ABSTRACT
The antiviral effects of hepatitis C virus (HCV)-specific CD8 T cells have been shown in an HCV replicon system but not in an authentic infectious HCV cell culture (HCVcc) system. Here, we developed tools to examine the antigenicity of HCV-infected HLA-A2-positive Huh7.5 hepatoma cells (Huh7.5A2 cells) in activating HCV-specific CD8 T cells and the downstream antiviral effects. Infectious HCV epitope mutants encoding the well-defined genotype 1a-derived HLA-A2-restricted HCV NS3-1073 or NS5-2594 epitope were generated from a genotype 2a-derived HCV clone (Jc1Gluc2A) by site-directed mutagenesis. CD8 T-cell lines specific for NS3-1073 and NS5-2594 were expanded from HCV-seropositive persons by peptide stimulation in vitro or engineered from HCV-seronegative donor T cells by transduction of a lentiviral vector expressing HCV-specific T-cell receptors. HCV-specific CD8 T cells were cocultured with Huh7.5 cells that were pulsed with titrating doses of HCV epitope peptides or infected with HCV epitope mutants. HCV-specific CD8 T-cell activation (CD107a, gamma interferon, macrophage inflammatory protein 1β, tumor necrosis factor alpha) was dependent on the peptide concentrations and the relative percentages of HCV-infected Huh7.5A2 cells. HCV-infected Huh7.5A2 cells activated HCV-specific CD8 T cells at levels comparable to those achieved with 0.1 to 2 μM pulsed peptides, providing a novel estimate of the level at which endogenously processed HCV epitopes are presented on HCV-infected cells. While HCV-specific CD8 T-cell activation with cytolytic and antiviral effects was blunted by PD-L1 expression on HCV-infected Huh7.5A2 cells, resulting in the improved viability of Huh7.5A2 cells, PD-1 blockade reversed this effect, producing enhanced cytolytic elimination of HCV-infected Huh7.5A2 cells. Our findings, obtained using an infectious HCVcc system, show that the HCV-specific CD8 T-cell function is modulated by antigen expression levels, the percentage of HCV-infected cells, and the PD-1/PD-L1 pathways and has antiviral and cytotoxic effects.
IMPORTANCE We developed several novel molecular and immunological tools to study the interactions among HCV, HCV-infected hepatocytes, and HCV-specific CD8 T cells. Using these tools, we show the level at which HCV-infected hepatoma cells present endogenously processed HCV epitopes to HCV-specific CD8 T cells with antiviral and cytotoxic effects. We also show the marked protective effect of PD-L1 expression on HCV-infected hepatoma cells against HCV-specific CD8 T cells.
KEYWORDS: CD8 T cells, Huh7.5 cells, lentiviral transduction, HCV-specific TCR, coculture, PD-1 blockade
INTRODUCTION
CD8 T cells are critical antiviral effector cells that mediate both viral clearance and the pathogenesis of liver disease in hepatitis C virus (HCV) infection. In a chimpanzee model of acute hepatitis C, the onset of liver inflammation and viral decline coincided with the detection of increased levels of CD8 T cells in the liver (1), whereas CD8 T-cell depletion prolonged the period of viremia with attenuated liver inflammation upon reinoculation of previously HCV-immune animals (2). In patients with acute hepatitis C, successful viral clearance has been associated with a vigorous and broad HCV-specific CD8 T-cell response (3). For example, CD8 T cells mediated the gamma interferon (IFN-γ)-mediated noncytolytic control of HCV replication, and the relevance of cytotoxic T-lymphocyte escape variants was shown by direct coculture of HCV-specific CD8 T cells with Huh7 cells transfected with HCV replicons (4–6). In contrast, HCV persists with antiviral effector CD8 T-cell dysfunction (7–9).
T-cell dysfunction in chronic hepatitis C is associated with the induction of multiple intrinsic and extrinsic inhibitory pathways, such as the PD-1, CTLA-4, and Tim-3 pathways, as well as regulatory T cells and cytokines (10–14). These regulatory pathways also contribute to other chronic viral infections and tumor immunity. For example, PD-1 expression on CD8 T cells specific for HCV as well as hepatitis B virus (HBV) or human immunodeficiency virus (HIV) has been associated with functional impairment that can be restored by PD-1 blockade in vitro, with further synergy occurring through the CTLA-4, Tim-3, and 4-1BB pathways (10, 11, 14). The therapeutic effect of PD-1 blockade was also seen by a transient decline in the viral load with an enhanced intrahepatic HCV-specific CD4 and CD8 T-cell response in 1/3 chimpanzees (15). Furthermore, a single infusion of an anti-PD-1 monoclonal antibody resulted in a 4-log reduction in HCV RNA titers in 3/10 patients with chronic hepatitis C, including 1 that experienced an elevation of alanine aminotransferase levels to levels 17-fold above the upper limit of normal (16).
Interest in the development of immunotherapy for HCV infection has declined due to the availability of highly effective oral antivirals. However, interactions between antiviral CD8 T cells and virus-infected hepatocytes may be relevant to treatment approaches for other conditions, including liver cancer due to HCV or HBV, as well as chronic hepatitis B (17, 18). In this regard, differential levels of cytolytic and noncytolytic HCV suppression were displayed by T-cell receptor (TCR)-redirected human T cells targeting HCV NS3 and NS5 upon coculture with HCV replicon cells (19). However, little is known about CD8 T-cell interactions with Huh7.5 cells that are truly infected (rather than transfected) with HCV.
In this study, we wished to examine the interactions between HCV-specific CD8 T cells and HCV-infected Huh7.5 cells and to define the factors involved in HCV-specific CD8 T-cell activation with antiviral and cytolytic effects. To this end, we established a coculture system with HCV-infected HLA-A2-positive (HLA-A2+) Huh7.5 cells (Huh7.5A2 cells) and CD8 T cells specific for 2 different HLA-A2-restricted HCV epitopes that were naturally expanded from HCV-seropositive persons or engineered by transduction of a lentiviral vector expressing the HCV-specific TCR. We further examined the impact of the PD-1/PD-L1 pathway on these interactions.
RESULTS
Natural and engineered CD8 T cells are activated by HLA-A2-transduced Huh7.5 cells presenting exogenously pulsed cognate HCV epitope peptides.
To develop a coculture system with HCV-infected hepatoma cells and well-defined HLA-A2-restricted HCV-specific CD8 T cells, HCV-permissive Huh7.5 cells were transduced with a lentivirus that encodes HLA-A2 (Fig. 1A). The resulting HLA-A2+ Huh7.5 cells (Huh7.5A2 cells) could present exogenously pulsed known HLA-A2-restricted HCV epitope peptides to activate HCV-specific CD8 T cells in a peptide-specific manner (Fig. 2). Figure 2A shows HLA-A2/peptide tetramer staining (left) and the peptide-specific activation (right) of CD8 T cells with a natural HCV-specific TCR (nT cells) that were expanded from spontaneous HCV resolvers. CD8 T cells isolated from healthy HCV-seronegative donors were also engineered to express HCV-specific TCR by in vitro transduction with lentiviruses that encode HCV-specific T-cell receptors, as previously described (20, 21). These CD8 T cells with an engineered HCV-specific TCR (eT cells) could also bind specific HLA-A2/peptide tetramers with peptide-specific chemokine production and CD107a mobilization (Fig. 2B), thereby enabling the use of seronegative donor T cells in our study. Both nT cells and eT cells were used in our study.
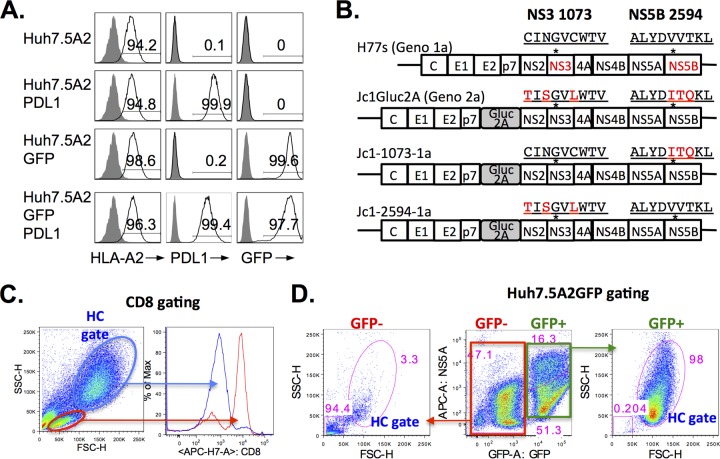
FIG 1.
Huh7.5 target cell lines and HCV clones and their derivatives. (A) High-level expression of HLA-A2, PD-L1, and/or GFP in transduced Huh7.5 cell lines. Huh7.5 cells were transduced with lentiviruses to express HLA-A2, PD-L1, and GFP and further purified by FACS. A high level of expression of greater than 94% was confirmed by FACS. (B) Full-length HCV clones and their variants. The H77s (genotype [Geno] 1a) construct was kindly provided by Stanley Lemon (University of North Carolina) and Min-Kyung Yi (University of Texas, Galveston, TX). The Jc1Gluc2A (genotype 2a) construct was kindly provided by Brett Lindenbach (Yale University) and Charles Rice (Rockefeller University). Jc1-1073-1a and Jc1-2594-1a were derived from the Jc1Gluc2A clone by site-directed mutagenesis and encode immunogenic genotype 1a-derived HLA-A2-restricted CD8 T-cell epitopes NS3-1073 and NS5B-2594, respectively. Asterisks overlying the schematic map of HCV constructs indicate the general locations of NS3-1073 and NS5B-2594 epitopes. The amino acid sequences of NS3 from residues 1073 to 1082 and NS5 from residues 2594 to 2602 are shown, with the genotype 1a-derived sequence being shown in black font and the genotype 2a-derived sequences being shown in red font. Correct substitutions were confirmed by sequence analysis. (C) Gating strategy for CD8 T cells following coculture. The red gate shows that the population in the conventional lymphoid/live gate is enriched for CD8 T cells, whereas the blue gate (the hepatocyte [HC] gate), which has higher FSC-H/SSC-H values, mostly contains CD8-negative cells. (D) Identification of Huh7.5A2GFP target cells by GFP expression in coculture. The green box gate for GFP+ cells on the middle FACS panel identifies most events in the HC gate (right FACS panel) and HCV+ population. The red box gate for GFP− cells shows mostly low FSC-H/SSC-H values without HCV expression outside the HC gate, as shown in the left FACS panel. Numbers in the FACS plots indicate the frequency of the parent for gated cells.
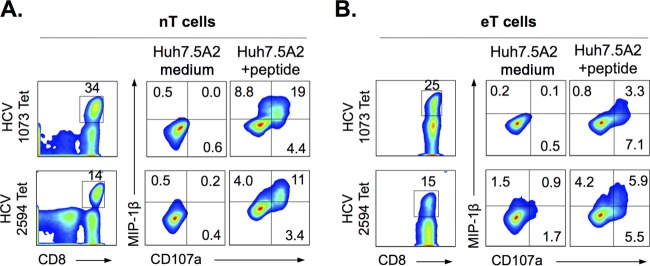
FIG 2.
Natural and engineered CD8 T cells are activated by HLA-A2-transduced Huh7.5 cells presenting exogenously pulsed cognate HCV epitope peptides. HCV-specific CD8 T cells specific for well-defined HLA-A2-restricted HCV NS3-1073 or NS5B-2594 epitopes were expanded from natural HCV resolvers (nT cells) (A) or engineered from seronegative donor by lentiviruses expressing HCV-specific T cell receptors (eT cells) (B), as shown by MHC/peptide tetramer (Tet) staining (left panels) as well as peptide-specific activation with CD107a mobilization and/or MIP-1β expression upon exposure to Huh7.5A2 cells pulsed exogenously with cognate peptides in vitro (right panels). To stimulate HCV-specific CD8 T cells, Huh7.5A2 cells were seeded overnight at 1 × 105 cells in a 48-well plate or 2 × 105 cells in a 24-well plate, pulsed with or without 10 μM cognate peptide for 1 h, and washed 3 times before the addition of 1 × 105 nT or eT cells. After 6 h of coculture, CD8 T cells were collected, washed, stained, and analyzed by FACS. Numbers in the FACS plots indicate the frequency of the parent for gated cells.
Natural and engineered HCV-specific CD8 T cells are activated by HCV-transfected or HCV-infected Huh7.5A2 cells that express endogenously processed and presented genotype 1a-derived cognate HCV epitopes.
We next examined if Huh7.5A2 cells are (i) permissive for HCV replication and infection and (ii) capable of processing and presenting endogenously synthesized HLA-A2-restricted HCV epitopes. As shown in Fig. 3A, Huh7.5A2 cells were readily transfected by genotype 2a-derived Jc1 and genotype 1a-derived H77s constructs. The culture supernatant of Jc1-transfected Huh7.5A2 cells could efficiently infect naive Huh7.5A2 cells, as shown in Fig. 3B, but minimal infectivity was shown for the culture supernatant of H77s-transfected Huh7.5A2 cells (data not shown). Nonetheless, HCV-specific CD8 T cells were activated upon coculture with H77s-transfected Huh7.5A2 cells (Fig. 3C), indicating that HCV epitopes can be endogenously processed and presented in the context of HLA-A2 by Huh7.5A2 cells.
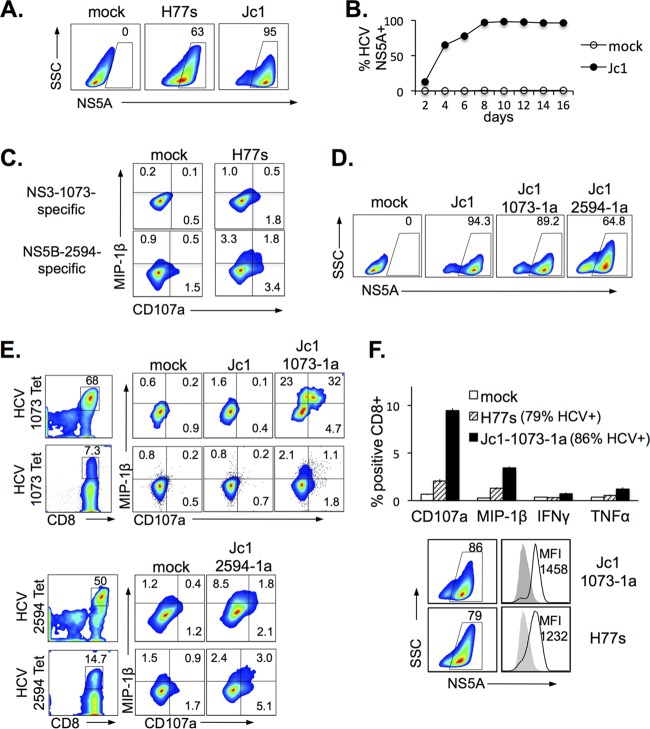
FIG 3.
Natural and engineered HCV-specific CD8 T cells are activated by HCV-transfected or HCV-infected Huh7.5A2 cells that express endogenously processed and presented genotype 1a-derived cognate HCV epitopes. (A) HCV expression, detected by anti-HCV NS5A, in Huh7.5A2 cells following transfection with the H77s (genotype 1a) or Jc1Gluc2A (genotype 2a) construct. (B) Kinetics of HCV NS5A expression in Huh7.5A2 cells after infection with the culture supernatant of Jc1Gluc2A-transfected Huh7.5A2 cells. (C) Representative results of FACS showing HCV-specific activation of HCV NS3-1073-specific (top) and HCV NS5B-2594-specific (bottom) nT cells upon coculture with HCV H77-transfected but not mock-transfected Huh7.5A2 cells. (D) Representative FACS showing HCV NS5A expression in Huh7.5A2 cells following infection with Jc1, Jc1-1073-1a, and Jc1-2594-1a. (E) Activation of CD8 T cells specific for genotype 1a-derived HCV NS3-1073 or NS5B-2594 by Huh7.5A2 cells infected with Jc1 epitope mutants but not with the original Jc1 clone. Results are shown for nT cells (top) and eT cells (bottom). NS3-1073- or NS5B-2594-specific CD8 T cells were detected by MHC/peptide tetramer (left) and activated by endogenous epitope presentation from HCV-infected (but not mock- or Jc1-infected) Huh7.5A2 cells (right). To examine HCV-specific CD8 T-cell activation, HCV-infected or -transfected Huh7.5A2 cells were seeded at 1 × 105 cells in a 48-well flat-bottom plate or 2 × 105 cells in a 24-well flat-bottom plate and incubated overnight to 24 h before coculture with 0.5 × 105 to 1 × 105 nT or eT cells for 6 h in 3 to 4 replicates, followed by FACS analysis. (F) (Top) Comparison of HCV-specific CD8 T-cell effector function between mock-infected, H77s-transfected (79% HCV+), and Jc1-1073-1a-infected (86% HCV+) Huh7.5A2 cells. Error bars indicate standard deviations. (Bottom) HCV expression of H77s-transfected and Jc1-1073-1a-infected Huh7.5A2 target cells by FACS multicolor plots and histogram overlays (the gray-shaded histogram represents the mock-infected control). Numbers in the FACS plots indicate the frequency of the parent for gated cells.
The infectious HCV clone Jc1 is based on genotype 2a and does not encode the immunogenic genotype 1a-derived NS3-1073 and NS5B-2594 sequences. Therefore, the Jc1 construct was modified by site-directed mutagenesis to encode the genotype-1a-derived NS3-1073 (CINGVCWTV) or NS5B-2594 (ALYDVVSKL) sequence as described in Materials and Methods. Huh7.5A2 cells could be transfected with these epitope mutants (designated Jc1-1073-1a and Jc1-2594-1a, respectively), and the mutants showed a high level of replication and infectivity similar to those of the original Jc1 strain (Fig. 3D). NS3-1073-specific CD8 T cells could be activated by Huh7.5A2 cells infected with Jc1-1073-1a but not with the original Jc1 virus (Fig. 3E). Similarly, NS5B-2594-specific CD8 T cells were readily activated by Jc1-2594-1a-infected Huh7.5A2 cells (Fig. 3E, bottom). Furthermore, Huh7.5A2 cells infected with Jc1-1073-1a were more immunogenic than those transfected with H77s (Fig. 3F, top), despite comparable HCV NS5A expression levels according to both the percentage of cells expressing NS5A and the mean fluorescence intensity (MFI) (Fig. 3F, bottom).
HCV-infected Huh7.5A2 cells activate HCV-specific CD8 T cells at levels comparable to those achieved with 0.1 to 2 μM peptide concentrations.
Little is known about the level of endogenous antigen presentation in HCV-infected cells. Therefore, we compared the levels of HCV-specific CD8 T-cell activation between cocultures with HCV-infected Huh7.5A2 cells (i.e., Jc1 epitope mutants) and cocultures with HCV-infected Huh7.5A2 cells pulsed with titrating doses of HCV peptides. As shown in Fig. 4A, HCV-specific CD8 T cells showed increasing effector function as the peptide concentration increased, although they were heterogeneous in their functional hierarchy (Fig. 4B). For example, engineered HCV NS3-1073-specific eT cells showed a greater IFN-γ response than CD107a mobilization, whereas the other two CD8 T-cell lines showed higher levels of macrophage inflammatory protein 1β (MIP-1β) expression and CD107a mobilization. As shown in Fig. 4B, maximal activation up to the level of the percentage of tetramer-positive (tetramer+) CD8 T cells (shown as dashed line) and/or a plateau could be reached at peptide concentrations of between 1 and 10 μM (Fig. 4B). Importantly, HCV-infected Huh7.5A2 cells activated HCV-specific CD8 T cells at levels comparable to those achieved with 0.1 to 2 μM peptide concentrations, providing novel estimates of the level of endogenous processing and presentation of two well-defined HLA-A2-restricted HCV CD8 T-cell epitopes in HCV-infected cells.
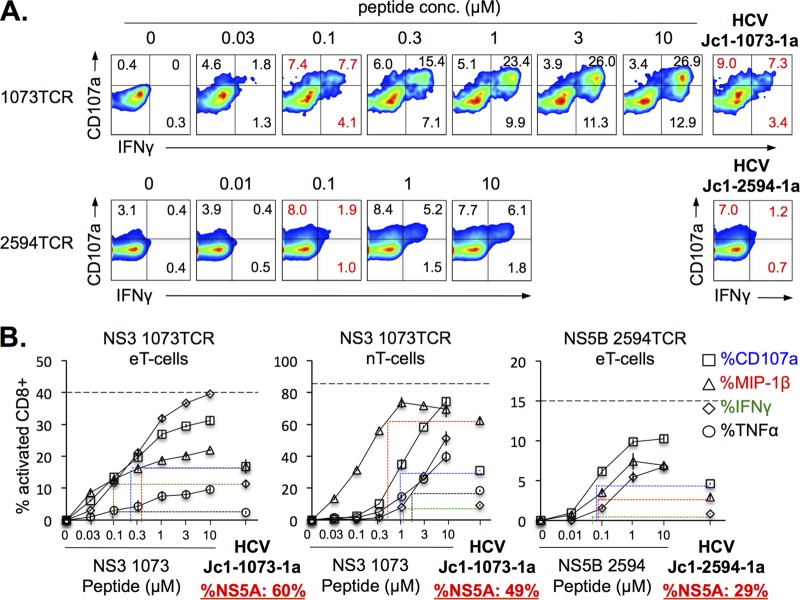
FIG 4.
HCV-infected Huh7.5A2 cells can activate HCV-specific CD8 T cells to levels comparable to those achieved with 0.1 to 2 μM peptide concentrations. (A) Representative FACS plots of NS3-1073-specific (top) and NS5B-2594-specific (bottom) TCR-transduced eT cells cocultured with Huh7.5A2 cells pulsed with various concentrations of peptides or with Huh7.5A2 cells infected by Jc1-1073-1a or Jc1-2594-1a (far right, top and bottom, respectively). Specifically, Huh7.5A2 cells were plated at 1 × 105 cells in a 48-well flat-bottom plate, cultured overnight, pulsed for 1 h with titrating doses of peptides or medium, and washed 3 times before 6 h of coculture with 0.5 × 105 nT or eT cells in 3 to 4 replicates (anti-CD107a was added at the start of coculture; GolgiStop protein transport inhibitor was added after 1 h), followed by surface and intracellular staining for FACS. (B) HCV-specific CD8 T-cell activation after coculture with Huh7.5A2 cells pulsed with titrating doses of HCV peptides and with HCV-infected Huh7.5A2 cells (29 to 60% HCV NS5A-positive cells). The y axes show HCV-specific CD8 T-cell activation levels. The x axes show peptide concentrations. The levels of activation of CD8 T cells cocultured with HCV-infected Huh7.5A2 cells are shown on the far right of each panel, with colored dotted lines extrapolating comparable CD8 T-cell activation levels and the corresponding peptide concentrations in cocultures with peptide-pulsed Huh7.5A2 cells. Error bars indicate standard deviations. The horizontal dashed lines show the percentage of tetramer-positive CD8 T cells in each eT cell or nT cell line. Numbers in the FACS plots indicate the frequency of the parent for gated cells.
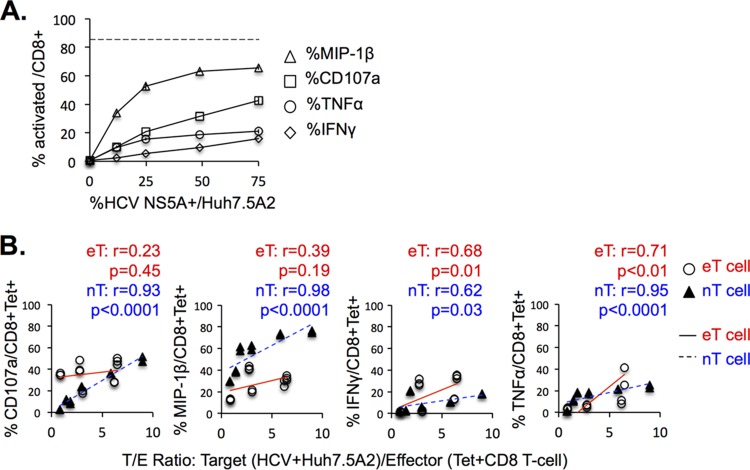
The level of HCV-specific CD8 T-cell activation is also associated with the relative percentage of HCV-infected Huh7.5A2 cells.
We examined if the percentage of HCV-positive (HCV+) Huh7.5A2 cells (as measured by flow cytometry) correlated with the HCV-specific effector function. As shown in Fig. 5A, HCV-specific IFN-γ, tumor necrosis factor alpha (TNF-α), and MIP-1β expression as well as CD107a mobilization increased as the frequency of HCV-infected Huh7.5A2 target cells increased. The HCV-specific CD8 T-cell effector function was also compared to target cell/effector cell (T/E) ratios, calculated as the number of HCV NS5A-positive Huh7.5A2 target T cells/number of HCV-specific tetramer+ CD8 effector T cells (Fig. 5B). As shown in Fig. 5B, the HCV-specific CD8 T-cell activation level increased as the T/E ratio increased. As an example, at a T/E ratio of 5 (i.e., five HCV-infected Huh7.5A2 target cells to one HCV-specific CD8 T cell), approximately ∼30% of HCV-specific CD8 T cells showed CD107a mobilization and ∼15% showed IFN-γ production (Fig. 5B). As shown in Fig. 5B, nT cells showed significant correlations between the T/E ratio and all four effector functions examined, whereas eT cells showed significant correlations for IFN-γ and TNF-α expression but not for MIP-1β expression or CD107a mobilization. Collectively, these findings suggest that HCV-specific CD8 T-cell activation is influenced by the relative frequency of HCV-infected target cells.
FIG 5.
The level of HCV-specific CD8 T-cell activation is associated with the percentage of HCV-infected Huh7.5A2 cells. (A) The HCV-specific effector function is compared to the percentage of cells expressing NS5A in cocultures with Huh7.5A2 cells (the percent NS5A expression was modified by serial dilution of HCV-infected Huh7.5A2 cells). (B) Comparison of HCV-specific CD8 effector T-cell function with the target cell/effector cell ratio (the ratio between the number of HCV NS5A-positive Huh7.5A2 target cells and the number of tetramer-positive CD8 effector T cells). Infected Huh7.5A2 cells were plated at 1 × 105 cells in a 48-well flat-bottom plate or 2 × 105 cells in a 24-well flat-bottom plate for 24 h and cocultured with 0.5 to 2 × 105 nT or eT cells for 6 h in 3 to 4 replicates. The number of HCV+ target cells per well was calculated from the total number of Huh7.5A2 cells plated times the percentage of HCV NS5A-positive Huh7.5A2 cells. The number of tetramer-positive CD8+ T cells was calculated from the percentage of tetramer-positive CD8 T cells times the number of CD8 T cells added per well. Results consisting of 25 data points from 8 independent experiments are shown. Open circles, eT cells (from three healthy donors); black triangles, nT cells (from one HCV resolver). P values were calculated by the nonparametric Spearman test.
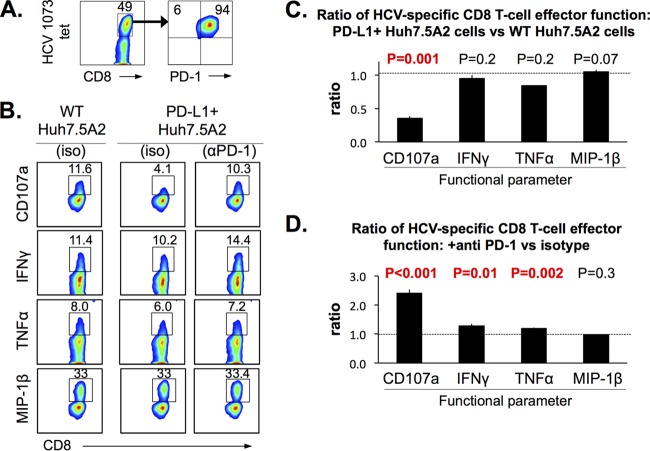
HCV-specific CD8 effector T-cell function levels are blunted by PD-L1 expression in Huh7.5A2 cells and enhanced by PD-1 blockade.
We then examined the effect of PD-1/PD-L1 interactions in our HCV coculture system, using HCV-specific CD8 T cells that express PD-1 and Huh7.5A2 cells with and without PD-L1 expression. Figure 6A shows a high level of PD-1 expression in NS3-1073-specific CD8 nT cells expanded with cognate peptide stimulation (without concurrent PD-L1 or PD-L2 expression; data not shown), whereas PD-L1 was expressed in 99.9% of PD-L1-positive (PD-L1+) Huh7.5A2 cells (Fig. 1A). Surprisingly, CD107a mobilization (but not IFN-γ, TNF-α, or MIP-1β expression) in NS3-1073-specific CD8 nT cells was significantly reduced in cocultures with PD-L1+ Huh7.5A2 cells compared to cocultures with wild-type (WT) Huh7.5A2 cells (Fig. 6B and C). This suggested that the PD-1/PD-L1 interaction has a greater impact on cytolytic capacity than cytokine production by HCV-specific CD8 T cells in this system.
FIG 6.
The HCV-specific CD8 effector T-cell function is blunted by PD-L1 expression in Huh7.5A2 cells and enhanced by PD-1 blockade. (A) A representative FACS plot showing high levels of PD-1 expression in the HCV-specific CD8+ T-cell line used in the assay. (B) Representative FACS plots of HCV-specific CD8 T cells cocultured with peptide-pulsed Huh7.5A2 cells without PD-L1 expression (WT Huh7.5A2) and with PD-L1 expression (PD-L1+ Huh7.5A2, 99.9% PD-L1+ cells) pretreated with isotype IgG (iso) or anti-PD-1 blocking antibody (αPD-1). (C) Ratio of the HCV-specific CD8 T-cell effector function upon coculture with PD-L1+ Huh7.5A2 cells and WT Huh7.5A2 cells. (D) Ratio of HCV-specific CD8 T-cell effector functions (CD107a mobilization and expression of IFN-γ, TNF-α, and MIP-1β) between cocultures of PD-L1+ Huh7.5A2 cells that were pretreated with anti-PD-1 or with isotype antibody. Huh7.5A2 cells were plated at 1 × 105 cells in a 48-well flat-bottom plate and incubated for 24 h before the addition of 0.3 × 105 nT cells for 6 h. The Huh7.5A2 cells in selected wells were pulsed with 10 μg/ml peptides for 1 h and washed 2 times with medium before the addition of T cells. Dotted horizontal lines indicate a ratio of 1.0. Error bars indicate standard deviations. P values were calculated by the Student t test (n = 3). Numbers in the FACS plots indicate the frequency of the parent for gated cells.
In contrast, pretreatment of CD8 T cells with anti-PD-1 before coculture with peptide-pulsed PD-L1+ Huh7.5A2 cells enhanced HCV-specific CD107a mobilization as well as IFN-γ and TNF-α production (Fig. 6D). As expected, PD-1 blockade did not promote a similar functional enhancement of PD-L1-negative Huh7.5A2 cells (data not shown). Although >90% of HCV-specific nT cells were CTLA-4 positive, CTLA-4 blockade did not enhance HCV-specific CD8 T-cell function since B7 expression was lacking in Huh7.5A2 cells in this system (data not shown).
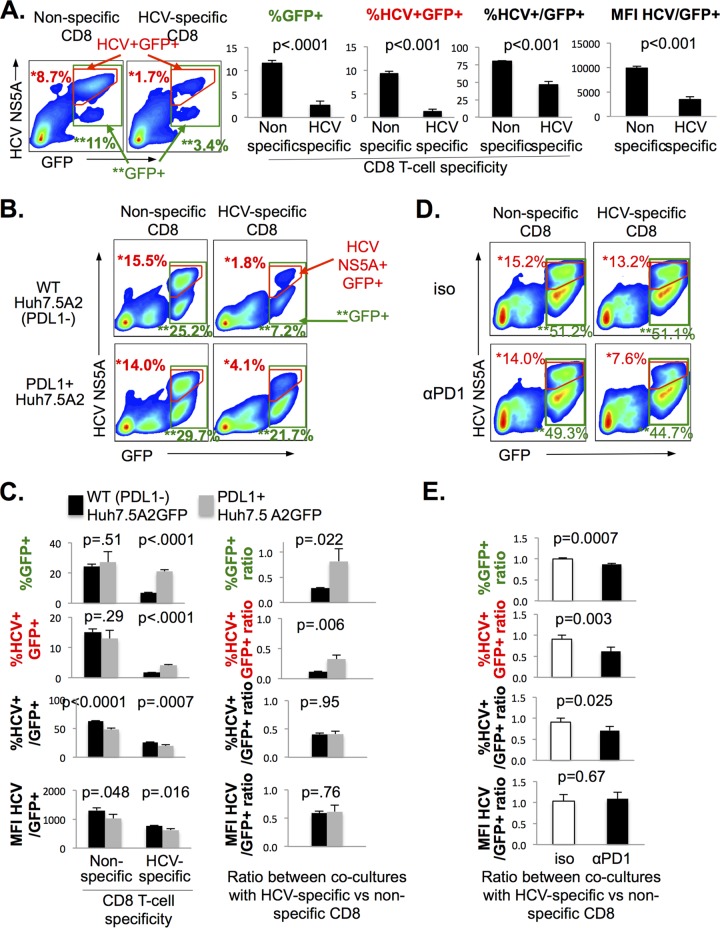
PD-L1 expression on HCV-infected Huh7.5A2 cells protects their viability in response to HCV-specific CD8 T cells.
Based on the cytolytic potential detected in HCV-specific CD8 T cells, we then compared the frequencies of and HCV expression levels (on a per cell basis) for HCV-infected Huh7.5A2 cells cocultured with HCV-specific and nonspecific CD8 T cells. To distinguish between viable Huh7.5A2 cells from CD8+ effector T cells and debris in the cocultures, Huh7.5A2 cells were transduced with green fluorescent protein (GFP) and sorted to a high purity (>99%) (Fig. 1A). As shown in Fig. 1D, GFP-positive (GFP+) and HCV+ cells were detected largely in the hepatocyte gate, whereas GFP-negative (GFP−) cells were smaller than hepatocytes according to their forward scatter (FSC) and side scatter (SSC) characteristics. As shown in representative fluorescence-activated cell sorting (FACS) plots and bar graphs in Fig. 7A, cocultures with HCV-specific CD8 T cells retained significantly fewer GFP+ cells (both total and HCV+ GFP+ cells) and showed lower levels of HCV expression in GFP+ cells on the basis of the percentage of HCV+/GFP+ cells and the MFI of HCV NS5A/GFP+ cells than cocultures with nonspecific CD8 T cells (Fig. 7A). These findings are consistent with the cytolytic elimination of HCV-infected GFP+ Huh7.5A2 cells by HCV-specific CD8 T cells.
FIG 7.
PD-L1 expression on HCV-infected Huh7.5A2 cells protects their viability in response to HCV-specific CD8 T cells. (A) Elimination of HCV-infected Huh7.5 cells by HCV-specific CD8 T cells following coculture. (Left) Representative FACS plots showing GFP and HCV NS5A expression in HCV Jc1-1073-1a-infected GFP-labeled Huh7.5A2 cells following coculture with nonspecific and HCV NS3-1073-specific CD8 T cells. Green-boxed gates are total GFP+ cells (consisting mostly of the population with high FSC/SSC characteristics consistent with hepatocytes, as shown in Fig. 1D). The red-outlined gate includes GFP+ cells that were also positive for HCV NS5A expression. (Right) Bar graphs show significantly reduced mean percentages of GFP+, HCV+ GFP+, and HCV+/GFP+ cells and the MFI of HCV NS5A/GFP+ cells in cocultures with HCV-specific CD8 T cells compared to those of nonspecific CD8 T cells. Error bars indicate standard deviations, and P values were calculated by the Student t test (n = 3). (B) Representative FACS plots of HCV-infected WT and PD-L1+ Huh7.5A2GFP target cells after coculture with nonspecific or HCV-specific CD8 T cells. (C) (Left) Bar graphs compare the average percentages of GFP+ cells in the hepatocyte gate, HCV+ GFP+ cells in the hepatocyte gate, and HCV+ cells in GFP+ cells in the hepatocyte gate, and the MFI of HCV+ GFP+ cells between cocultures with HCV-specific and nonspecific CD8 T cells with WT (black bars) and PD-L1+ (gray bars) Huh7.5A2GFP-positive target cells. The percentages of GFP+ and HCV+ GFP+ target cells were greater when HCV-specific CD8 T cells were cocultured with PD-L1+ than WT Huh7.5A2GFP target cells. (Right) Bar graphs show the average ratios for the percentages of GFP+ cells in the hepatocyte gate, HCV+ GFP+ cells in the hepatocyte gate, and HCV+ cells in GFP+ cells, and the MFI of HCV+ GFP+ cells between cocultures with HCV-specific and nonspecific CD8 T cells. PD-L1 expression was associated with increased ratios between cocultures with HCV-specific and nonspecific CD8 T cells for the percentages of GFP+ and HCV+ GFP+ cells but not for the percentages of HCV+ cells among GFP+ cells or the MFI of HCV+ GFP+ cells. Error bars indicate standard deviations, and P values were calculated by the Student t test (n = 3). (D) (Top) Representative FACS plots of HCV-infected PD-L1+ Huh7.5A2 cells following coculture with nonspecific or HCV-specific CD8 T cells that were pretreated with isotype or anti-PD-1 (αPD1) antibodies. (E) Bar graphs compare average ratios for the percentages of GFP+, HCV+ GFP+, and HCV+/GFP+ cells and the MFI of HCV+ GFP+ cells between cocultures with HCV-specific or nonspecific CD8 T cells. Error bars indicate standard deviations, and P values were calculated by the Student t test (n = 4). In these experiments, Huh7.5A2 cells were plated at 1 × 105 cells in a 24-well flat-bottom plate overnight, followed by the addition of 1 × 106 nT cells for 24 h of coculture. Numbers in the FACS plots indicate the frequency of the parent for gated cells.
We then examined if PD-L1 expression in Huh7.5A2 target cells modified their elimination by CD8 T cells. As shown in Fig. 7B, the percentages of GFP+ and HCV+ GFP+ cells were significantly higher for PD-L1+ than WT Huh7.5A2 cells following coculture with HCV-specific CD8 T cells (P < 0.0001). These differences persisted when they were compared as the ratios between cocultures with HCV-specific and nonspecific CD8 T cells, suggesting that PD-L1 expression on HCV-infected Huh7.5A2 cells protects against their elimination by HCV-specific CD8 T cells. However, PD-L1 expression on Huh7.5A2 cells was not associated with increased HCV expression (by both the percentage of HCV+/GFP+ cells and the MFI of HCV NS5A/GFP+ cells), suggesting that PD-L1 may have a greater impact against the cytotoxic activity than the antiviral activity of HCV-specific CD8 T cells in our system.
To examine if PD-1 blockade may reverse the cytoprotective effect of PD-L1, Jc1-1073-1a-infected PD-L1+ Huh7.5A2 cells were cocultured with HCV NS3-1073-specific or nonspecific CD8 T cells that had been pretreated with anti-PD-1 blocking or isotype antibody. As shown in Fig. 7D and E, anti-PD-1 pretreatment of HCV-specific CD8 T cells significantly reduced the total percentages of GFP+, HCV+ GFP+, and HCV+/GFP+ cells among Huh7.5A2 cells but did not have a significant effect on HCV expression according to the MFI. Similar results were obtained in three independent experiments with various T/E ratios (data not shown). Thus, PD-L1 expression on HCV-infected cells promoted the survival of HCV-infected Huh7.5A2 cells by attenuating the HCV-specific CD8 T-cell cytolytic effector function, whereas PD-1 blockade enhanced the cytolytic elimination of HCV-infected cells.
DISCUSSION
Much is known about the phenotype, function, and mechanisms of dysfunction of HCV-specific CD8 T cells isolated from HCV-infected patients (22). However, due to a lack of convenient in vitro and in vivo models of HCV infection, there are limited data regarding the direct impact of HCV-specific CD8 T cells on HCV and HCV-infected hepatocytes. For example, CD8 T-cell-mediated cytolytic and noncytolytic control of HCV replication was shown in vitro using HCV-specific CD8 T cells from an HCV-infected patient with HCV replicon cells (4, 5), including a study examining CD8 T-cell responses to well-defined HLA-A2-restricted HCV epitopes expressed by HLA-A2-transduced HCV replicon cells. More recently, the antiviral efficacy of HCV-specific CD8 T cells against HCV replicon cells was associated with their expression of not only IFN-γ but also CD127 and PD-1 (23). In our study, we examined the antiviral effect of HCV-specific CD8 T cells and their regulation in an authentic infectious HCV cell culture (HCVcc) system using (i) infectious HCV clones encoding well-defined HCV CD8 T-cell epitopes and (ii) HCV-specific CD8 T cells expanded from HCV resolvers with natural TCR or engineered from healthy controls by transduction of the lentiviral vector expressing HCV-specific TCR.
A key feature of our study was the use of an infectious HCVcc system. Although it was not directly compared with an HCV replicon system, the HCVcc system may more closely resemble the in vivo scenario due to continued viral spread and adaptation. Notably, genotype 2a-derived Jc1 mutant clones retained their infectivity and replicative capacity, despite genotypic substitutions of NS3-1073 or NS5A-2594 epitope sequences. Consistent with the mutable quasispecies nature of HCV, epitope mutations were tolerated without compromising infectivity and replicative capacity. Moreover, the substituted genotype 1a epitope sequences were processed and presented in the context of HLA-A2, resulting in the activation of HCV-specific CD8 T cells in an epitope-specific and HLA-A2-restricted manner. This was not bystander activation, since HCV-specific CD8 T-cell activation did not occur in Huh7.5A2 cells infected with wild-type genotype 2a virus or HLA-A2-negative Huh7.5 cells.
Our HCVcc system provided a novel opportunity to examine the level at which HCV-infected hepatocytes can present endogenously processed HCV epitopes and activate HCV-specific CD8 T cells. HCV-specific CD8 T cells showed a dose-dependent increase in antigen-specific effector function when they were cocultured with Huh7.5 cells pulsed with titrating doses of exogenous HCV peptides. This was not due to expansion of HCV-specific CD8 T cells from HCV-seropositive donors with a diverse affinity for TCR, since a similar dose dependence was also observed for engineered CD8 T cells expressing the same HCV-specific TCR. Compared to peptide-pulsed Huh7.5A2 cells, HCV-infected Huh7.5A2 cells activated HCV-specific CD8 T cells at levels similar to those achieved with 0.1 to 2 μM peptide concentrations (Fig. 4). Huh7.5A2 cells infected with the Jc1-1073-1a epitope mutant were more immunogenic than H77s-transfected Huh7.5A2 cells, despite similar HCV expression levels (Fig. 3F). Increased HCV-specific CD8 T-cell activation by Jc1 virus was also intriguing since Jc1 was derived from the JFH1 clone, which was isolated from a unique patient with fulminant hepatitis C. While Huh7.5 cells are not primary hepatocytes and have a deficient interferon response, our findings show that they can efficiently process and present HCV epitopes, including the genotype 1a-derived HCV NS3-1073 epitope in the context of a genotype 2a-derived flanking sequence.
We speculate that the percentage of HCV-infected hepatocytes and their levels of endogenous HCV epitope expression are lower in vivo in HCV-infected patients than in the HCVcc system in vitro. Indeed, the estimated percentage of HCV-infected hepatocytes in patients with chronic hepatitis C varies widely and ranges from 1% to 50% in association with serum HCV RNA titers (24), whereas the percentages of HCV+ Huh7.5 cells were generally higher (29 to 95%) in our assays. Since the HCV-specific CD8 T-cell activation level was associated with the available number of HCV-infected target cells (Fig. 4), we further speculate that only a small percentage of HCV-specific CD8 T cells may be fully activated in vivo due to the limited number of HCV-infected cells with high levels of epitope expression, thus contributing to further HCV persistence.
Another distinct aspect of our study was the use of CD8 T cells that were transduced by lentiviruses to express T-cell receptors specific for well-defined HLA-A2-restricted HCV-specific CD8 T-cell epitopes. These engineered HCV-specific eT cells were derived with variable transduction efficiencies (15 to 40%) from total CD8 T cells from HCV-seronegative healthy donors. Similar to CD8 T cells transduced with TCRs specific for HIV and HBV (18, 21), HCV-specific eT cells showed antigen-specific activation and antiviral activities that were comparable to those of nT cells. The eT cells from healthy seronegative donors and nT cells from spontaneous HCV resolvers may be more functionally competent than functionally exhausted HCV-specific CD8 T cells in patients with chronic hepatitis C (10–14), despite the high level of induction of PD-1 and CTLA-4 expression after in vitro expansion. Somewhat surprisingly, in our system, HCV-specific CD8 T cells exerted a more cytolytic function (e.g., CD107a mobilization, elimination of HCV-infected GFP+ Huh7.5 cells) than an antiviral function. These eT cells (and lentiviruses expressing HCV-specific TCRs) may prove useful in further probing host-virus interactions and their regulation to dissect their various functions. Furthermore, while their use against HCV in this era of highly effective direct antiviral drugs is unlikely (25), TCR-engineered T cells and other immunotherapeutic approaches provide exciting new avenues to treat cancer, chronic infection, and autoimmunity (26, 27).
In conclusion, we developed a novel system to examine CD8 T-cell interactions with an infectious HCVcc system using both naturally expanded and engineered CD8 T cells. We further show that the HCV-specific CD8 T-cell function is modulated by antigen expression levels, the percentage of HCV-infected cells, and the PD-1/PD-L1 pathways and has antiviral and cytotoxic effects. These findings with the HCVcc system may be relevant in HCV immune pathogenesis and have potential relevance to the development of immunotherapeutics against chronic infection and cancer.
MATERIALS AND METHODS
Research subjects as sources for T-cell cocultures.
The research subjects were recruited from the Liver Clinic at the Philadelphia Corporal Michael J. Crescenz Veterans Affairs (VA) Medical Center (CMCVAMC) and the Clinical Translational Research Center at the University of Pennsylvania and provided informed consent. The study was approved by the institutional review board of each of those institutions. The subjects included three anti-HCV-positive, HCV RNA-negative spontaneous HCV resolvers previously shown to be HLA-A2+ and to have an HLA-A2-restricted CD8 T-cell response to the NS3-1073 and NS5B-2594 epitopes (7, 10). In addition, eight healthy HCV-seronegative control subjects without a known liver disease were included. We excluded persons who had had acute or chronic hepatitis C within the previous 1 year and individuals with human immunodeficiency virus (HIV) or hepatitis B virus (HIV) infection, autoimmune diseases, or conditions that precluded phlebotomy.
Lymphocyte isolation.
Peripheral blood lymphocytes (PBLs) were isolated from blood by density centrifugation using Ficoll-Histopaque (Sigma-Aldrich, St. Louis MO) before use, as previously described (28, 29).
Expansion of HCV-specific nT cells from HCV-seropositive subjects.
Short-term HCV-specific CD8 T-cell lines were established from spontaneous HCV resolvers by stimulating their PBLs with antigenic peptides (10 μM) and recombinant interleukin-2 (rIL-2; 100 IU/ml), with the further addition of rIL-2 every 3 to 4 days and weekly restimulation using autologous feeder cells as previously described (30). HCV-specific CD8 T cells were detected by flow cytometry using major histocompatibility complex (MHC)/peptide tetramers and/or intracellular cytokine staining (11). These CD8 T cells with a natural HCV-specific TCR (nT cells) showed HLA-A2-restricted and peptide-specific activation upon coculture with HLA-A2+ antigen-presenting cells.
Lentiviral vectors expressing HCV-specific TCR.
HCV-specific TCR α and β chains were isolated and cloned into a lentiviral vector to transduce primary CD8 T cells as described before (20, 21). Briefly, HCV-specific CD8 T cells were expanded from the PBLs of HCV resolvers by NS3-1073 or NS5B-2594 peptide stimulation, stained with specific MHC/peptide tetramers, and sorted by flow cytometry to >90% purity. The resulting HCV-specific CD8 T cells were used to generate a cDNA library to isolate HCV-specific TCR α and β chains, which were cloned into the lentiviral vector.
Expansion of HCV-specific eT cells from healthy control CD8 T cells by transduction of a lentiviral vector expressing HCV-specific TCR.
HCV-seronegative healthy donor CD8 T cells were purified with a CD8 T-cell isolation kit (Miltenyi Biotech), stimulated overnight with anti-human CD3/CD28 beads (Invitrogen) in a cell/bead ratio of 1:1 and 100 IU/ml rIL-2, and transduced with the lentiviral vector expressing the TCR specific for HCV NS3-1073 or NS5B-2594 (multiplicity of infection, 50) in the presence of 8 μg/ml SureENTRY transduction reagent (SABiosciences). Transduced cells were cultured in 5% CO2 at 37°C. The cell concentration was maintained at 0.5 million to 1 million cells per ml with frequent medium changes and the addition of rIL-2 (100 IU/ml) every 2 to 3 days. Anti-human CD3/CD28-specific beads were removed on day 5. The HCV-specific T-cell frequency was determined after 7 to 10 days of culture by the use of an MHC/peptide tetramer specific for HCV NS3-1073 or HCV NS5B-2594 (3, 31). These CD8 T cells with an engineered HCV-specific TCR (eT cells) showed HLA-A2-restricted and peptide-specific activation upon coculture with HLA-A2+ antigen-presenting cells.
HCV-replicating Huh7.5 hepatoma cells.
Huh7.5 cells (generously provided by Charles Rice, Rockefeller University) were transduced with a lentiviral vector expressing HLA-A2 (pELNS HLA-A2) (32) and further purified by flow cytometry with stable HLA-A2 expression (>94%) (Fig. 1). Further transduction of HLA-A2+ Huh7.5 cells with lentiviral vectors expressing GFP and/or PD-L1 (pELNS GFP and pELNS PD-L1) resulted in three additional cell lines, the Huh7.5A2PDL1, Huh7.5A2GFP, and Huh7.5A2GFP+PDL1 cell lines, with >97% stable expression (Fig. 1A).
Full-length HCV clones.
HCV plasmids included genotype 2a-derived Jc1Gluc2A (called Jc1 in this article) (33) expressing the Gaussia luciferase from Brett Lindenbach (Yale University) and Charles Rice (Rockefeller University, New York, NY), genotype 1a-derived H77s from Min-Kyung Yi (University of Texas, Galveston, TX) (34), and the genotype 2a-derived JFH1 clone from Takaji Wakita (National Institute of Infectious Diseases, Japan) (35). The Jc1Gluc2A clone was further modified by site-directed mutagenesis to express well-defined genotype 1a-derived HLA-A2-restricted HCV CD8 T-cell epitope NS3-1073 (CINGVCWTV) or NS5B-2594 (ALYDVVSKL) derived from H77s using a QuikChange II XL site-directed mutagenesis kit (Agilent Technologies), resulting in clones Jc1-1073-1a and Jc1-2594-1a, respectively. The forward and reverse primers used for site-directed mutagenesis were as follows: NS3-1073F (5′-GTCCTTCCTCGGAACATGCATCAATGGGGTATGCTGGACTGTCTACCACGGAGCTGGC-3′), NS3-1073R (5′-GCCAGCTCCGTGGTAGACAGTCCAGCATACCCCATTGATGCATGTTCCGAGGAAGGAC-3′), NS5B-2594F (5′-GAAAATGGCCCTCTATGACGTGGTTAGCAAGCTTCCTCAGGCGGTAATGG-3′), and NS5B-2594R (5′-CCATTACCGCCTGAGGAAGCTTGCTAACCACGTCATAGAGGGCCATTTTC-3′). The boldface in the sequences represents genotype 1a-derived sequences, and underlining represents optimum epitope sequences. Figure 1B shows the HCV constructs and the corresponding sequences of NS3-1073 and NS5B-2594.
To establish a culture supernatant with infectious HCV, HCV RNA was transcribed from the available HCV clones and electroporated into Huh7.5 cells as previously described (36). The culture supernatant was harvested, filtered through a 0.45-μm-pore-size filter, concentrated, examined for infectivity by determination of the 50% tissue culture infective dose, and stored in aliquots at −80°C.
Detection of HCV expression in Huh7.5 cells by flow cytometry.
HCV expression in HCV-infected or -transfected Huh7.5 cells was detected using a protocol adapted from Blight et al. (37). Briefly, Huh7.5 cells were (i) detached from the culture plate by adding trypsin-EDTA (0.05%) for 1 to 2 min, (ii) washed with culture medium, (iii) fixed with 2% paraformaldehyde (PFA) for 30 min, (iv) permeabilized by 0.1% saponin for 15 min, (v) stained with primary anti-NS5A antibody (monoclonal antibody 9E10, kindly provided by Charles Rice) for 30 min, (vi) washed twice with phosphate-buffered saline (PBS), and (vii) stained with the secondary antibody goat anti-mouse IgG (Alexa Fluor 647; Life Technologies) for 30 min. Cells were fixed with 2% PFA, acquired with a FACSCanto flow cytometer (Becton Dickinson), and analyzed with FlowJo software (TreeStar, Ashland, OR).
Coculture of HCV-specific CD8 T cells and HCV-expressing Huh7.5 target cells.
HCV-infected or uninfected Huh7.5 target cells were seeded (1 × 105 cells in a 48 well-plate or 2 × 105 cells in a 24-well plate) overnight before the addition of effector CD8 T cells at various target cell-to-tetramer+ effector cell ratios for 6 to 24 h of coculture. In selected experiments, Huh7.5 cells were pulsed with HCV peptide at various concentrations for at least 1 h and washed 3 times with complete medium before being cocultured with effector CD8 T cells.
CD8 T cells were examined for antigen-specific expression of IFN-γ, TNF-α, and MIP-1β as well as CD107a mobilization after 6 h of coculture/stimulation. Anti-CD107a was added at the start of coculture, whereas 0.7 μg/ml GolgiStop was added after 1 h of coculture. After coculture, nonadherent cells were aspirated from the plate, washed, and stained with live/dead cell stain (Invitrogen) and surface markers, followed by permeabilization, intracellular staining, and FACS analysis as previously described (11). In selected experiments, effector T cells were pretreated with blocking antibodies for 1 h before coculture. For analysis of Huh7.5 hepatoma cells, adherent Huh7.5 cells were detached from the culture plate by adding trypsin-EDTA (0.05%) for 1 to 2 min, washed with culture medium, and stained for HCV expression as described above.
Gating strategy in FACS analysis of cocultured CD8 T cells and Huh7.5 cells.
Acquired FACS data were analyzed using FlowJo software (TreeStar, Ashland, OR). HCV-specific CD8 T-cell frequency and function were examined by gating on live CD8 T cells. Figure 1D shows the enrichment of CD8 T cells in a lymphoid/live gate defined by forward scatter (FSC) and side scatter (SSC) appearance. To distinguish the Huh7.5 target cells from CD8 T cells and cellular debris in the cocultures, Huh7.5A2GFP cells (>99% GFP+ cells) were used to identify Huh7.5 target cells on the basis of GFP expression. As shown in Fig. 1D, most GFP+ cells were identified within the hepatocyte gate and had higher forward and side scatter values than GFP−, cells which showed low forward-scatter-height (FSC-H) and side-scatter-height (SSC-H) values, consistent with smaller cells or debris.
Detection of HCV replication in Huh7.5 cells.
Infected Huh7.5 cells were fixed with 2% PFA for 30 min, permeabilized by 0.1% saponin for 15 min, and then stained by primary anti-NS5A antibody (monoclonal antibody 9E10; kindly provided by Charles Rice) for 30 min. Cells were washed twice with PBS and secondarily stained with goat anti-mouse IgG (Alexa Fluor 647; Life Technologies) for 30 min. Cells were subsequently fixed again with 2% PFA and then acquired by FACS.
Antibodies and peptide.
Anti-human CD8 (conjugated to allophycocyanin [APC]-H7), CD107a (fluorescein isothiocyanate), IFN-γ (phycoerythrin [PE]-Cy7), MIP-1β (PE), and TNF-α (APC) were purchased from BD Biosciences. Anti-human CD3 (Alexa Fluor 700), HLA-A2 (APC), and PD-L1 (APC) were purchased from eBioscience. Purified anti-PD-1, purified mouse IgG1 isotype, and PD-1 (PE) were purchased from BioLegend. HCV HLA-A2-restricted NS3-1073 peptide (CINGVCWTV) and NS5B-2594 peptide (ALYDVVTKL) were synthesized by Invitrogen. MHC/peptide tetramers were purchased from Life Technologies.
Statistics.
Immunologic parameters were compared by the Student t test or nonparametric Wilcoxon test. Correlations were tested by the Spearman rank correlation test. P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
We thank Charles Rice (Rockefeller University, New York, NY), Brett Lindenbach (Yale University, New Haven, CT), Takaji Wakita (National Institute of Infectious Diseases in Japan), Min-Kyung Yi (University of Texas, Galveston, TX), and Stanley Lemon (University of North Carolina, Chapel Hill, NC) for generously sharing their valuable reagents and knowledge for our study; our study subjects and research staff who participated in this study, including Mary E. Valiga for recruitment of the study subjects; and Paul Hallberg, Hank Pletcher, and Jonni Moore at the Flow Cytometry and Cell Sorting Resource Laboratory of the University of Pennsylvania Abramson Cancer Center and the biosafety level 3 Cell Sorting Core Facility at the Children's Hospital of Philadelphia for support and technical expertise.
This work was supported by the resources and facilities at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, the NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases (P30DK50306) and its Molecular Biology and Cell Culture Core Facilities, and NIH Public Health Service research grant M01-RR00040. Funding support was provided through NIH grants AI47519 (to K.-M.C.), AA12849 (to K.-M.C.), and VA Merit Review award 5I01BX000649 (to K.-M.C.). H.C. was also supported through tumor virology training grant T32-CA-115299-09 at the University of Pennsylvania.
REFERENCES
- 1.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A 99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med 191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo J, Aichele U, Kersting N, Klein R, Aichele P, Bisse E, Sewell AK, Blum HE, Bartenschlager R, Lohmann V, Thimme R. 2009. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology 136:1391–1401. doi: 10.1053/j.gastro.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Zhu H, Tu Z, Xu YL, Nelson DR. 2003. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology 37:1335–1342. doi: 10.1053/jhep.2003.50207. [DOI] [PubMed] [Google Scholar]
- 6.Uebelhoer L, Han JH, Callendret B, Mateu G, Shoukry NH, Hanson HL, Rice CM, Walker CM, Grakoui A. 2008. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog 4:e1000143. doi: 10.1371/journal.ppat.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437–1448. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, Reddy KR, McKeating JA, Chang KM. 2007. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology 132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan DE, Sugimoto K, Ikeda F, Stadanlick J, Valiga M, Shetty K, Reddy KR, Chang KM. 2005. T-cell response relative to genotype and ethnicity during antiviral therapy for chronic hepatitis C. Hepatology 41:1365–1375. doi: 10.1002/hep.20706. [DOI] [PubMed] [Google Scholar]
- 10.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. 2008. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 134:1927–1937, 1937.e1–e2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, Tobias J, Kwok WW, Chang KM. 2008. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol 82:5043–5053. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. 2008. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol 48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. 2010. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest 120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, Ahmed R, Walker CM. 2013. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc Natl Acad Sci U S A 110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. 2013. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One 8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sastry KS, Too CT, Kaur K, Gehring AJ, Low L, Javiad A, Pollicino T, Li L, Kennedy PT, Lopatin U, Macary PA, Bertoletti A. 2011. Targeting hepatitis B virus-infected cells with a T-cell receptor-like antibody. J Virol 85:1935–1942. doi: 10.1128/JVI.01990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, Moriconi F, Farzhenah F, Mazzoni A, Chan L, Morris E, Thrasher A, Maini MK, Bonino F, Stauss H, Bertoletti A. 2015. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 62:486–491. doi: 10.1016/j.jhep.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Pasetto A, Frelin L, Aleman S, Holmstrom F, Brass A, Ahlen G, Brenndorfer ED, Lohmann V, Bartenschlager R, Sallberg M, Bertoletti A, Chen M. 2012. TCR-redirected human T cells inhibit hepatitis C virus replication: hepatotoxic potential is linked to antigen specificity and functional avidity. J Immunol 189:4510–4519. doi: 10.4049/jimmunol.1201613. [DOI] [PubMed] [Google Scholar]
- 20.Aleksic M, Liddy N, Molloy PE, Pumphrey N, Vuidepot A, Chang KM, Jakobsen BK. 2012. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur J Immunol 42:3174–3179. doi: 10.1002/eji.201242606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. 2008. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehermann B. 2009. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seigel B, Bengsch B, Lohmann V, Bartenschlager R, Blum HE, Thimme R. 2013. Factors that determine the antiviral efficacy of HCV-specific CD8(+) T cells ex vivo. Gastroenterology 144:426–436. doi: 10.1053/j.gastro.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Wieland S, Makowska Z, Campana B, Calabrese D, Dill MT, Chung J, Chisari FV, Heim MH. 2014. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology 59:2121–2130. doi: 10.1002/hep.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlotsky JM. 2014. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Barrett DM, Grupp SA, June CH. 2015. Chimeric antigen receptor- and TCR-modified T cells enter Main Street and Wall Street. J Immunol 195:755–761. doi: 10.4049/jimmunol.1500751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.June CH, Riddell SR, Schumacher TN. 2015. Adoptive cellular therapy: a race to the finish line. Sci Transl Med 7:280ps287. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto K, Kaplan DE, Ikeda F, Ding J, Schwartz J, Nunes FA, Alter HJ, Chang KM. 2005. Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection. J Virol 79:6976–6983. doi: 10.1128/JVI.79.11.6976-6983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimoto K, Stadanlick J, Ikeda F, Brensinger C, Furth EE, Alter HJ, Chang KM. 2003. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology 37:590–599. doi: 10.1053/jhep.2003.50103. [DOI] [PubMed] [Google Scholar]
- 30.Cho H, Kikuchi M, Li Y, Nakamoto N, Amorosa VK, Valiga ME, Chang KM. 2014. Induction of multiple immune regulatory pathways with differential impact in HCV/HIV coinfection. Front Immunol 5:265. doi: 10.3389/fimmu.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward S, Lauer G, Isba R, Walker B, Klenerman P. 2002. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin Exp Immunol 128:195–203. doi: 10.1046/j.1365-2249.2002.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J, Riley JL. 2008. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J Virol 82:11117–11128. doi: 10.1128/JVI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. 2009. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol 83:8379–8395. doi: 10.1128/JVI.00891-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci U S A 103:2310–2315. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blight KJ, McKeating JA, Rice CM. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]