Abstract
An 84-year-old male complained of fever, cough, sputum, and appetite loss. His renal function rapidly worsened, and he had hypoalbuminemia and hypocomplementemia. His condition worsened and C-reactive protein levels were elevated. Vasculitis syndrome was suspected and he was administered 40 mg of prednisolone, although myeloperoxidase antineutrophil cytoplasmic antibody (ANCA), proteinase-3 ANCA and antiglomerular basement membrane antibody tests were negative. His body temperature decreased and fatigue promptly resumed. On renal biopsy, light microscopy revealed endocapillary and extracapillary glomerulonephritis. Vasculitis was detected in interlobular arteries. Immunofluorescence studies revealed granular deposits of C3 and IgG along capillary walls. Electron microscopy revealed dome-shaped small electron-dense granular subepithelial deposits. Acute post-infectious glomerulonephritis was suspected. Although his renal function improved, he developed hemoptysis and was diagnosed with pulmonary hemorrhage. He received methylprednisolone and plasma exchange, and his respiratory status improved gradually. This is an extremely rare case and suggests the importance of considering a differential diagnosis.
Keywords: Acute post-infectious glomerulonephritis, Crescentic formations, Vasculitis, Pulmonary hemorrhage
Introduction
Non-streptococcal post-infectious or infection-related glomerulonephritis often affects the elderly, and shows acute nephritis syndrome and hypocomplementemia similar to that seen in children and young adults. Pulmonary hemorrhage usually does not accompany post-infectious glomerulonephritis. The characteristic histological patterns of post-infectious glomerulonephritis are diffuse endocapillary hypercellularity with numerous neutrophil and C3 and IgG depositions with immunofluorescence study, and subepithelial hump-shaped deposits with electron microscopy. Vasculitis of intrarenal arteries is rarely observed in post-infectious glomerulonephritis.
Clinically, the combination of pulmonary hemorrhage and fulminant renal failure is often empirically thought to be antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis or Goodpasture’s syndrome. Here, we report an extremely rare case of pulmonary hemorrhage with acute post-infectious glomerulonephritis accompanied with crescentic glomerulonephritis and vasculitis of the interlobular arteries.
Case report
An 84-year-old male complained of fever, cough, sputum and appetite loss, and was referred to our hospital when fever and appetite loss persisted despite taking oral antibiotics. He had been treated for hypertension, hyperlipidemia and prostatomegaly, and had a past history of ischemic heart disease, which was treated by percutaneous coronary interventions. His serum creatinine level was 0.84 mg/dL 10 months earlier and urinalysis showed no abnormality 2 months earlier. On admission, the patient had pedal edema, with blood pressure of 95/71 mmHg, an irregular heartbeat of 80 beats/min, and a temperature of 37.2 °C. Tonsillar swelling was not observed. Chest auscultation revealed coarse crackles at right lower lung fields.
Laboratory studies revealed an elevated white blood cell count (16,100/μL) and C-reactive protein (5.54 mg/dL). His hemoglobin level was slightly low (11.9 g/dL). Serum total protein was 5.8 g/dL and serum albumin was 2.1 g/dL. His serum creatinine level was 1.79 mg/dL, and blood urea nitrogen level was 36 mg/dL. Serum IgG, IgA, and IgM were within normal ranges (1534, 406, and 213 mg/dL, respectively). Serum complement levels were extremely low: C3 was 42 mg/dL (normal range 86–160 mg/dL), C4 was 2.5 mg/dL (normal range 17–45 mg/dL), and CH50 was 8 IU/mL (normal range 30–45 IU/mL). Anti-streptolysin-O titer was not elevated (34 IU/mL, normal range 0–239 IU/mL). An antinuclear antibody test was slightly positive (1:80) and an anti-double stranded DNA antibody test was negative. Myeloperoxidase ANCA, proteinase-3 ANCA, and antiglomerular basement membrane antibody tests were negative, as were hepatitis B surface antigen and anti-hepatitis C virus antibody tests. Blood and urine cultures were negative. Urinalysis showed a urine pH of 6.0, specific gravity of 1.030, 3+ test for protein and 3+ test for occult blood; the urine sediment contained >100 red blood cells per high-power field. Urinary protein was 1.58 g/g Cr.
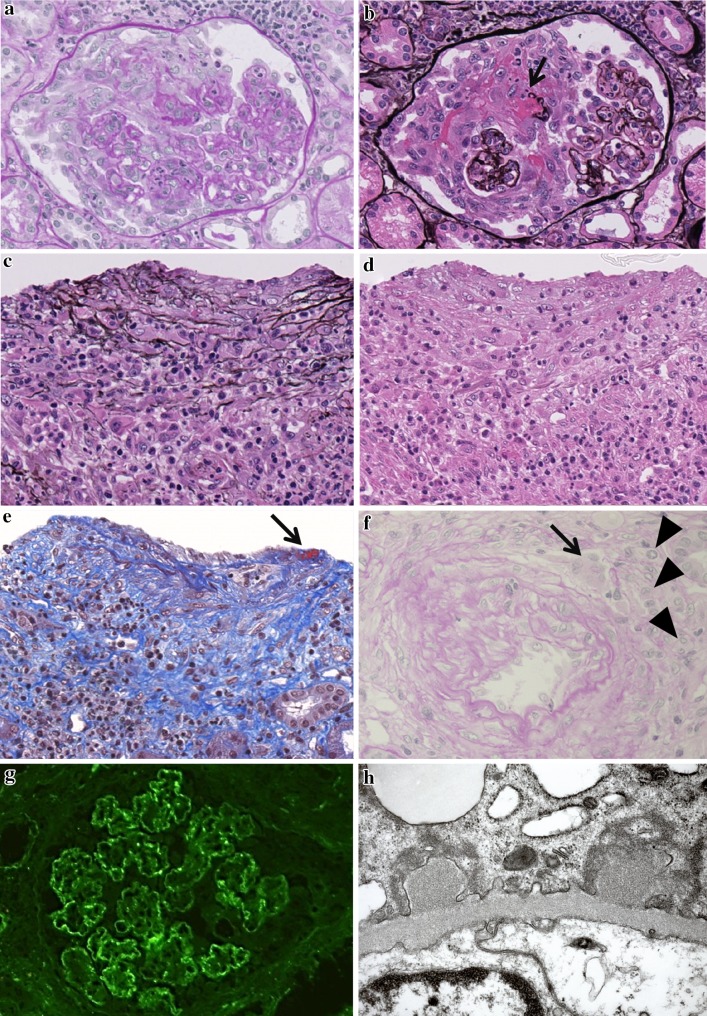
On the 6th hospital day, his urine volume gradually decreased and his renal function declined, with the serum creatinine values increasing to 8.9 mg/dL and blood urea nitrogen to 104 mg/dL. Concurrently, his body temperature rose to 40.0 °C and severe respiratory distress appeared. Vasculitis syndrome was suspected, and he received 40 mg of prednisolone. The next day his high fever resolved and renal function gradually improved. On the 10th hospital day, a percutaneous renal biopsy was performed. Light microscopy revealed that all 25 glomeruli were enlarged and showed diffuse severe hypercellularity with neutrophilic infiltrates and swollen endothelial cells (Fig. 1a, b). Large cellular crescents were observed in 10 glomeruli (Fig. 1a, b), and fibrinoid necrosis was observed in 3 glomeruli (Fig. 1b). Two interlobular arteries were densely surrounded by inflammatory cells, including neutrophils (Fig. 1c, d), and also showed mural infiltration of neutrophils and mononuclear cells (Fig. 1c–e). A focus of fibrinous exudate was observed in one artery (Fig. 1e). Granulomatous inflammation by multinuclear giant cells and epithelioid cells with focal wall destruction was observed in another smaller interlobular artery (Fig. 1f). From these findings, acute vasculitis was confirmed. The interstitium was involved with patchy dense inflammatory infiltrates including neutrophils. Immunofluorescence studies revealed coarse granular and band-like depositions of C3 (Fig. 1g) and IgG along the capillary walls. IgM, IgA, C4 and C1q were not detected. With electron microscopy, cellular crescents and endocapillary hypercellularity in endothelial cells and neutrophils were observed. Dome-shaped small electron-dense granular subepithelial deposits were observed (Fig. 1h), and there was an absence of mesangial, intramembranous, and subendothelial deposits in glomeruli.
Fig. 1.
a Marked endocapillary hypercellularity and an influx of neutrophils and karyorrhexis (PAS stain, original magnification ×20). b The same glomerulus as in a showing marked cellular crescent formation and rupture of the glomerular basement membrane with fibrinoid necrosis (indicated by arrow) (PAM stain, original magnification ×20). c, d Marked infiltration of neutrophils and epithelioid cells (macrophages) and lymphocytes in an interlobular artery with PAM stain (c) and H&E stain (d) (original magnification ×20). e Disruption of media by cellular infiltration and fibrin deposition in endothelium (indicated by arrow) (MT stain, original magnification ×20). f Mural cellular infiltration of multinuclear giant cells (indicated by arrow) and plump macrophages (epithelioid cells) (indicated by arrowhead), and disruption of medial muscular wall (PAS stain, original magnification ×20). g Immunofluorescence for C3. Coarse granular and band-like depositions of C3 are present along peripheral capillary walls. h Two dome-shaped electron-dense subepithelial deposits (original magnification ×30,000)
On the 19th hospital day, the patient suddenly developed hemoptysis and his respiratory distress rapidly worsened. He was transferred to the intensive care unit and mechanical ventilation was started. A chest X-ray showed dense consolidation of the bilateral lung. A chest CT revealed alveolar consolidation in both middle dorsal lung fields. Diffuse pulmonary hemorrhage was confirmed by broncho-alveolar lavage. Methylprednisolone (250 mg/day for 2 days) was administered. The steroid dose was reduced in consideration of the risk of severe infection. Despite steroid treatment, pulmonary hemorrhage persisted. On the 32nd hospital day, the patient received plasma exchange three times and methylprednisolone (250 mg/day for 3 days). Pulmonary hemorrhage did not completely resolve with these treatments but gradually improved around the 40th hospital day. Re-analyses for ANCA and antiglomerular basement membrane antibodies were negative. Around the 60th hospital day, his respiratory status improved further and he was able to discontinue ventilation during the day. On the 62nd hospital day, serum complement levels increased: C3 was 50 mg/dL, C4 was 16.4 mg/dL, and CH50 was 21 IU/mL. His serum creatinine level was 1.02 mg/dL. On the 70th hospital day, his temperature unexpectedly rose to 39 °C. His central catheter (peripherally inserted central catheter) was removed and he was treated immediately with antibiotics as a catheter-related blood stream infection had occurred. Despite using vasopressors, his blood pressure dropped and he died on the 71st hospital day. His blood culture grew Pseudomonas aeruginosa and Candida species. An autopsy revealed that glomerular crescents were observed in only 5 % of glomeruli and endocapillary proliferative glomerulonephritis had disappeared. No vasculitis was observed in alveolar capillaries. Immunofluorescence studies were not performed.
Discussion
In this case, it was hard to assess whether the pathogenic mechanism was ANCA-negative vasculitis or post-infectious glomerulonephritis. The clinical course of rapidly progressive renal failure with fever, frequent pulmonary hemorrhage, and the renal histological findings, such as crescentic formations and vasculitis, suggested the presence of vasculitis syndrome. Conversely, endocapillary proliferative glomerulonephritis, depositions of C3 and IgG along the capillary walls, and subepithelial deposits suggested post-infectious glomerulonephritis together with hypocomplementemia.
Observation of crescentic formations is not rare in acute post-infectious glomerulonephritis. Moroni et al. [1] reported on the formation of crescents in 36 % of 50 adult cases of acute post-infectious glomerulonephritis. In children with acute post-streptococcal glomerulonephritis, 41 % of patients were reported to have crescents, and they had significantly higher serum creatinine values and a greater tendency for needing acute dialysis [2]. Nasr et al. [3] reported post-infectious glomerulonephritis in the elderly and found that 40 of 109 patients (36 %) presented with cellular crescents. The researchers suggested that older men and patients with diabetes or malignancies are at particular risk for crescentic formation. Prognosis for older patients is poor, with fewer than 25 % recovering full renal function.
Vasculitis in interlobular arteries is usually not observed in post-infectious glomerulonephritis. However, Moroni et al. [1] reported vascular fibrinoid necrosis in 3 of 50 adult cases with infection-associated glomerulonephritis. Bodaghi et al. [4] described vasculitis in four children with acute streptococcal glomerulonephritis, and other cases have been reported [5, 6]. Although it is very rare, renal vasculitis may occur as a phenotype of severe acute post-infectious glomerulonephritis.
Life-threatening pulmonary hemorrhage is an unusual phenomenon in acute post-infectious glomerulonephritis, with only four cases being reported globally [7–10]. Although the pathogenesis has not been clearly defined, an immunological mechanism is suspected. Fukuda et al. [11] reviewed a case of hemorrhagic interstitial pneumonia with acute nephritis, granular patterns of IgG and C3 along lung alveoli, and also IgG depositions along glomerular capillary walls. They confirmed IgG in subepithelial deposits by immunoelectron microscopy and concluded that renal and pulmonary lesions might have occurred through the same immune-mediated mechanism. Masson et al. [12] reported pulmonary hemorrhage in a patient with fibrillary glomerulonephritis. Postmortem examination revealed the presence of similar immunoglobulins and fibrillary deposits in both renal glomeruli and pulmonary alveolar-capillary interstitium. The authors suggested that a circulating factor may have been responsible for the deposition of the fibrillary material.
Usually, in poststreptococcal glomerulonephritis, low serum C3 and C3 depositions on glomeruli are evident. Cases showing depression of both C3 and C4 are occasionally observed in older post-infectious glomerulonephritis patients [3]. In the present case, low serum C4 was observed, but C4 deposition was not detected with immunofluorescence microscopy. It is possible that C4 depositions were harder to detect because of the intense glomerular inflammation.
In post-infectious glomerulonephritis, hypocomplementemia should resolve within 2 months except in those with persistent infection [13]. In this case, serum complement levels increased but hypocomplementemia persisted. We suggest the following reasons for these findings: (1) the clinical course was drastic; (2) glomeruli revealed intense diffuse endocapillary and extracapillary proliferative glomerulonephritis; and (3) the vessels revealed vasculitis.
In the present case, we diagnosed this case as severe post-infectious glomerulonephritis with pulmonary hemorrhage. Although it is extremely rare, this case is interesting as it suggests the importance of considering a differential diagnosis.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Moroni G, Pozzi C, Quaglini S, Segagni S, Banfi G, Baroli A, et al. Long-term prognosis of diffuse proliferative glomerulonephritis associated with infection in adults. Nephrol Dial Transplant. 2002;17:1204–1211. doi: 10.1093/ndt/17.7.1204. [DOI] [PubMed] [Google Scholar]
- 2.Wong W, Morris MC, Zwi J. Outcome of severe acute post-streptococcal glomerulonephritis in New Zealand children. Pediatr Nephrol. 2009;24:1021–1026. doi: 10.1007/s00467-008-1086-5. [DOI] [PubMed] [Google Scholar]
- 3.Nasr SH, Fidler ME, Valeri AM, Cornell LD, Sethi S, Zoller A, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187–195. doi: 10.1681/ASN.2010060611. [DOI] [PubMed] [Google Scholar]
- 4.Bodaghi E, Kheradpir KM, Maddah M. Vasculitis in acute streptococcal glomerulonephritis. Int J Pediatr Nephrol. 1987;8:69–74. [PubMed] [Google Scholar]
- 5.Ingelfinger JR, McCluskey RT, Schneeberger EE, Grupe WE. Necrotizing arteritis in acute poststreptococcal glomerulonephritis: report of a recovered case. J Pediatr. 1977;91:228–232. doi: 10.1016/S0022-3476(77)80817-2. [DOI] [PubMed] [Google Scholar]
- 6.Zeledon JI, McKelvey RL, Servilla KS, Hofinger D, Konstantinov KN, Kellie S, et al. Glomerulonephritis causing acute renal failure during the course of bacterial infections. Histological varieties, potential pathogenetic pathways and treatment. Int Urol Nephrol. 2008;40:461–470. doi: 10.1007/s11255-007-9323-6. [DOI] [PubMed] [Google Scholar]
- 7.Sung HY, Lim CH, Shin MJ, Kim BS, Kim YO, Song HC, et al. A case of post-streptococcal glomerulonephritis with diffuse alveolar hemorrhage. J Korean Med Sci. 2007;22:1074–1078. doi: 10.3346/jkms.2007.22.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilboa N, McIntire S, Hopp L, Ellis D. Acute noncrescentic poststreptococcal glomerulonephritis presenting with pulmonary hemorrhage. Pediatr Nephrol. 1993;7:147–150. doi: 10.1007/BF00864381. [DOI] [PubMed] [Google Scholar]
- 9.Chugh KS, Gupta VK, Singhal PC, Sehgal S. Case report: poststreptococcal crescentic glomerulonephritis and pulmonary hemorrhage simulating Goodpasture’s syndrome. Ann Allergy. 1981;47:104–106. [PubMed] [Google Scholar]
- 10.Lam M, Krous HF, Llach F. Massive pulmonary hemorrhage and fulminant renal failure associated with immune complex glomerulonephritis. South Med J. 1981;74:1338–1342. doi: 10.1097/00007611-198111000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda Y, Yamanaka N, Ishizaki M, Suzuki T, Masugi Y, Yajima G, et al. Immune complex-mediated glomerulonephritis and interstitial pneumonia simulating Goodpasture’s syndrome. Acta Pathol Jpn. 1982;32:361–370. doi: 10.1111/j.1440-1827.1982.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 12.Masson RG, Rennke HG, Gottlieb MN. Pulmonary hemorrhage in a patient with fibrillary glomerulonephritis. N Engl J Med. 1992;326:36–39. doi: 10.1056/NEJM199201023260106. [DOI] [PubMed] [Google Scholar]
- 13.Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]