ABSTRACT
Syrian hamsters are permissive for the replication of species C human adenoviruses (HAdV-C). The virus replicates to high titers in the liver of these animals after intravenous infection, while respiratory infection results in virus replication in the lung. Here we show that two types belonging to species C, HAdV-C5 and HAdV-C6, replicate to significantly different extents and cause pathology with significantly different severities, with HAdV-C6 replicating better and inducing more severe and more widespread lesions. The virus burdens in the livers of HAdV-C6-infected hamsters are higher than the virus burdens in HAdV-C5-infected ones because more of the permissive hepatocytes get infected. Furthermore, when hamsters are infected intravenously with HAdV-C6, live, infectious virus can be isolated from the lung and the kidney, which is not seen with HAdV-C5. Similarly to mouse models, in hamsters, HAdV-C6 is sequestered by macrophages to a lesser degree than HAdV-C5. Depletion of Kupffer cells from the liver greatly increases the replication of HAdV-C5 in the liver, while it has only a modest effect on the replication of HAdV-C6. Elimination of Kupffer cells also dramatically increases the pathology induced by HAdV-C5. These findings indicate that in hamsters, pathology resulting from intravenous infection with adenoviruses is caused mostly by replication in hepatocytes and not by the abortive infection of Kupffer cells and the following cytokine storm.
IMPORTANCE Immunocompromised human patients can develop severe, often lethal adenovirus infections. Respiratory adenovirus infection among military recruits is a serious problem, in some cases requiring hospitalization of the patient. Furthermore, adenovirus-based vectors are frequently used as experimental viral therapeutic agents. Thus, it is imperative that we investigate the pathogenesis of adenoviruses in a permissive animal model. Syrian hamsters are susceptible to infection with certain human adenoviruses, and the pathology accompanying these infections is similar to what is observed with adenovirus-infected human patients. We demonstrate that replication in permissive cells in a susceptible host animal is a major part of the mechanism by which systemic adenovirus infection induces pathology, as opposed to the chiefly immune-mediated pathology observed in nonsusceptible hosts. These findings support the use of compounds inhibiting adenovirus replication as a means to block adenovirus-induced pathology.
KEYWORDS: adenoviruses, hamster, viral pathogenesis
INTRODUCTION
Human adenoviruses (Ads) form 7 species (human adenoviruses A to G [HAdV-A to HAdV-G]) that include over 70 types (previously serotypes) (reviewed in references 1 and 2). Species C Ads comprise HAdV-C1, -C2, -C5, and -C6 (designated Ad1, Ad2, etc. in this article) and, possibly, the newly described HAdV-C57 (3). Species C Ads are of particular interest because they are among the most common Ads, they are ubiquitous, typically causing upper respiratory tract infections in infants and young children, and they are frequently observed in immunosuppressed patients, especially children. Of further interest, Ad5 and, to a lesser extent, Ad2 are the types that have been studied very extensively as models for molecular adenovirology. A great deal is known about nearly all the ∼35 genes of these DNA viruses and how the products of these genes function in the entire cycle of replication in cell culture.
Although the replication cycles of Ad5 and Ad2 in cell culture are well described, much less is known about the pathogenesis of Ad5 and Ad2 in replication-permissive animal models. Ad5 is the backbone for many genetically engineered vectors for use in gene therapy and cancer gene therapy. These vectors have been characterized nearly exclusively in mice, and most of what is known about Ad5's behavior in animals comes from studies with Ad5-based vectors in mice. However, mice are not a useful model for understanding Ad5's replication (i.e., production of progeny virus) and pathogenesis because mice are not permissive for replication of Ad5 or other human Ads (4).
In contrast to mice, two small animal models, namely, Syrian hamsters (5, 6) and cotton rats (4, 7, 8), are quite permissive for Ad5 replication. Our laboratory (6, 9–17) and other laboratories (18–28) have developed the Syrian hamster as a tumor model to characterize Ad5-based oncolytic Ad vectors (reviewed in references 29 and 30). The advantage of this model is that tumors formed, e.g., by subcutaneous injection of Syrian hamster cancer cell lines are permissive for replication of oncolytic Ad5-based vectors, as are most of the tissues of the hamster. Also, the Syrian hamster has an intact immune system, such that immunity develops against the vector and the tumor. As such, the behavior of oncolytic Ad vectors in the Syrian hamster should reflect their behavior in human cancer patients.
Syrian hamsters also serve as a general model for species C Ad pathogenesis. In immunocompetent Syrian hamsters, following intravenous (i.v.) administration, Ad5 is disseminated to and replicates in the liver and most other organs (17). While immunocompetent Syrian hamsters are permissive for replication of Ad5 following i.v. or intranasal administration of the virus, a robust innate and adaptive immune response develops that eliminates most of the virus within a week or two (13, 17, 31). Immunocompetent hamsters do not develop extensive pathology after adenovirus infection; in a dose escalation toxicity study with Ad5, we found that the no observable adverse effect level (NOAEL) for immunocompetent hamsters was 3 × 1010 virus particles per kg of body weight (10). However, when Syrian hamsters are immunosuppressed by treatment with high-dose cyclophosphamide (CP), Ad5 replicates to very high levels in the liver and other organs following i.v. administration and causes pathology similar to that seen with immunocompromised human patients, including lethal multiorgan disease (32). Using immunosuppressed Syrian hamsters, we have successfully evaluated compounds that may inhibit Ad5 replication and pathogenicity. We found that brincidofovir (previously named CMX-001) (32, 33), cidofovir (33), ganciclovir (34), and valganciclovir (35) are very effective.
To further develop the Syrian hamster as a model, we (36) and others (37) have sequenced the Syrian hamster transcriptome. We identified 42,707 unique transcripts representing 34,191 unique genes. Using these data, a custom microarray was developed and employed to analyze gene expression in the Syrian hamster liver at 18 h following i.v. injection of Ad5. We found that about 20% of transcripts were up- or downregulated, with prominent upregulation of genes involved in the innate immune response to virus infection (36). The latter finding is consistent with our recent study in Syrian hamsters with the STAT2 gene knocked out (STAT2 KO hamsters). We found that STAT2 KO hamsters are much more permissive for disseminated Ad5 replication and pathogenesis than are wild-type Syrian hamsters (31). In contrast to the wild-type Syrian hamsters infected i.v. with Ad5, i.v.-infected STAT2 KO hamsters were defective in the expression of type I interferon response genes, such as PKR, OAS, and Mx2. This study indicated that the type I interferon response is crucial to controlling acute disseminated Ad5 infections (31).
As discussed above, Ad5 has been used as the backbone virus in the vast majority of gene therapy and cancer gene therapy studies in mice. A potential problem with the use of Ad5 as a vector in humans is that it is a common type, as indicated by serological studies (reviewed in reference 2). Ad5 neutralizing antibodies, often of high titer, range in various studies from 30% to 70% occurrence in individuals in the United States, 50% to 60% in Europe, and generally much higher in other parts of the world (2). These high levels of neutralizing antibodies might be expected to neutralize Ad5-based vectors. This issue has prompted one laboratory to explore the use of Ad6 as a vector for gene therapy and vaccine development (38–41). About half the population of the world has neutralizing antibodies to Ad6, but mostly of low titer (42). It is not known whether these differences reflect the incidence of Ad6 in the human population or a stronger immune response against Ad5. Another potential problem with the use of Ad5 as a vector for studies in mice (and probably in humans) is that following i.v. administration in mice, at least 90% of the injected particles of Ad5 or Ad5-based vectors are eliminated by Kupffer cells (resident liver macrophages), thereby reducing the efficacy of Ad5-based vectors (43–45). In contrast, Ad6 vectors or Ad5/Ad6 chimeric vectors containing the Ad6 hexon hypervariable region interact with Kupffer cells to a lower degree than does Ad5 (46); this observation provides further support for the use of Ad6 as a vector backbone (41).
Prompted by these differences between Ad6- and Ad5-based vectors in mice, we compared the degrees of pathogenicity of Ad6 and Ad5 in Syrian hamsters. As we report here, we found that wild-type Ad6 is considerably more pathogenic than wild-type Ad5.
RESULTS
Intravenously injected Ad6 but not Ad5 is pathogenic in immunocompetent Syrian hamsters.
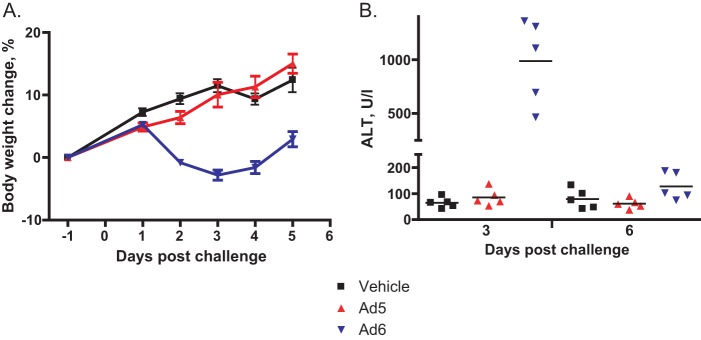
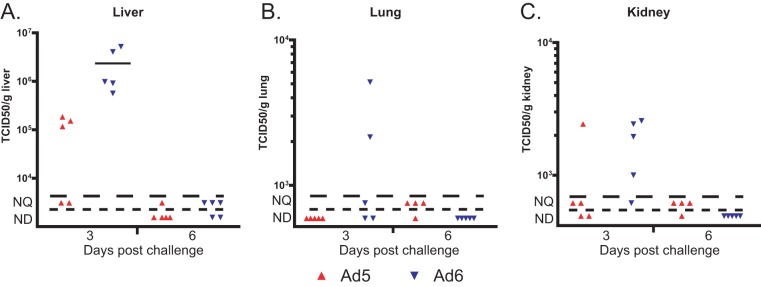
When injected i.v., 6 × 1010 PFU/kg of Ad6 caused weight loss for hamsters, while the same dose of Ad5 did not affect weight gain (Fig. 1A). Ad6 caused significant liver damage, as evidenced by serum alanine transaminase (ALT) levels, while Ad5 did not cause any elevation of serum ALT levels (Fig. 1B). To investigate the cause of the increased pathology, we determined the virus burdens in the livers, lungs, and kidneys of the animals. We found that Ad6-infected hamsters had consistently higher virus burdens, which were especially high in the livers (Fig. 2). The host immune response to Ad6 was also higher than the response to Ad5; there was more severe infiltration of the liver by T lymphocytes for Ad6-infected hamsters than for Ad5-infected ones (Fig. 3).
FIG 1.
Ad6 but not Ad5 induced morbidity in immunocompetent hamsters. A dose of 6 × 1010 PFU/kg of virus was injected intravenously. (A) Body weight changes. Each symbol represents the group mean; the whiskers symbolize the standard deviations. Vehicle versus Ad6, P < 0.0001 (two-way ANOVA). (B) Serum alanine transaminase levels. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Day 3, vehicle versus Ad6, P = 0.0079, and Ad5 versus Ad6, P = 0.0079; day 6, vehicle versus Ad6, P = 0.2222, and Ad5 versus Ad6, P = 0.0159 (Mann-Whitney U test).
FIG 2.
The virus burdens in immunocompetent, Ad6-infected hamsters are higher than those in Ad5-infected ones. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. NQ, nonquantifiable; ND, nondetectable.
FIG 3.
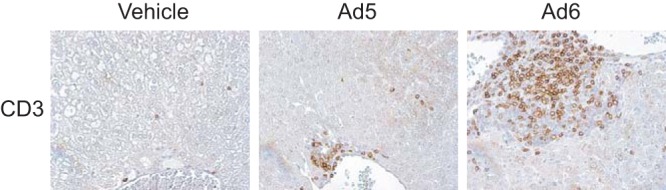
Infiltration by T lymphocytes is more pronounced in Ad6-infected animals than in Ad5-infected ones. Liver samples were collected at 3 days postchallenge. Immunohistochemical staining for the CD3 protein is shown.
Intravenously injected Ad5 and Ad6 replicate in the liver of immunosuppressed Syrian hamsters.
To demonstrate that both Ads replicate in hamsters, we injected the viruses i.v. into immunosuppressed animals. Immunosuppression enhances Ad replication in hamsters, providing a better opportunity to trace virus burdens. To equalize the pathogenicity of the two viruses, 5 times less Ad6 than Ad5 was injected (2 × 1010 PFU/kg versus 1 × 1011 PFU/kg). At 6 h postinfection (p.i.), approximately 2 × 107 50% tissue culture infective doses (TCID50)/g of liver tissue of infectious Ad5 could be recovered from the livers, which decreased by approximately 2 orders of magnitude at the 24-h time point, indicating that the virus recovered at 6 h p.i. was derived from the input bolus (Fig. 4). Practically no infectious virus was found in the livers of Ad6-infected hamsters at 6 h p.i.; however, at 24 h p.i., the virus burdens reached levels similar to those of the Ad5-infected animals (Fig. 4). At 5 days p.i., high infectious virus burdens (2 × 109 and 5 × 109 TCID50/g of liver tissue for Ad5 and Ad6, respectively) were detected in the livers of animals infected with either virus (Fig. 4), indicating ongoing virus infection.
FIG 4.
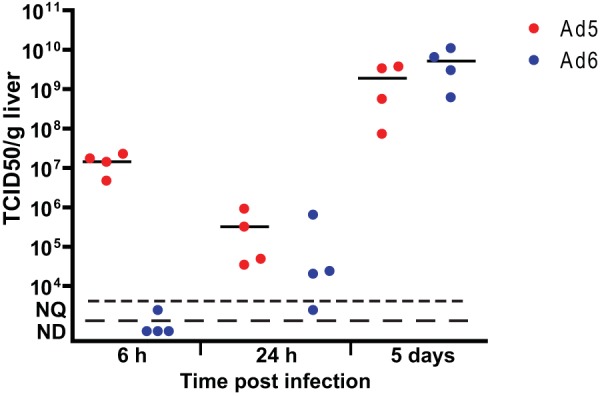
Both Ad5 and Ad6 replicate in the livers of immunosuppressed hamsters. Virus burdens in the livers increased significantly from 6 h p.i. to 5 days p.i. (P = 0.0286; Mann-Whitney U test). Symbols represent values from individual animals; the horizontal bars signify the mean value for each group. NQ, not quantifiable; ND, not detectable.
Intravenously injected Ad6 is more pathogenic than Ad5 in immunosuppressed Syrian hamsters.
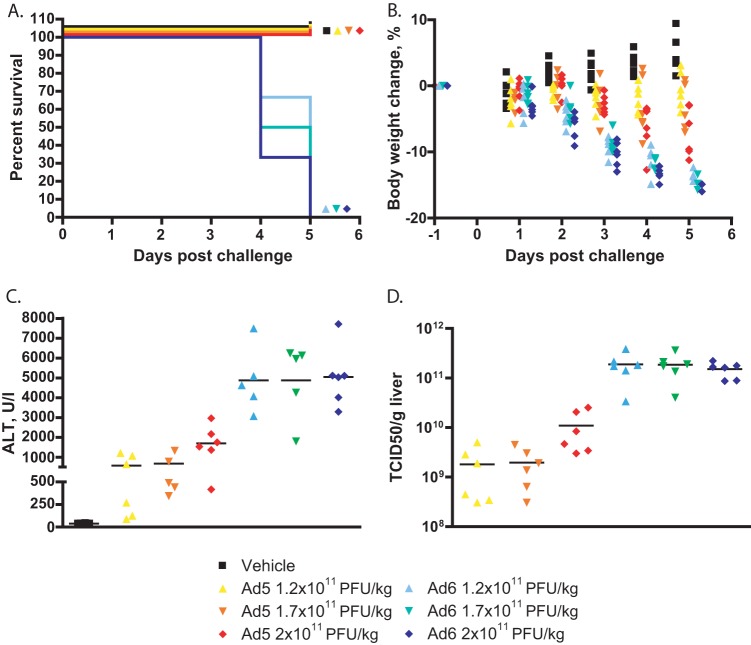
Both the increased replication of Ad6 and the more intense immune response can contribute to enhanced pathogenicity. To investigate whether the increased replication alone can cause more pathology, we infected immunocompromised hamsters with various doses of Ad5 and Ad6, ranging from 1.2 × 1011 to 2 × 1011 PFU/kg. By 4 to 5 days postchallenge, all Ad6-infected hamsters had lost about 15% of their body weight (Fig. 5B) and were sacrificed because they were moribund (Fig. 5A). We sacrificed the Ad5-infected hamsters at this time as well, in order to have time-matched samples. While some of these latter animals were losing weight (Fig. 5B), they were not moribund at sacrifice. At necropsy, all Ad6-infected hamsters showed the gross pathological signs of advanced disseminated Ad infection (yellow, mottled, friable liver); conversely, only animals infected with the highest dose of Ad5 showed lesions in their livers, and even these lesions were much less severe than for Ad6-infected animals (data not shown). All sera collected from Ad-infected hamsters, with the exception of 3 hamsters infected with the lowest dose of Ad5, showed elevated ALT levels; however, the ALT levels in the sera of Ad6-infected hamsters were 3- to 5-fold higher than for animals infected with the matching dose of Ad5 (Fig. 5C). Similarly to immunocompetent hamsters, the virus burdens in the livers of Ad6-infected animals were 10- to 100-fold higher than for Ad5-infected ones (Fig. 5D).
FIG 5.
Ad6 is more pathogenic than Ad5 in CP-treated hamsters. (A) Survival. Ad5 versus Ad6 at all dose levels, P < 0.0001 (Log rank). (B) Body weight changes. Each symbol represents the value from an individual animal. Ad5 versus Ad6 at all dose levels, P < 0.0001 (two-way ANOVA). (C) Serum alanine transaminase levels. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Ad5 versus Ad6 at 1.2 × 1011 PFU/kg, P = 0.0043; Ad5 versus Ad6 at 1.7 × 1011 PFU/kg, P = 0.0079; Ad5 versus Ad6 at 2 × 1011 PFU/kg, P = 0.0022 (Mann-Whitney U test). (C) Virus burdens in livers. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Ad5 versus Ad6 at all dose levels, P = 0.0022 (Mann-Whitney U test).
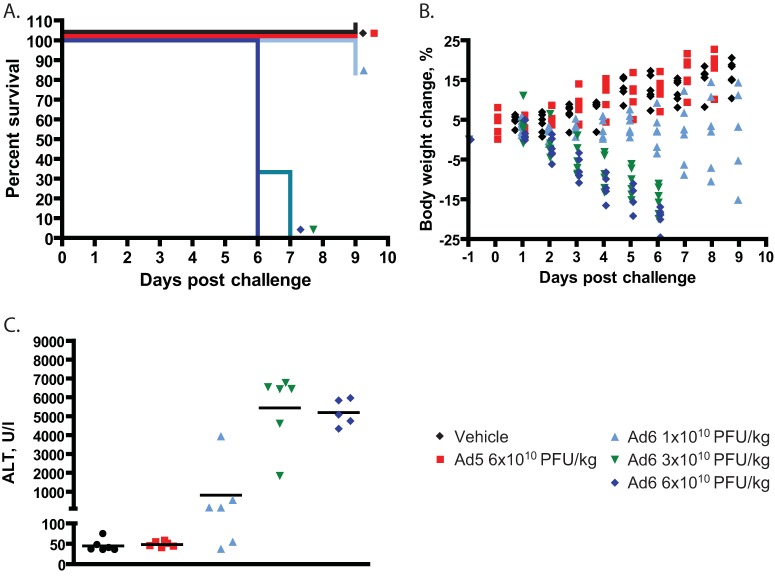
We repeated this experiment using lower infectious doses (1 × 1010 to 6 × 1010 PFU/kg) of both viruses. The 6 × 1010 and 3 × 1010 PFU/kg doses of Ad6 caused 100% mortality, and 1 of 6 hamsters became moribund in the 1 × 1010 PFU/kg group (Fig. 6A). Conversely, we observed no pathology with any of the Ad5-infected hamsters (Fig. 6A and B; only the results for the highest dose of Ad5 are shown). Ad6 at all doses caused body weight loss, but Ad5 at the highest dose did not (Fig. 6B). As expected, the serum ALT levels for the animals in the groups infected with the two higher doses of Ad6 were very high, and even hamsters infected with the lowest dose of Ad6 had markedly elevated serum ALT levels (Fig. 6C). No elevation of serum ALT levels was detected in any of the Ad5-infected hamsters (Fig. 6C; only the results for the highest dose of Ad5 are shown).
FIG 6.
Ad6 is more pathogenic than Ad5 in CP-treated hamsters. (A) Survival. Ad5 versus Ad6 at 6 × 1010 PFU/kg, P < 0.0001 (Log rank). (B) Body weight changes. Each symbol represents the value from an individual animal. Significance could not be computed because of early deaths from the Ad6-infected groups. (C) Serum alanine transferase levels. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Ad5 versus Ad6 at 6 × 1010 PFU/kg, P = 0.0043 (Mann-Whitney U test).
Based on these data, the intravenous 50% lethal dose (LD50) of Ad6 in immunosuppressed Syrian hamsters is approximately 2 × 1010 PFU/kg, which is about 10-fold lower than the LD50 established for Ad5 in this model, indicating that the main cause of the more severe pathology caused by Ad6 is the increased virus burden.
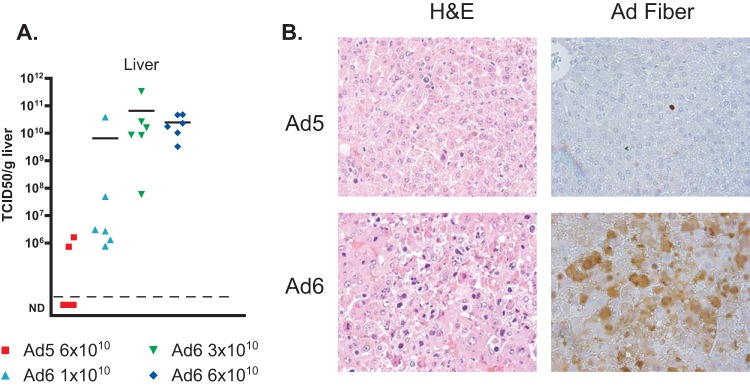
The virus burdens are higher in the organs of Ad6-infected hamsters than in the organs of Ad5-infected ones.
As we noted before, hamsters infected with Ad6 had higher virus burdens in the liver than Ad5-infected ones (Fig. 5D). To further investigate this finding, we analyzed liver, lung, and kidney samples collected from the experiment whose results are shown in Fig. 6. We found that the virus burdens in the livers of Ad6-infected hamsters were at least 10,000-fold higher than those in the livers of animals infected with the matching dose of Ad5 (Fig. 7A). In accordance with this finding, histopathological analysis revealed more extensive and more severe pathology (Fig. 7B, left) and much more widespread infection (Fig. 7B, right) in the livers of Ad6-infected hamsters. Similarly, at the 6 × 1010 PFU/kg dose, the virus burdens were approximately 108 to 109 TCID50/g of tissue for the kidneys (Fig. 8A) and lungs (Fig. 8C) of the Ad6-infected animals, while no infectious virus was detected in these organs collected from hamsters infected with the same dose of Ad5. While Ad6-infected cells could be found in the kidney and lung (Fig. 8B and D), no pathology was apparent in either organ, with the exception of distended tubules in the kidney.
FIG 7.
Ad6 replicates better than Ad5 in the livers of immunosuppressed Syrian hamsters. (A) Virus burdens in livers. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Significance could not be computed because of nonscalar (not detectable [ND]) values for Ad5. (B) Histopathology (left) and Ad fiber immunohistochemistry (right) of liver sections. Results from animals infected with 6 × 1010 PFU/kg are shown. H&E, hematoxylin and eosin.
FIG 8.
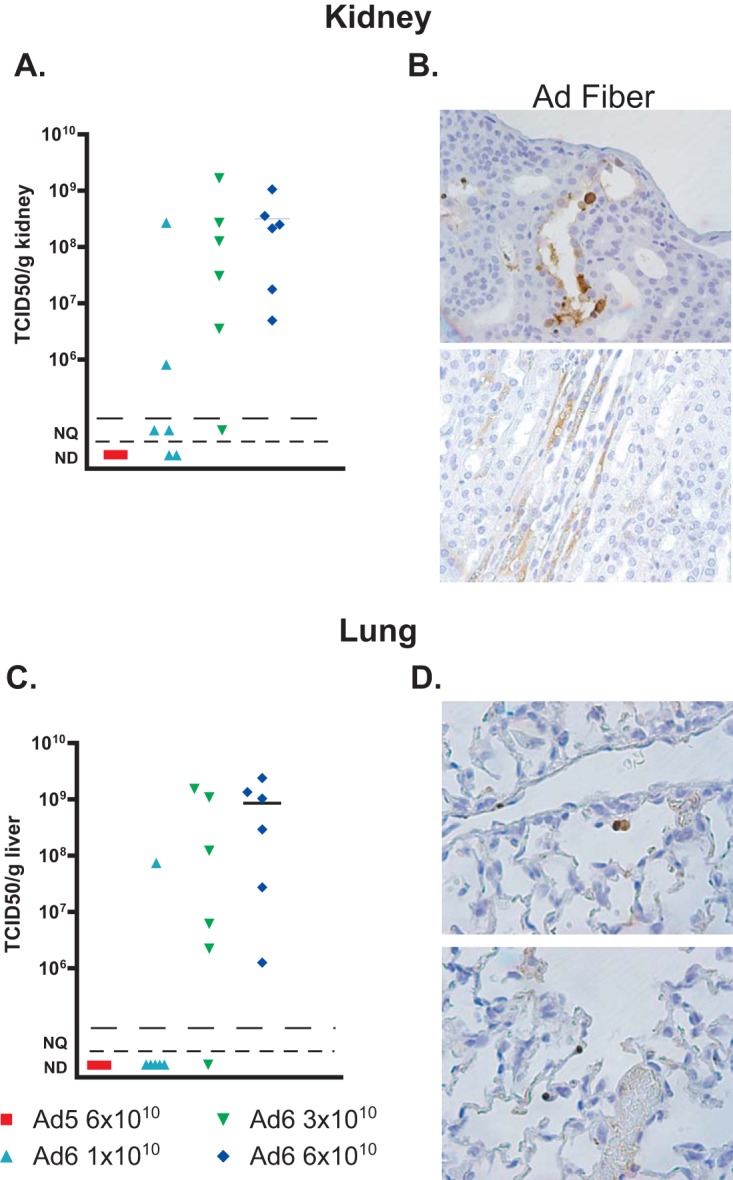
The virus burdens in the kidneys and lungs of Ad6-infected immunosuppressed Syrian hamsters are higher than those in Ad5-infected ones. (A, C) Infectious virus loads in kidneys (A) and lungs (C). Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. Significance could not be computed because of nonscalar (not detectable) values for Ad5. NQ, nonquantifiable; ND, not detectable. (B, D) Immunohistochemistry for Ad fiber in kidney (B) and lung (D) sections of Ad6-infected animals. Results from animals infected with 6 × 1010 PFU/kg are shown.
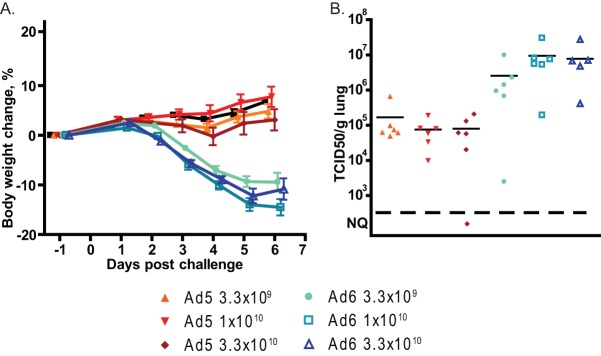
Intranasally administered Ad6 replicates more and causes more pathology in the lung than Ad5.
We infected immunosuppressed hamsters intranasally with Ad6 or Ad5, with doses ranging from 3.3 × 109 to 3.3 × 1010 PFU/kg. There were 12 hamsters per dose level; 6 were sacrificed at 3 days postchallenge, and the other 6 were sacrificed at 7 days postchallenge. All Ad6-infected hamsters lost body weight after challenge, while the weight gains of Ad5-infected animals were indistinguishable from those of vehicle-treated hamsters (Fig. 9A). At 3 days postchallenge, the virus burdens in the lungs of Ad6-infected animals were approximately 100-fold higher than in the lungs of Ad5-infected ones (Fig. 9B). Generally, more hamsters in the Ad6-infected groups had lung pathology than in the Ad5-infected group, especially in the lower-dose groups (Table 1). Immunohistochemical staining for the Ad fiber protein showed that both viruses infected bronchial epithelial cells; the infection was more widespread for Ad6 than for Ad5, resulting in widespread damage to the epithelial layer of the bronchi (Fig. 10A and B). Marked mononuclear/granulocytic infiltration was detected for animals infected with either virus, with no discernible differences. This infiltration increased both in dissemination and severity from day 3 to day 6 postchallenge (Fig. 10C to F).
FIG 9.
Intranasally administered Ad6 is more pathogenic and replicates better in the lung than Ad5. (A) Body weight changes. Mean values ± standard errors of the means (SEM) for each group are shown. Vehicle versus Ad5 at 3.3 × 108, P = 0.2804; vehicle versus Ad5 at 1 × 109, P = 0.0771; vehicle versus Ad5 at 3.3 × 109, P = 0.0017; vehicle versus any Ad6 group and Ad5 groups versus respective Ad6 groups, P < 0.0001 (two-way ANOVA). (B) Virus burdens in lungs. Each symbol represents the value from an individual animal; the horizontal bars signify the mean value for each group. NQ, nonquantifiable.
TABLE 1.
Proportions of animals with gross lung pathology after intranasal infection
| Virus, dose (PFU/kg) | No. of hamsters with gross pathology/total no. in group at indicated day p.i. |
|
|---|---|---|
| 3 | 6 | |
| Ad5, 3.3 × 109 | 1/6 | 0/6 |
| Ad5, 1 × 1010 | 3/6 | 2/6 |
| Ad5, 3.3 × 1010 | 4/6 | 3/6 |
| Ad6, 3.3 × 109 | 4/6 | 4/6 |
| Ad6, 1 × 1010 | 6/6 | 1/6 |
| Ad6, 3.3 × 1010 | 4/6 | 3/6 |
FIG 10.
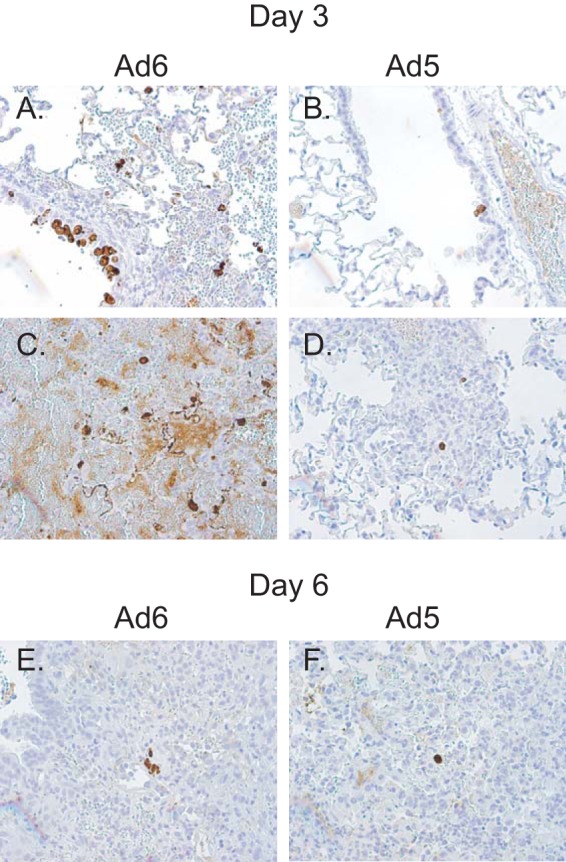
Intranasally administered Ad6 replicates better than Ad5 in the lung. Results from animals infected with 3.3 × 1010 PFU/kg are shown. Immunohistochemical staining of lung tissues of Ad6-infected (A, C, E) and Ad5-infected (B, D, F) hamsters for the Ad fiber protein at 3 (A to D) and 6 (E, F) days postchallenge.
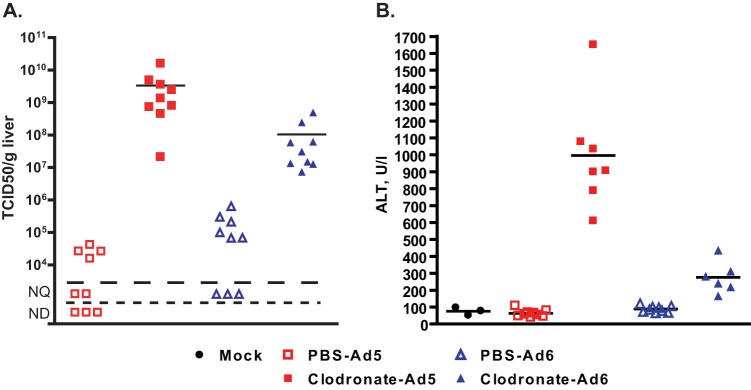
Depletion of Kupffer cells increases the replication of Ad5 but not Ad6 in the liver of Syrian hamsters.
It has been shown before that Kupffer cells sequester a large portion of intravenously injected Ad in mice and that Ad6 is less susceptible to this sequestering than Ad5 (47). To test whether this was happening in hamsters as well, we depleted the Kupffer cells of hamsters using clodronate-loaded liposomes. As expected, intravenously injected Ad6 (6 × 1010 PFU/kg) infected the livers of untreated animals better than Ad5 (Fig. 11A) at 3 days postchallenge; however, when clodronate-treated animals were infected with Ad5, the virus burdens in the livers increased approximately 100,000-fold compared to those in untreated hamsters (Fig. 11A). On the other hand, the increases in the virus burdens for the clodronate-treated, Ad6-infected hamsters were much more modest, about 1,000-fold (Fig. 11A). The increased virus burdens in the clodronate-treated, Ad5-infected hamsters caused severe liver damage (average serum ALT levels of 1,000 U/liter, Fig. 11B), while the elevation of serum ALT levels in the Ad6-infected counterparts of these animals was less prominent (Fig. 11B).
FIG 11.
Ablation of Kupffer cells by clodronate liposomes increases the virus load and enhances pathogenicity more in the livers of Ad5-infected than in those of Ad6-infected hamsters. (A) Virus burdens in livers at 3 days postchallenge. (B) Serum alanine transaminase levels. PBS-Ad5 versus PBS-Ad6, P = 0.0244; clodronate-Ad5 versus clodronate-Ad6, P = 0.0012; PBS-Ad5 versus clodronate-Ad5, P = 0.0002; PBS-Ad6 versus clodronate-Ad6, P = 0.0004 (Mann-Whitney U test). Symbols signify values from individual animals; horizontal bars signify the mean value for each group. NQ, not quantifiable; ND, not detectable.
DISCUSSION
We have shown here that Ad6 replicates to higher levels in the liver and other organs and is much more pathogenic than Ad5 in Syrian hamsters. We compared infection by Ad6 and Ad5 in immunocompetent hamsters using an i.v. dose of 6 × 1010 PFU/kg. Ad5 did not cause weight loss or elevation of serum ALT, although some virus (∼105 TCID50/g of liver tissue) was found in the livers of 3 of 5 animals. With Ad6, ∼3 × 106 TCID50/g of liver tissue of Ad6 was found in all 5 hamsters at 3 days, the ALT levels were elevated, and the hamsters lost weight starting at 2 days postinfection (Fig. 1 and 2). Furthermore, there was much more infiltration of T cells into the liver with Ad6 than with Ad5 (Fig. 3). By 6 days, neither Ad6 nor Ad5 was found in the liver and ALT levels were nearly normal. In other studies, we have shown that there is considerable neutralizing antibody response by 6 days (31), and this immune response is likely responsible for eliminating the virus. We have demonstrated that both Ad5 and Ad6 replicate in the liver of hamsters to high levels (Fig. 4). Notably, we found that part of the input bolus of Ad5 was still infectious at 6 h p.i., while no infectious Ad6 could be recovered from the livers of infected animals at this time. This may indicate a slower uptake and/or uncoating for Ad5 than for Ad6 and may contribute to the decreased pathogenicity of Ad5 compared to that of Ad6. However, the interpretation of these data is complicated by the fact that, to avoid pathogenicity with Ad6, we injected 5 times less Ad6 than Ad5. Clearly, further investigation is needed to elucidate this mechanism.
Most of our experiments were conducted in hamsters immunosuppressed by treatment with CP. This treatment prevents the elimination of the virus by the adaptive immune response and thereby allows replication of the virus to continue for much longer than in immunocompetent hamsters. Also, the CP-treated hamsters provide a model for disseminated Ad infection and Ad pathogenesis in immunosuppressed human patients. In our studies, following i.v. administration and depending on the dose of virus used, both Ad6 and Ad5 infected the liver, replicated, caused extensive liver pathology, and were lethal. However, about 10-fold more Ad5 than Ad6 was required to cause these effects. In fact, the LD50 of Ad5 was greater than 2 × 1011 PFU/kg (hamsters weigh about 100 g), whereas the LD50 of Ad6 was about 2 × 1010 PFU/kg (Fig. 6A). At a dose of 6 × 1010 PFU/kg, Ad5 barely replicated in the liver, did not cause an increase in serum ALT, and did not affect the weight gain of the hamsters. In contrast, Ad6 at this dose infected many (most) hepatocytes, replicated to high levels in the liver (>1010 TCID50/g of liver tissue), caused massive liver pathology, and was lethal by 6 days postinfection (Fig. 6 and 7). Ad6 at doses of 6 × 1010 and 3 × 1010 PFU/kg was also found in the kidneys and lungs, whereas Ad5 at 6 × 1010 PFU/kg was not detected in these organs (Fig. 8).
Ad6 and Ad5 commonly cause respiratory infections, so we examined replication and pathogenesis following intranasal administration of these viruses in CP-treated hamsters. We found that Ad6 replicated to ∼100-fold-higher levels than Ad5: ∼107 TCID50/g of lung tissue for Ad6 compared to ∼105 TCID50/g of lung tissue for Ad5 (Fig. 9B). Also, Ad6 at a dose of 3.3 × 109 PFU/kg caused greater weight loss than an Ad5 dose of 3.3 × 1010 PFU/kg (Fig. 9A).
Considering our data, the question arises as to why Ad6 replicates to higher levels and is more pathogenic than Ad5. Ad6 has barely been studied compared to Ad5 (and Ad2). As is the case with Ad5 and Ad2, the Ad6 genome has been sequenced, and it was found to be 94% and 98% identical to those of Ad5 and Ad2, respectively (3, 48). The transcription units and the genes appear to be very similar to those of Ad5 and Ad2. Also, the Ad6 transcriptome has been sequenced and the early (6 h p.i.) and later (12 h p.i.) RNAs quantitated; this study did not reveal notable differences between Ad6 and Ad5/Ad2 (49).
One possible explanation for the difference in pathogenicities between Ad6 and Ad5 is that macrophages appear to have different affinities for these viruses. It has been known for some time that in mice, the majority of intravenously injected Ad5 is taken up by resident macrophages in the liver, named Kupffer cells (47, 50), and sinusoidal endothelial cells (51). It is known that Ads can bind to scavenger receptors and complement receptors present on phagocytic cells and that blocking this interaction results in increased infection of hepatocytes (52). Furthermore, circulating natural antibodies can bind to Ad virions, and the resulting complexes can be taken up by cells displaying Fc receptors (47). In both mice (53) and hamsters (Fig. 2), Kupffer cells are responsible for the nonlinear dose response for hepatocyte transduction or infection; i.e., the Kupffer cell compartment needs to be saturated before a significant number of hepatocytes can get infected. Thus, Kupffer cells are a major barrier to infection of hepatocytes by Ads. It was demonstrated that in mice, Ads 6, 11, and 35 are less susceptible to Kupffer cell sequestration than Ad5 (41). This difference is mouse strain specific, and it is dependent on the levels of circulating IgM (47). We have shown that a similar difference between Ad5 and Ad6 exists in hamsters and that, similarly to mice with high levels of IgM (54), the mechanism involves Kupffer cells in hamsters as well. Depletion of Kupffer cells by treatment with clodronate-containing liposomes increased the infectious virus burden in the liver of Ad5-infected hamsters. It is not known whether natural antibody levels play a role in the process of virus uptake into Kupffer cells in hamsters. Kupffer cells die shortly after getting infected, and thus, the infection is abortive, effectively sequestering the infecting virus and preventing the infection of hepatocytes. With mice, in which human Ads replicate very poorly, this means that the liver pathology after intravenous Ad injection is mostly the result of the cytokine storm released by the infected Kupffer cells (55). Similar symptoms were observed when large doses of replication-defective Ad vectors were injected intravenously into human patients (56). With Syrian hamsters, the situation is reversed; the liver pathology is the result of the replication of Ads in permissive hepatocytes (Fig. 11), and it can be alleviated with drugs inhibiting virus replication (32–35). In this model, the uptake of virus by Kupffer cells reduces infection of the hepatocytes and, thus, mitigates pathogenesis. This result is in accordance with findings described in a permissive mouse model of hepatitis B infection, in which Kupffer cells were shown to accelerate the resolution of pathology (57). Presently, it is not known whether Ad5 and Ad6 are sequestered at a different rate by human Kupffer cells. However, it is reported that Kupffer cells play a major role in clearing intravenously injected Ad5-based vectors from the bloodstream (56). It stands to reason that during a natural Ad infection, these cells perform a protective role. We believe that the pathology seen after i.v. infection of Syrian hamsters with replication-competent Ads reflects the pathogenesis of systemic Ad infection in human patients.
MATERIALS AND METHODS
Animals.
Female Syrian hamsters (Mesocricetus auratus) were purchased from Envigo (Indianapolis, IN) at approximately 100 g of body weight. All studies were approved by the Institutional Animal Care and Use Committee of Saint Louis University and were conducted according to federal and institutional regulations.
Cells and viruses.
HEK293 human embryonic kidney cells were purchased from Microbix (Mississauga, Ontario, Canada) and cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum at 37°C. A wild-type human Ad5, named Ad5 wt500, was isolated by our laboratory from an Ad5 stock purchased from the ATCC (Manassas, VA). The virus was purified by isopycnic gradient centrifugation, and the titer was determined by plaque assay on A549 cells (ATCC), as described in reference 58.
Infection of hamsters with adenovirus.
Immunocompetent hamsters were infected by the intravenous (i.v.) route by injection of 6 × 1010 PFU of Ad5 or Ad6 into the jugular vein. In other experiments, the hamsters were immunosuppressed using cyclophosphamide (32). CP was administered intraperitoneally at a dose of 140 mg/kg and then twice weekly at a dose of 100 mg/kg for the duration of the study. CP used in this manner reduced nearly all leukocyte types more than 7-fold within a few days (9, 16, 32). Virus challenge was performed 5 to 7 days after the first administration of CP. For i.v. administration, the animals were anesthetized with a ketamine-xylazine mixture, and Ad5 was injected i.v. (via the jugular vein) in 200 μl of phosphate-buffered saline (PBS) (59). Intranasal inoculation was performed by anesthetizing the hamsters with isoflurane (Isothesia; Henry Schein Animal Health) and then pipetting the virus into the nostrils in 100 μl PBS. For both routes, control animals were administered PBS.
After challenge, hamsters were observed and weighed daily. Moribund animals and animals that lost more than 20% of their body weight were sacrificed as needed. At necropsy, livers, lungs, and kidneys were collected and the infectious virus burden was determined using a 50% tissue culture infectious dose (TCID50) assay as described previously (32). TCID50 assays are less laborious than plaque assays and enabled us to determine the virus load in a large number of samples. Generally, 1 PFU equals 2 TCID50. Sera were assayed for alanine aminotransferase (ALT) (Advanced Veterinary Laboratory, St. Louis, MO).
Portions of the collected tissues were preserved in formalin and processed for histopathology (Seventh Wave Laboratories, St. Louis, MO). Immunohistochemistry was provided by Deborah Berry and the team at Histopathology & Tissue Shared Resource (HTSR) (via Science Exchange), using a 1:1,000 dilution of adenovirus Ab-4 antibody (4D2) (Lab Vision, Fremont, CA) and a 1:200 dilution of CD3-ε antibody (M-20) (Santa Cruz Biotechnology, Santa Cruz, CA) to stain for Ad fiber and hamster CD3 protein, respectively.
Depletion of Kupffer cells with clodronate liposomes.
Hamsters were injected i.v. with 200 μl of clodronate liposomes (Foundation Clodronate Liposomes, Amsterdam, Netherlands) or PBS liposomes (control). At 24 h after the administration of clodronate, the animals were injected i.v. with Ad5 or Ad6 as described above.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software). Two-way analysis of variance (ANOVA) was used to compare body weight changes. For serum transaminase levels and virus burdens in the liver, the overall effects were calculated using the Kruskal-Wallis test and comparisons between groups were performed using the Mann-Whitney U test. A P value of ≤0.05 was considered significant.
ACKNOWLEDGMENT
The authors declare no conflict of interest.
This project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (www.niaid.nih.gov), under contract no. HHSN2722001000021. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Berk AJ. 2013. Adenoviridae, p 1704–1731. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Wold WSM, Ison MG. 2013. Adenoviruses, p 1732 In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Walsh MP, Seto J, Liu EB, Dehghan S, Hudson NR, Lukashev AN, Ivanova O, Chodosh J, Dyer D, Jones MS, Seto D. 2011. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol 49:3482–3490. doi: 10.1128/JCM.00156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg HS, Lundholm-Beauchamp U, Horswood RL, Pernis B, Wold WSM, Chanock RM, Prince GA. 1989. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A 86:3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjorth RN, Bonde GM, Pierzchala WA, Vernon SK, Wiener FP, Levner MH, Lubeck MD, Hung PP. 1988. A new hamster model for adenoviral vaccination. Arch Virol 100:279–283. doi: 10.1007/BF01487691. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, Wold WS. 2006. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 7.Pacini DL, Dubovi EJ, Clyde WA Jr. 1984. A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis 150:92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- 8.Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, La Regina MC, Prince GA, Wold WSM. 2005. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther 16:139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips N, Wold WSM. 2008. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol Ther 16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer J, Shashkova EV, Kuppuswamy M, Dhar D, Thomas MA, Tollefson AE, Zumstein LA, Wold WSM, Toth K. 2009. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther 16:644–654. doi: 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shashkova EV, Spencer JF, Wold WSM, Doronin K. 2007. Targeting interferon-α increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther 15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 12.Spencer JF, Sagartz JE, Wold WSM, Toth K. 2009. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Ther 16:912–922. doi: 10.1038/cgt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhar D, Spencer JF, Toth K, Wold WSM. 2009. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol Ther 17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young BA, Spencer JF, Ying B, Tollefson AE, Toth K, Wold WS. 2013. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther 20:521–530. doi: 10.1038/cgt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young BA, Spencer JF, Ying B, Toth K, Wold WS. 2013. The effects of radiation on antitumor efficacy of an oncolytic adenovirus vector in the Syrian hamster model. Cancer Gene Ther 20:531–537. doi: 10.1038/cgt.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar D, Toth K, Wold WS. 2014. Cycles of transient high-dose cyclophosphamide administration and intratumoral oncolytic adenovirus vector injection for long-term tumor suppression in Syrian hamsters. Cancer Gene Ther 21:171–178. doi: 10.1038/cgt.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, Dhar D, Shashkova EV, Kuppuswamy M, Doronin K, Thomas MA, Zumstein LA, Wold WS, Lichtenstein DL. 2009. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther 16:625–637. doi: 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortolanza S, Alzuguren P, Bunuales M, Qian C, Prieto J, Hernandez-Alcoceba R. 2007. Human adenovirus replicates in immunocompetent models of pancreatic cancer in Syrian hamsters. Hum Gene Ther 18:681–690. doi: 10.1089/hum.2007.017. [DOI] [PubMed] [Google Scholar]
- 19.Sonabend AM, Ulasov IV, Han Y, Rolle CE, Nandi S, Cao D, Tyler MA, Lesniak MS. 2009. Biodistribution of an oncolytic adenovirus after intracranial injection in permissive animals: a comparative study of Syrian hamsters and cotton rats. Cancer Gene Ther 16:362–372. doi: 10.1038/cgt.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, Nokisalmi P, Raki M, Laasonen L, Sarkioja M, Rajecki M, Kangasniemi L, Guse K, Helminen A, Ahtiainen L, Ristimaki A, Raisanen-Sokolowski A, Haavisto E, Oksanen M, Karli E, Karioja-Kallio A, Holm SL, Kouri M, Joensuu T, Kanerva A, Hemminki A. 2010. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Ryan MJ, Estep JE, Miniard BM, Rudge TL, Peggins JO, Broadt TL, Wang M, Preuss MA, Siegal GP, Hemminki A, Harris RD, Aurigemma R, Curiel DT, Alvarez RD. 2011. A new generation of serotype chimeric infectivity-enhanced conditionally replicative adenovirals: the safety profile of ad5/3-delta24 in advance of a phase I clinical trial in ovarian cancer patients. Hum Gene Ther 22:821–828. doi: 10.1089/hum.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bortolanza S, Bunuales M, Otano I, Gonzalez-Aseguinolaza G, Ortiz-de-Solorzano C, Perez D, Prieto J, Hernandez-Alcoceba R. 2009. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther 17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, Ranki T, Oksanen M, Holm SL, Haavisto E, Karioja-Kallio A, Laasonen L, Partanen K, Ugolini M, Helminen A, Karli E, Hannuksela P, Joensuu T, Kanerva A, Hemminki A. 2010. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bortolanza S, Bunuales M, Alzuguren P, Lamas O, Aldabe R, Prieto J, Hernandez-Alcoceba R. 2009. Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters. Cancer Gene Ther 16:703–712. doi: 10.1038/cgt.2009.12. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa N, Abei M, Yokoyama KK, Fukuda K, Seo E, Kawashima R, Nakano Y, Yamada T, Nakade K, Hamada H, Obata Y, Hyodo I. 2013. Cyclophosphamide enhances antitumor efficacy of oncolytic adenovirus expressing uracil phosphoribosyltransferase (UPRT) in immunocompetent Syrian hamsters. Int J Cancer 133:1479–1488. doi: 10.1002/ijc.28132. [DOI] [PubMed] [Google Scholar]
- 26.Bunuales M, Garcia-Aragoncillo E, Casado R, Quetglas JI, Hervas-Stubbs S, Bortolanza S, Benavides-Vallve C, Ortiz-de-Solorzano C, Prieto J, Hernandez-Alcoceba R. 2012. Evaluation of monocytes as carriers for armed oncolytic adenoviruses in murine and Syrian hamster models of cancer. Hum Gene Ther 23:1258–1268. doi: 10.1089/hum.2012.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaRocca CJ, Han J, Gavrikova T, Armstrong L, Oliveira AR, Shanley R, Vickers SM, Yamamoto M, Davydova J. 2015. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery 157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TV, Heller GJ, Barry ME, Crosby CM, Turner MA, Barry MA. 2016. Evaluation of polymer shielding for adenovirus serotype 6 (Ad6) for systemic virotherapy against human prostate cancers. Mol Ther Oncolytics 3:15021. doi: 10.1038/mto.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wold WS, Toth K. 2012. Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res 115:69–92. doi: 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 30.Dhar D, Toth K, Wold WS. 2012. Syrian hamster tumor model to study oncolytic Ad5-based vectors. Methods Mol Biol 797:53–63. doi: 10.1007/978-1-61779-340-0_4. [DOI] [PubMed] [Google Scholar]
- 31.Toth K, Lee SR, Ying B, Spencer JF, Tollefson AE, Sagartz JE, Kong IK, Wang Z, Wold WS. 2015. STAT2 knockout Syrian hamsters support enhanced replication and pathogenicity of human adenovirus, revealing an important role of type I interferon response in viral control. PLoS Pathog 11:e1005084. doi: 10.1371/journal.ppat.1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, Wold WSM. 2008. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A 105:7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tollefson AE, Spencer JF, Ying B, Buller RM, Wold WS, Toth K. 2014. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res 112:38–46. doi: 10.1016/j.antiviral.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Ying B, Tollefson AE, Spencer JF, Balakrishnan L, Dewhurst S, Capella C, Buller RM, Toth K, Wold WS. 2014. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob Agents Chemother 58:7171–7181. doi: 10.1128/AAC.03860-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth K, Ying B, Tollefson AE, Spencer JF, Balakrishnan L, Sagartz JE, Buller RM, Wold WS. 2015. Valganciclovir inhibits human adenovirus replication and pathology in permissive immunosuppressed female and male Syrian hamsters. Viruses 7:1409–1428. doi: 10.3390/v7031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying B, Toth K, Spencer JF, Aurora R, Wold WSM. 2015. Transcriptome sequencing and development of an expression microarray platform for liver infection in adenovirus type 5-infected Syrian golden hamsters. Virology 485:305–312. doi: 10.1016/j.virol.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tchitchek N, Safronetz D, Rasmussen AL, Martens C, Virtaneva K, Porcella SF, Feldmann H, Ebihara H, Katze MG. 2014. Sequencing, annotation and analysis of the Syrian hamster (Mesocricetus auratus) transcriptome. PLoS One 9:e112617. doi: 10.1371/journal.pone.0112617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. 2006. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-Ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol 80:1688–1699. doi: 10.1128/JVI.80.4.1688-1699.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CY, Weaver EA, Khare R, May SM, Barry MA. 2011. Mining the adenovirus virome for oncolytics against multiple solid tumor types. Cancer Gene Ther 18:744–750. doi: 10.1038/cgt.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crosby CM, Nehete P, Sastry KJ, Barry MA. 2015. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J Virol 89:669–675. doi: 10.1128/JVI.02184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shashkova EV, May SM, Barry MA. 2009. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology 394:311–320. doi: 10.1016/j.virol.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 43.Wolff G, Worgall S, vanRooijen N, Song WR, Harvey BG, Crystal RG. 1997. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol 71:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alemany R, Balague C, Curiel DT. 2000. Replicative adenoviruses for cancer therapy. Nat Biotechnol 18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 45.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 8:37–77. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 46.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P, Barry MA. 2011. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther 19:1254–1262. doi: 10.1038/mt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khare R, Hillestad ML, Xu Z, Byrnes AP, Barry MA. 2013. Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J Virol 87:3678–3686. doi: 10.1128/JVI.01392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver EA, Hillestad ML, Khare R, Palmer D, Ng P, Barry MA. 2011. Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412:19–27. doi: 10.1016/j.virol.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner MA, Middha S, Hofherr SE, Barry MA. 2015. Comparison of the life cycles of genetically distant species C and species D human adenoviruses Ad6 and Ad26 in human cells. J Virol 89:12401–12417. doi: 10.1128/JVI.01534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alemany R, Suzuki K, Curiel DT. 2000. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 51.Ganesan LP, Mohanty S, Kim J, Clark KR, Robinson JM, Anderson CL. 2011. Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium. PLoS Pathog 7:e1002281. doi: 10.1371/journal.ppat.1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piccolo P, Annunziata P, Mithbaokar P, Brunetti-Pierri N. 2014. SR-A and SREC-I binding peptides increase HDAd-mediated liver transduction. Gene Ther 21:950–957. doi: 10.1038/gt.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 54.Alzuguren P, Hervas-Stubbs S, Gonzalez-Aseguinolaza G, Poutou J, Fortes P, Mancheno U, Bunuales M, Olague C, Razquin N, Van Rooijen N, Enguita M, Hernandez-Alcoceba R. 2015. Transient depletion of specific immune cell populations to improve adenovirus-mediated transgene expression in the liver. Liver Int 35:1274–1289. doi: 10.1111/liv.12571. [DOI] [PubMed] [Google Scholar]
- 55.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol 71:8798–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Sitia G, Iannacone M, Aiolfi R, Isogawa M, van Rooijen N, Scozzesi C, Bianchi ME, von Andrian UH, Chisari FV, Guidotti LG. 2011. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog 7:e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K, Wold WS. 2007. Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med 130:223–235. [DOI] [PubMed] [Google Scholar]
- 59.Thomas MA, Spencer JF, Wold WSM. 2007. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med 130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]