Abstract
The cryopyrin-associated periodic syndrome (CAPS) is an autosomal dominant autoinflammatory disease characterized by fever, skin rash, and joint involvement with acute inflammatory response. The genetic defect involves the NLRP3 gene that encodes cryopyrin and leads to an abnormal production of interleukin-1 (IL-1). Therefore, anti-IL-1 treatment represents an effective therapy. One of the most severe manifestations of the disease is secondary amyloidosis that causes renal failure. We present a patient with CAPS who underwent renal transplantation for renal insufficiency caused by amyloidosis. The function of the transplanted kidney deteriorated because of the late administration of IL-1 receptor antagonist, anakinra. This case may indicate the importance of early initiation of anti-IL-1 treatment in CAPS patients who have undergone kidney transplantation.
Keywords: Cryopyrin-associated periodic syndrome, Autoinflammatory disease, Interleukin-1, Amyloidosis, Kidney transplantation, Anakinra
Introduction
Cryopyrin-associated periodic syndrome (CAPS) is a rare autosomal dominant autoinflammatory disease. Its population frequency has been reported to be 1–3 per million [1, 2]. CAPS is caused by mutations in the NLRP3 [3]. NLRP3 is a gene that encodes cryopyrin. Cryopyrin is one of the components of NLRP3 inflammasome that converts pro-interleukin-1β (IL-1β) to its biologically active form through the activation of procaspase-1. Cryopyrin detects numerous bacterial products or intracellular danger signals, with the resulting production of IL-1β inducing local or systemic responses against noxious agents such as microorganisms [4]. However, mutations in the cryopyrin gene induce inappropriate activation of inflammatory responses [5], leading to clinical manifestations of fever, urticarial skin rash, joint symptoms, and increased levels of the acute-phase plasma proteins [4].
The clinical phenotype of CAPS is diverse. Familial cold autoinflammatory syndrome (FCAS) is the mildest type, Muckle–Wells syndrome (MWS) has intermediate severity, and the most severe type is neonatal-onset multisystem inflammatory disease (NOMID) or chronic infantile neurologic cutaneous articular syndrome (CINCA) [6]. FACS is characterized by recurrent intermittent fever and skin rash induced by cold exposure. NOMID/CINCA is the most severe type with involvement of the central nervous system and characteristic arthropathy. In patients with NOMID/CINCA, clinical symptoms are persistent with an elevation of acute-phase reactants and leukocytosis [4].
A proportion of patients with CAPS develop secondary AA amyloidosis that leads to progressive chronic kidney disease. As IL-1β plays a pivotal role in the pathogenesis of CAPS, anti- IL-1 treatment has been reported to be effective in patients with the syndrome. Thus, successful anti-IL-1 treatment of renal amyloidosis in patients with CAPS has been reported [7–14]. In this paper, we describe a patient with CAPS who received a renal transplant due to progressive renal insufficiency caused by amyloidosis. The function of the transplanted kidney deteriorated despite anti-IL-1 treatment. This case may indicate the importance of the early diagnosis and institution of anti-IL-1 treatment in CAPS with a severe phenotype.
Case report
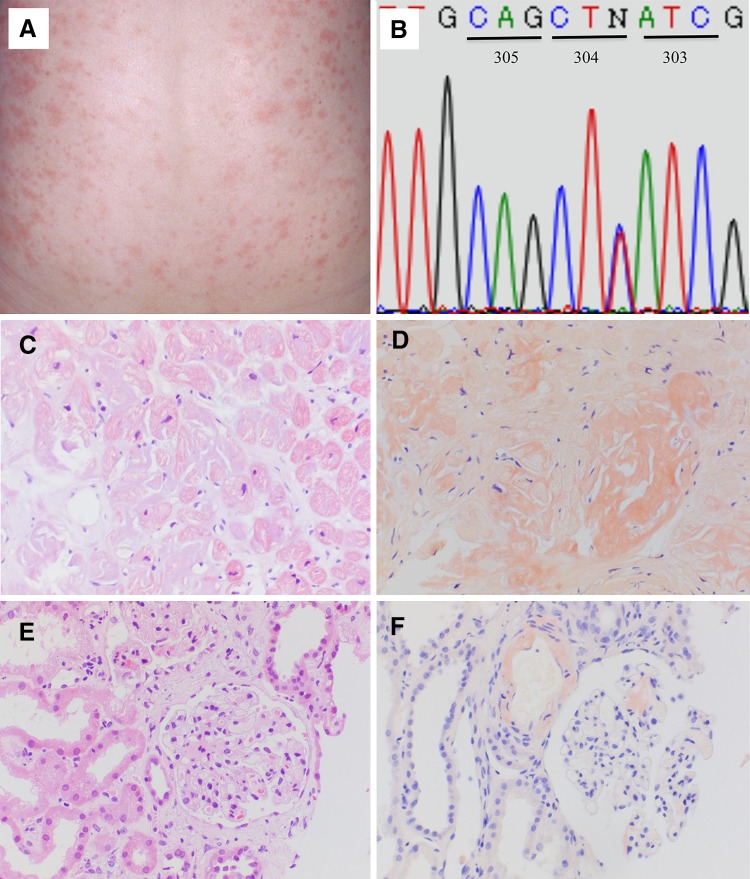
In 2005, a Japanese female aged 33 years visited our outpatient clinic with chronic renal failure. During early childhood, she had developed an urticarial skin rash and polyarthralgia with intermittent febrile episodes. The fever and skin rash (Fig. 1a) were triggered by cold exposure, although they also occurred without any stimuli. Anterior uveitis and elbow joint deformity leading to ulnar dislocation developed at 12 years of age, and at 23 years of age, she visited the rheumatology department of our hospital, and was diagnosed with Still’s disease. Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoid failed to improve her symptoms with a persistent elevation of CRP and serum amyloid A protein (SAA) levels being observed. At the age of 26 years, she developed proteinuria due to renal amyloidosis and was treated with NSAIDs and herbal medicines. However, renal function deteriorated and hemodialysis was introduced at the age of 32 years. In March 2008, a living-related kidney transplant was carried out using a standard protocol of immunosuppression that included tacrolimus, mycophenolate mofetil, and prednisolone. After transplantation, she was admitted again to the rheumatology department. CAPS was suspected and genetic testing was performed following approval from the Institutional Genome Ethics Committee. Sequencing of the NLRP3 gene showed that the patient was heterozygous for the p.E304 K mutation (Fig. 1b). At that time, the recombinant human IL-1 receptor antagonist, anakinra, was not initiated because of consent reasons. The skin rash, intermittent fever, and elevated inflammatory reactions continued despite treatment with immunosuppressants. Seventeen months after transplantation, she developed congestive heart failure and function of the transplanted kidney deteriorated. Biopsies of the cardiac muscle (Fig. 1c, d) and the transplanted kidney (Fig. 1e, f) confirmed the diagnosis of cardiac and allograft amyloidosis. In view of her serious clinical course, informed consent for the use of anakinra was obtained based on the approval of the Institutional Ethics Committee. Treatment with anakinra was started 23 months after transplantation. The dose of anakinra was reduced to 100 mg every other day because of the decreased renal function. The skin rash and intermittent fever were improved. The levels of CRP also decreased with the exception of a short period when a left radical nephroureterectomy was performed for urothelial carcinoma of the middle calyx. The SAA levels before treatment with anakinra ranged between 49.8 and 163.0 μg/ml and decreased to 10.1–25.7 μg/ml 2 months after the initiation of anakinra (normal range of SAA ≤ 8.0 μg/ml). However, the function of the allograft decreased gradually and hemodialysis was introduced again 34 months after transplantation (Fig. 2).
Fig. 1.
A typical urticarial skin rash was observed on the back of the patient (a). Sequencing of NLRP3 revealed that the patient was heterozygous for c.910G > A leading to a p.E304 K mutation. Codon numbers are described based on reference sequence NM_001243133.1 (b). Light microscopy of cardiac muscles in the right ventricle (c, d). A hematoxylin and eosin stain showing hypertrophy and disarrangement of cardiac muscles of various sizes (c, ×200). Extensive interstitial deposition of amorphous material positive for Congo-red staining (d, ×200). Hematoxylin and eosin stain of the allograft (e, f). Mesangial lesions with focal segmental dilatation and deposition of amorphous material are shown (e, ×200). Congo-red stain showing amyloid deposition in the mesangium and vasculature (f, ×200)
Fig. 2.
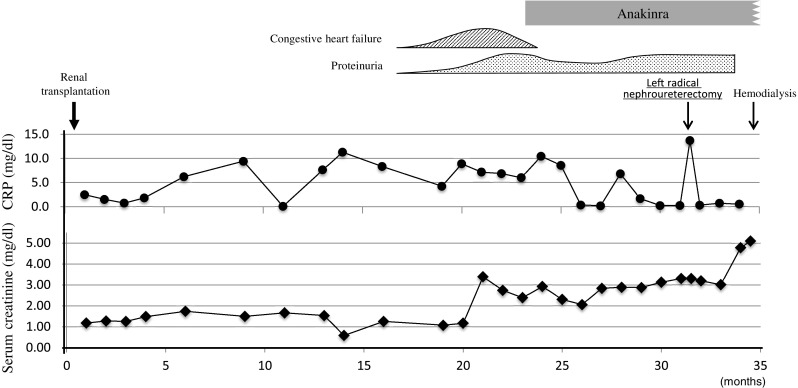
The clinical course of the patient highlighting serum levels of CRP and creatinine before and after the treatment with anakinra
Discussion
The primary therapeutic strategy in CAPS is anti-IL-1 treatment. Anakinra is a recombinant IL-1 receptor antagonist that is not approved in Japan for any diseases, despite being approved for rheumatoid arthritis in some other countries. Its efficacy in patients with CAPS is characterized by rapid resolution of disease symptoms [15]. To date, eight reports on the efficacy of anti-IL-1 agents for treatment of amyloidosis in patients with CAPS have been published [7–14]. In 17 patients reported in the eight references, a lack of efficacy was reported in two patients treated with anakinra [9, 13].
Two cases with CAPS complicated with AA amyloidosis who underwent renal transplantation have been reported previously, although these reports did not include a detailed description of clinical course. The case reported by Leslie et al. [7] underwent renal transplantation and began taking anakinra 18 months later. No involvement of the graft with amyloid deposition was detected by serum amyloid P component scintigraphy [7]. Lane et al. [12] conducted a case-series study of patients with the hereditary periodic fever syndrome complicated with AA amyloidosis. In their study, one of 13 cases who underwent renal transplantation was a CAPS patient. The patient underwent renal transplantation prior to initiation of an anti-IL-1 agent and no graft failure was reported. In the present case, treatment with anakinra failed to suppress the deterioration of the allograft function. Anakinra was not administrated until 23 months after renal transplantation due to issues with consent. At that time, anakinra was the only drug available for anti-IL-1 treatment and had not been approved in Japan for any diseases. The use of anakinra without medical insurance became an obstacle to early initiation of anakinra in this case. Pathological examination of the left native kidney where nephroureterectomy was performed revealed massive deposition of AA amyloid (data not shown). The finding may suggest a lack of efficacy of anakinra on amyloid deposition in this case although careful interpretation will be needed. Recently, it has been suggested that progressive dose adjustment of anti-IL-1 agents is needed in patients with a more severe phenotype [16]. Kuemmerle-Deschner et al. [17] analyzed the efficacy of anti-IL-1 agents in 21 patients with Muckle–Wells syndrome and suggested switching to canakinumab was effective after secondary failure to anakinra. Although those reports did not mention the effect of anti-IL-1 treatment on amyloidosis, dose adjustment of anakinra or switching to canakinumab may have been a therapeutic option in our case.
Pinney et al. [18] investigated the outcome of renal transplantation in systemic amyloidosis and reported that 19.5 % of patients with AA amyloidosis showed recurrent amyloid in their allografts with a median of 5.3 years after transplantation. In that study, 23.2 % of the subjects had a hereditary periodic fever syndrome, although detailed information of each case was not described. In our case, amyloid deposited in the allograft with unexpected rapid progression. From transplantation to the initiation of anakinra, inflammatory response was persistent as evidenced by sustained high levels of CRP (Fig. 2). Rapid deposition of amyloid in the allograft may be characteristic of CAPS although further investigations are needed.
The decrease in serum levels of CRP and SAA observed during treatment with anakinra may indicate anakinra is effective for suppressing inflammation (Fig. 2). The function of the allograft was deteriorated by rapid amyloid deposition before administration of anakinra. Therefore, anakinra should be started immediately after renal transplantation. Renal amyloidosis can be complicated by various autoinflammatory diseases such as familial Mediterranean fever (FMF). Moser et al. [19] reported a patient with FMF who developed renal amyloidosis. In that case, anakinra was started before renal transplantation and was continued successfully thereafter. The efficacy of early initiation of anakinra in CAPS patients with renal amyloidosis has been reported [9]. The clinical course of this case showed that congestive heart failure caused by amyloidosis also accounted for allograft dysfunction (Fig. 2). Taken together, these results may indicate early diagnosis and treatment with IL-1 inhibitor is important for a favorable outcome of CAPS.
This case was complicated with cardiac amyloidosis (Fig. 1c, d). Cardiac amyloidosis is a rare complication of AA amyloidosis, however, it has been reported to be associated with an unfavorable outcome [14]. Tanaka et al. [20] analyzed 42 cases of AA amyloidosis and revealed the association between survival rate and amyloid-related cardiac involvement. Anti-IL-1 agents might be a potential treatment for cardiac amyloidosis in CAPS although cardiac amyloidosis in CAPS has not been extensively studied. In this case, treatment for cardiac failure was initiated before the administration of anakinra and we could not evaluate the efficacy of anakinra on cardiac amyloidosis.
CAPS includes three phenotypes: FCAS, MWS, and NOMID/CINCA, although those boundaries between these three conditions remain unclear [4]. In fact, our patient showed some overlapping features of MWS and NOMID/CINCA. Genetic analysis of our patient revealed the p.E304 K in the NLRP3. The same mutation has been reported previously in an Italian patient [21] although the clinical presentations were not identical in the two cases. The Italian case showed persistent arthritis, bony overgrowth, lymphadenopathy, and hepatosplenomegaly that were not seen in our patient, while our patient had characteristic face, fever, and mild hearing loss. In our patient, a right ulnar dislocation at the elbow joint and hypoplasia of the distal end of the right ulna were observed. It is not clear whether these were characteristic lesions of CAPS. Further investigation of the genotype–phenotype correlation of CAPS is required.
Successful treatment of CAPS with canakinumab was reported in 2009 [22] and its use was approved in Japan in November 2011 [23]. The efficacy and tolerability of canakinumab in CAPS patients with a severe phenotype have also been reported [24], indicating that canakinumab has the potential to contribute to favorable outcomes in patients with CAPS in Japan.
Conflict of interest
None.
References
- 1.Cuisset L, Jeru I, Dumont B, Fabre A, Cochet E, Le Bozec J, Delpech M, Amselem S, Touitou I, French CAPS study group Mutations in the autoinflammatory cryopyrin-associated periodic syndrome gene: epidemiological study and lessons from 8 years of genetic analysis in France. Ann Rheum Dis. 2011;70:495–499. doi: 10.1136/ard.2010.138420. [DOI] [PubMed] [Google Scholar]
- 2.Tilson H, Primatesta P, Kim D, Rauer B, Hawkins PN, Hoffman HM, Kuemmerle-Deschner J, van der Poll T, Walker UA. Methodological challenges in monitoring new treatments for rare diseases: lessons from the cryopyrin-associated periodic syndrome registry. Orphanet J Rare Dis. 2013;8:139. doi: 10.1186/1750-1172-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldbach-Mansky R. Current status of understanding the pathogenesis and management of patients with NOMID/CINCA. Curr Rheumatol Rep. 2011;13:123–131. doi: 10.1007/s11926-011-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim PW, Aksentijevich I, Colburn NT, Kastner DL. Hereditary recurrent fevers. In: Hockberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. Philadelphia: Elsevier; 2011. pp. 1637–1657. [Google Scholar]
- 5.Aksentijevich I, Putnam CC, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–1285. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 7.Leslie KS, Lachmann HJ, Bruning E, McGrath JA, Bybee A, Gallimore JR, Roberts PF, Woo P, Grattan CE, Hawkins PN. Phenotype, genotype, and sustained response to anakinra in 22 patients with autoinflammatory disease associated with CIAS-1/NALP3 mutations. Arch Dermatol. 2006;142:1591–1597. doi: 10.1001/archderm.142.12.1591. [DOI] [PubMed] [Google Scholar]
- 8.Thornton BD, Hoffman HM, Bhat A, Don BR. Successful treatment of renal amyloidosis due to familial cold autoinflammatory syndrome using an interleukin 1 receptor antagonist. Am J Kidney Dis. 2007;49:477–481. doi: 10.1053/j.ajkd.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, Pinto G, Pagnier A, Bodemer C, Bodaghi B, Tardieu M, Prieur AM, Quartier P. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2010;62:258–267. doi: 10.1002/art.25057. [DOI] [PubMed] [Google Scholar]
- 10.Aït-Abdesselam T, Lequerré T, Legallicier B, François A, Le Loët X, Vittecoq O. Anakinra efficacy in a Caucasian patient with renal AA amyloidosis secondary to cryopyrin-associated periodic syndrome. Jt Bone Spine. 2010;77:616–617. doi: 10.1016/j.jbspin.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, Wittkowski H, Bialkowski A, Tzaribachev N, Lohse P, Koitchev A, Deuter C, Foell D, Benseler SM. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle–Wells syndrome. Arthritis Rheum. 2011;63:840–849. doi: 10.1002/art.30149. [DOI] [PubMed] [Google Scholar]
- 12.Lane T, Loeffler JM, Rowczenio DM, Gilbertson JA, Bybee A, Russell TL, Gillmore JD, Wechalekar AD, Hawkins PN, Lachmann HJ. AA amyloidosis complicating the hereditary periodic fever syndromes. Arthritis Rheum. 2013;65:1116–1121. doi: 10.1002/art.37827. [DOI] [PubMed] [Google Scholar]
- 13.Enríquez R, Sirvent AE, Padilla S, Noguera-Pons R, Andrada E, Ardoy F, Millán I, Amorós F. Nephrotic syndrome and AA amyloidosis revealing adult-onset cryopyrin-associated periodic syndrome. Ren Fail. 2013;35:738–741. doi: 10.3109/0886022X.2013.790300. [DOI] [PubMed] [Google Scholar]
- 14.Scarpioni R, Rigante D, Cantarini L, Ricardi M, Albertazzi V, Melfa L, Lazzaro A. Renal involvement in secondary amyloidosis of Muckle–Wells syndrome: marked improvement of renal function and reduction of proteinuria after therapy with human anti-interleukin-1β monoclonal antibody canakinumab. Clin Rheumatol. (2014) [Epub ahead of print]. [DOI] [PubMed]
- 15.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. New Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caorsi R, Lepore L, Zulian F, Alessio M, Stabile A, Insalaco A, Finetti M, Battagliese A, Martini G, Bibalo C, Martini A, Gattorno M. The schedule of administration of canakinumab in cryopyrin associated periodic syndrome is driven by the phenotype severity rather than the age. Arthritis Res Ther. 2013;15:R33. doi: 10.1186/ar4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuemmerle-Deschner JB, Wittkowski H, Tyrrell PN, Koetter I, Lohse P, Ummenhofer K, Reess F, Hansmann S, Koitschev A, Deuter C, Bialkowski A, Foell D, Benseler SM. Treatment of Muckle–Wells syndrome: analysis of two IL-1-blocking regimens. Arthritis Res Ther. 2013;15:R64. doi: 10.1186/ar4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinney JH, Lachmann HJ, Sattianayagam PT, Gibbs SD, Wechalekar AD, Venner CP, Whelan CJ, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD. Renal transplantation in systemic amyloidosis—importance of amyloid fibril type and precursor protein abundance. Am J Transpl. 2013;13:433–441. doi: 10.1111/j.1600-6143.2012.04326.x. [DOI] [PubMed] [Google Scholar]
- 19.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, Lachmann HJ, Lang U, Kovarik J. Successful treatment of familial Mediterranean fever with anakinra and outcome after renal transplantation. Nephrol Dial Transpl. 2009;24:676–678. doi: 10.1093/ndt/gfn646. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka F, Migita K, Honda S, Fukuda T, Mine M, Nakamura T, Yamasaki S, Ida H, Kawakami A, Origuchi T, Eguchi K. Clinical outcome and survival of secondary (AA) amyloidosis. Clin Exp Rheumatol. 2003;21:343–346. [PubMed] [Google Scholar]
- 21.Caroli F, Pontillo A, D’Osualdo A, Travan L, Ceccherini I, Crovella S, Alessio M, Stabile A, Gattorno M, Tommasini A, Martini A, Lepore L. Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatology. 2007;46:473–478. doi: 10.1093/rheumatology/kel269. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN, Canakinumab in CAPS Study Group Use of canakinumab in the cryopyrin-associated periodic syndrome. New Engl J Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 23.Yokota S, Nishikomori R, Takada H, Kikuchi M, Nozawa T, Kanetaka T, Kizawa T, Miyamae T, Mori M, Heike T, Hara T, Imagawa T. Guidance on the use of canakinumab in patients with cryopyrin-associated periodic syndrome in Japan. Mod Rheumatol. 2013;23:425–429. doi: 10.3109/s10165-012-0769-8. [DOI] [PubMed] [Google Scholar]
- 24.Kuemmerle-Deschner JB, Hachulla E, Cartwright R, Hawkins PN, Tran TA, Bader-Meunier B, Hoyer J, Gattorno M, Gul A, Smith J, Leslie KS, Jiménez S, Morell-Dubois S, Davis N, Patel N, Widmer A, Preiss R, Lachmann HJ. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Ann Rheum Dis. 2011;70:2095–2102. doi: 10.1136/ard.2011.152728. [DOI] [PubMed] [Google Scholar]