ABSTRACT
Respiratory syncytial virus (RSV) causes severe respiratory disease in young children. Antibodies specific for the RSV prefusion F protein have guided RSV vaccine research, and in human serum, these antibodies contribute to >90% of the neutralization response; however, detailed insight into the composition of the human B cell repertoire against RSV is still largely unknown. In order to study the B cell repertoire of three healthy donors for specificity against RSV, CD27+ memory B cells were isolated and immortalized using BCL6 and Bcl-xL. Of the circulating memory B cells, 0.35% recognized RSV-A2-infected cells, of which 59% were IgA-expressing cells and 41% were IgG-expressing cells. When we generated monoclonal B cells selected for high binding to RSV-infected cells, 44.5% of IgG-expressing B cells and 56% of IgA-expressing B cells reacted to the F protein, while, unexpectedly, 41.5% of IgG-expressing B cells and 44% of IgA expressing B cells reacted to the G protein. Analysis of the G-specific antibodies revealed that 4 different domains on the G protein were recognized. These epitopes predicted cross-reactivity between RSV strain A (RSV-A) and RSV-B and matched the potency of antibodies to neutralize RSV in HEp-2 cells and in primary epithelial cell cultures. G-specific antibodies were also able to induce antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis of RSV-A2-infected cells. However, these processes did not seem to depend on a specific epitope. In conclusion, healthy adults harbor a diverse repertoire of RSV glycoprotein-specific antibodies with a broad range of effector functions that likely play an important role in antiviral immunity.
IMPORTANCE Human RSV remains the most common cause of severe lower respiratory tract disease in premature babies, young infants, the elderly, and immunocompromised patients and plays an important role in asthma exacerbations. In developing countries, RSV lower respiratory tract disease has a high mortality. Without an effective vaccine, only passive immunization with palivizumab is approved for prophylactic treatment. However, highly potent RSV-specific monoclonal antibodies could potentially serve as a therapeutic treatment and contribute to disease control and mortality reduction. In addition, these antibodies could guide further vaccine development. In this study, we isolated and characterized several novel antibodies directed at the RSV G protein. This information can add to our understanding and treatment of RSV disease.
KEYWORDS: neutralization, RSV G protein, antibody function, antibody repertoire, respiratory syncytial virus
INTRODUCTION
Human respiratory syncytial virus (RSV) remains the most common cause of hospital admissions for lower respiratory tract disease (LRTD) in the United States, and RSV LRTD is accompanied by high mortality in low- and middle-income countries (1, 2). Without an approved vaccine, prevention of severe RSV LRTD has focused on RSV fusion (F) protein-specific antibodies, especially since the prefusion F structure has been solved using stabilizing prefusion F-specific antibodies (3, 4). The F protein-specific antibody palivizumab has been shown to be able to prevent severe disease in high-risk infants. However, it is not cost-effective to administer it to all young children and is currently used only in a high-dose, prophylactic manner in high-risk infants (5). Therefore, the search for more potent antibodies continues, as effective therapeutic options are still lacking. In this context, the role of other RSV surface proteins, such as the G protein, in the prevention of severe disease is less well defined, and knowledge of this role could prove useful in the development of new therapies.
The G protein is produced in two forms: a membrane-bound form and a soluble form (sG). The latter is composed of the extracellular domain of the G protein and is shed within hours after infection in vitro (6). Although modified RSV strains lacking G protein are still infectious in vitro, infection in vivo is highly attenuated, underscoring the importance of the G protein (7, 8). Successful infection in vivo thus seems to depend on the presence of a functional G protein. Compared to the highly conserved F protein, the G protein is highly variable, with low identity (53%) between RSV strain A (RSV-A) and RSV-B. The extracellular domains (amino acids [aa] 66 to 298) of sG are even less well conserved (44%) (9). Despite this variability, the extracellular domains of sG have one central conserved region between aa 164 and 176, followed by a region with four conserved cysteine residues (aa 173 to 186) which form a cysteine noose containing a CX3C motif (10). This motif is similar to the only known CX3C chemokine, called fractalkine (11). Tripp and colleagues (11, 12) have shown that the G protein can influence immune signaling by interaction with the fractalkine receptor (CX3CR1), a receptor present on leukocytes (13), and that blocking this interaction abrogates inflammation and viral replication in mice. Recent reports support the hypothesis that CX3CR1 is a cellular receptor for RSV in primary human epithelial cell cultures (14–16).
In this study, we evaluated the diversity of the RSV-specific B cell repertoire in healthy child day care providers (adults) using a flow cytometry-based screening assay. Our aim was to map RSV-specific antibody diversity and to search for highly potent neutralizing G protein-specific antibodies with immune-modulating properties.
RESULTS
Isolation and characterization of RSV-specific antibodies.
The frequency of RSV-specific memory B cells in the CD27+ IgG-expressing (IgG+) and CD27+ IgA-expressing (IgA+) memory B cell fraction of the child day care providers was determined. After immortalization of the B cells with BCL6 and Bcl-xL, the potency of binding of antibodies present in the culture supernatant to RSV-A2-infected HEp-2 cells was tested by flow cytometry. From the total number of IgG+ memory B cells (∼57,000 cells) and IgA+ memory B cells (∼54,000 cells) screened, 208 cultures produced IgG specific for RSV-infected cells and 185 cultures produced IgA specific for RSV-infected cells (Table 1). In these child day care providers, who most likely encounter RSV regularly, the frequency of RSV-A2-specific B cells was thus approximately 1 in 282. In two donors, we could compare the immunoglobulin isotype distribution of RSV-specific antibodies. As shown in Table 1, circulating IgA+ memory B cells dominated the RSV response (59% for IgA+ memory B cells versus 41% for IgG+ memory B cells).
TABLE 1.
RSV-A2-specific antibody repertoire and repertoire of strong RSV-A2-binding clones
| Donor | RSV-A2-specific antibody repertoire |
Repertoire of strong RSV-A2-binding clonesa |
|||||
|---|---|---|---|---|---|---|---|
| No. of B cells screened | No. (%) of RSV-A2 reactive MBCsb | No. (%) of IgA+/IgG+ cells | No. of clones selected | No. of IgA+/IgG+ cells | No. (%) of IgA+ cells reactive to G/F/unknown | No. (%) of IgG+ cells reactive to G/F/unknown | |
| 1 | 46,000 | 153 (0.33) | 94 (61)/59 (39) | 21 | 6/15 | 3/3/0 | 6/5/4 |
| 2 | 46,000 | 161 (0.35) | 91 (57)/70 (43) | 19 | 3/16 | 1/2/0 | 4/9/3 |
| 3 | 19,000 | 79 (0.42) | NDc/79 (100) | 34 | ND/34 | ND | 17/15/2 |
| Total | 111,000 | 393 (0.35) | 185/208 | 74 | 9/65 | 4/5/0 | 27/29/9 |
Clones were selected by their binding to RSV-A2-infected intact cells.
MBC, mini-bulk cultures (20 cells per well).
ND, not determined.
We selected the most potent RSV-A2 binders of the IgA mini-bulk cultures (MBCs; 20 cells per well) (9 clones) and IgG MBCs (65 clones) and determined their reactivity against RSV surface proteins (F, G, and SH), which was found to be divided between reactivity against F protein and reactivity against G protein (Fig. 1), while no antibodies specific for RSV SH protein were found. Of nine IgG antibodies (14%, 9/65; Fig. 1B), we were unable to determine the antigen specificity. Since they specifically bind RSV-infected cells, the antigen is probably one of the other RSV proteins.
FIG 1.
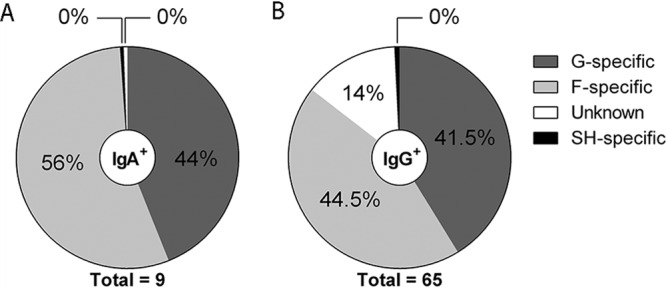
Specificity of selected RSV-reactive antibodies. (A) Distribution of selected IgA+ B cells (n = 9) specific for G protein or F protein. (B) Distribution of selected IgG+ B cells (n = 65) specific for G protein, F protein, or SH protein or directed against an unknown antigen.
Since RSV G-specific antibodies derived from humans have been less well studied than F-specific antibodies, we chose to focus on G-specific antibodies in our study. Based on the binding response to RSV-A2-infected cells determined by flow cytometry, we selected seven G-specific IgG+ B cell clones, of which six were of the IgG1 subclass and one was of the IgG2 subclass. All antibodies showed high levels of somatic hypermutation in the variable heavy chain (VH) and variable light chain (VL) regions (average, 15.1 and 7.6 mutations, respectively), indicative of antigen selection (Table 2). Regarding VH usage, it has been described that for infectious disease targets, such as the influenza virus hemagglutinin stem region (17), RSV (18), and hepatitis C virus (HCV) (19), the VH1-69 family is predominantly used. In this study, however, we did not observe this, since only one of the seven selected clones used VH1-69 (Table 2).
TABLE 2.
MAb gene segment analysis of IgG and IgA RSV G-specific antibodies
| RSV G-specific antibody and clone | Immunoglobulina |
Amino acid replacement variable region |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heavy chain |
Light chain |
||||||||||||
| Isotype | V | D | J | CDR3 | λ orκ | V | J | CDR3 | Heavy chain |
Light chain |
|||
| Replacement | Silent | Replacement | Silent | ||||||||||
| AT32 | IgG1 | 1-24*01 | 6-13*01(1) | 4*02 | CAAEARYCDNSRCSPNFDHW | κ | KV4-1*01 | KJ5*01 | CQQYYDPLITF | 17 | 5 | 7 | 4 |
| AT33 | IgG1 | 1-69*01 | 5-24*01(3) | 4*02 | CARDAEWAAGSDYFFDYW | λ | LV3-25*03 | LJ2*01 | CQSTDTSGPLF | 18 | 10 | 10 | 2 |
| AT34 | IgG1 | 3-30*03 | 3-22*01(2) | 6*02 | CASQGAKGGHELSFYCALDVW | κ | KV1-5*03 | KJ1*01 | CQQYNSHTWTF | 11 | 11 | 8 | 14 |
| AT40 | IgG1 | 3-30-3*01 | 4-17*01(2) | 4*02 | CARGRALDDFADYGGYYFDYW | κ | KV1-12*01 | KJ3*01 | CQQANTFPFTF | 24 | 8 | 7 | 13 |
| AT42 | IgG2 | 4-39*01 | 2-21*01(2) | 4*02 | CARHWAGLYFDSW | κ | KV3-20*01 | KJ4*01 | CQYYGDSPGSF | 17 | 9 | 9 | 8 |
| AT50 | IgG1 | 1-18*01 | 5-24*01(1) | 6*02 | CARGGAQEMVRIHYYYYGMDVW | κ | KV1-9*01 | KJ3*01 | CQQLNTYPLTF | 6 | 3 | 6 | 5 |
| AT51 | IgG1 | 1-18*01 | 3-3*01(2) | 4*02 | CARPATSYDDLRSGYLNYCDYW | κ | KV1-9*01 | KJ4*01 | CQQFHTYPLTF | 13 | 6 | 6 | 2 |
| AT61 | IgA1 | 3-33*01 | 3-22*01(2) | 4*02 | CARDGYDSSGPYPYW | λ | LV3-21*03 | LJ3*02 | CQVWDSSSDHWVF | 0 | 1 | 0 | 0 |
| AT62 | IgA1 | 3-30*03 | 2-8*01(2) | 4*02 | CATDPPKFYDIDGLPDPVW | κ | KV2-24*01 | KJ4*01 | CMQATHLLTF | 9 | 3 | 6 | 4 |
| AT63 | IgA1 | 4-31*03 | 3-10*01(1) | 2*01 | CAREVLFWVGEQNPPNWYFDLW | λ | LV7-43*01 | LJ2*01 | CLLYFGGNRVF | 19 | 4 | 16 | 3 |
| AT64 | IgA1 | 4-4*02 | 3-10*01(3) | 3*01 | CARSRTIVPVAKGGGADW | λ | ND | ND | ND | 15 | 9 | ||
Displayed is the immunoglobulin buildup separated in V-D-J sections of the heavy and light chains, including somatic hypermutation. ND, not determined.
From the RSV-specific IgA repertoire we selected the B cell clones that were the strongest binders to intact RSV-A2-infected cells, measured by flow cytometry. We selected 9 IgA+ B cell clones. From these 9 antibodies, 2 were of the IgA2 subclass, 7 were of the IgA1 subclass (Table 2), and only AT62 used a kappa light chain. Of the IgA antibodies, 5 were specific for the RSV F protein and 4 were specific for the RSV G protein. Compared to the IgG clones, the majority of the IgA clones had an amino acid composition more closely matching that of germ line sequences. Although IgA antibodies can activate complement via the mannose-binding lectin pathway (20), none of the IgA antibodies neutralized, irrespective of whether complement was added (data not shown). Because IgA antibodies lack a potent neutralizing capacity, we chose to focus on G-specific IgG antibodies in the subsequent functional experiments. However, to determine whether the absence of neutralization of G-specific IgA antibodies was due to the IgA isotype, we recloned two IgA antibodies (AT61G and AT64G) into an IgG background.
G-specific antibody affinity.
We determined the affinity of our G-specific IgG antibody panel by surface plasmon resonance (SPR) analysis. A chip was coated with the G-specific antibodies, and full-length secreted G protein was injected over the chip. The values of the corresponding kinetic parameters of antibody-antigen binding (association constant [ka], dissociation constant [kd], and equilibrium dissociation constant [KD]) are shown in Table 3. AT33, AT64G, and AT34 showed the highest affinity to the RSV-A2 G protein, indicated by low KDs of 11.1, 14.7, and 12.8 pM, respectively. Among the G-specific antibodies, 4 (AT34, AT40, AT42, and AT64G) recognized both the RSV-A2 and RSV-B G proteins. Except for AT42, the affinity of the RSV-A/B cross-reactive antibodies was higher for the RSV-A2 G protein than for the RSV-B G protein (Table 3). 131-2G, a mouse G-specific antibody, displayed a low affinity for both the RSV-A2 and RSV-B G proteins (KDs, 3,640 and 1,420 pM, respectively) (Table 3).
TABLE 3.
Kinetic constants for binding RSV-A2 G proteina
| Strain and epitope | Antibody | ka (s−1 · M−1 [104]) | kd (s−1 [105]) | KD (pM) |
|---|---|---|---|---|
| RSV-A2 | ||||
| A | AT42 | 79.8 ± 3.3 | 65.0 ± 13.2 | 809 ± 132 |
| B | AT34 | 31.3 ± 4.6 | 0.4 ± 0.2 | 12.8 ± 6.9 |
| B | AT40 | 59.4 ± 12.4 | 5.8 ± 0.4 | 106 ± 29 |
| B | AT64G | 35.7 ± 6.2 | 0.5 ± 0.2 | 14.7 ± 2.8 |
| B | 131-2G | 28.5 ± 2.0 | 103 ± 2 | 3,640 ± 320 |
| C | AT50 | 21.8 ± 2.5 | 35.1 ± 7.7 | 1,610 ± 340 |
| C | AT51 | —b | — | — |
| C | AT61G | 18.9 ± 4.0 | 200 ± 40 | 10,800 ± 3,000 |
| D | AT32 | 42.5 ± 8.3 | 18.2 ± 2.3 | 459 ± 122 |
| D | AT33 | 73.5 ± 5.2 | 0.8 ± 0.4 | 11.1 ± 4.8 |
| RSV-B | ||||
| A | AT42 | 22.2 ± 1.2 | 4.6 ± 0.5 | 208 ± 33 |
| B | AT34 | 3.76 ± 0.42 | 2.7 ± 0.7 | 719 ± 174 |
| B | AT40 | 5.99 ± 0.50 | 2.3 ± 0.4 | 379 ± 45 |
| B | AT64G | 6.33 ± 0.99 | 4.8 ± 1.1 | 776 ± 169 |
| B | 131-2G | 111 ± 5 | 159 ± 20 | 1,420 ± 120 |
| C | AT50 | — | — | — |
| C | AT51 | — | — | — |
| C | AT61G | — | — | — |
| D | AT32 | — | — | — |
| D | AT33 | — | — | — |
Data represent means ± standard deviations from at least two independent measurements. —, no binding detected.
AT51 did not bind in this setup (the immobilized antibody is inactive).
RSV G-specific antibody epitope mapping.
The fact that not all antibodies in our panel had similar specificities and affinities indicates that they recognize different epitopes. To determine the epitope recognized by the G-specific antibodies, we used a library of short (12-aa) peptide fragments of the RSV-A2 strain (aa 164 to 199, in which each of the peptide fragments was shifted by 1 residue from the previous peptide), which were immobilized on an SPR chip. Antibody mapping revealed four different binding domains on the G protein (Fig. 2). One antibody, AT42, did not recognize any of the peptides and did not react with denatured G protein in Western blotting assays (data not shown). However, this antibody did bind to G protein-transfected cells and to G protein in an enzyme-linked immunosorbent assay and by SPR analysis. These results suggest a conformational epitope that cannot be reproduced in a 12-aa peptide. We have named this conformational epitope epitope A. The 3 other regions consisted of linear epitopes surrounding the CX3C binding domain (aa 169 to 191) (Fig. 2). Epitope B consists of the amino acid sequence NDFHFEVFNF and was recognized by AT34, AT40, and AT64G but also by well-known antibodies, like 131-2G and 3D3 (21). The epitope B sequence is highly conserved between RSV strains (9), which explains why antibodies specific for epitope B recognize RSV strains A and B (Fig. 2 and Table 3). A third epitope (epitope C), which was recognized by AT50, AT51, and AT61G, overlaps the last 2 cysteine residues that flank the cysteine noose region (Fig. 2). Yet another epitope, epitope D, was located just outside the cysteine noose region. This epitope is specifically recognized by AT32 and AT33. Antibodies specific for epitopes C and D only recognized RSV-A (Table 3).
FIG 2.
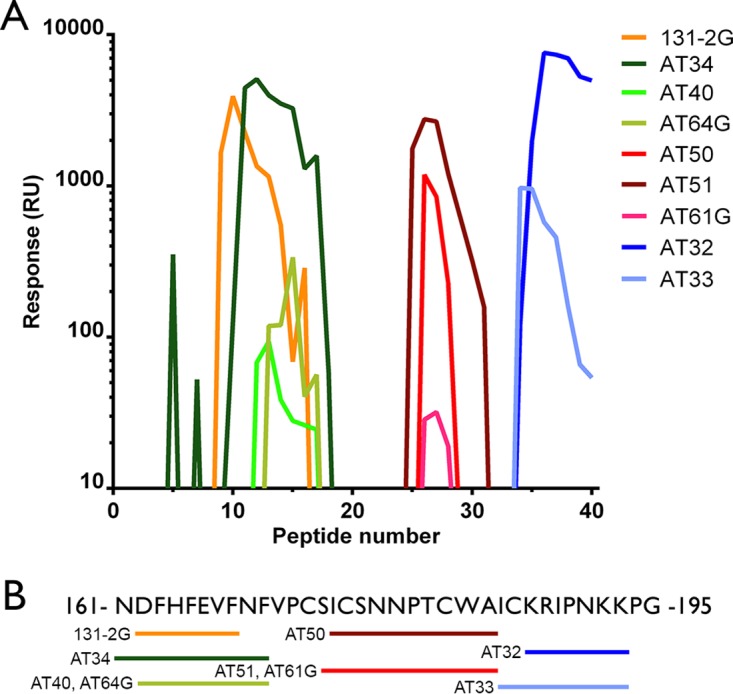
Epitope mapping of RSV G-specific antibodies. (A) An SPR array of 12-aa peptides that were partially overlapping was used to determine the binding epitope of G-specific antibodies. The lines depict the binding response (in relative units [RU]; 1 RU is 0.1 millidegrees) to the corresponding peptide numbers. Three linear epitopes can be distinguished: epitope B (131-2G, AT34, AT40, AT64G), epitope C (AT50, AT51, AT61G), and epitope D (AT32, AT33). (B) Amino acid sequence of the RSV-A2 G protein, indicating the binding epitope of each antibody.
Strain-specific neutralization of G-specific antibodies correlates with epitope specificity.
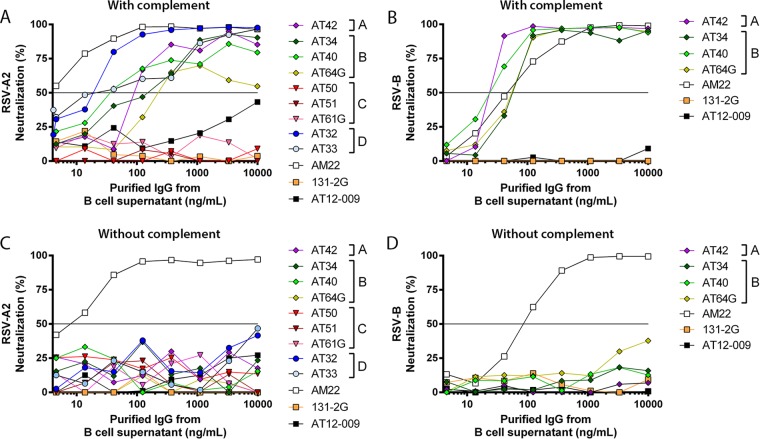
Antibody efficacy is determined by multiple variables, ranging from antibody and target glycosylation, binding on and off rate, the epitope, and the angle that the target is approached by the antibody (4, 22–24). Here, we hypothesized that for the G-specific antibodies, the neutralization potency may be determined by the binding site on the G protein. Therefore, we performed in vitro complement-dependent neutralization (CDN). The seven selected IgG antibodies and the two G-specific IgA antibodies recloned into an IgG background were tested (Fig. 3 and Table 4). All antibodies binding to epitopes A, B, and D neutralized in the presence of complement on HEp-2 cells (Fig. 3). There were differences in the 50% inhibitory concentrations (IC50s) (Table 4) between the selected antibodies, with AT32 being the most potent in the presence of complement (IC50, 14.7 ng/ml; Table 4) and AT33 being the least potent (IC50, 249 ng/ml; Table 4). Both the IgG1 antibodies and the IgG2 antibody neutralized RSV in the presence of complement when purified directly from the B cell supernatant. On the contrary, antibodies specific for epitope C did not neutralize in the presence of complement; this included the IgA antibody (AT61G) that was recloned onto an IgG backbone and that was specific for epitope C. However, the AT64G antibody, which originated from an IgA epitope B-specific antibody, did neutralize, although neutralization never reached >70% (Fig. 3) (mean IC50, 109 ng/ml; 95% confidence interval, 36.3 to 329 ng/ml). The RSV-B-reactive antibodies (AT34, AT40, AT42, AT64G) were also tested for neutralization of RSV-B-infected HEp-2 cells. Neutralization with complement resulted in IC50s even lower than those for RSV-A neutralization (Table 4; Fig. 3B). Together these results may suggest that the binding domain of the antibodies on the G protein contributes to the complement-induced neutralization potency. None of the G-specific antibodies neutralized RSV-A or RSV-B >50% in the absence of complement in the HEp-2 cell assay, whereas the prefusion F-specific antibody AM22 did potently neutralize (Fig. 3C and D).
FIG 3.
Complement-dependent and -independent RSV neutralization by G-specific antibodies. RSV-A2 or RSV-B on HEp-2 cells was neutralized in vitro by increasing the concentration of antibodies specific for the RSV G protein with 10% rabbit complement (A and B) and without complement (C and D). AT12-009, an HCV E2-specific antibody, was used as a negative control. AM22 is a prefusion F protein-specific antibody. All experiments were performed twice in triplicate.
TABLE 4.
CDN, ADCC, and ADCPa
| Epitope | Clone | Strain(s) specificity |
KD (pM) for G protein from: |
IC50 (ng/ml) for RSV A/B CDN | % neutralization of HAE cells in culture at 0.2 μg/ml | EC50 (ng/ml) |
||
|---|---|---|---|---|---|---|---|---|
| RSV-A | RSV-B | ADCC | ADCP | |||||
| A | AT42 | A, B | 809 ± 132 | 208 ± 33 | 90.3/11.1 | 69 ± 7 | — | 609 |
| B | AT34 | A, B | 12.8 ± 6.9 | 719 ± 174 | 158/37.2 | 1 ± 0 | 1,352 | 304 |
| B | AT40 | A, B | 106 ± 29 | 379 ± 45 | 30.6/15.1 | 7 ± 2 | 456 | 336 |
| B | AT64G | A, B | 14.7 ± 2.8 | 776 ± 169 | 109/39.9 | — | ND | ND |
| B | 131-2G | A, B | 3,640 ± 320 | 1,420 ± 120 | —/— | 92 ± 11 | ND | ND |
| C | AT50 | A | 1,610 ± 340 | — | —/— | 92 ± 4 | 791 | 798 |
| C | AT51 | A | — | — | —/— | 68 ± 13 | — | — |
| C | AT61G | A | 10,800± 3,000 | — | —/— | — | ND | ND |
| D | AT32 | A | 459 ± 122 | — | 14.7/— | 23 ± 33 | 3,866 | 265 |
| D | AT33 | A | 11.1 ± 4.8 | — | 249/— | 41 ± 21 | — | 279 |
| AM22 | A, B | — | — | 2.6/41.1 | 99 ± 2 | 222 | 119 | |
Neutralization and opsonization experiments were performed with antibodies purified from B cell supernatants. Data represent means ± standard deviations from at least three independent measurements. IC50, 50% inhibitory concentration, the concentration of antibody which inhibits 50% of RSV plaque formation in vitro; EC50; 50% effective concentration, the concentration of antibody which results in 50% of neutrophils with phagocytized FITC-labeled RSV-A2-infected HEp-2 cells or 50% ADCC; —, no binding to RSV G protein was detected in the SPR experiment or <50% neutralization, ADCC, or phagocytosis was measured; ND, not determined; AM22, antibody specific for the RSV prefusion F protein.
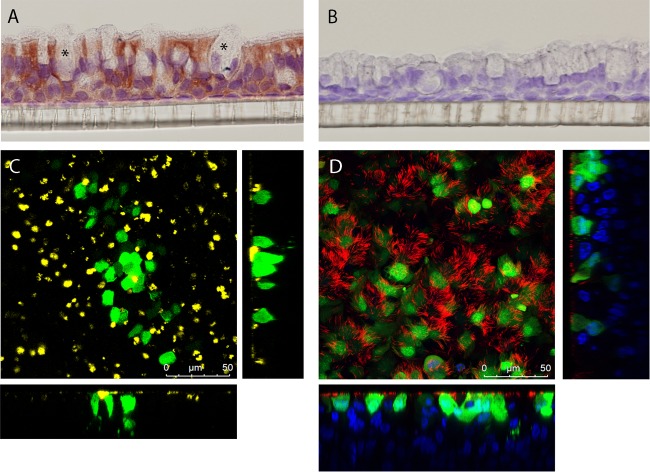
Because HEp-2 cells probably lack the RSV-binding receptor CX3CR1 and infection depends on the surrogate receptors (e.g., heparan sulfates), we tested complement-independent neutralization on primary human airway epithelial (HAE) cells that express CX3CR1 (16). After we confirmed the expression of CX3CR1 on ciliated cells but not goblet cells in our HAE cell culture system (Fig. 4A and B), we then evaluated RSV-A2 infection and found infection of only apical ciliated cells (Fig. 4C to E). We found complete neutralization for all the G-specific antibodies at 20 μg/ml, including 131-2G (Fig. 5), and partial neutralization at 0.2 μg/ml for antibodies binding to epitopes A (AT42, 69%) and C (AT50, 92%; AT51, 68%) (Fig. 5). Antibodies binding to epitopes B and D did not neutralize at low concentrations (Fig. 5). In contrast to the mechanism of complement-induced neutralization, which probably relies on destroying virus particles, the neutralization of RSV on HAE cells is likely dependent on blocking of the G protein-CX3CR1 interaction. Consistent with this idea, epitope C-specific antibodies showed potent neutralization since they bound an epitope very close to the CX3CR1 binding domain on the G protein (Fig. 1). Altogether these findings suggest that antibodies against different epitopes on the G protein exhibit different neutralization potencies in the absence of complement.
FIG 4.
RSV-A2 infection of primary human airway epithelial cell cultures. (A) Immunohistochemistry staining of CX3CR1 (5 μg/ml, indicated by red staining) on the HAE cell membrane. Asterisks, goblet cells. (B) IgG isotype control staining (5 μg/ml). (C) Top and side views of RSV-A2-infected HAE cells, showing RSV-infected cells in green (GFP), goblet cells in yellow (MUC5B), and nuclei in blue (Hoechst 33342). (D) Top and side views of RSV-A2-infected HAE cells, showing RSV-infected cells in green (GFP), cilia in red (β-tubulin), and nuclei in blue (Hoechst 33342).
FIG 5.
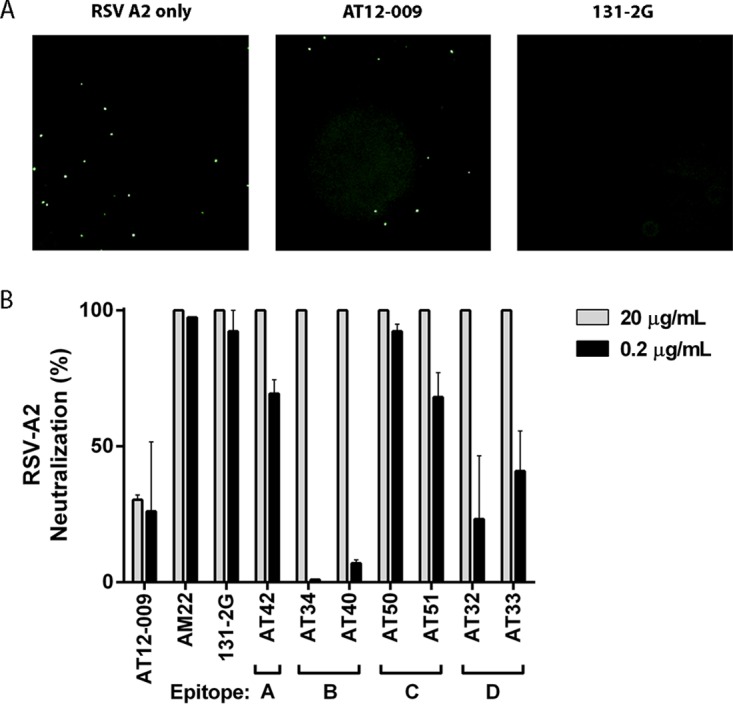
Complement-independent RSV neutralization by G-specific antibodies on primary epithelial cell cultures. (A) Representative images of RSV-A2-GFP-infected HAE cell cultures 30 h after infection. RSV infection is indicated by green GFP-positive cells in the left panel and green GFP-positive cells after preincubation with AT12-009 (negative control) in the middle panel or after preincubation with 131-2G (positive control), which resulted in 100% neutralization (i.e., no RSV-infected cells), in the right panel. (B) Quantification of the percent RSV neutralization using 20 μg/ml and 0.2 μg/ml of the G-specific antibodies. All G-specific antibodies neutralized at 20 μg/ml. Antibodies specific for epitopes A and C still neutralized at 0.2 μg/ml. Data are expressed as means ± SEMs from two experiments performed in duplicate. Data were generated using an Operetta system (PerkinElmer) and analyzed using Columbus software (PerkinElmer).
RSV G-specific antibodies facilitate ADCC and ADCP.
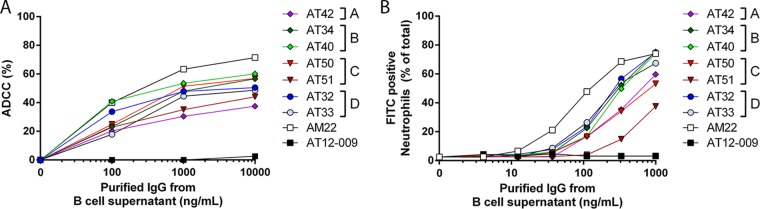
Besides direct neutralization, antibodies can also facilitate the destruction of infected cells, for example, by antibody-dependent cellular cytotoxicity (ADCC), where antibodies bind to infected cells and flag natural killer (NK) cells to lyse the infected cells. The efficacy of ADCC induction depends on the antibody subclass (IgG1 > IgG2; Table 2) (25). We determined the capacity of the G-specific antibodies to induce ADCC using RSV-A2-infected HEp-2 cells and human peripheral blood mononuclear cells (PBMCs). All G-specific antibodies induced ADCC, albeit with different potencies (Fig. 6A). Antibodies directed against epitope B (50% effective concentrations [EC50s], 1,352 ng/ml for AT34 and 456 ng/ml for AT40) and AT50 (EC50, 791 ng/ml) were the most potent inducers of ADCC, while AT40 was as potent as AM22 at low antibody concentrations (Fig. 6A). AT42 (epitope A), AT33 (epitope D), and AT51 (epitope C) had the lowest levels of ADCC induction and did not reach >50% target cell killing (Fig. 6A).
FIG 6.
ADCC and phagocytosis of RSV-A2-infected HEp2 cells. (A) ADCC assay. Lines depict the mean percentage of RSV-A2-infected HEp-2 cells killed by human PBMCs. AT12-009 was used as a negative control. Experiments were performed twice in duplicate. (B) ADCP assay. Lines depict the mean percentage of neutrophils that had phagocytized FITC-labeled RSV-A2-infected HEp-2 cells after 24 h of incubation in the presence of increasing concentration of antibodies. All experiments were performed in triplicate with neutrophils from three different donors.
Infected cells or infectious debris can also be phagocytized by neutrophils. Thus, besides ADCC, we also tested the antibody-dependent cellular phagocytosis (ADCP) of RSV-infected HEp-2 cells by neutrophils, finding that G-specific antibodies induced phagocytosis with EC50 levels of between 200 and 900 ng/ml, with the high-affinity antibodies AT32 and AT33 demonstrating higher phagocytosis activity than the antibodies with lower affinities (Table 4; Fig. 6B). The IgG2 antibody induced phagocytosis at a level similar to the level of phagocytosis induced by the G-specific IgG1 antibodies. Antibodies from epitopes A (AT42) and C (AT50 and AT51) demonstrated opsonization activity, although with a capacity lower than that for the other epitopes (Fig. 6B). The results from the ADCC and ADCP assay suggest that, in contrast to the neutralization ability, the ability of G-specific antibodies to promote effector cell functions is probably not epitope dependent.
DISCUSSION
In this study, we investigated the RSV-specific B cell repertoire of three healthy donors in order to find potential therapeutic antibodies. All donors had high numbers (∼0.35%) of memory B cells producing antibodies against intact RSV-A2-infected cells. These antibodies mainly targeted RSV-A2 surface proteins G and F. The majority of RSV-specific antibodies were of the IgA isotype (59%), while 41% were of the IgG isotype.
Other groups that studied naturally occurring RSV-specific antibodies in healthy adults have found either frequencies (<0.01%) of RSV G-reactive B cells much lower than those that we found (21) or frequencies (0.04 to 0.18%) of B cells producing F-specific antibodies (26) comparable to the frequency of 1 in 282 (0.35%) memory B cells that we found. The variation might be explained by the efficiency of our B cell culture method combined with the sensitivity of the flow cytometry-based selection method but could also be due to the donors that we selected. By using B cells from child day care providers, who are almost certainly exposed to multiple RSV carriers each year, we aimed to increase the chance of finding highly potent antibodies. In addition, we may even have underestimated the frequency of RSV-specific B cells, since we screened on RSV-A2-infected cells but not on RSV-B-infected cells. Others have found that serum neutralizing capacity mainly depends on prefusion F protein-specific antibodies and not on G protein-specific antibodies (27). However, this study determined neutralization on HEp-2 cells without addition of complement, thereby missing the G-specific antibodies which neutralize in the presence of complement and in HAE cell cultures.
In our study, we focused on the G-specific IgG antibody panel. We found four dominant epitopes with distinct functional properties. Antibodies specific for epitopes A and B are cross-reactive between RSV strains A and B, but antibodies specific for epitopes C and D only recognize RSV-A (Table 4). This could be explained by the high amino acid sequence homology between strains A and B around epitopes A and B, also termed the “conserved region,” whereas the amino acid sequence after this region is more variable (9).
We determined the epitopes of the selected G-specific antibodies by their binding to short 12-aa-long peptides. It is likely that most peptides have a linear or minimal conformational structure; therefore, it is well possible that the binding domain of the antibodies is outside the G protein part that we studied (aa 150 to 200) or covers an area larger than the 12-aa sequences. For example, 131-2G has an epitope between aa 163 and 168 which is similar to the epitope found by Kauvar and colleagues (aa 164 to 168) (28). However, Johnson et al. found defective binding of 131-2G to RSV with a mutated 186 aa, indicating that the epitope might span a larger portion of the G protein (14). This could explain how 131-2G is able to neutralize in our HAE cell system, while other antibodies binding epitope B do not at lower concentrations.
All human RSV G-specific IgG antibodies specific for epitopes A, B, and D are strong neutralizers in the presence of complement (Table 4). Complement components play a critical role in antiviral antibody efficacy. For instance, component C1q enhances the neutralization activity of monoclonal antibodies (MAbs) against influenza virus (29). These antibodies may induce potent complement activation because they form hexameric clusters (30). Antibodies binding epitope C and the mouse 131-2G antibody do not neutralize epitopes on HEp-2 cells in the presence of complement. This could be the result of their lower affinity for the G protein than for the other epitopes, which could result in less antigen-driven antibody hexameric clustering. The epitope C-specific antibodies may thus not be able to fully activate complement and induce neutralization. CDN relies on lysis of the virion membrane after signaling and activation of the complement system by virus-bound antibodies (31). A high antibody affinity results in high levels of bound antibody potentiating CDN. This relation is most clear in RSV-B CDN, where a higher affinity translates to more potent neutralization (Table 4). This phenomenon is less clear for RSV-A CDN, where some lower-affinity antibodies still potently neutralize (Table 4). This suggests that, although affinity certainly potentiates the neutralization capacity, there are other factors (e.g., antibody cross-linking, Fc tail presentation, and epitope) influencing the availability and presentation of the Fc tail to complement.
Fc tail presentation could also play a role during other immune effector functions activated through bound antibodies, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) of infected cells, as has been shown in influenza disease (24). Some of our antibodies were able to induce substantial ADCC (mainly epitopes B [AT34] and C [AT40 and AT50]), while others were more potent inducers of ADCP (mainly epitope D, AT32 and AT33). ADCP especially seems to depend on affinity rather than epitope, as antibodies to different epitopes induce similar EC50s (Table 4). Similar to CDN, both ADCC and ADCP require an accessible epitope on the target protein and antibodies bound to the epitope to activate effector cells to initiate cellular cytotoxicity or phagocytosis. Taken together, G-specific antibodies may play an important role in antiviral immunity in vivo via ADCC and ADCP.
The role of the RSV G-protein in cell tropism, infectivity, and attachment of RSV to its target cells still has to be fully elucidated. Recent studies reported an important role for G protein and its receptor, CX3CR1, in the pathogenesis of RSV (14, 32). In addition, it was shown that genetic ablation of the G protein reduces RSV infectivity in mice and cotton rats (7, 8), indicating that viral entry of airway epithelial cells might be hampered without a functional G protein. The G protein expresses a CX3C motif which can bind to the CX3CR1 receptor and, hence, can facilitate viral entry in vivo in airway epithelial cells and in vitro in HAE cell cultures (14–16). Inhibition of this interaction by G-specific antibodies may thus lead to reduced viral infection in HAE cell cultures. Indeed, we found complete neutralization at 20 μg/ml, while at lower concentrations (0.2 μg/ml), neutralization potency seemed grouped by antibody epitope specificity. Interestingly, the antibodies specific for epitope C, which did not show strong efficacy and had a relatively low binding affinity, showed potent neutralization in the HAE cell culture system. The epitope C-specific antibodies seemed to recognize an epitope located on the apex of the cysteine noose, and therefore, they may be best suited to inhibit the G-CX3CR1 interaction. Together, this suggests that antibody-induced inhibition of the RSV G protein interaction with the CX3CR1 receptor does prevent viral entry into airway epithelial cells. Notwithstanding that, besides the G-specific antibodies, AM22, a prefusion F protein-specific antibody (3, 33), also completely neutralized virus infection in HAE cell cultures, suggesting that a functional F protein is also required for HAE cell infection.
Furthermore, the fractalkine binding motif in the G protein is also thought to play a role in modulating the immune response, as it is able to block fractalkine signaling through binding to the CX3CR1 receptor on human PBMCs (11). In vivo and in vitro studies have shown that inhibition of this interaction can inhibit immune cell trafficking and dampens viral replication and pathology in mice infected with RSV (11, 12). This also suggests that the G-specific antibodies could play an immune-modulating role by inhibition of soluble G-CX3CR1 signaling in vivo.
In conclusion, healthy adults harbor a diverse repertoire of high-affinity RSV-specific antibodies, with a predominant IgA+ B cell response. The G-specific IgG antibodies are divided into four epitopes, and antibodies to two epitopes, A and B, cross-react with both RSV strains A and B, but antibodies to epitopes C and D are specific to strain A. The antibodies have a broad range of distinct functions, including CDN, ADCC, ADCP, and complement-independent neutralization in primary HAE cell cultures. While neutralization in HAE cell cultures is highly dependent on the epitope, ADCC and ADCP are more related to affinity. Our results indicate that G-specific antibodies are part of the natural antibody response to RSV and that these antibodies could play an important part in antiviral immunity. Future studies should analyze the potency of these G-specific antibodies in vivo to boost therapeutic antibody regime development and structure-based vaccine design.
MATERIALS AND METHODS
Viruses and cells.
RSV reference strain A2 (subgroup A; VR-1540; ATCC), RSV-X (subgroup A; GenBank accession number FJ948820.1; kindly provided by M. Widjojoatmodjo, RIVM, The Netherlands), green fluorescent protein (GFP)-expressing RSV-A2 (rgRSV224; which was originally described by Hallak et al. [34] and which was a kind gift from L. Bont, WKZ, Utrecht, The Netherlands), and RSV-B strain (VR-1580; kindly provided by F. Coenjaerts, University of Utrecht, Utrecht, The Netherlands) were grown on HEp-2 cells in Iscove's modified Dulbecco's medium (IMDM; Gibco) with 8% fetal calf serum (FCS), 100 IU/ml penicillin, and 100 μg/ml streptomycin.
HAE cell culture.
Primary human airway epithelial (HAE) cells were obtained from patients undergoing an elective lobectomy in the Academic Medical Center, Amsterdam, The Netherlands. Healthy tracheobronchial tissue was obtained from the margin of the surgical resection by a pathologist. Informed consent was obtained from the patient before sampling. The Institutional Review Board of the Academic Medical Center approved the study protocol (2015_122#A2301550). Epithelial cells were isolated following a protocol modified from that of Fulcher and Randell (35). Briefly, the tissue was cleaned by removing the excess connective tissue and sliced into 1- to 2-cm segments. These segments were treated for 48 h with 0.001% DNase and 0.1% protease in 40 ml minimal essential medium (Life Technologies) with 0.25 μg/ml amphotericin B (Sigma), 50 μg/ml gentamicin (Sigma), 100 U/ml penicillin (Sigma), and 100 μg/ml streptomycin (Sigma) at 4°C. Then, 4 ml fetal bovine serum (Thermo Scientific) was added, and epithelial cells were gently scraped off with a number 10 scalpel blade, followed by washing with Dulbecco's phosphate-buffered saline and resuspension in bronchial epithelial cell growth medium (medium was generated based on a protocol from Fulcher and Randell [35]). The primary epithelial cells were cultured either in VitroCol type I human collagen solution (Advanced BioMatrix)-coated T75 flasks for expansion or in human type IV placental collagen (Sigma)-coated porous support Transwell inserts in 24-well plates (Corning). When the cells were confluent, the medium in the lower chamber was replaced by PneumaCult ALI medium (StemCell Technologies) and the medium in the upper chamber (the medium at the air-liquid interface) was removed. After 31 to 32 days of culture, the cells were fully differentiated and ready for neutralization experiments.
B cell isolation, culture, and immortalization.
The use of peripheral blood samples was approved by the medical ethical committee of the Academic Medical Center, Amsterdam, The Netherlands (NL.19303.018.07), and the samples were obtained after written informed consent was provided. Memory B cells were isolated from the peripheral blood of three healthy child day care providers. The B cells were immortalized using BCL6 and Bcl-xL and were sorted into CD27+ IgA+ and CD27+ IgG+ populations using flow cytometry (FACSAria flow cytometer; BD Biosciences) (33). The transduced IgA+ and IgG+ B cell populations were maintained in IMDM with 8% FCS in the presence of mouse recombinant interleukin-21 and irradiated CD40L-expressing L cells for prolonged periods of time, and supernatants of the mini-bulk cultures (MBCs; 20 B cells per well) were tested for specificity against RSV-A2 by binding to intact RSV-A2-infected HEp-2 cells, which was analyzed by flow cytometry (FACSCanto II flow cytometer; BD Biosciences). Single cells of the RSV-reactive MBCs were cultured and retested for RSV-A2 antigen binding.
Sequencing and cloning of selected antibodies.
A selection of the MBCs producing antibodies with the highest level of binding to RSV-A2-infected cells on the basis of flow cytometry was obtained for further cloning. cDNA was generated from the total RNA of the selected B cell clones, and a variable heavy chain (VH) and variable light chain (VL) PCR was performed. The VH and VL regions were sequenced, and antibodies of interest were cloned into the pCR2.1 TA cloning vector (Invitrogen). Sequences were checked for reverse transcriptase- or DNA polymerase-induced mutations, before the VH and VL regions were cloned in frame with human IgG1 and kappa or lambda constant domains into pcDNA3.1 (Invitrogen). For protein production, the vectors were transiently transfected into 293 cells. Antibodies were subsequently purified using HiTrap protein A or G columns on an Äkta instrument (GE Healthcare).
Antibody specificity.
The RSV-reactive clones were screened for binding to RSV surface protein F, G, or SH by flow cytometry on intact transfected 293 cells. The 293 cells were transfected with a construct expressing the RSV-A2 F protein, G protein (RSV-X derived), or SH protein (a pCAGGS SH-RSV-A-containing construct; a kind gift of X. Saelens, University of Ghent, Ghent, Belgium). In a separate experiment, all antibodies were tested for cross-reactivity to the RSV-B strain. The RSV-specific antibody panel was added to the transfected cells, and binding was detected with a phycoerythrin (PE)-labeled goat anti-human F(ab′)2 antibody (Southern Biotech). The data were analyzed using FlowJo software (FlowJo LLC).
SPR analysis.
Surface plasmon resonance (SPR) analysis was performed on an Ibis Mx96 instrument (Ibis Technologies). Purified antibodies were chemically immobilized on amine-functionalized chips (Ssens, Enschede, The Netherlands) as described before (36).
Epitope mapping was done by immobilizing biotinylated peptides on a streptavidin-coated SPR chip in a continuous-flow microspotter (Wasatch Microfluidics), using a 2.0 μM peptide solution diluted in capture buffer (phosphate-buffered saline [PBS], 0.05% Tween 20, 0.1% sodium azide). Before injection of the samples, the chips were extensively washed with capture buffer. All injected samples were prepared in capture buffer containing 0.01% human serum albumin. During the course of the experiments, the temperature in the Ibis flow cell was kept at 25°C. The data were processed with SprintX software (Ibis Technologies). Kinetic constants were fitted to the binding curves, by making a global fit to all curves using a 1:1 binding model, with Scrubber2 software (BioLogic).
Murine antibody 131-2G was purchased from EMD Millipore. For binding to purified RSV G protein, we used the extracellular domains of strains A2 (aa 66 to 297; catalog number 11070-V08H) and B (aa 67 to 299; catalog number 13029-V08H) from Sino Biological and a library of N-terminally biotinylated 12-mer peptides, comprising amino acids 150 to 200 of the RSV-A2 G protein (NKI, Dutch Cancer Institute, Amsterdam, The Netherlands).
Complement-dependent and -independent RSV neutralization.
Complement-dependent neutralization (CDN) was performed in vitro by using a method similar to that described by Zielinska et al. (37). In short, 25 PFU/well of RSV-A2 was mixed and preincubated with G-specific antibodies (from B cell culture supernatants) with 10% rabbit complement serum (Sigma-Aldrich) for 60 min at 37°C, and the mixture was added to HEp-2 cells. After 48 h of culture at 37°C, the monolayers were fixed with 1:1 acetone-methanol. Infected cells were visualized by incubating the samples overnight with polyclonal goat RSV-specific Alexa Fluor-647-labeled antibody (Biodesign). Fluorescent images were acquired and analyzed using an Operetta imaging system (PerkinElmer). Complement-independent neutralization on primary HAE cell cultures was done in a similar fashion by incubating 50 PFU/well of RSV-GFP with the G-specific antibody panel (final concentrations, 20 and 0.2 μg/ml) for 60 min at 37°C, after which the inoculum was added to HAE cell cultures for 30 min at 37°C. Next, the inoculum was removed from the surface of the HAE cell cultures and GFP expression was evaluated after 30 h of infection, using the Operetta system (PerkinElmer). Membranes were fixed in 4% paraformaldehyde for 30 min. Confocal microscopy (with a Leica SP-8 X microscope) was performed on whole membranes stained with anti-β-tubulin (Sigma), MUC5B (Santa Cruz), and Hoechst 33342 (Sigma) overnight at 4°C. Other membranes were embedded in paraffin, and 5-μm-thick sections were cut. Immunohistochemical staining of CX3CR1 on HAE cell cultures was performed as described before (38) with an anti-CX3CR1 antibody (eBioscience; kind gift of C. Verseijden, Tytgat Institute, Amsterdam, The Netherlands).
ADCC assay.
We determined antibody-dependent cellular cytotoxicity (ADCC) as described before (39). Briefly, PBMCs were thawed and rested overnight in IMDM with 8% FCS at 37°C. Serial dilutions of the G-specific antibody panel were added to 1.25 × 104 calcein acetoxymethyl (Corning)-labeled RSV-A2-infected cells in 96-well plates. Then, PBMCs (5 × 105 cells) were added at a ratio of 40 PBMCs per 1 RSV-infected HEp-2 cell, and the mixture was incubated for 4 h at 37°C. The cells were washed once with PBS–1 mM EDTA, detached with PBS–0.5% trypsin, and transferred to a CellCarrier Spheroid ULA 96-well plate (PerkinElmer). DAPI (4′,6-diamidino-2-phenylindole) and Accudrop beads (BD Biosciences) were added, and the cells were analyzed by flow cytometry (LRS Fortessa flow cytometer; BD Biosciences). The stopping gate was set to 5,000 beads per sample. The number of remaining RSV-A2-infected HEp-2 cells under each condition was related to the number of RSV-A2 infected HEp-2 cells in the control wells without antibody.
Antibody-dependent cellular phagocytosis assay.
RSV-A2-infected HEp-2 cells were green fluorescent labeled by use of a PKH kit (Sigma-Aldrich). Next, 2 × 104 RSV-A2-infected HEp-2 cells were added to a 96-well plate containing serial dilutions of the G-specific antibody panel and incubated for 1 h at 37°C. AT12-009 (a hepatitis C virus E2-specific IgG1 antibody) was used as a negative control. This was followed by the addition of 2 × 104 Ficoll-isolated human blood neutrophils. The plates were incubated overnight at 37°C. On the next day, the neutrophils were stained with DAPI and CD45-PE-Cy7 (BD Biosciences) for 30 min on ice, and the percentage of fluorescein isothiocyanate (FITC)-positive neutrophils (viable/CD45 positive) was calculated.
Accession number(s).
The antibodies have been deposited in GenBank under accession numbers KY249684 to KY249697 for the IgG antibodies and KY249698 to KY249704 for the IgA antibodies.
ACKNOWLEDGMENTS
AIMM Therapeutics employed E. Yasuda, K. Wagner, Y. B. Claassen, A. Q. Bakker, and T. Beaumont. The funders had no role in study design, data collection, and analysis, the decision to publish, or preparation of the manuscript, except that AIMM Therapeutics discovered, patented, and furthered the development of the described RSV G-specific antibodies. AIMM Therapeutics agreed to publish these results. The research was supported by the Amsterdam Economic Board (EZ1311, ALOHA grant).
We thank K. de Haan for excellent support in setting up the HAE cell cultures and H. Hilkmann for 12-mer peptide generation.
REFERENCES
- 1.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, Zhu Y, Patel A, Hymas W, Chappell JD, Kaufman RA, Kan JH, Dansie D, Lenny N, Hillyard DR, Haynes LM, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, Wunderink RG, Edwards KM, Pavia AT, McCullers JA, Finelli L, CDC EPIC Study Team. 2015. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilman MS, Moin SM, Mas V, Chen M, Patel NK, Kramer K, Zhu Q, Kabeche SC, Kumar A, Palomo C, Beaumont T, Baxa U, Ulbrandt ND, Melero JA, Graham BS, McLellan JS. 2015. Characterization of a prefusion-specific antibody that recognizes a quaternary, cleavage-dependent epitope on the RSV fusion glycoprotein. PLoS Pathog 11:e1005035. doi: 10.1371/journal.ppat.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resch B, Gusenleitner W, Nuijten MJ, Lebmeier M, Wittenberg W. 2008. Cost-effectiveness of palivizumab against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther 30:749–760. doi: 10.1016/j.clinthera.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks DA, McIntosh K, Patterson JL. 1988. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol 62:2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng MN, Whitehead SS, Collins PL. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283–296. doi: 10.1006/viro.2001.1138. [DOI] [PubMed] [Google Scholar]
- 8.Widjojoatmodjo MN, Boes J, van Bers M, van Remmerden Y, Roholl PJ, Luytjes W. 2010. A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats. Virol J 7:114. doi: 10.1186/1743-422X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PR, Spriggs MK, Olmsted RA, Collins PL. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A 84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langedijk JP, Schaaper WM, Meloen RH, van Oirschot JT. 1996. Proposed three-dimensional model for the attachment protein G of respiratory syncytial virus. J Gen Virol 77(Pt 6):1249–1257. [DOI] [PubMed] [Google Scholar]
- 11.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA. 2010. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. 1997. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91:521–530. doi: 10.1016/S0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, Walsh EE, Peeples ME. 2015. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog 11:e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chirkova T, Lin S, Oomens AG, Gaston KA, Boyoglu-Barnum S, Meng J, Stobart CC, Cotton CU, Hartert TV, Moore ML, Ziady AG, Anderson LJ. 2015. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen Virol 96:2543–2556. doi: 10.1099/vir.0.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong KI, Piepenhagen PA, Kishko M, DiNapoli JM, Groppo RP, Zhang L, Almond J, Kleanthous H, Delagrave S, Parrington M. 2015. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS One 10:e0130517. doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, Piralla A, Percivalle E, Sallusto F, Baldanti F, Lanzavecchia A. 2013. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 19.Chan CH, Hadlock KG, Foung SK, Levy S. 2001. V(H)1-69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood 97:1023–1026. doi: 10.1182/blood.V97.4.1023. [DOI] [PubMed] [Google Scholar]
- 20.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. 2001. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167:2861–2868. doi: 10.4049/jimmunol.167.5.2861. [DOI] [PubMed] [Google Scholar]
- 21.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, Tripp RA, Walsh EE, Keyt BA, Kauvar LM. 2009. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 183:6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 22.Misasi J, Gilman MS, Kanekiyo M, Gui M, Cagigi A, Mulangu S, Corti D, Ledgerwood JE, Lanzavecchia A, Cunningham J, Muyembe-Tamfun JJ, Baxa U, Graham BS, Xiang Y, Sullivan NJ, McLellan JS. 2016. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 351:1343–1346. doi: 10.1126/science.aad6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietersz GA, Wenjun L, Krauer K, Baker T, Wreschner D, McKenzie IF. 1997. Comparison of the biological properties of two anti-mucin-1 antibodies prepared for imaging and therapy. Cancer Immunol Immunother 44:323–328. doi: 10.1007/s002620050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otterstrom JJ, Brandenburg B, Koldijk MH, Juraszek J, Tang C, Mashaghi S, Kwaks T, Goudsmit J, Vogels R, Friesen RH, van Oijen AM. 2014. Relating influenza virus membrane fusion kinetics to stoichiometry of neutralizing antibodies at the single-particle level. Proc Natl Acad Sci U S A 111:E5143–E5148. doi: 10.1073/pnas.1411755111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 26.Gilman MSA, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, Mas V, Melero JA, Wright PF, Graham BS, McLellan JS, Walker LM. 2016. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 1(6):eaaj1879. doi: 10.1126/sciimmunol.aaj1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang GY, Druz A, Georgiev IS, Rundlet EJ, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Nason MC, Capella C, Peeples ME, Ledgerwood JE, McLellan JS, Kwong PD, Graham BS. 2015. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauvar LM, Collarini EJ, Keyt B, Foord O. 24 October 2008, filing date Anti-RSV G protein antibodies. Patent WO2009055711.
- 29.Feng JQ, Mozdzanowska K, Gerhard W. 2002. Complement component C1q enhances the biological activity of influenza virus hemagglutinin-specific antibodies depending on their fine antigen specificity and heavy-chain isotype. J Virol 76:1369–1378. doi: 10.1128/JVI.76.3.1369-1378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW. 2014. Complement is activated by IgG hexamers assembled at the cell surface. Science 343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain P-O, Schandene L, Casartelli N, Rameix-Welti M-A, Hervé P-L, Dériaud E, Beitz B, Ripaux-Lefevre M, Miatello J, Lemercier B, Lorin V, Descamps D, Fix J, Eléouët J-F, Riffault S, Schwartz O, Porcheray F, Mascart F, Mouquet H, Zhang X, Tissières P, Lo-Man R. 2017. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 46:301–314. doi: 10.1016/j.immuni.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, van Bleek GM, Widjojoatmodjo MN, Bogers WM, Mei H, Radbruch A, Scheeren FA, Spits H, Beaumont T. 2010. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med 16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallak LK, Spillmann D, Collins PL, Peeples ME. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74:10508–10513. doi: 10.1128/JVI.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulcher ML, Randell SH. 2013. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 945:109–121. [DOI] [PubMed] [Google Scholar]
- 36.Wagner K, Kwakkenbos MJ, Claassen YB, Maijoor K, Bohne M, van der Sluijs KF, Witte MD, van Zoelen DJ, Cornelissen LA, Beaumont T, Bakker AQ, Ploegh HL, Spits H. 2014. Bispecific antibody generated with sortase and click chemistry has broad antiinfluenza virus activity. Proc Natl Acad Sci U S A 111:16820–16825. doi: 10.1073/pnas.1408605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zielinska E, Liu D, Wu HY, Quiroz J, Rappaport R, Yang DP. 2005. Development of an improved microneutralization assay for respiratory syncytial virus by automated plaque counting using imaging analysis. Virol J 2:84. doi: 10.1186/1743-422X-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Pineros YS, Lutter R, van Woensel JB, Bem RA. 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol 238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 39.Gillissen MA, Yasuda E, de Jong G, Levie SE, Go D, Spits H, van Helden PM, Hazenberg MD. 2016. The modified FACS calcein AM retention assay: a high throughput flow cytometer based method to measure cytotoxicity. J Immunol Methods 434:16–23. doi: 10.1016/j.jim.2016.04.002. [DOI] [PubMed] [Google Scholar]