | 1967 | Ts | I, I/E | I, I/E | + | 5, 7 |
| CC2152 | Au | Qld | Malagargag | 1960 | Ms | E | I | +/− | 7 | |
| 32 | CC2326 | Au | Qld | Windorah | 1995 | Ts | ND | I | + | |
| 33 | CC5011 | Au | NSW | Lake Tandou | 2006 | Ms | E | I | + | 7 |
| 34 | CC5002 | Au | NSW | Lake Tandou | 2006 | Ms | I/E | I | + | 7 |
| CC5003 | Au | NSW | Lake Tandou | 2006 | Ts | E | I | + | 7 | |
| 35 | CC5009 | Au | NSW | Lake Tandou | 2006 | Ms | I/E | I | + | 7 |
| 36 | CC5025 | Au | SA | Margaret Creek | 2000 | Ts | ND | ND | + | |
| 37 | CC5001 | Au | NSW | Lake Tandou | 2006 | Ts | I/E | I | + | 7 |
| 38 | CC5007 | Au | NSW | Lake Tandou | 2006 | Ms | E | I | + | 7 |
| 39 | CC5008 | Au | NSW | Lake Tandou | 2006 | Ms | E | I | + | 7 |
| 40 | 102F51 | US | ND | ND | 1927 | Ms | ND | E | 0 | 3, 18 |
| 41 | CC5012 | Au | NSW | Menindee | 2006 | Ms | I | E | 0 | 7 |
| 42 | 15A6 | Pa | NA | ND | ND | Ms | ND | E | 0 | 3, 18 |
| 43 | 15B4 | Pa | NA | ND | ND | Ms | ND | ND | 0 | 3, 18 |
| 44 | 74B12 | Pa | NA | ND | ND | Ms | ND | ND | 0 | 3, 18 |
| 45 | CC5052 | Au | NSW | Wentworth | 2000 | Ts | ND | ND | 0 | |
| 46 | CC2282b | Is | NA | Shikmaa | 1965 | Ta | I | I | 0 | 9 |
| USDA1114 | US | CA | ND | ND | Tb | ND | ND | 0 | 6 | |
| USDA1115 | US | CA | ND | ND | Tc | ND | ND | 0 | 6 | |
| 47 | M119 | Sy | NA | ND | ND | Mp | ND | ND | 0 | 3, 18 |
| 48 | CC164 | ND | NA | ND | ND | ND | ND | ND | 0 | |
| CC2019 | Au | Qld | Bollon | 1958 | Ms | I | E | 0 | 4, 7, 9 | |
| CC2053 | Au | Qld | Mitchell | 1958 | Ms | I | E | +/− | 4, 7, 9 | |
| CC5013 | Au | NSW | Menindee | 2006 | Ms | I | E | 0 | 7 | |
| USDA1176 | ND | NA | NA | ND | ND | ND | ND | 0 | ||
| USDA1177 | US | ND | NA | ND | Tf | ND | ND | 0 | 6 | |
| CC2003 | Au | Qld | Narromine | 1958 | Ms | I | I | 0 | 4, 7, 18, 19, 26 | |
| 49 | CC5010 | Au | NSW | Lake Tandou | 2006 | Ms | I | I/E | 0 | 7 |
| 50 | USDA1002T | US | VA | Washington, DC | 1919 | Ms | ND | E | 0 | 3, 18, 19 |
| 51 | LMG 14919T | Su | NA | Kosti | 1987 | Pc | ND | ND | + | 29, 30 |
| 52 | M254 | Jo | NA | ND | ND | Mr | ND | ND | 0 | 3, 18 |
| 53 | CC5051 | Au | NSW | Wentworth | 2000 | Ts | ND | ND | 0 | |
| 54 | A321T | Fr | NA | Aude | ND | Mt | ND | ND | 0 | 13 |
| 55 | M3 | Sy | NA | ND | ND | Mo | ND | ND | 0 | 3, 18 |
| 56 | M102 | Sy | NA | ND | ND | Mt | ND | ND | 0 | 3, 18 |
| 57 | M161 | Sy | NA | ND | ND | Mn | ND | ND | 0 | 3, 18 |
| 58 | M205 | Tu | NA | ND | ND | Mt | ND | ND | 0 | 3, 18 |
| 59 | M1 | Sy | NA | ND | ND | Mo | ND | ND | 0 | 3, 18 |
| 60 | M280 | Jo | NA | ND | ND | Mo | ND | ND | 0 | 3, 18 |
| 61 | LMG 24690T (ORS 1407) | Tu | NA | Infra-arid zone | ND | Au | ND | ND | 0 | 12, 31 |
CST, multilocus chromosomal sequence type. The corresponding allelic profiles are listed in Table S1 in the supplemental material.
Au, Australia; Is, Israel; Fr, France; Jo, Jordan; Pa, Pakistan; Sy, Syria; Su, Sudan; Tu, Tunisia; US, United States.
Au, Argyrolobium uniflorum; Mn, Medicago noeana; Mo, M. orbicularis; Mp, Medicago spp.; Mr, M. rotata; Mt, M. truncatula; Ms, M. sativa; Pc, Prosopis chilensis; Ta, Trigonella arabica; Tb, T. balansae; Tc, T. corniculata; Tf, T. foenum-graecum; Ts, T. suavissima.
Symbiotic N-fixing effectiveness with T. suavissima: I, ineffective; I/E, partly effective; E, effective.
Symbiotic N-fixing effectiveness with M. sativa: I, ineffective; I/E, partly effective; E, effective.
Ability to utilize 2-oxoglutarate (alpha-ketoglutarate) as a sole carbon source after 72h. +, strong growth; +/−, moderate-to-weak growth; 0, poor growth.
Name of the property from which the strain originated.
ND, not determined.
NA, not applicable.
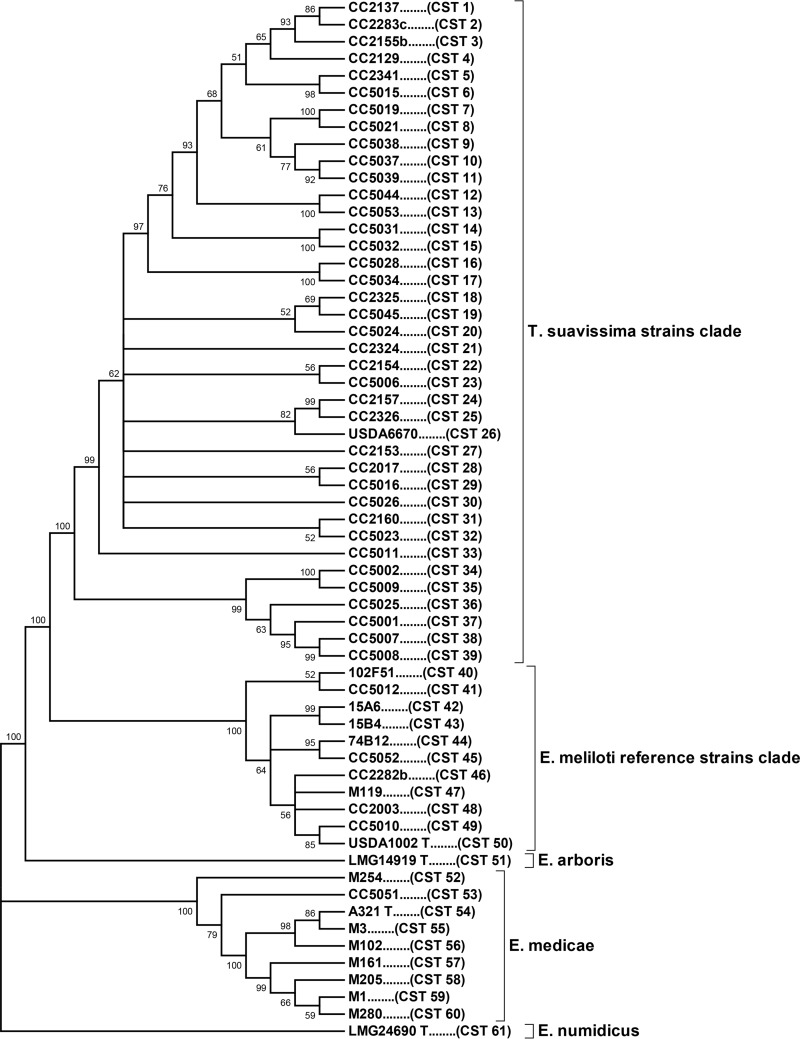
Nucleotide sequence-based phylogenetic analysis of the 61 CSTs by the maximum likelihood (ML) method revealed that the CSTs were clustered on four primary branches in an ML tree. Each of these branches corresponds to a named Ensifer species (Fig. 1). The largest cluster, consisting of CST 1 through CST 50, will be referred to as the E. meliloti cluster. The E. meliloti cluster is subdivided into two distinct clades, the T. suavissima strain clade (CST 1 through CST 39) and the E. meliloti reference strain clade (CST 40 through CST 50). With one exception, the 54 strains in the T. suavissima strain clade are of Australian origin. The single exception is strain CC2283c (CST 2), a strain isolated from T. arabica in Israel in 1965. That particular CST is also shared by two T. suavissima strains that were isolated from field soils in Australia (Table 2). The E. meliloti reference strain clade, which is also within the E. meliloti cluster, contains 11 CSTs that represent seven Australian field soil isolates and 12 reference strains from other countries (Table 2). The second largest cluster, which will be referred to as the E. medicae cluster (Fig. 1), consists of nine CSTs representing nine diverse E. medicae strains (CST 52 through CST 60). Only one of these strains (CC5051) was isolated from Australian soils, while the other seven were isolated from locations in the Mediterranean region (3, 13). The two remaining major branches in Fig. 1 represent the type strains for E. arboris (CST 51) and E. numidicus (CST 61), which were isolated from soils in Sudan and Tunisia, respectively.
FIG 1.
Chromosomal maximum likelihood tree based on concatenated nucleotide sequences of 10 loci in 84 strains. Alignment of the sequences revealed 61 distinct chromosomal sequence types (CSTs); one representative strain for each CST is shown. Bootstrap values of greater than 50% (of 500 replicates) are indicated at nodes. T., Trigonella; E., Ensifer.
The numbers of alleles observed for each locus within the T. suavissima strain clade (CST 1 through CST 39) are summarized in Table 3. The number of alleles per locus ranged from seven for nuoE1 to 17 for edd, with an average of 12.4 alleles per locus. The corresponding estimates of allelic diversity reported previously for a global collection of 230 non-Australian Medicago sp. Ensifer strains (18) are also shown in Table 3. It is evident that the numbers of alleles observed for most of the T. suavissima strain loci are roughly equivalent to, and in some cases greater than, the numbers of alleles observed for the corresponding loci in the global collection of Medicago sp. Ensifer strains. It is also evident that only a small number of alleles are shared between the two geographically divergent groups of strains. The extensive allelic diversity among the loci of the T. suavissima strains is noteworthy, considering that the corresponding data for the global collection of Medicago sp. Ensifer strains were obtained from a much larger number of strains, collectively representing two genetically divergent species of Ensifer (E. meliloti and E. medicae) (Fig. 1). Furthermore, the global strains were isolated from a wide variety of geographic locations, primarily from the Mediterranean Basin and western Asia.
TABLE 3.
The number of distinct Ensifer alleles according to strain geographic origin and trap host
| Locus | No. of alleles for: |
No. alleles shared/total | |
|---|---|---|---|
| Australian T. suavissima strain CSTsa | Global (non-Australian) Medicago sp. CSTsb | ||
| asd | 14 | 15 | 0/29 |
| edd | 17 | 17 | 2/32 |
| zwf | 16 | 9 | 0/25 |
| gap | 14 | 8 | 1/21 |
| glnD | 12 | 9 | 0/21 |
| gnd | 12 | 7 | 1/18 |
| nuoE1 | 7 | 11 | 1/17 |
| ordL2 | 11 | 8 | 0/19 |
| recA | 8 | 9 | 0/17 |
| sucA | 13 | 11 | 0/24 |
| Average = 12.4 | Average = 10.4 | Sum = 5/223 | |
Thirty-nine chromosomal sequence types (CSTs) reported for the 54 strains in the current study.
Ninety CSTs reported for 230 Medicago sp. strains from other continents in a previous study (18).
Estimated phylogenetic relationships among the extrachromosomal replicons.
The phylogenetic relationships among the extrachromosomal replicons in four T. suavissima strains and in 18 reference strains were also estimated using the MLST method. The nucleotide sequences of three concatenated chromid loci and three concatenated symbiotic megaplasmid loci were aligned for these comparisons. The multilocus sequence profiles for each of the replicons in the 22 strains revealed 16 distinct sequence types (STs) for their chromids and 20 distinct STs for the symbiotic megaplasmids in the same strains (Table 4). Some strains share the same ST for one extrachromosomal replicon but not the other (e.g., E. meliloti strains 15B4 and 74B12), while other strains share STs for both extrachromosomal replicons (e.g., E. medicae strains M254 and A321T) but do not share their chromosomal profiles (e.g., CST 52 and CST 54, respectively). Also, in several cases, the same chromid ST was shared across multiple strains having differing chromosomal genotypes. For example, the E. medicae reference strains A321T, M3, M102, M161, and M254, which were chosen to represent the range of chromosomal genotypes identified in a previous study (18), all have the same chromid ST but have different chromosomal STs. The same is true for the E. meliloti reference strains 102F51, CC2003, and 74B12.
TABLE 4.
Extrachromosomal replicon sequence types for 22 strains representing four closely related species of Ensifer (Sinorhizobium)
| Species | Strain | Chromid locusa |
Symbiotic megaplasmid locusb |
||||
|---|---|---|---|---|---|---|---|
| dak | gabTc | idhA | nifD | nodCc | sma0198 | ||
| E. meliloti | USDA1002T | 1 | 1 | 10 | 1 | 3 | 13 |
| 102F51 | 1 | 1 | 11 | 9 | 3 | 32 | |
| CC2003 | 1 | 1 | 11 | 15 | 32 | 3 | |
| 15A6 | 5 | 1 | 4 | 3 | 4 | 14 | |
| 15B4 | 1 | 4 | 10 | 3 | 4 | 6 | |
| M119 | 3 | 1 | 11 | 12 | 15 | 4 | |
| 74B12 | 1 | 1 | 11 | 3 | 4 | 6 | |
| CC5052 | 19 | 6 | 11 | 2 | 22 | 3 | |
| USDA6670d | 9 | 10 | 25 | 6 | 7 | 33 | |
| CC2155bd | 20 | 25 | 28 | 6 | 7 | 34 | |
| CC5011d | 21 | 26 | 29 | 51 | 7 | 33 | |
| CC5034d | 22 | 10 | 30 | 6 | 7 | 3 | |
| E. medicae | A321T | 2 | 2 | 2 | 2 | 2 | 2 |
| M1 | 2 | 9 | 2 | 7 | 11 | 18 | |
| M3 | 2 | 2 | 2 | 2 | 11 | 2 | |
| M102 | 2 | 2 | 2 | 2 | 11 | 20 | |
| M161 | 2 | 2 | 2 | 17 | 17 | 31 | |
| M205 | 16 | 15 | 24 | 22 | 2 | 22 | |
| M254 | 2 | 2 | 2 | 2 | 2 | 2 | |
| M280 | 2 | 20 | 2 | 7 | 2 | 2 | |
| E. arboris | LMG 14949T | 23 | 27 | 31 | 52 | 52 | 35 |
| E. numidicus | LMG 24690T | 24 | 28 | 32 | 53 | 53 | 36 |
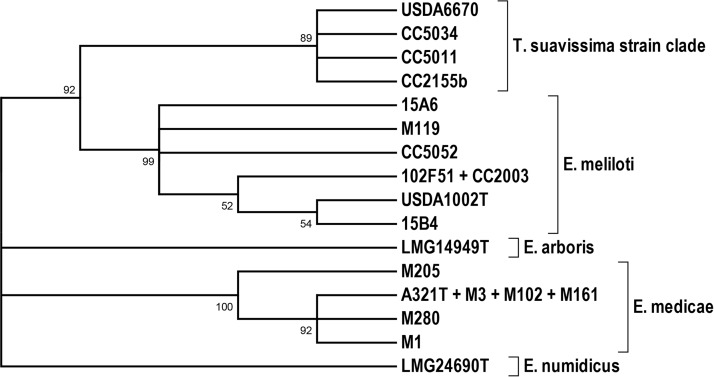
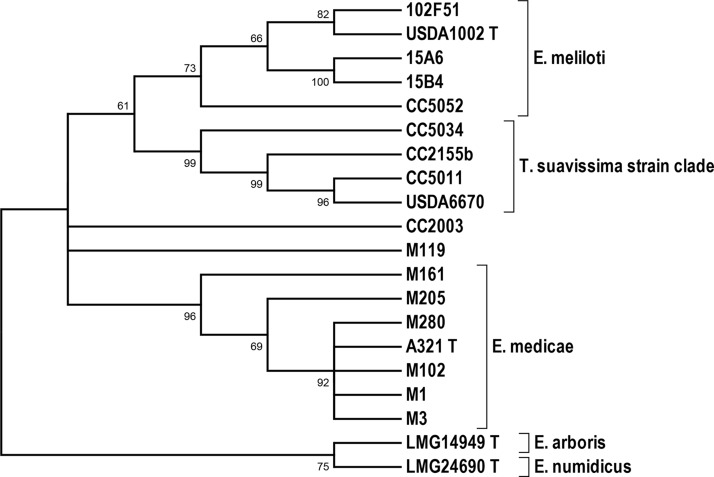
The phylogenetic relationships among the extrachromosomal replicons in the 22 strains were also estimated using the maximum likelihood (ML) method. Three-locus concatenated sequences were aligned and compared for each of the replicons in each of the strains. An ML tree illustrating the relationships among the chromid replicons is shown in Fig. 2, and a corresponding ML tree illustrating the relationships among the symbiotic megaplasmid replicons is shown in Fig. 3. Assuming that the strains of the T. suavissima clade are indeed conspecific with E. meliloti, then a species-level congruence is evident between the topology of the chromosomal tree (Fig. 1) and the topology of the chromid tree (Fig. 2). However, in the symbiotic megaplasmid ML tree, the divergence between the E. meliloti STs and the E. medicae STs is not well supported (Fig. 3).
FIG 2.
Chromid maximum likelihood tree based on concatenated nucleotide sequences of three loci in 22 strains (listed in Table 4). Alignment of the sequences revealed 16 distinct sequence types; representative strains for each sequence type are shown. Bootstrap values of greater than 50% (of 500 replicates) are indicated at nodes. T., Trigonella; E., Ensifer.
FIG 3.
Symbiotic megaplasmid maximum likelihood tree based on concatenated nucleotide sequences of three loci in 22 strains (listed in Table 4). Alignment of the sequences revealed 20 distinct sequence types; representative strains for each sequence type are shown. Bootstrap values of greater than 50% (of 500 replicates) are indicated at nodes. T., Trigonella; E., Ensifer.
Phenotypic characteristics of the T. suavissima strains.
Transmission electron micrographs of the Australian T. suavissima strains CC2017 and CC2155b are shown in Fig. S1 and S2 in the supplemental material, respectively. The ultrastructural characteristics of the cells, including their size, shape, flagellation, and budding morphology, are consistent with the characteristics normally associated with other members of the genus Ensifer (Sinorhizobium) (24).
The symbiotic nitrogen-fixing effectiveness ratings for 30 of the strains on T. suavissima and M. sativa are listed in Table 2. These results were compiled from the results of the current study (see Table S3) and also from the results from three previous studies (4, 7, 9). In general, members of the T. suavissima strain clade (CST 1 through CST 39) are symbiotically more effective on T. suavissima than they are on M. sativa (Table 2). Conversely, members of the E. meliloti reference strain clade (CST 40 through CST 50) are generally more effective on M. sativa than they are on T. suavissima (Table 2).
Trigonella anguina was included as a potential host in the current effectiveness study because it is a sister species to T. suavissima (25) and it was unclear whether the T. suavissima strains would be symbiotically effective on T. anguina. The results shown in Table S2 indicate that most of the strains that are symbiotically effective on T. suavissima are also effective on its sister species, T. anguina.
The four strains with the consistently highest levels of symbiotic effectiveness with T. suavissima are strains CC2017, CC2155b, USDA6670, and CC5037 (Table 2; see also Table S2). The symbiotic effectiveness results compiled in Table 2 also revealed that different strains sharing the same CST (e.g., CST 2 strains and CST 31 strains) may express significantly different levels of symbiotic effectiveness on the same host.
The results of the initial comparative biochemical profiling of three of the T. suavissima strains (CC2017, CC2155b, and USDA6670), two of the E. meliloti reference strains (USDA1002T and USDA1021), and two of the E. medicae reference strains (A321T and CC169) revealed that the only biochemical test that consistently differentiated the T. suavissima strains from the other strains was that which showed their ability to utilize 2-oxoglutarate (α ketoglutarate) as a sole carbon source. Neither the two E. meliloti reference strains nor the two E. medicae reference strains possessed this ability.
To substantiate this observation, an expanded study was initiated to examine the ability of all the strains in the collection to utilize 2-oxoglutarate as a sole carbon source. Of the 54 strains in the T. suavissima strain clade (CST 1 through CST 39), 44 showed strong growth on 2-oxoglutarate, 4 showed moderate-to-weak growth, and four showed poor growth (Table 2). The only other strain in the collection showing strong growth on this carbohydrate was the type strain for E. arboris (LMG 14949T). Two other strains in the collection also showed moderate-to-weak growth on 2-oxoglutarate; these were E. kostiense (HAMBI 1489T) and one of the strains in the E. meliloti strain clade (CC2053). Although the latter strain is not symbiotically effective with T. suavissima (Table 2), it is effective on two other species of Trigonella (7, 9). None of the nine representatives of the E. medicae strain cluster (CST 52 through CST 60) were able to grow on the 2-oxoglutarate broth media nor were any of the additional Ensifer type strains that were examined, which included E. adhaerens (ATCC 33499T), E. americanum (CFNEI 156T), E. chiapanecum (IITG S70T), E. fredii (USDA205T), E. mexicanus (IITG R7T), E. adhaerens (Lc04T), E. numidicus (LMG 24690T), E. saheli (HAMBI 215T), E. terangae (HAMBI 220T), and E. xingiangensis (CCBAU 110T). To determine whether the failure of the strains to grow on 2-oxoglutarate may have been due to an inhibitory effect of that compound on growth, the strains were also grown on 2-oxoglutarate medium supplemented with 5 g liter−1 glycerol. The additional carbon source resulted in normal growth of the strains (data not shown), suggesting that their poor growth on 2-oxoglutarate was probably not due to an inhibitory effect of the 2-oxoglutarate.
Since the locus encoding 2-oxoglutarate dehydrogenase (sucA) was sequenced in all of the strains in the chromosomal MLST study, it was of interest to determine if there was a correlation between the ability of certain strains to utilize 2-oxoglutarate and the specific sucA alleles in those strains. No such correlation was evident, other than the fact that the T. suavissima strains, most of which could grow on 2-oxoglutarate, did not share sucA alleles with any of the other strains in the study (Table S1).
DISCUSSION
One of the primary goals of this study was to provide a taxonomic placement for the group of Australian T. suavissima microsymbionts that are represented by the strain USDA6670 (CC2013). In a previous study which examined the abundance, symbiotic effectiveness, and host range of native populations of rhizobia nodulating T. suavissima (7), it was postulated that these rhizobia may be members of a distinct taxon within the genus Ensifer. Our initial results, which were based on pairwise 16S RNA gene and atpD nucleotide sequence comparisons and also on a comparative ANI analysis, suggested that USDA6670 is conspecific with the type strain of E. meliloti. The subsequent results of our population-scale chromosomal MLST analyses supported this placement; however, they also indicated that the T. suavissima strains and the E. meliloti reference strains each constitute monophyletic clades within the species E. meliloti. The results also confirmed that E. meliloti is clearly differentiated from the other closely related species of Ensifer (i.e., E. medicae, E. arboris, and E. numidicus).
The distinctiveness of the strains in the T. suavissima clade was substantiated by the fact that only five of the 228 allelic variants within the T. suavissima strain CSTs were found within the CSTs representing a global collection of 230 E. meliloti and E. medicae reference strains. The distinctiveness of the T. suavissima strains is also supported by the monophyletic clustering of the chromid STs of four representative T. suavissima strains (Fig. 2). The topological congruence between chromosomal and chromid ML trees is perhaps not surprising, considering that chromids usually share intrinsic characteristics (e.g., codon usage patterns) with the chromosomes of the cells in which they reside (17). By contrast, the topology of the symbiotic megaplasmid tree differed somewhat from those of the chromosomal and chromid trees, in that two of the E. meliloti genotypes were dispersed on divergent nodes of the symbiotic megaplasmid tree, and that these nodes received little bootstrap support (Fig. 3). Previous studies have shown that certain loci (e.g., nod genes) that are mapped to the symbiotic megaplasmids in E. meliloti often have complex evolutionary histories, apparently because of frequent horizontal transfer and recombination (19, 20, 26).
In addition to their distinct multilocus genotypes, the T. suavissima strains also possess some significant differences in phenotype compared with those of most other Ensifer strains. For example, the results of the symbiotic nitrogen-fixation effectiveness tests revealed that, even though the T. suavissima strains are capable of nodulation and nitrogen fixation with M. sativa, they are generally more symbiotically effective with T. suavissima. Their modest symbiotic effectiveness with M. sativa might be an important agronomic consideration if these strains are shown to be competitive for nodulation in soils where alfalfa is grown.
The initial metabolic and biochemical screening of three of the T. suavissima strains revealed that, unlike the E. meliloti and E. medicae reference strains, the T. suavissima strains were able to utilize 2-oxoglutarate (α-ketoglutarate) as a sole carbon source. When the entire collection of T. suavissima strains, reference strains, and type strains were screened for this phenotype, 48 of the 54 strains in the T. suavissima strain clade were able to grow on 2-oxoglutarate as a sole carbon source, while only two of the (twelve) more divergent Ensifer type strains (E. arboris and E. kostiense) were able to do so. Furthermore, only a single strain in the E. meliloti clade possessed this phenotype. Although that particular strain is symbiotically ineffective with T. suavissima (7, 9) and only marginally effective with M. sativa (4), it is effective with two other species of Trigonella (9). The presence of the 2-oxoglutarate utilization phenotype in three of the more divergent species examined (E. meliloti, E. arboris, and E. kostiense) suggests that this may be an ancestral trait among members of the genus Ensifer.
The limited natural distribution of T. suavissima and its symbionts to remote parts of the Australian continent provides an opportunity to address some biogeographical questions on the evolution of the T. suavissima-Ensifer symbiosis. For example, did both symbionts coevolve in isolation on the continent, and if so, for how long? Since most species of the genus Trigonella are native to the Mediterranean region and to western Asia (2, 10), and since there are no other members of the family Trifolieae that are native to Australia, it would seem reasonable to suggest that the early ancestors of T. suavissima may have been introduced into Australia from the Mediterranean region or western Asia. Indeed, the similarity of T. suavissima to southern European legumes was first noted in the initial description of the species in 1835 (8).
Whether the rhizobial symbionts of T. suavissima may have originated elsewhere is still open to question. However, our observation of the extensive allelic diversity within the Australian T. suavissima strains, together with our observation of only very limited allele sharing between the T. suavissima strain CSTs and those observed in a global Medicago sp. reference collection, would argue against the proposition that T. suavissima strains likely share a very recent common ancestor with strains from the Mediterranean region or western Asia. However, on the contrary, one might reasonably question whether the reference collection in the above comparison can be expected to reflect the full range of allelic diversity in E. meliloti. Furthermore, it should be noted that in the development of the global collection, no Trigonella species were used as trap hosts. Although species of Medicago and Trigonella are both members of the same cross-inoculation group (27), the validity of this group has been questioned (28). Thus, it is possible that the Medicago species used as trap hosts in the development of the global reference collection may have biased its composition.
If indigenous populations of rhizobia that are closely related to the Australian T. suavissima strains do indeed exist naturally in soils outside Australia, then a more targeted isolation strategy might be necessary to expose them. One approach might be to sample field sites at locations where large natural populations of T. anguina and other close relatives of T. suavissima are known to exist. To avoid any potential trap host bias, it would be advisable to rely on T. suavissima as the primary trap host. Subsequent screening and identification of T. suavissima-like strains from among the resulting nodule isolates could be facilitated using a two-step approach, involving an initial 2-oxoglutarate enrichment screening, followed by a PCR-restriction fragment length polymorphism (RFLP) screening of the 2-oxoglutarate-positive strains. Diagnostic restriction sites for the latter could be identified among the allelic sequences reported in this study.
MATERIALS AND METHODS
Bacterial strains, DNA samples, and PCR.
In this study, a total of 84 strains were examined, 64 of which were from the personal collection of J. Brockwell. The latter strains were isolated mostly from T. suavissima trap hosts and several M. sativa trap hosts grown in Australian soils (Table 2). Of the remaining 20 strains, 10 were E. meliloti reference strains, eight were E. medicae reference strains, and the type strains for E. arboris and E. numidicus were also included (18, 19, 26, 29–31). The reference strains were chosen to represent the range of chromosomal genetic diversity revealed in previous studies examining chromosomal genotypes of E. meliloti and E. medicae strains from geographically diverse locations (3, 13, 18). Prior to DNA extraction, all strains were plated to check for purity and then were grown for 3 days in modified arabinose-gluconate broth (32). Genomic DNAs were extracted using a QIAamp DNeasy minikit (Qiagen, Valencia, CA). DNA samples were stored at −20°C and the parent cultures were maintained in glycerol suspensions at −70°C.
Loci to be sequenced for chromosomal MLST were selected by referring to the chromosome sequence of E. meliloti strain USDA1021 (23). Ten loci distributed around the chromosome of USDA1021 were selected for PCR amplification and sequencing (18). The entire open reading frame for each locus was examined to aid in the design of primers that would amplify a portion of the gene, ranging in size from 200 to 500 bp. Five loci were chosen to represent the first half of the E. meliloti chromosome (USDA1021 genome annotation positions 1 to 1,735,000), which contains a higher degree of nucleotide diversity and a more positive GC skew (20). These loci, their products, and the respective fragment sizes that were selected for comparison were as follows: edd, phosphogluconate dehydratase, 501 bp; glnD, protein-PII uridylyltransferase, 309 bp; nuoE1, NADH dehydrogenase I chain E protein, 222 bp; ordL2, putative oxidoreductase protein, 432 bp; and zwf, glucose-6-phosphate 1-dehydrogenase, 453 bp. Five additional loci were selected to represent the second half of the E. meliloti chromosome (USDA1021 genome annotation positions 1,735,000 to 3,654,135), which contains a lower degree of nucleotide diversity and a more negative GC skew (20). These loci, their products, and the respective fragment sizes were as follows: asd, aspartate-semialdehyde dehydrogenase, 456 bp; gap, glyceraldehyde 3-phosphate dehydrogenase, 414 bp; gnd, 6-phosphogluconate dehydrogenase, 303 bp; recA, DNA strand exchange and recombination protein, 327 bp; and sucA, α-ketoglutarate (2-oxoglutarate) dehydrogenase E1, 429 bp.
Loci to be sequenced for the symbiotic megaplasmid MLST analyses were also selected by referring to the genome sequence of E. meliloti strain USDA1021 (15). The three pSymA loci, their products, and the respective fragment sizes were as follows: nifD, nitrogenase molybdenum-iron protein alpha chain, 420 bp; nodC, acetylglucosaminyltransferase, 348 bp; and SMa0198, ABC transporter permease, 312 bp (19). Similarly, three chromid (pSymB) loci were selected for amplification and analysis. These loci, their products, and the respective fragment sizes were as follows: dak, glycerone kinase, 465 bp; gabT, 4-aminobutyrate aminotransferase, 522 bp; and idh, myo-inositol dehydrogenase, 528 bp.
The details describing the PCR primer sequences and amplification reactions are described in detail elsewhere (18, 19). Briefly, amplification conditions were optimized using the FailSafe PCR PreMix selection kit protocol and reagents (Epicentre, Madison, WI). The PCR thermocycling reaction mixtures were incubated in an MJ Research PTC-225 Peltier thermal cycler (MJ Research, Waltham, MA). Following amplification, the PCR products were purified to remove PCR primers using the AMPure PCR purification system (Agincourt Bioscience Corp., Beverly, MA).
DNA sequences and data analyses.
Initial estimates of the phylogenetic relatedness between the representative T. suavissima strain USDA6670 and several Ensifer type strains were obtained through pairwise comparisons of published sequences for two conserved “housekeeping” loci (the 16S rRNA gene and atpD). The six type strains that were included in these initial comparisons were chosen on the basis of previous studies indicating their close phylogenetic relatedness to E. meliloti (11, 12, 31, 33–35). Pairwise similarity matrices were developed for each locus using published sequences that were aligned using the program Muscle, as implemented in MEGA v. 6.0 (36). The numbers of sequence differences were determined for a 1,404-bp core segment of the 16S rRNA locus and for a 420-bp segment of the atpD locus. The initial relatedness estimates obtained through these comparisons were then used as a guide to select four reference species for the more in-depth MLST analyses. In a separate comparison, the average nucleotide identity (ANI) between the chromosome of E. meliloti genome reference strain USDA1021 (accession no. AL591688) and the draft genome sequence of the T. suavissima strain USDA6670 (DOE JGI GEBA Root Nodulating Bacteria project, identification [ID] Ga0002169, accession no. ATWE01) was calculated (37).
The multilocus allelic profiles and corresponding nucleotide sequences for the E. meliloti and E. medicae reference strains in this study are described elsewhere (18, 19). To obtain sequences for the MLST alleles in the Trigonella strains and the other reference strains in the MLST analysis, purified PCR product templates were sequenced using nested PCR primers in both forward and reverse directions. Sequencing reactions were performed using an Applied Biosystems Dye Deoxy terminator cycle sequencing kit in conjunction with an Applied Biosystems 3130 genetic analyzer (Applied Biosystems, Foster City, CA). Homologous chromosomal and extrachromosomal allelic sequences for E. arboris LMG 14919T, E. meliloti USDA6670, and E. numidicus LMG 27395T were obtained by a BLAST search of their respective draft genome sequences. Draft sequences for the E. numidicus reference strain LMG 27395T were obtained through a commercial sequencing lab (Molecular Research LP, Shallowater, TX). Briefly, a library pool for strain LMG 27395T was paired-end sequenced for 300 cycles using the HiSeq 2500 system. Resulting fragments were assembled into 56 contigs.
A Microsoft Access database was created to compile the MLST sequences from the various sources. The strategy for the design and manipulation of the database is described elsewhere (18). Briefly, the different alleles were identified using the program Sequence Comparator version 2.0.1 written by Keith Jolley. Alleles from previous studies had already been assigned identification number codes. Alleles that were identified in this study were assigned new allele identification number codes. The number codes for all of the chromosomal alleles included in the study are listed in Table S1 in the supplemental material. Similarly, the number codes for all of the extrachromosomal replicon alleles are listed in Table 4. For each replicon in each strain, a composite multilocus profile was developed. Strains possessing the same multilocus profile for a given replicon were assigned to the same sequence type (ST) for that particular replicon.
To estimate the phylogenetic relatedness between homologous replicons, the allelic sequences for the loci within each ST were aligned and the alleles were concatenated. To estimate chromosomal phylogenetic relatedness through the use of the concatenated sequences, the maximum likelihood (ML) method was implemented using MEGA 6.0 (36). Model selection was facilitated using the corrected Akaike information criterion. The best fits for the chromosomal data were obtained using the Tamura 3-parameter model of DNA substitutions with gamma-distributed rate variations among sites, under the assumption that a certain fraction of sites were evolutionarily invariable (T92 + G + I). The best fits for the chromid and symbiotic megaplasmid data were obtained using the Tamura 3-parameter model of DNA substitutions with gamma-distributed rate variations among sites (T92 + G). The final trees were obtained using the ML heuristic method employing the nearest-neighbor-interchange tree-searching strategy. A multiparametric bootstrap resampling of 500 pseudoreplicates was plotted onto the previously selected best-scored ML tree for each replicon.
Phenotypic characterizations of the strains.
In a previous study (7), the symbiotic effectiveness of 20 of the T. suavissima strains was evaluated using a plant-tube method. In this study, the effectiveness of five additional strains was examined using a similar method (38). Briefly, axenically grown seedlings of M. sativa, T. suavissima, and T. anguina were inoculated with 100 μl of a dilute broth culture suspension of a single strain. The shoot dry matter production of the seedlings was measured after 35 days of growth. The effectiveness of each strain was assessed by comparing the effect of the strain on shoot dry matter production relative to that observed for nitrate-fertilized control seedlings and uninoculated control seedlings. In this study, the symbiotic effectiveness of four of the isolates from previous studies (7, 9) (CC2017, CC2155b, CC2157, CC2283c) was included, in addition to those of five isolates that were not examined in those studies (CC2325, CC5033, CC5037, CC5043, and USDA6670). Trigonella anguina D. was included as a host in this study because it is a sister species to T. suavissima (25). However, unlike T. suavissima, T. anguina is native to the Mediterranean region. The seeds for this study were obtained through the USDA ARS National Genetic Resource Program's Genetic Resources Network. The cultivars used and their accession numbers are as follows: M. sativa subsp. sativa, PI 673733; T. anguina, PI 227394; and T. suavissima, PI 198170.
An initial assessment of the metabolic diversity and chemical sensitivity among three representative Australian T. suavissima strains and four Ensifer reference strains was obtained using an automated Biolog microplate ID system (Biolog, Hayward, CA). A subsample of seven strains was examined, including three Australian T. suavissima strains (CC2017, CC2155b, and USDA6670), two E. meliloti reference strains (USDA1002T and USDA1021), and two E. medicae reference strains (A321T and CC169). A dilute suspension of each strain was inoculated into 96 wells of a GEN III microplate. The results of the 94 biochemical tests were recorded after 6 days.
Based on the results of the microplate biochemical analysis, a subsequent study was undertaken to examine the specific ability of each of the strains in the collection to utilize 2-oxoglutarate (α-ketoglutarate) as a sole carbon source. The following Ensifer type strains were also included in this analysis: E. adhaerens (ATCC 33499T), E. americanum (CFNEI 156T), E. arboris (HAMBI 1552T), E. chiapanecum (IITG S70T), E. fredii (USDA205T), E. kostiense (HAMBI 1489T), E. mexicanus (IITG R7T), E. adhaerens (Lc04T), E. numidicus (LMG 24690T), E. saheli (HAMBI 215T), E. terangae (HAMBI 220T), and E. xingiangensis (CCBAU 110T). The inoculants for these assimilation assays were grown for 72 h on tryptone-yeast agar supplemented with 5.3 mM calcium chloride (39). A small portion of a colony was then diluted in 4 ml of sterile phosphate-buffered saline. The resulting suspension was mixed, and an aliquot was collected that was sufficient to provide an optical density at 600 nm (OD600) of 0.010 to 0.015 after addition to a defined broth medium (40) containing 2.5 g liter−1 2-oxoglutarate (α-ketoglutarate) (K1875; Sigma-Aldrich) as the sole carbon source. The cultures were shaken at 200 rpm at 28°C for 72 h, at which time their optical densities were recorded. Strains having OD600 values of >0.075 were scored as having strong growth, those with values between 0.025 and 0.075 were scored as having moderate-to-weak growth, and those with values <0.025 were scored as having poor growth.
The ultrastructure of two of the Australian T. suavissima strains (CC2017 and CC2155b) was examined by transmission electron microscopy. Cells were grown for 3 days in modified arabinose-gluconate broth (32). Specimens were then placed on grids and stained with 4% uranyl acetate for 5 min prior to microscopy.
Accession number(s).
Accession numbers for the nucleotide sequences in this study are listed alphabetically according to the locus name and increasing allele code numbers in Table S3. Those for newly determined sequences are KX896263 through KX896397.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles Murphy (USDA ARS) for electron microscopy and Jeff Newman (Lycoming College) for the initial metabolic and biochemical profiling (Biolog) analyses.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03446-16.
REFERENCES
- 1.Small E. 2011. Alfalfa and relatives: evolution and classification of Medicago. NRC Research Press, Ottawa, ON, Canada. [Google Scholar]
- 2.Allen ON, Allen EK. 1981. Papilionoideae: Trifolieae. Trigonella L., p 666–668. In Allen ON, Allen EK (ed), The Leguminosae, a source book of characteristics, uses, and nodulation. The University of Wisconsin Press, Madison, WI. [Google Scholar]
- 3.Eardly BD, Materon LA, Smith NH, Johnson DA, Rumbaugh MD, Selander RK. 1990. Genetic structure of natural populations of the nitrogen-fixing bacterium Rhizobium meliloti. Appl Environ Microbiol 56:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockwell J, Hely FW. 1966. Symbiotic characteristics of Rhizobium meliloti: an appraisal of the systematic treatment of nodulation and nitrogen fixation interactions between hosts and rhizobia of diverse origins. Aust J Agric Res 17:885–899. doi: 10.1071/AR9660885. [DOI] [Google Scholar]
- 5.Gordon DM, Wexler M, Reardon TB, Murphy PJ. 1995. The genetic structure of Rhizobium populations. Soil Biol Biochem 27:491–499. doi: 10.1016/0038-0717(95)98624-W. [DOI] [Google Scholar]
- 6.Wernegreen JJ, Riley MA. 1999. Comparison of the evolutionary dynamics of symbiotic and housekeeping loci: a case for the genetic coherence of rhizobial lineages. Mol Biol Evol 16:98–113. doi: 10.1093/oxfordjournals.molbev.a026041. [DOI] [PubMed] [Google Scholar]
- 7.Brockwell J, Evans CM, Bowman AM, McInnes A. 2010. Distribution, frequency of occurrence and symbiotic properties of the Australian native legume Trigonella suavissima Lindl. and its associated root-nodule bacteria. Rangeland J 32:395–406. doi: 10.1071/RJ09080. [DOI] [Google Scholar]
- 8.Mitchell TL. 1838. Chapter 2.6, Expedition sent to explore the course of the river Darling, in 1835: Australian shamrock, p 254–255. Three expeditions into the interior of eastern Australia, 2nd ed, vol 1 T. & W. Boone, London, UK: http://gutenberg.net.au/ebooks/e00035.html. [Google Scholar]
- 9.Brockwell J. 1971. Patterns of symbiotic behavior in Trigonella L. Aust J Agric Res 22:917–921. [Google Scholar]
- 10.Petropoulos GA. (ed). 2002. Fenugreek: the genus Trigonella. Taylor and Francis, New York, NY. [Google Scholar]
- 11.Martens M, Delaere M, Coopman R, De Vos P, Gillis M, Willems M. 2007. Multilocus sequence analysis of Ensifer and related taxa. Int J Syst Evol Microbiol 57:489–503. doi: 10.1099/ijs.0.64344-0. [DOI] [PubMed] [Google Scholar]
- 12.Merabet C, Martens M, Mahdi M, Zakhia F, Sy A, Le Roux C, Domergue O, Coopman R, Bekki A, Mars M, Willems A, de Lajudie P. 2010. Multilocus sequence analysis of root nodule isolates from Lotus arabicus (Senegal), Lotus creticus, Argyrolobium uniflorum and Medicago sativa (Tunisia) and description of Ensifer numidicus sp. nov. and Ensifer garamanticus sp. nov. Int J Syst Evol Microbiol 60:664–674. doi: 10.1099/ijs.0.012088-0. [DOI] [PubMed] [Google Scholar]
- 13.Rome S, Fernandez MP, Brunel B, Normand P, Cleyet-Marel JC. 1996. Sinorhizobium medicae sp. nov., isolated from annual Medicago spp. Int J Syst Microbiol 46:972–980. doi: 10.1099/00207713-46-4-972. [DOI] [PubMed] [Google Scholar]
- 14.Reeve W, Chain P, O'Hara G, Ardley J, Nandesena K, Brau L, Tiwari R, Malfatti S, Kiss H, Lapidus A, Copeland A, Nolan M, Land M, Hauser L, Chang Y-J, Ivanova N, Mavromatis K, Markowitz V, Krypides N, Gollagher M, Yates R, Dilworth M, Howieson J. 2010. Complete genome sequence of the Medicago microsymbiont Ensifer (Sinorhizobium) medicae strain WSM419. Stand Genomic Sci 2:77–86. doi: 10.4056/sigs.43526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Deano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Golding B, Gouzy J, Gurjal M, Hernanadez-Lucas I, Hong A, Huizar L, Hyman RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thebault P, Vandenbol M, . et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn S, Stiens M, Puhler A, Schluter A. 2008. Prevalence of pSmeSM11a-like plasmids in indigenous Sinorhizobium meliloti strains isolated in the course of a field release experiment with genetically modified S. meliloti strains. FEMS Microbiol Ecol 63:118–131. doi: 10.1111/j.1574-6941.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 17.Harrison PW, Lower RP, Kim NK, Young JP. 2010. Introducing the bacterial ‘chromid’: not a chromosome, not a plasmid. Trends Microbiol 18:141–148. doi: 10.1016/j.tim.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.van Berkum P, Elia P, Eardly BD. 2006. Multilocus sequence typing as an approach for population analysis of Medicago-nodulating rhizobia. J Bacteriol 188:5570–5577. doi: 10.1128/JB.00335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Berkum P, Elia P, Eardly BD. 2010. Application of multilocus sequence typing to study the genetic structure of megaplasmids in Medicago-nodulating rhizobia. Appl Environ Microbiol 76:3967–3977. doi: 10.1128/AEM.00251-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein B, Branca A, Mudge J, Bharti AK, Briskine R, Farmer AD, Sugawara M, Young ND, Sadowsky MJ, Tiffin P. 2012. Population genomics of the facultatively mutualistic bacteria Sinorhizobium meliloti and S. medicae. PLoS Genet 8:e1002868. doi: 10.1371/journal.pgen.1002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-R LM, Konstantinidis K. 2014. Bypassing cultivation to identify species. Microbe Wash DC 9:111–118. doi: 10.1128/microbe.9.111.1. [DOI] [Google Scholar]
- 22.Kim M, Oh H-S, Park S-C, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 23.Capela D, Barloy-Hubler F, Gouzy J, Bothe G, Ampe F, Batut J, Boistard P, Becker A, Boutry M, Cadieu E, Dreano S, Gloux S, Godrie T, Goffeau A, Kahn D, Kiss E, Lelaure V, Masuy D, Pohl T, Portetelle D, Puhler A, Purnelle B, Ramsperger U, Renard C, Thebault P, Vandenbol M, Weidner S, Galibert F. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc Natl Acad Sci U S A 98:9877–9882. doi: 10.1073/pnas.161294398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuykendall LD, Hashem FM, Wang ET. 2005. Genus VII. Sinorhizobium Chen Yan and Li 1988b, 396VP emend. de Lajudie, Willems, Pot, Dewettinck, Maestrojuan, Neyra, Collins, Dreyfus, Kersters and Gillis 1994, 732, p 358–362. In Brenner DJ, Krieg NR, Staley JT, Garrity GM (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY. [Google Scholar]
- 25.Maureira-Butler IJ, Pfeil BE, Muangprom A, Osborn TC, Doyle J. 2008. The reticulate history of Medicago (Fabaceae). Syst Biol 57:466–482. doi: 10.1080/10635150802172168. [DOI] [PubMed] [Google Scholar]
- 26.Bailly X, Olivieri I, Brunel B, Cleyet-Marel J-C, Bena G. 2007. Horizontal gene transfer and recombination drive the evolution of the nitrogen-fixing symbionts of Medicago species. J Bacteriol 189:5223–5236. doi: 10.1128/JB.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fred EB, Baldwin IL, McCoy E. 1932. Root nodule bacteria and leguminous plants. University Wisconsin Press, Madison, WI. [Google Scholar]
- 28.Lieberman MT, Mallory LM, Simkins S, Alexander M. 1985. Numerical taxonomic analysis of cross-inoculation patterns of legumes and Rhizobium. Plant Soil 84:225–244. doi: 10.1007/BF02143186. [DOI] [Google Scholar]
- 29.Reeve W, Tian R, Brau L, Goodwin L, Munk C, Detter C, Tapia R, Han C, Liolios K, Huntemann M, Pati A, Woyke T, Mavrommatis K, Markowitz V, Ivanova N, Krypides N, Willems A. 2013. Genome sequence of Ensifer arboris strain LMG 14919T; a microsymbiont of the legume Prosopis chilensis growing in Kosti, Sudan. Stand Genomic Sci 9:473–483. doi: 10.4056/sigs.4828625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nick G, de Lajudie P, Eardly BD, Suomalainen S, Paulin L, Zhang X, Gillis M, Lindstrom K. 1999. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int J Syst Bacteriol 49:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- 31.Degefu T, Wolde-Meskel E, Frostegard A. 2012. Phylogenetic multilocus sequence analysis identifies seven novel Ensifer genospecies isolated from a less-well-explored biogeographical region in East Africa. Int J Syst Evol Microbiol 62:2286–2295. doi: 10.1099/ijs.0.039230-0. [DOI] [PubMed] [Google Scholar]
- 32.van Berkum P. 1990. Evidence for a third uptake hydrogenase phenotype among the soybean bradyrhizobia. Appl Environ Microbiol 56:3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloret L, Ormeno-Orillo E, Rincon R, Martinez-Romero J, Rogel-Hernandez MA, Martinez-Romero E. 2007. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst Appl Microbiol 30:280–290. doi: 10.1016/j.syapm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Martens M, Dawyndt P, Coopman R, Gillis M, De Vos P, Willems A. 2008. Advantage of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium). Int J Syst Evol Microbiol 58:200–214. doi: 10.1099/ijs.0.65392-0. [DOI] [PubMed] [Google Scholar]
- 35.Rincon-Rosales R, Lloret L, Ponce E, Martinez-Romero E. 2009. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol Ecol 67:103–117. doi: 10.1111/j.1574-6941.2008.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 38.Vincent JM. 1970. A manual for the practical study of root-nodule bacteria. IBP handbook no. 15. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom. [Google Scholar]
- 39.Beringer JE. 1974. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198. [DOI] [PubMed] [Google Scholar]
- 40.Manhart JR, Wong PP. 1979. Nitrate reductase activities of rhizobia and the correlation between nitrate reduction and nitrogen fixation. Can J Microbiol 25:1169–1174. doi: 10.1139/m79-181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.