ABSTRACT
Simian T-lymphotropic virus 1 (STLV-1) enters human populations through contact with nonhuman primate (NHP) bushmeat. We tested whether differences in the extent of contact with STLV-1-infected NHP bushmeat foster regional differences in prevalence of human T-lymphotropic virus 1 (HTLV-1). Using serological and PCR assays, we screened humans and NHPs at two Sub-Saharan African sites where subsistence hunting was expected to be less (Taï region, Côte d'Ivoire [CIV]) or more (Bandundu region, Democratic Republic of the Congo [DRC]) developed. Only 0.7% of human participants were infected with HTLV-1 in CIV (n = 574), and 1.3% of humans were infected in DRC (n = 302). Two of the Ivorian human virus sequences were closely related to simian counterparts, indicating ongoing zoonotic transmission. Multivariate analysis of human demographic parameters and behavior confirmed that participants from CIV were less often exposed to NHPs than participants from DRC through direct contact, e.g., butchering. At the same time, numbers of STLV-1-infected NHPs were higher in CIV (39%; n = 111) than in DRC (23%; n = 39). We conclude that similar ultimate risks of zoonotic STLV-1 transmission—defined as the product of prevalence in local NHP and human rates of contact to fresh NHP carcasses—contribute to the observed comparable rates of HTLV-1 infection in humans in CIV and DRC. We found that young adult men and mature women are most likely exposed to NHPs at both sites. In view of the continued difficulties in controlling zoonotic disease outbreaks, the identification of such groups at high risk of NHP exposure may guide future prevention efforts.
IMPORTANCE Multiple studies report a high risk for zoonotic transmission of blood-borne pathogens like retroviruses through contact with NHPs, and this risk seems to be particularly high in tropical Africa. Here, we reveal high levels of exposure to NHP bushmeat in two regions of Western and Central tropical Africa. We provide evidence for continued zoonotic origin of HTLV-1 in humans at CIV, and we found that young men and mature women represent risk groups for zoonotic transmission of pathogens from NHPs. Identifying such risk groups can contribute to mitigation of not only zoonotic STLV-1 transmission but also transmission of any blood-borne pathogen onto humans in Sub-Saharan Africa.
KEYWORDS: HTLV-1, STLV-1, bushmeat, retroviruses, Sub-Saharan Africa, zoonoses
INTRODUCTION
Retroviruses originating in nonhuman primates (NHP) have spilled over to human populations at multiple occasions and today impact human health on a global scale (1). The hunting, handling, and consumption of NHP have been put forward as likely source of zoonotic infection with blood-borne viruses, such as simian immunodeficiency virus (SIV, giving rise to human immunodeficiency virus [HIV]), simian T-lymphotropic virus 1 (STLV-1), and simian foamy virus (SFV) (2, 3).
While HIV is the most momentous example of retroviral spread in humans, HTLV-1 (the counterpart of STLV-1 in humans) is also carried by 10 to 20 million people around the globe (4). Of those, 2 to 7% develop HTLV-1-associated symptoms of adult T-cell leukemia and neurological disorders like tropical spastic paraparesis or HTLV-1-associated myelopathy (5). In Sub-Saharan Africa (SSA), seroprevalence can reach 3%, e.g., among Pygmy groups in southern Cameroon (6). The area is also a hot spot of HTLV-1 diversity: eight of the nine phylogenetically distinct subtypes (A to J) have been detected in SSA (7, 8). Another striking feature of HTLV-1 epidemiology in SSA is that human strains sometimes form regional composite clades with simian-derived (STLV-1) sequences (6, 8, 9). While human-to-human transmission of HTLV-1 through, e.g., sexual intercourse, is a well-acknowledged route of spread (7), such an interspersion indicates that regional HTLV-1 diversity probably partly arose and may still be arising from zoonotic transmission events.
The risk of transmission of retroviruses from NHP is suggested to be greatest during activities involving direct blood contact (8, 10–13). Hence, if STLVs are prevalent among local NHP, differences in the extent and type of contact with NHP among human populations may contribute to differences in local HTLV-1 prevalence and diversity. To test this hypothesis, we investigated STLV-1/HTLV-1 epidemiology at two regions in SSA with predicted differences in human NHP exposure. Rates of contact of the local human population with live and dead NHP are high near the Taï National Park in Côte d'Ivoire (CIV) (8, 13), and NHP species are frequently infected with STLV-1 (up to 50% [14]). Yet Taï National Park is by now an isolated forest refugium, hunting is prohibited within the park, and bushmeat is often purchased already processed at local bushmeat markets (15–17). Compared to Taï, the Bandundu region in the predominantly forested central African Congo basin provides ample opportunity for direct contact of humans with live or freshly killed NHP, as people still extensively rely on NHP bushmeat for subsistence (18). As for the Taï region, previous evidence from several locations in Democratic Republic of the Congo (DRC) indicates that STLV-1 is circulating among local NHP (up to 20% of NHP are infected with the virus [19]), while prevalence among humans is unknown.
For this study, we (i) sampled and screened NHP and humans in CIV and DRC to infer STLV-1/HTLV-1 prevalence and phylogeny and (ii) surveyed human demographic factors and behavior in order to assess the risk of human NHP-borne STLV-1 infections in the study regions.
RESULTS
Human contact to NHP bushmeat and individual level risk factors.
We assessed factors potentially affecting the risk of human exposure to NHP using multivariate analysis. The model including cooking great ape as response variable was not statistically significant. All other models explained 19 to 64% of the variance as indicated by the conditional R2 (Table 1). Only for the models using dismembering, cooking, and eating monkeys as response was the interaction term between sex and age included; in the other cases, this full model was not significantly better than a reduced model excluding the interaction term.
TABLE 1.
Specifications of statistical models derived from general linear mixed model analysisa
| Term for included response variable | Estimate | SE | Lower CLb | Upper CLb | χ2 | DF | P |
|---|---|---|---|---|---|---|---|
| Hunting monkeyc | |||||||
| Intercept | −6.299 | 1.014 | −9.177 | −4.773 | |||
| Sex: male | 4.838 | 1.016 | 3.305 | 7.717 | 40.095 | 1 | <0.001 |
| Aged | 0.064 | 0.145 | −0.257 | 0.437 | 0.195 | 1 | 0.659 |
| Country: DRC | 0.706 | 0.315 | 0.023 | 1.493 | 4.051 | 1 | 0.044 |
| Dismembering monkeye | |||||||
| Intercept | −2.428 | 0.236 | −2.966 | −2.000 | |||
| Sex: male | 2.222 | 0.283 | 1.606 | 2.794 | |||
| Aged | 0.170 | 0.201 | −0.238 | 0.595 | |||
| Country: DRC | 0.643 | 0.330 | −0.086 | 1.380 | 3.120 | 1 | 0.077 |
| Sex and age | −0.663 | 0.218 | −1.094 | −0.233 | 9.031 | 1 | 0.003 |
| Preparing monkeyf | |||||||
| Intercept | 0.395 | 0.228 | −0.070 | 0.905 | |||
| Sex: male | −1.849 | 0.249 | −2.450 | −1.347 | |||
| Aged | 0.293 | 0.196 | −0.135 | 0.682 | |||
| Country: DRC | 2.445 | 0.504 | 1.437 | 3.533 | 14.541 | 1 | <0.001 |
| Sex and age | −0.766 | 0.233 | −1.228 | −0.310 | 10.821 | 1 | 0.001 |
| Eating monkeyg | |||||||
| Intercept | 0.465 | 0.244 | −0.051 | 1.010 | |||
| Sex: male | 0.668 | 0.219 | 0.241 | 1.122 | |||
| Aged | 0.426 | 0.163 | 0.095 | 0.769 | |||
| Country: DRC | 2.986 | 0.552 | 1.910 | 4.244 | 16.967 | 1 | <0.001 |
| Sex and age | −0.903 | 0.223 | −1.345 | −0.467 | 16.470 | 1 | <0.001 |
| Hunting great apeh | |||||||
| Intercept | −6.045 | 1.024 | −8.933 | −4.498 | |||
| Sex: male | 2.789 | 1.048 | 1.131 | 5.697 | 11.243 | 1 | 0.001 |
| Aged | 0.292 | 0.266 | −0.240 | 0.814 | 1.182 | 1 | 0.277 |
| Country: DRC | 0.111 | 0.588 | −1.189 | 1.314 | 0.035 | 1 | 0.851 |
| Dismembering great apei | |||||||
| Intercept | −4.716 | 0.531 | −5.946 | −3.805 | |||
| Sex: male | 2.089 | 0.555 | 1.099 | 3.335 | 15.451 | 1 | <0.001 |
| Aged | −0.053 | 0.206 | −0.474 | 0.359 | 0.066 | 1 | 0.797 |
| Country: DRC | 0.284 | 0.425 | −0.646 | 1.251 | 0.438 | 1 | 0.508 |
| Eating great apej | |||||||
| Intercept | −2.293 | 0.232 | −2.797 | −1.841 | |||
| Sex: male | 1.600 | 0.236 | 1.132 | 2.169 | 18.919 | 1 | <0.001 |
| Aged | −0.137 | 0.114 | −0.366 | 0.099 | 1.430 | 1 | 0.232 |
| Country: DRC | −0.909 | 0.352 | −2.152 | −0.158 | 5.266 | 1 | 0.022 |
Participants' sex, age, and country of residence were included as test predictors, and sampling village was included as random effect. The model including preparing great ape as a response variable was not significantly better than the null model comprising only the random effects (likelihood ratio test: χ2 = 7.974; P = 0.093; n = 684) and was therefore not considered further. DF, degrees of freedom; DRC, Democratic Republic of the Congo. Significant results are shown in bold.
Based on a model without the correlation between random slope and intercept.
The full model was highly significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 42.269; DF = 4; P < 0.001; n = 696) but not significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 1.083; DF = 1; P = 0.298). Therefore, results from the reduced model excluding the interaction are reported. It explained 64.11% of the variance (conditional R2). Intercept of random effects: variance = 0.00; SE = 0.00.
Age was z-transformed to a mean of 0 and standard error of 1.
The full model was highly significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 30.899; DF = 4; P < 0.001; n = 702) and significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 9.031; DF = 1; P = 0.003). Therefore, results from the full model including the interaction are reported. It explained 30.24% of the variance (conditional R2). Intercept of random effects: variance = 0.04; SE = 0.20.
The full model was highly significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 47.620; DF = 4; P < 0.001; n = 702) and highly significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 10.821; DF = 1; P = 0.001). Therefore, results from the full model including the interaction are reported. It explained 41.90% of the variance (conditional R2). Intercept of random effects: variance = 0.22; SE = 0.47.
The full model was highly significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 40.332; DF = 4; P < 0.001; n = 716) and highly significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 16.470; DF = 1; P < 0.001). Therefore, results from the full model including the interaction are reported. It explained 41.94% of the variance (conditional R2). Intercept of random effects: variance = 0.29; SE = 0.54.
The full model was significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 14.740; DF = 4; P = 0.005; n = 689), but not significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 1.766; DF = 1; P = 0.184). Therefore, results from the reduced model excluding the interaction are reported. It explained 39.05% of the variance (conditional R2). Intercept of random effects: variance = 0.00; SE = 0.00.
The full model was significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 16.022; DF = 4; P = 0.003; n = 690), but not significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 0.531; DF = 1; P = 0.466). Therefore, results from the reduced model excluding the interaction are reported. It explained 24.48% of the variance (conditional R2). Intercept of random effects: variance = 0.00; SE = 0.00.
The full model was highly significant compared to the null model comprising only the random effects (likelihood ratio test: χ2 = 30.346; DF = 4; P < 0.001; n = 714), but not significant compared to a reduced model excluding the interaction term between sex and age (likelihood ratio test: χ2 = 0.434; DF = 1; P = 0.510). Therefore, results from the reduced model excluding the interaction are reported. It explained 19.42% of the variance (conditional R2). Intercept of random effects: variance = 0.11; SE = 0.33.
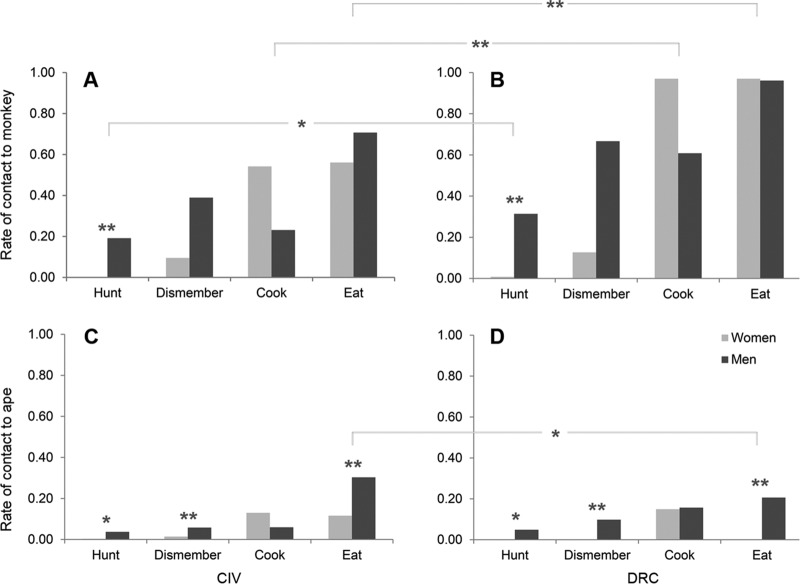
Residency in DRC was a clear positive predictor of the frequency to hunt, cook, and eat monkeys, and a trend was observed for dismembering monkeys (Fig. 1; Table 1). For instance, nearly all Congolese participants consumed monkey species, while only 56% of Ivorian women and 71% of Ivorian men did so. In contrast, more participants in CIV (women, 12%; men, 30%) ate great apes than in DRC (women, 0%; men, 21%) (Fig. 1; Table 1). Overall contact with NHP increased along a gradient of bushmeat processing. For instance, nearly only men hunted, but most members of both sexes ate monkeys. The effect of sex was dependent on age for the models including dismembering, cooking, and eating monkeys as a response: combined for both countries, the probability to dismember monkeys slightly increased with age in women but decreased with age in men (interaction sex-age: estimate + standard error [SE] = −0.663 + 0.218; χ2 = 9.031; degrees of freedom [DF] = 1; P = 0.003 [Table 1; Fig. 2]). Likewise, the probability to cook monkeys increased with age in women but decreased in men (interaction sex-age: estimate + SE = −0.766 + 0.233; χ2 = 10.821; DF = 1; P = 0.001 [Table 1; Fig. 2]). Lastly, women had lower levels of monkey consumption at younger ages than men, but at older ages women ate monkey more frequently than men (interaction sex-age: estimate + SE = −0.903 + 0.223; χ2 = 16.470; DF = 1; P < 0.001 [Table 1; Fig. 2]).
FIG 1.
Activities resulting in contact with nonhuman primate bushmeat from monkeys (A and B) and great apes (C and D) by inhabitants of the Taï region in CIV (A and C) and the Bandundu region in DRC (B and D). P values were derived from GLMM analysis. *, P < 0.05; **, P < 0.001.
FIG 2.
Visualization of the interaction between the fixed affects sex and age (in years) included as terms in GLMM analyses. The size of the circles is proportional to the number of participants representing that data point (smallest circle = 1 individual). Graphs on the left side represent results for female participants, those on the right side for male participants. Models included dismembering monkeys (A), cooking monkeys (B), and eating monkeys (C) as response variables. Dashed lines depict the model (fitted at the average of the respective parameter for both countries), and dotted lines depict bootstrapped 95% confidence intervals.
Prevalence of HTLV-1/STLV-1 among humans and NHP in the study region.
Of the 574 human sera tested from CIV and 302 human sera tested from DRC, 22 (3.8%) and 10 (3.3%) were enzyme-linked immunosorbent assay (ELISA) positive, respectively. Three of those samples from CIV (0.5%) and also three samples from DRC (1%) revealed confirmatory Western blot profiles typical of HTLV-1 (including one sample from DRC showing a pattern consistent with HTLV-1/2 dual infection [Table 2]). Another nine specimens from CIV and four specimens from DRC were seroreactive while producing indeterminate Western blot profiles. An interesting finding was the detection of HTLV-2-compatible patterns in two individuals from CIV. However, those two were negative by tax-specific PCR.
TABLE 2.
Characteristics of participants positive for STLV-1/HTLV-1 at the study sites in Côte d'Ivoire and the Democratic Republic of the Congo, determined in the present and a previous studya
| Country | Study participant/accession no. for LTR/accession no. for env | Sex | Age (yrs) at sampling | HTLV-1 Western blot result | Infecting subtype HTLV-1 PCR | Minimum patristic distance to any STLV-1, % |
NHP contacted via indicated means |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LTR | env | Hunting | Dismembering | Cooking | Eating | ||||||
| CIVb | Gah050/HE667747/HE667769 | M | 32 | STLV-1I/SM | 0.65 | 0.76 | None | Monkeys, chimps | Monkeys, chimps | Monkeys, chimps | |
| CIVb | Gul014/HE667748/HE667760 | F | 42 | HTLV-1A | 3.24 | 4.11 | None | None | Monkeys, chimps | Monkeys, chimps | |
| CIVb | Kei005/HE667749/HE667761 | F | 78 | STLV-1I/SM | 0.65 | 0.72 | None | Monkeys | Monkeys | Monkeys | |
| CIVb | Kei025/HE667750/HE667762 | M | 63 | STLV-1J | 0 | 0.22 | None | Monkeys | None | Monkeys | |
| CIVb | Kei075/HE667751/HE667763 | F | 50 | HTLV-1A | 3.63 | 3.7 | None | None | Monkeys | Monkeys | |
| CIVb | Pau002/HE667752/HE667764 | F | 55 | HTLV-1A | 2.79 | 3.66 | None | None | Monkeys | Monkeys | |
| CIVb | Pau009/HE667755/HE667765 | M | 76 | STLV-1I/SM | 0.19 | 0.44 | Monkeys, chimps | Monkeys, chimps | None | Monkeys, chimps | |
| CIVb | Pon002/HE667753/HE667766 | F | 68 | HTLV-1A | 3.16 | 3.88 | None | Monkeys | Monkeys | Monkeys | |
| CIVb | Tie005/HE667754/HE667767 | F | 55 | HTLV-1A | 5.18 | 3.01 | None | Monkeys | Monkeys | Monkeys | |
| CIVb | Tie011/HE667756/HE667768 | F | 52 | HTLV-1A | 5.18 | 3.01 | None | Monkeys | Monkeys | Monkeys | |
| CIV | Pon167c | M | 52 | HLTV-1 | HTLV-1A | 3.87 | None | None | None | None | |
| CIV | Pau490c | F | 35 | HLTV-1 | HTLV-1A | 3.01 | None | None | None | None | |
| CIV | Gah590 | F | 26 | HLTV-1 | STLV-1I/SM | 0.65 | 2.61 | None | None | None | None |
| CIV | Gah618d | F | 73 | ID | STLV-1I/SM | 1.39 | None | Monkeys, chimps | Monkeys, chimps | ||
| DRC | N276 | M | 49 | HLTV-1 | HTLV-1B | 1.52 | 0.43 | None | Monkeys | Monkeys | Monkeys |
| DRC | N305 | F | 53 | ID | HTLV-1B | 1.52 | 0.65 | None | Monkeys | Monkeys | |
| DRC | N307e | M | 57 | HTLV-1/2 | HTLV-1B | 1.71 | 0.65 | None | Monkeys | Monkeys | Monkeys |
| DRC | L047 | F | 10 | HLTV-1 | HTLV-1B | 2.1 | 1.3 | ||||
Letters in study participants' identification codes stand for the different sampling villages. M, male; F, female; ID, indeterminate.
Published in reference 8.
Only env sequence determined.
Only LTR sequence determined.
env sequence duplicate of N305.
During quantitative PCR, we detected HTLV-1/STLV-1 positives with a minimum copy number of 3.4 molecules per microliter, and from those we also successfully amplified env and long terminal repeat (LTR) fragments using seminested PCR. We therefore conclude that our assays were adequately sensitive to detect low copy numbers of the target. Out of all ELISA-positive samples, four DNA extracts amplified during tax-specific PCR in each CIV and DRC and env and/or LTR fragments were successfully generated. Combining serological and genetic evidence, HTLV-1 infection rates were 0.7% in CIV and 1.3% in DRC.
Among NHP, over a third of the 111 samples from CIV from this and previous studies (total, 38.7%) (8, 9, 47–49) were STLV-1 positive by env and/or LTR PCR, while 23.1% of the 39 NHP samples from DRC were positive. Looking closer at the prevalence of STLV-1 among individual primate species, several species' samples from CIV revealed positivity of over a third (sooty mangabey, Cercocebus atys, 8/20; Western red colobus, Piliocolobus badius, 18/43; and Western chimpanzee, Pan troglodytes verus, 17/42). Similarly, in DRC, a colobine species (Tshuapa red colobus, Piliocolobus tholloni) had a relatively high rate of STLV-1 infection (6/17 [Table 3]).
TABLE 3.
Prevalence of STLV-1 among the NHP population at the study sites in Côte d'Ivoire and the Democratic Republic of the Congo
| NHP speciesa | No. | HTLV-1 env and/or LTR |
|
|---|---|---|---|
| No. positive | Total % positive | ||
| Tested in CIV | |||
| Cercocebus atysb | 20 | 8 | |
| Cercopithecus campbelli | 3 | 0 | |
| Cercopithecus diana | 2 | 0 | |
| Cercopithecus petaurista | 1 | 0 | |
| Piliocolobus badiusc | 43 | 18 | |
| Pan troglodytes verusd | 42 | 17 | |
| Total | 111 | 43 | 38.7 |
| Tested in DRC | |||
| Lophocebus aterrimus | 1 | 0 | |
| Cercopithecus ascanius | 10 | 2 | |
| Cercopithecus pogonias | 3 | 0 | |
| Cercopithicus neglectus | 1 | 1 | |
| Colobus guereza | 4 | 0 | |
| Perodicticus potto | 1 | 0 | |
| Piliocolobus tholloni | 17 | 6 | |
| Cercopithecidaee | 1 | 0 | |
| Galagidaee | 1 | 0 | |
| Total | 39 | 9 | 23.1 |
The difference in number of HTLV-1 infections between the human populations was not statistically significant (Fisher's exact test: 4/574 positive in CIV and 4/302 positive in DRC; P = 0.457), while we observed a trend toward significance for NHP (43/111 positive in CIV and 9/39 positive in DRC; P = 0.082).
Phylogenetic analyses.
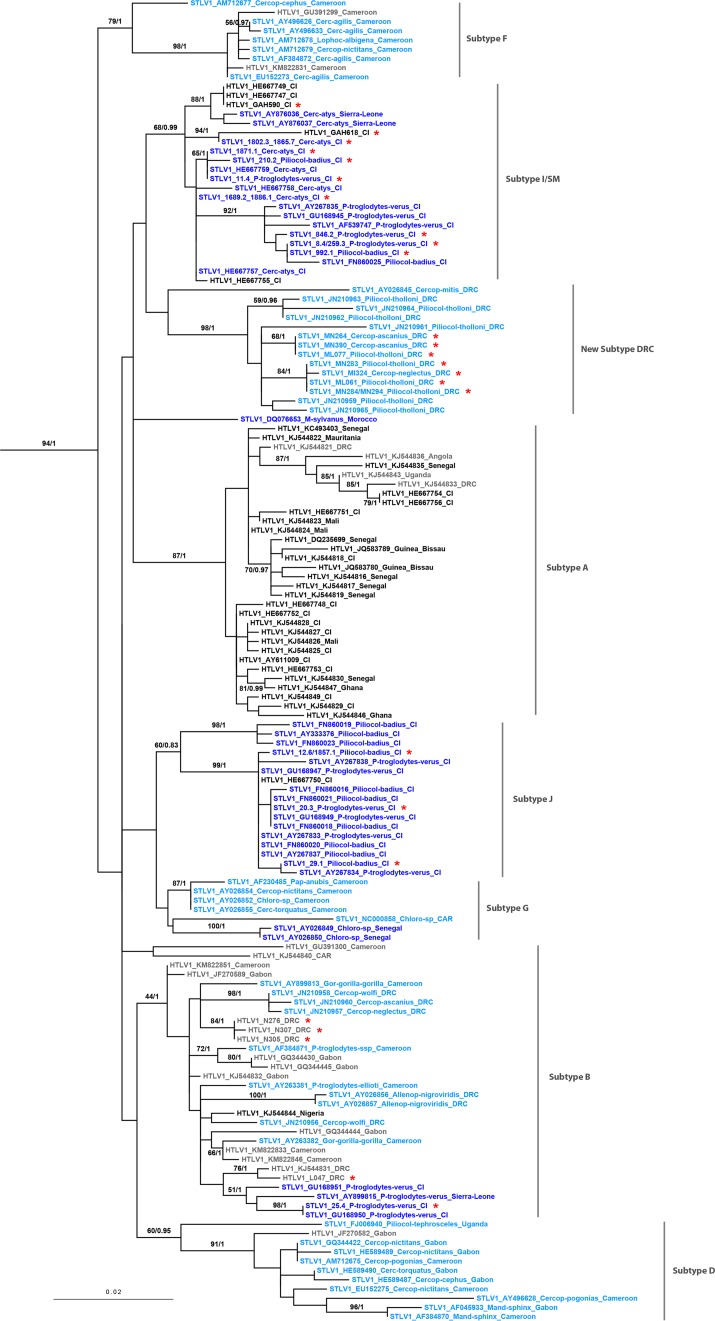
Maximum likelihood (ML) and Bayesian methods delivered comparable tree topologies for the LTR (Fig. 3) and env (Fig. 4). In detail, in the LTR phylogeny two of the newly generated Ivorian human sequences (Gah590 and Gah618) were closely related to sequences of the STLV-1 I/SM subtype previously described from this geographic region (bootstrap, 68; posterior probabilities, 0.99 [Table 2; Fig. 3]) (8). Both sequences originated from female participants of the village Gahably. Only Gah618 was reported to have had contact with NHP bushmeat previously (Table 2). The three new sooty mangabey sequences (1802.3, 1871.1, and 1689.2), two Western red colobus sequences (210.2 and 992.1), and three Western chimpanzee sequences (11.4, 846.2, and 8.4) from CIV fell into the same sequence cluster (Fig. 3). The two remaining human sequences from CIV (only generated for env; one woman from Pauleoula [Pau490] and one man from Ponan [Pon167]) clustered closely with known sequences of the HLTV-1A subtype (bootstrap, 82; posterior probabilities, 1 [Table 2; Fig. 4]). The four new human sequences from DRC (two men [N276 and N307] and one woman [N305] from Nganda and one woman from Lompole [L047]) were closely related to a weakly supported clade containing human and simian sequences previously assigned to subtype HTLV-1 B, to which also one new chimpanzee sequence from CIV (25.4) was attributed (bootstrap, 44; posterior probabilities, 1 [Table 2; Fig. 3]). The remaining simian sequences from CIV (two from red colobus [12.6 and 29.1] and one from Western chimpanzee [20.3]) clustered with published simian and human sequences from the Taï region, described as STLV-1 J/HTLV-1 (8) (bootstrap, 60; posterior probabilities, 0.83 [Fig. 3]). All simian sequences from DRC (two from red-tailed monkey [MN264 and MN390], four from Tshuapa red colobus [ML061, ML077, MN283, and MN284], and one from De Brazza's monkey [MI324]) were closely related to simian sequences described as a new subtype from DRC previously (19, 20) (bootstrap, 98; posterior probabilities, 1 [Fig. 3]). This subtype contains sequences from Kole (e.g., Pth95 and Pth73 included in our phylogenetic LTR tree [Fig. 4] [20]) and from Mbandaka (Cas463 and Cag432 [19]; locations shown in Fig. 5).
FIG 3.
Maximum likelihood tree generated from the alignment of 145 LTR sequences (557 bp) in a study examining the prevalence of STLV-1/HTLV-1 among humans and nonhuman primates in western Côte d'Ivoire (CIV) and central Democratic Republic of the Congo (DRC). Bayesian methods resulted in similar tree topologies. Branch labels include bootstrap values if >50 (except for HTLV-1B clade) and posterior probability values if >0.95 (except for STLV-1 J/HTLV-1 clade). Assignment of molecular HTLV-1 subtypes was done on the basis of reference sequences included in this study, which were assigned to a given subtype previously. The scale bar represents nucleotide substitutions per site. To increase legibility, branches leading to outgroup sequences (KC786907 and DQ076656) were removed after rooting. Subtype G was formerly termed Central and West African subtype. HTLV-1 sequences originating from human specimens from West Africa are shown in black and those from central Africa in gray. STLV-1 sequences determined from simian specimens from West Africa are shown in dark blue and those from Central Africa in light blue. Sequences generated in this study are marked with a red asterisk. Sequence names are rendered as HTLV/STLV_ GenBank accession number/study identifier of new sequences_ host species (for primates)_ country of origin. Country abbreviations: CIV, Côte d'Ivoire; DRC, Democratic Republic of the Congo; CAR, Central African Republic. Primate genus abbreviations: Allenop, Allenopithecus; Cerc, Cercocebus; Cercop, Cercopithecus; Chloro, Chlorocebus; Gor, Gorilla; Lophoc, Lophocebus; M, Macaca; Mand, Mandrillus; Pap, Papio; Piliocol, Piliocolobus; P, Pan.
FIG 4.
Maximum likelihood tree generated from the alignment of 117 env sequences (495 bp) in a study examining the prevalence of STLV-1/HTLV-1 among humans and nonhuman primates in western Côte d'Ivoire and central Democratic Republic of the Congo. Bayesian methods resulted in similar tree topologies. Branch labels include bootstrap values if >50 and posterior probability values if >0.95. Assignment of molecular HTLV-1 subtypes was done on the basis of reference sequences included in this study, which were assigned to a given subtype previously. The scale bar represents nucleotide substitutions per site. To increase legibility, branches leading to outgroup sequences (KF242505 and DQ076646) were removed after rooting. Subtype G was formerly termed Central and West African subtype. HTLV-1 sequences originating from human specimens from West Africa are shown in black and those from central Africa in gray. STLV-1 sequences determined from simian specimens from West Africa are shown in dark blue and those from Central Africa in light blue. Sequences generated in this study are marked with a red asterisk. Sequence names are rendered as HTLV/STLV_ GenBank accession number/study identifier of new sequences_ host species (for primates)_ country of origin. Abbreviations are as for Fig. 3.
FIG 5.
Sampling locations (yellow dots) at the Taï region near Taï National Park, Côte d'Ivoire and at the Bandundu region near Salonga National Park, Democratic Republic of the Congo. Red dots indicate sampling locations from previous studies (19, 20), from which simian sequences closely related to sequences generated in this study stem.
DISCUSSION
Combining genetic, serological, and epidemiological data, we show that STLV-1/HTLV-1 is prevalent among NHP in CIV and DRC, that humans are frequently in contact with NHP through various activities, and that humans carry HTLV-1 strains closely related to simian ones.
Humans are highly exposed to NHP.
Contact with NHP was frequent at both study sites, but people from DRC were more frequently exposed to, e.g., monkey bushmeat than participants from CIV (Fig. 1). Similar to previous findings from the Taï region, Uganda, and Cameroon (11, 13, 18), contact of women decreased along a gradient of bushmeat freshness (e.g., most women ate, but nearly never hunted, NHP), while men primarily dismembered and hunted NHP. Although this indicates that men are in contact with NHP body fluids more often than women, such contact turned out to be age dependent: for dismembering, cooking, and eating monkeys, contact rates decreased with age in men and increased with age in women. This is in line with the observation by one of us (Grit Schubert) that it is often a younger male community member that butchers the monkey prey of a hunter. This age-sex interaction has previously been overlooked, but it is important in specifying risk groups: besides higher male engagement in activities involving freshly killed primates, men also potentially come into contact with simian pathogens earlier in life than women.
Evidence for zoonotic transmission of STLV-1 in CIV and potentially DRC.
Three male study participants (aged 32, 63, and 76 years), and three female participants (aged 26, 73, and 78 years) from CIV were found to carry STLV-1 sequences closely related to sequences found in local sooty mangabey, Western red colobus, and Western chimpanzees (STLV-1I/SM and STLV-1J [Fig. 3 and 4; Table 2]). This corroborates previous findings that zoonotic transmission of STLVs (and more generally retroviruses) to humans is an ongoing process in the Taï region (8, 21). However, inference on a link between sex and age is impossible from this low number of positives. One of the STLV-1-infected female participants (Gah590 [Table 2]), for instance, was relatively young (26 years old) and did not fall into the risk group of mature women we identified. In order to find evidence for a connection between risk assignment and actual HTLV-1/STLV-1 infection rates, a substantially larger data set would be required. All four human sequences from DRC fell into subtype B clade, which contains STLV-1/HTLV-1 sequences of both human and simian origins at high frequencies. In this clade, it is therefore difficult to disentangle zoonotic or subsequent transmission among humans (9). Interestingly, two of our highly related human sequences (N305 and N307 [Fig. 3 and 4]) originated from a married couple, strongly pointing to intrafamily transmission.
Why are HTLV-1 infection rates equally low at CIV and DRC?
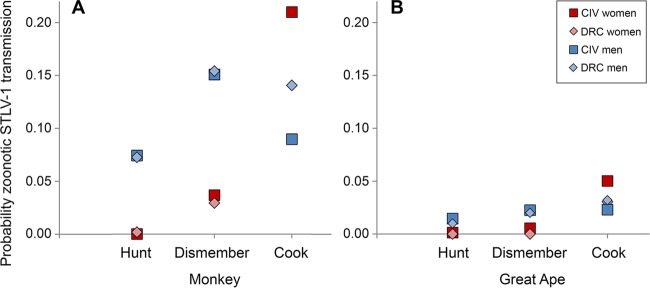
Unlike predicted from mere differences in behavioral exposition rates to NHP, overall rates of human infection with HTLV-1 were similarly low in the Taï region (0.7%) and the Bandundu region (1.3%). A relatively low rate of (zoonotic) HTLV-1 in humans despite extensive contact with NHP can, to some degree, be explained by the necessity of cell contact upon virus transmission (22). However, prevalence in local simian hosts is also expected to affect the ultimate risk of acquiring STLV-1 from NHP. In both CIV and DRC, red colobus monkey, a species that is hunted frequently by humans (15), also carries STLV-1 most often, with prevalences of 13.9 to 29.4% in DRC (20) (Table 3) and 41.9% in CIV (Table 3). Of all NHP sampled, more than one-third were infected with STLV-1 in CIV, but only one-fifth were infected in DRC. Intriguingly, when multiplying this difference in prevalence with contact rates during activities that can involve direct blood contact (hunting, butchering, and cooking NHP), the resulting risks are nearly identical in CIV and DRC (Fig. 6). Our sample size of NHP was small in DRC, but evidence from throughout DRC reveals that, on average, 5.4% (20) to 15.4% (19) of all NHP carry STLV-1, which is even lower than the 23.1% we found. This may suggest that the risk of acquiring STLV-1 from NHP is lower in DRC (despite high average contact rates) and that most transmission events result from human-to-human contacts. Notably, all newly generated Congolese NHP sequences belong to the “new subtype DRC” (19, 20). This subtype forms a well-supported, thus far exclusively simian clade (bootstrap, 98 to 100; posterior probabilities, 1 [Fig. 3 and 4]). Consistent with the clustering of sequences generated in this study and of sequences stemming from previously sampled locations at DRC (Kole and Mbandaka [Fig. 5]), there is no major riverine barrier that could prevent gene flow between those sites.
FIG 6.
STLV-1 transmission risk from NHP during hunting, dismembering, and cooking monkeys (A) and great apes (B). Risk is illustrated separately for women and men living near Taï National Park, Côte d'Ivoire, or Salonga National Park, Democratic Republic of the Congo, and was calculated as product of individual rates of contact to NHP and STLV-1 prevalence in NHP in the study regions in Côte d'Ivoire (on average 38.7%) and Democratic Republic of the Congo (on average 23.1% [Table 3]).
Taken together, our results lead us to conclude that the ultimate risks of zoonotic STLV-1 transmission—the product of prevalence in local NHP and high-risk contact of humans to NHP—are similar for the West and Central African human populations we investigated. This may contribute to a similarly low HTLV-1 prevalence in humans sampled cross-sectionally in both regions. Others have, however, detected variation among ethnic groups in HTLV-1 occurrence in individuals belonging to a high-risk group (being bitten by an NHP), and up to 21% of Pygmies of Cameroon were infected with the virus (6). Nevertheless, the high-risk age and sex groups—young men and older women—identified in this study may serve as primary targets of research, sensitization, and education campaigns. Future study comparing potential risk factors in exposition of human populations to, e.g., zoonotic blood-borne pathogens, must take into account also the prevalence of such pathogens in nonhuman hosts in order to draw sensible conclusions on the risk of zoonotic transmission.
MATERIALS AND METHODS
The study.
During 2011 to 2013, inhabitants from seven villages in Western CIV, Taï region, nearby Taï National Park, and from three villages in Central DRC, Bandundu region, close to Salonga National Park, were asked to participate in a study on zoonotic pathogen transmission among humans and NHP (Fig. 5). The study objectives and procedures were first introduced to local health authorities/health workers and, with their help, explained to the local population. This included also procedures for sampling NHP bushmeat. Prior to sampling, the objectives and procedures were again explained individually.
Ethics.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration (http://www.wma.net/en/30publications/10policies/b3/) and its later amendments or comparable ethical standards. Study approval for human sampling was given by the Ivorian ethics commission (Comité national d'éthique et de la recherche [CNER], permit number 101 10/MSHP/CNER/P) and the Congolese ethics commission (Comité d'Éthique, Ministère de l'Enseignement Supérieur et Universiaire, permit number ESO/CE/018/11). Informed consent was obtained from all individual participants included in the study. All questionnaire data were treated anonymously.
Permits for NHP sampling were obtained from the Office Ivoirien des Parcs et Réserves and the Ministère de la Recherche of Côte d'Ivoire. Ethical approval for samples from bushmeat in DRC was not required, as samples were taken only from dead animals; hunting of primates was not encouraged by researchers.
Questionnaire analysis. (i) Data collection.
A questionnaire was developed that provided personal demographic information (sex, age, country of residence, and village of residence) and NHP contact data, allowing us to assess factors affecting the risk of pathogen transmission from NHP to humans (for detailed questionnaire development, see also reference 13). Contact with NHP was subdivided into hunting, dismembering, cooking, and eating NHP. At both study sites, monkeys as well as great apes (Western chimpanzees [Pan troglodytes verus] in Taï and bonobos [Pan paniscus] in Bandundu) are present (17, 23), and these two categories are reliably distinguished by the local population. Contact with NHP was therefore recorded separately for monkeys and great apes. The questionnaire was in French, but if necessary, interviews were also conducted in local languages (e.g., Guéré in CIV and Nkundu in DRC). Interviews were conducted by trained local interviewers and supervised by a scientist.
For CIV, questionnaire data on nutritional habits and contact with NHP were gathered from 504 participants from the villages Daobly, Ponan, Gouleako, Pauleoula, Gahably, Zaipobly, and Keibly (also used in the study described in reference 13). For DRC, data were collected from 236 volunteering participants from the villages Lompole, Ipope, and Nganda. While the data set was larger for CIV than for DRC, it contained comparable proportions of male (CIV, 39.4%; DRC, 43.2%) and female (CIV, 60.6%; DRC, 56.8%) participants, and the mean ages were also similar for both countries (CIV, 39.7 years; DRC, 40.4 years).
Questionnaire analysis. (ii) Exposure to NHP and individual-level risk factors.
The proportions of participants that had been exposed to NHP (monkeys and great apes) through hunting, dismembering, cooking, and eating were summarized by their sex and country of residence. We did not consider ethnicity here (but see reference 13) because there was no variation among the Congolese participants (all were Bantu).
To investigate what influenced the probability to be in contact with NHP, we used generalized linear mixed models (GLMM) (24) with binomial error structure and logit link function. The four binary response variables were hunting, dismembering, cooking, and eating NHP, and each of the four models was calculated for monkeys and for great apes separately. The fixed effects tested in the models were the factors sex (male/female) and country of residence (CIV/DRC) and the covariate age. Since changes in contact with NHP over age potentially differ between the sexes, we included also the interaction between age and sex into the model. Sampling village was included as random effect (25). To keep the type I error rate at the nominal level of 5% (25, 26), random slopes of the fixed effects sex and age were accounted for, but not the correlation parameters between random intercepts and random slopes terms because respective null models were unidentifiable. Age was z-transformed (to a mean of 0 and a standard deviation of 1).
All models were fitted as logistic models in R (27) using the function glmer of the R-package lme4 (28, 29). We checked for model stability by excluding subjects one at a time from the data and comparing the model estimates derived for these data with those derived for the full data set. This indicated no influential subjects to exist. Variance inflation factors (30) were derived using the function vif of the R-package car (31) on a standard linear model excluding the random effects, and they did not indicate collinearity to be an issue.
We examined the significance of the full model compared to the null model comprising only the random effect and random slopes through a likelihood ratio test (implemented through the R-function anova with “argument test” set to “Chisq” [32, 33]). Using the same procedure, we compared the fit of the full model with that of a reduced model that excluded the interaction term between age and sex. To allow for a likelihood ratio test, we fitted the models using maximum likelihood (rather than restricted maximum likelihood [34]). Confidence intervals were derived using the function confint.merMod of the package lme4. P values for the individual effects were based on likelihood ratio tests comparing the full with respective reduced models (R function drop1 [25]).
Lastly, we calculated the variance explained by the model (conditional R2 [35]). For each model, we excluded participants with missing values. Sample sizes are given in Table 1.
Human and NHP sample collection and storage.
For humans, 9 ml of blood was collected into EDTA tubes by venipuncture from 574 participants in CIV and 302 participants in DRC (numbers are higher than for questionnaire analyses because not all participants completed a questionnaire). This included 325 female and 216 male participants from CIV (for 33 individuals, sex information was lost) and 159 female and 143 male participants from DRC. Blood samples were separated into serum, leukocytes (buffy coat), and erythrocytes, and respective aliquots were snap-frozen in liquid nitrogen in the field and for laboratory analyses transferred to −80°C at the Laboratoire National d'appui au Développement Agricole, CIV, and Robert Koch Institute, Germany.
Fifty-two anesthetized NHP and NHP carcasses from CIV were sampled as described in reference 14, and blood from three additional pet monkeys was collected by a veterinarian. Thirty-nine primate bushmeat samples from DRC were taken by trained veterinarians during butchering of freshly hunted monkey carcasses in the local villages or a hunter's camp and also from freshly smoked NHP. Prior to sampling, local hunters were asked for permission. To not encourage hunting, never did hunters receive any payment (money or goods) for sampling, and primate meat was never purchased from, e.g., passing bushmeat traders.
Serological screening.
Human serum samples were tested in duplicate for reactivity to HTLV-1 and -2 antigens by an HTLV-1/2 ELISA (Murex Biotech, Dartford, UK), allowing detection of IgA, IgG, and IgM antibodies. Reactive samples were then subjected to Western blot analysis (HTLV Blot 2.4; MP Diagnostics/Biomedicals/Singapore) as a confirmatory assay, and interpretation was done according to the manufacturer's instructions.
DNA extraction and amplification.
DNA was extracted from buffy coats of all ELISA-reactive human samples by using a QIAamp DNA blood minikit and from all NHP samples by using a DNeasy tissue kit (Qiagen, Hilden, Germany), following the manufacturer's instructions.
For human DNA extracts, we then attempted to amplify HTLV-1 sequences in a quantitative PCR that targets a short fragment of the diagnostic tax region (∼180 bp), using 200 ng of template DNA. We therefore followed the protocol described in reference 14, using the primers SK43 (CGG ATA CCC AGT CTA CGT GT) and SK44 (GAG CCG ATA ACG CGT CCA TCG), the probe HTLV TaxTM (6-carboxyfluorescein [FAM]-CGC CCT ATG GCC ACC TGT CCA GA XT P), and the following cycling conditions: 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 35 s.
In order to obtain phylogenetically informative sequences, tax-positive human DNA extracts as well as all NHP DNA extracts were then analyzed by a multiplex nested/seminested PCR system targeting proviral DNA fragments of env (∼570 bp) and the long terminal repeat (LTR; ∼640 bp). In the first round, 200 ng of DNA extract was used, along with two pairs of primers: S10 (GGC CCT AAT AAT TCT ACC CG) and R8906 (GAA CTT TCG ATC TGT AAC GGC G) for the LTR and HFL71 (CCA GTG GAT CCC GTG GAG A) and HFL72 (AGG AGG ATT TGA TGG GAG A) for env. Cycling conditions were 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 90 s, with a final step at 72°C for 10 min. After dilution of the first round's PCR products 1:40, two separate second-round reactions were performed using 2 μl of the diluted PCR product, with the primers Xho (GAG CTC GAG CAG ATG ACA ATG ACC ATG AG) and again R8906 in a seminested reaction that targeted the LTR and primers HFL75 (TCA AGC TAT AGT CTC CTC CCC CTG) and ENV2 (GGG AGG TGT CGT AGC TGA CGG AGG) in a nested reaction that targeted env. Cycling conditions for both assays were 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 58°C (LTR) or 62°C (env) for 30 s, and 72°C for 40 s, with a final step at 72°C for 10 min.
PCR products were visualized on a 1.5% agarose gel before being purified and Sanger sequenced in both directions. Prior to phylogenetic analyses, the obtained sequences were compared to publicly available sequences via the NCBI BLAST service (36) to confirm that the expected proviral DNA sequences had been amplified.
Specimens were considered HTLV-1 infected if they produced positive Western blot and/or PCR results. We calculated and plotted the ultimate risk of zoonotic STLV-1 transmission as the product of the overall prevalence of STLV-1 in local NHP and contact with NHP blood through hunting, dismembering, and cooking, respectively.
A subset of NHP from CIV (n = 60) and from DRC (n = 33) was also screened for STLV-3, which is known to occur in simian species and has potentially been transmitted to humans (37). We attempted to amplify proviral DNA fragments of the LTR (∼540 bp) using a multiplex nested/seminested PCR system (38). In the first round, 200 ng of DNA extract was used, with the primer pair AV51 (ACA ATT GCC TCG AGC TCA CCC) and pX-LTRAS (TTT ATA GGA CCC AGG GTT CTT). Cycling conditions were 94°C for 3 min followed by 14 cycles of 95°C for 20 s, 56°C for 30 s, and 72°C for 1 min and then 30 cycles of 95°C for 20 s, 52°C for 30 s, and 72°C for 1 min, with a final step at 72°C for 5 min. After dilution of the first round's PCR products 1:40, a second-round reaction was performed using 2 μl of the diluted PCR product, with the primers pX-LTRS (CRG GCA CAC RGG YCT ACT CCC) and pX-LTRAS. Cycling conditions were the same as in the first round. PCR products were visualized on a 1.5% agarose gel. All of the NHP specimens tested were STLV-3 negative.
NHP species confirmation.
For the DRC primate bushmeat and the CIV primate pet samples, species attribution was not always possible from field data (e.g., freshly smoked NHP). Taxonomic assignment of the samples was therefore conducted using a pan-mammal PCR assay targeting an ∼300-bp fragment of the mitochondrial 16S gene, as described in reference 8, with subsequent comparison to the NCBI's nonredundant database through an online BLAST search (36). Following the method of Calvignac-Spencer and colleagues (8), in cases where identity levels below 99% were observed (between 97% and 98%), identification was made combining BLAST information (i.e., second hit with a marked drop in E value) and available biological information (i.e., reported presence in the forest and photo evidence). In two instances, assignment was possible only at the family level.
Sequence analyses. (i) Data sets.
The LTR and env data sets included the sequences determined in this study, 2 outgroup sequences (one STLV-1 sequence from a Celebes crested macaque, Macaca nigra, and an HTLV-1 C sequence from a person from Australia), along with all publicly available HTLV-1/STLV-1 sequences from the study region in CIV (none were available for the study region in DRC), and a subset of the available sequences determined from humans or NHP in West and Central Africa. All published sequences were retrieved from NCBI. NHP sequences from captive individuals of uncertain geographic origin were excluded. Each data set was aligned by using MUSCLE (39) implemented in SeaView v4 (40). Duplicate sequences were identified using ALTER (41) and removed if originating from the same species. Final LTR and env sequence alignments comprised 145 and 117 sequences, respectively.
Sequence analyses. (ii) Phylogenetic analyses.
Phylogenetic analyses were performed both in a maximum likelihood (ML) and Bayesian framework on the env and LTR data sets, using nucleotide substitution models identified to explain the data best by running jModeltest v2.1.4 (42). On the basis of the comparison of Bayesian information criterion (BIC) scores derived from the model likelihoods, a Hasegawa-Kishino-Yano (HKY) model with γ-distributed rate heterogeneity (+G) was selected for the LTR data set, and a Tamura-Nei (TrN) model with equal base frequencies and γ-distributed rate heterogeneity was selected for the env data set.
ML analysis for the LTR data set was performed on the PhyML webserver (http://www.atgc-montpellier.fr/phyml/ [43, 44], including nucleotide equilibrium frequency optimization), and for the env data set by running PhyML v3.0 from the command line (including equal nucleotide equilibrium frequencies, which is not implemented on the PhyML webserver or the SeaView interface). The tree search started from a BioNJ tree and was performed using both nearest-neighbor interchange and subtree pruning-regrafting with optimization of tree topology and branch lengths. Branch robustness was measured by using nonparametric bootstrapping (250 pseudoreplicates).
Bayesian analyses were conducted in BEAST v1.8.2 (45), with the assumption of a relaxed molecular clock (uncorrelated lognormal) and a constant population size (following the description in reference 8). Two runs with each 20,000,000 generations were completed for each of the LTR and env data sets. Trees and numerical values taken by parameters of the model were sampled every 1,000 generations. We confirmed that chain mixing behavior was sufficient and that duplicate runs converged to the same parameter values using Tracer v1.6 (46). Trees from duplicate runs, for the LTR and env, respectively, were combined in LogCombiner v1.8.2 (part of the BEAST package) after removing a 40% burn-in period and by downsampling chains by a factor of 10. The resulting 2,400 trees per data set were combined in a single tree by using TreeAnnotator v1.8.2 (part of the BEAST package). Branch robustness measures of ML trees (bootstrap values) and Bayesian trees (posterior probabilities) are annotated on the respective tree.
Accession number(s).
Sequences generated in this study are deposited in GenBank with the accession numbers KY655277 to KY655301 for LTR sequences and KY655302 to KY655313 for env sequences.
ACKNOWLEDGMENTS
We thank all study participants in CIV and DRC and all members of the sampling teams at both countries, in particular Tapé Aimé Fréderic Bozoua, Eric Goueu, Joel Semporé and Ange H. Gnoukpoho from CIV, as well as Esperance Miezi and Désiré Muzuyu Muganza in DRC. We also thank Gottfried Hohmann for logistical support during field activities in DRC. Thanks go to Anna Löwa, Markus Ullrich, Verena Keil, Privat Gnabro, Yao Mathurin, and Kouakou Casimir for support during laboratory analyses, Martine Peeters for providing laboratory material, and Roger Mundry for statistical advice. We are grateful to local and national health authorities and ethics committees in CIV and DRC, as well as the Office Ivoirien des Parcs et Reserves of CIV for granting permission to conduct this research. Finally, we thank three anonymous reviewers for their helpful comments on a previous version of the manuscript.
We declare no conflict of interest.
B.F., C.A.-K., E.C.-H., F.H.L., G.S., J.-J.M.-T, L.G.N., R.M.W., S.C.-S., and S.K. conceived the study and designed the experiments; A.E.A., A.M., A.O.M., B.R.V., D.A.D., G.S., L.W., M.S.P., P.S., S.P., and U.T. performed the experiments; A.M., G.S., and S.C.-S. analyzed the data; and A.M., G.S., and S.C.-S. drafted the paper. All authors critically revised the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) with a grant to F. Leendertz (grant number LE1813/4-1).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Peeters M, D'Arc M, Delaporte E. 2014. The origin and diversity of human retroviruses. AIDS Rev 16:23. [PMC free article] [PubMed] [Google Scholar]
- 2.Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med 1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gessain A, Rua R, Betsem E, Turpin J, Mahieux R. 2013. HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology. Virology 435:187–199. doi: 10.1016/j.virol.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahieux R, Gessain A. 2003. HTLV-1 and associated adult T-cell leukemia/lymphoma. Rev Clin Exp Hematol 7:336–361. [PubMed] [Google Scholar]
- 6.Filippone C, Betsem E, Tortevoye P, Cassar O, Bassot S, Froment A, Fontanet A, Gessain A. 2015. A severe bite from a nonhuman primate is a major risk factor for HTLV-1 infection in hunters from Central Africa. Clin Infect Dis 60:1667–1676. doi: 10.1093/cid/civ145. [DOI] [PubMed] [Google Scholar]
- 7.Verdonck K, González E, Van Dooren S, Vandamme A-M, Vanham G, Gotuzzo E. 2007. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis 7:266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 8.Calvignac-Spencer S, Adjogoua EV, Akoua-Koffi C, Hedemann C, Schubert G, Ellerbrok H, Leendertz SA, Pauli G, Leendertz FH. 2012. Origin of human T-lymphotropic virus type 1 in rural Côte d'Ivoire. Emerg Infect Dis 18:830–833. doi: 10.3201/eid1805.111663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazanji M, Mouinga-Ondémé A, Lekana-Douki-Etenna S, Caron M, Makuwa M, Mahieux R, Gessain A. 2015. Origin of HTLV-1 in hunters of nonhuman primates in Central Africa. J Infect Dis 211:361–365. doi: 10.1093/infdis/jiu464. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe ND, Daszak P, Kilpatrick AM, Burke DS. 2005. Bushmeat hunting, deforestation, and prediction of zoonoses emergence. Emerg Infect Dis 11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paige S, Frost SW, Gibson M, Jones J, Shankar A, Switzer W, Ting N, Goldberg T. 2014. Beyond bushmeat: animal contact, injury, and zoonotic disease risk in western Uganda. Ecohealth 11:534–543. doi: 10.1007/s10393-014-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, Prosser AT, Torimiro JN, Wright A, Mpoudi-Ngole E, McCutchan FE, Birx DL, Folks TM, Burke DS, Heneine W. 2004. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363:932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]
- 13.Mossoun A, Pauly M, Akoua-Koffi C, Couacy-Hymann E, Leendertz SAJ, Anoh AE, Gnoukpoho AH, Leendertz FH, Schubert G. 2015. Contact to non-human primates and risk factors for zoonotic disease emergence in the Taï region, Côte d'Ivoire. Ecohealth 12:580–591. doi: 10.1007/s10393-015-1056-x. [DOI] [PubMed] [Google Scholar]
- 14.Leendertz SA, Junglen S, Hedemann C, Goffe A, Calvignac S, Boesch C, Leendertz FH. 2010. High prevalence, coinfection rate, and genetic diversity of retroviruses in wild red colobus monkeys (Piliocolobus badius badius) in Tai National Park, Côte d'Ivoire. J Virol 84:7427–7436. doi: 10.1128/JVI.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Refisch J, Inza K. 2005. Impact of commercial hunting on monkey populations in the Tai region, Côte d'Ivoire. Biotropica 37:136–144. doi: 10.1111/j.1744-7429.2005.03174.x. [DOI] [Google Scholar]
- 16.Covey RM, McGraw WS. 2014. Monkeys in a West African bushmeat market: implications for cercopithecid conservation in eastern Liberia. Trop Conserv Sci 7:115–125. doi: 10.1177/194008291400700103. [DOI] [Google Scholar]
- 17.Wild Chimpanzee Foundation. 2014. Annual report 2014. Activities of the Wild Chimpanzee Foundation for improved conservation of chimpanzees and their habitat in West Africa. http://www.wildchimps.org/fileadmin/content_files/pdfs/reports/2014_Yearly_Activity_Report_2014_English_final_18-02-2015.pdf.
- 18.Wolfe ND, Prosser TA, Carr JK, Tamoufe U, Mpoudi-Ngole E, Torimiro JN, LeBreton M, McCutchan FE, Birx DL, Burke DS. 2004. Exposure to nonhuman primates in rural Cameroon. Emerg Infect Dis 10:2094–2099. doi: 10.3201/eid1012.040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steve A-M, Ahidjo A, Placide M-K, Caroline F, Mukulumanya M, Simon-Pierre N-K, Octavie L-M, Valentin M-A, Jean-Jacques M-T, Eric D, Martine P. 3 January 2017. High prevalences and a wide genetic diversity of simian retroviruses in non-human primate bushmeat in rural areas of the Democratic Republic of Congo. Ecohealth doi: 10.1007/s10393-016-1202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahuka-Mundeke S, Mbala-Kingebeni P, Liegeois F, Ayouba A, Lunguya-Metila O, Demba D, Bilulu G, Mbenzo-Abokome V, Inogwabini B-I, Muyembe-Tamfum J-J. 2012. Identification and molecular characterization of new simian T cell lymphotropic viruses in nonhuman primates bushmeat from the Democratic Republic of Congo. AIDS Res Hum Retroviruses 28:628–635. doi: 10.1089/aid.2011.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayouba A, Akoua-Koffi C, Calvignac-Spencer S, Esteban A, Locatelli S, Li H, Li Y, Hahn BH, Delaporte E, Leendertz FH, Peeters M. 2013. Evidence for continuing cross-species transmission of SIVsmm to humans: characterization of a new HIV-2 lineage in rural Côte d'Ivoire. AIDS 27:2488–2491. doi: 10.1097/01.aids.0000432443.22684.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pais-Correia A-M, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze M-I. 2010. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med 16:83–89. doi: 10.1038/nm.2065. [DOI] [PubMed] [Google Scholar]
- 23.Grossmann F, Hart JA, Vosper A, Ilambu O. 2008. The bonobos, p 189–216. Springer, New York, NY. [Google Scholar]
- 24.Baayen RH. 2008. Analyzing linguistic data; a practical introduction to statistics. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 25.Barr DJ, Levy R, Scheepers C, Tily HJ. 2013. Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68:255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team. 2015. R: a language and environment for statistical computing. R Foundation, Vienna, Austria. [Google Scholar]
- 28.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 29.Reference deleted.
- 30.Fields A. 2005. Discovering statistics using SPSS. Sage Publications, Beverly Hills, CA. [Google Scholar]
- 31.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd ed Sage Publications, Beverly Hills, CA. [Google Scholar]
- 32.Forstmeier W, Schielzeth H. 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav Ecol Sociobiol 65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobson AJ, Barnett A. 2008. An introduction to generalized linear models. CRC Press, Boca Raton, FL. [Google Scholar]
- 34.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. [DOI] [PubMed] [Google Scholar]
- 37.Calattini S, Betsem E, Bassot S, Chevalier SA, Tortevoye P, Njouom R, Mahieux R, Froment A, Gessain A. 2011. Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology 410:48–55. doi: 10.1016/j.virol.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Courgnaud V, Van Dooren S, Liegeois F, Pourrut X, Abela B, Loul S, Mpoudi-Ngole E, Vandamme A, Delaporte E, Peeters M. 2004. Simian T-cell leukemia virus (STLV) infection in wild primate populations in Cameroon: evidence for dual STLV type 1 and type 3 infection in agile mangabeys (Cercocebus agilis). J Virol 78:4700–4709. doi: 10.1128/JVI.78.9.4700-4709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 41.Glez-Peña D, Gómez-Blanco D, Reboiro-Jato M, Fdez-Riverola F, Posada D. 2010. ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res 38:W14–W18. doi: 10.1093/nar/gkq321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posada D. 2008. jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 43.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 44.Guindon S, Lethiec F, Duroux P, Gascuel O. 2005. PHYML Online—a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33:W557–W559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rambaut A, Suchard M, Xie D, Drummond A. 2014. Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer.
- 47.Leendertz FH, Junglen S, Boesch C, Formenty P, Couacy-Hymann E, Courgnaud V, Pauli G, Ellerbrok H. 2004. High variety of different simian T-cell leukemia virus type 1 strains in chimpanzees (Pan troglodytes verus) of the Taï National Park, Côte d'Ivoire. J Virol 78:4352–4356. doi: 10.1128/JVI.78.8.4352-4356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leendertz FH, Boesch C, Ellerbrok H, Rietschel W, Couacy-Hymann E, Pauli G. 2004. Non-invasive testing reveals a high prevalence of simian T-lymphotropic virus type 1 antibodies in wild adult chimpanzees of the Tai National Park, Côte d'Ivoire. J Gen Virol 85:3305–3312. doi: 10.1099/vir.0.80052-0. [DOI] [PubMed] [Google Scholar]
- 49.Junglen S, Hedemann C, Ellerbrok H, Pauli G, Boesch C, Leendertz FH. 2010. Diversity of STLV-1 strains in wild chimpanzees (Pan troglodytes verus) from Côte d'Ivoire. Virus Res 150:143–147. doi: 10.1016/j.virusres.2010.02.020. [DOI] [PubMed] [Google Scholar]