Abstract
Many studies have established the central involvement of the Golgi apparatus in the transport and processing of plasma membrane, lysosomal, and secreted proteins. The Golgi apparatus of neurons is also involved in the axoplasmic flow of fast-moving macromolecules and in the orthograde, retrograde, and transsynaptic transport of exogenous ligands. Markers of the Golgi apparatus, based on traditional methods of enzyme cytochemistry, are not applicable to human tissues obtained at autopsy. For that reason, the Golgi apparatus of brain cells has not been examined adequately in diseases of the human nervous system. Here we report that an antiserum raised against MG-160, a 160-kDa sialoglycoprotein of medial cisternae of the Golgi apparatus of several rat cells, is a specific and easily reproducible immunocytochemical marker of the Golgi apparatus of human neurons and other cells obtained at autopsy. Application of this probe in amyotrophic lateral sclerosis has shown a fragmentation of the Golgi apparatus in motor neurons similar to that induced by depolymerization of microtubules. We suggest that the fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis has functional implications because significant reductions of secretion of insulin and immunoglobulins have been observed in islet cells and plasma cells, respectively, treated with microtubule-disrupting agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine J. C., Maurice M., Feldmann G., Avrameas S. In vivo and in vitro effects of colchicine and vinblastine on the secretory process of antibody-producing cells. J Immunol. 1980 Nov;125(5):1939–1949. [PubMed] [Google Scholar]
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Krasin F. A new hypothesis of the etiology of amyotrophic lateral sclerosis. The DNA hypothesis. Arch Neurol. 1982 Nov;39(11):677–680. doi: 10.1001/archneur.1982.00510230003001. [DOI] [PubMed] [Google Scholar]
- Croul S. E., Mezitis S. G., Gonatas N. K. An anti-organelle antibody in pathology. The chromatolytic reaction studied with a monoclonal antibody against the Golgi apparatus. Am J Pathol. 1988 Nov;133(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C. An update on long-term in vivo and in vitro studies designed to identify a virus as the cause of amyotrophic lateral sclerosis, parkinsonism dementia, and Parkinson's disease. Adv Neurol. 1982;36:343–353. [PubMed] [Google Scholar]
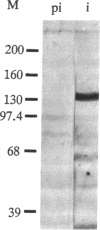
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag R., Stone G. C., Bolen F. A., Lindsey J. D., Ellisman M. H. Evidence that all newly synthesized proteins destined for fast axonal transport pass through the Golgi apparatus. J Cell Biol. 1982 Jun;93(3):568–575. doi: 10.1083/jcb.93.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. G., Gonatas J. O., Stieber A., Gonatas N. K. In vivo uptake of wheat germ agglutinin-horseradish peroxidase conjugates into neuronal GERL and lysosomes. Brain Res. 1980 Apr 28;188(2):465–472. doi: 10.1016/0006-8993(80)90045-1. [DOI] [PubMed] [Google Scholar]
- Hirano A. Aspects of the ultrastructure of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:75–88. [PubMed] [Google Scholar]
- Ho W. C., Allan V. J., van Meer G., Berger E. G., Kreis T. E. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989 Apr;48(2):250–263. [PubMed] [Google Scholar]
- Jasmin B. J., Cartaud J., Bornens M., Changeux J. P. Golgi apparatus in chick skeletal muscle: changes in its distribution during end plate development and after denervation. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Plaitakis A., Constantakakis E., Smith J. The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol. 1988 Sep;24(3):446–449. doi: 10.1002/ana.410240314. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Nunn P. B., Hugon J., Ludolph A. C., Ross S. M., Roy D. N., Robertson R. C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987 Jul 31;237(4814):517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Stieber A., Gonatas J. O., Gonatas N. K. Differences between the endocytosis of horseradish peroxidase and its conjugate with wheat germ agglutinin by cultured fibroblasts. J Cell Physiol. 1984 Apr;119(1):71–76. doi: 10.1002/jcp.1041190112. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Appel S. H., Crawford F., Sczcupak L. Immunoglobulins from amyotrophic lateral sclerosis patients enhance spontaneous transmitter release from motor-nerve terminals. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7371–7374. doi: 10.1073/pnas.85.19.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]