Abstract
Bleeding from the gastrointestinal tract is one of the common determinants of morbidity and mortality in the ordinary clinical setting. The gastrointestinal involvement of Henoch–Schönlein purpura (HSP) has often been described as self-limiting, with no long-term morbidity. In this report, we describe our experience with a male HSP patient who presented with abdominal pain, loss of appetite and deteriorated renal function associated with nephrotic syndrome. Despite the use of aggressive immunomodulatory treatments, including corticosteroids and plasmapheresis, he developed lethal gastrointestinal hemorrhage. We believe that the accumulation of more experience with additional cases similar to ours is mandatory for the establishment of optimal management for HSP patients with severe gastrointestinal manifestations.
Keywords: Henoch–Schönlein purpura, Gastrointestinal bleeding, Human albumin scintigraphy, Leukocytoclastic vasculitis, Entecavir
Introduction
Bleeding from the gastrointestinal tract is one of the common determinants of morbidity and mortality in various clinical settings [1]. This may also be the case in some subsets of patients with Henoch–Schönlein purpura (HSP), while the majority of abdominal manifestations of HSP may follow either a self-limiting course or show a favorable response to corticosteroid treatment [2–4]. In this report, we describe our serendipitous experience with a case of HSP in a male patient who developed lethal gastrointestinal hemorrhage.
Case report
A 60-year-old male patient was admitted to our hospital due to abdominal pain, loss of appetite and deteriorated renal function. He had been in stable health until 2 weeks prior to admission, when abdominal pain and anorexia developed. He was then presumptively diagnosed to have infectious colitis and was treated with antibiotics by a local physician. However, the medication did not work and brought about no improvement in his clinical symptoms, and thus, he was referred to our hospital.
At the time of admission, the patient had a temperature of 35.7 °C, a pulse of 82 beats/min and a blood pressure of 166/86 mmHg. The bowel sounds were normal. His abdomen was soft and flat, with lower abdominal tenderness, without rebound pain or palpable masses. A hematological analysis revealed a white blood cell (WBC) count of 19,900/µl (neutrophils, 85.7 %; lymphocytes, 7.5 %; monocytes, 5.5 %; eosinophils, 0.8 %; and basophils, 0.5 %), a normocytic red blood cell count of 349 × 104/µl, hematocrit level of 33.0 %, hemoglobin (Hb) of 11.1 g/dl, platelet count of 300,000/µl, increased fibrinogen at 389 mg/dl, fibrin/fibrinogen degradation products level of 89.4 (reference range 0–5.0) µg/ml, D-dimer level of 70.4 (reference range 0–1.5) µg/ml and decreased activity of blood coagulation factor XIII at 47 % (reference range 70–140 %). The laboratory tests revealed a serum creatinine (sCr) level of 3.31 mg/dl, serum albumin of 1.7 g/dl and a C-reactive protein level of 8.31 mg/dl. The serum immunoglobulin (Ig) G level was 462 mg/dl; IgA, 226 mg/dl; C3, 90 mg/dl; C4, 24 mg/dl; serum antistreptolysin, 187 (reference range <186) U/ml; and antistreptokinase titer, 1,280 (reference range <1,280). Hepatitis serology studies revealed that the patients was positive for the hepatitis B (HB) virus surface (HBs) antigen, anti-HB envelope (HBe) antibody and anti-HB core (HBc) antibody, while he was negative for the HBe antigen, anti-HBs antibody and antibody to hepatitis C virus. The level of serum HB virus-deoxyribonucleic acid (DNA), as determined on a polymerase chain reaction assay, was 5.4 (reference range <2.3) log copies/ml. Tests for anti-neutrophil cytoplasmic antibodies, anti-glomerular basement membrane antibodies and cryoglobulins were all negative. The urinalyses revealed microscopic hematuria associated with red blood cell casts, and proteinuria of 8.6 g/day, leading to a diagnosis of nephrotic syndrome. The patient’s creatinine clearance (Ccr) was 25.3 ml/min. The kidneys measured 11.0 cm (right) and 12.5 cm (left) in length by ultrasound, without evidence of hydronephrosis or a mass lesion. The guaiac test for fecal occult blood was positive. On plain abdominal computed tomography (CT) scans, segmental thickening in the small intestine (Fig. 1) and a slight amount of ascites were noted.
Fig. 1.
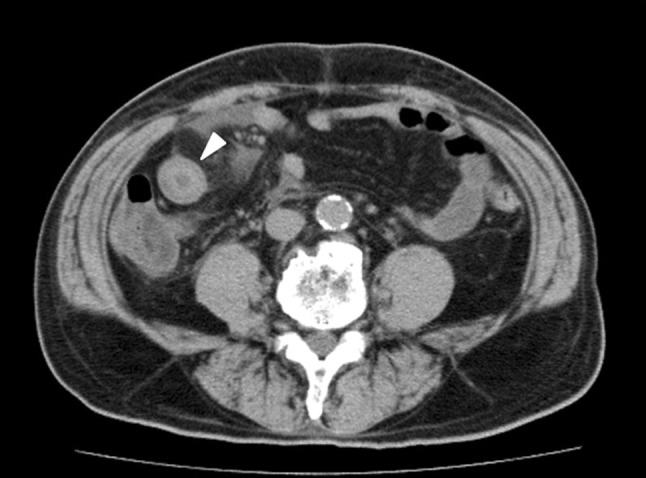
Abdominal CT scanning revealed circumferential bowel wall thickening (arrow) with no intussusception
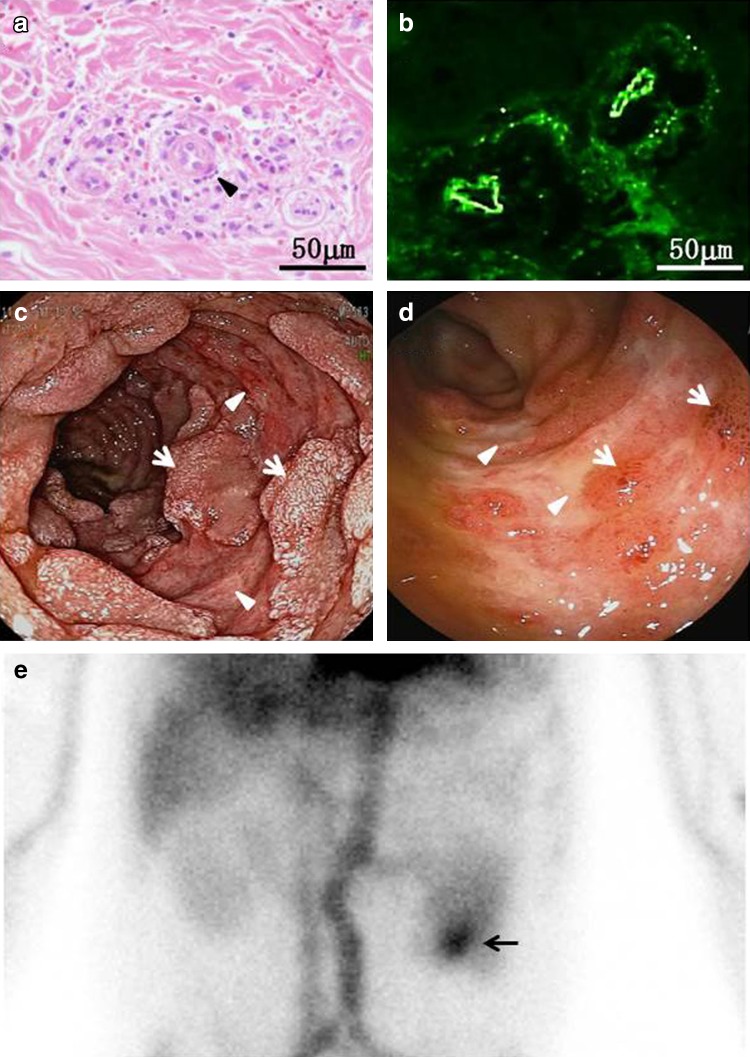
A few days after admission, the patient developed polyarthralgia involving the elbows and wrists with sparing of the small joint of the hands and feet. A palpable nonpruritic petechial rash then appeared over the distal portion of the upper and lower extremities. A skin biopsy at the site of one of the rashes showed pathological findings compatible with leukocytoclastic vasculitis associated with the vascular deposition of IgA (Fig. 2a, b). The patient also complained of increased abdominal pain and had several episodes of hematochezia or tarry stool. An endoscopic analysis found multiple raised petechiae associated with diffuse lineal ulcers in the descending duodenum (Fig. 2c) and scattered ulcerative lesions associated with a reddish mucosal erosion in the terminal ileum (Fig. 2d). 99mTechnetium-labeled human serum albumin (99mTc-HSA) scintigraphy revealed a marked enhancement of the radiotracer at the region of the jejunum 60 min after injection (Fig. 2e). After reviewing these diagnostic findings, the patient was diagnosed with HSP and concomitant gastrointestinal bleeding.
Fig. 2.
The skin biopsy (a, b), endoscopy (c, d) and scintigraphy findings (e). A punch-biopsy specimen of the skin obtained from the lower extremity revealed leukocytoclastic vasculitis in the superficial dermis, with the infiltration of polymorphonuclear leukocytes, leukocytoclasis (arrowhead) and extravasation of RBCs adjacent to small venules (a). Direct immunofluorescence studies revealed deposition of IgA in the walls of the involved dermal vessels (b). During upper gastrointestinal endoscopy, the antrum of the stomach was unremarkable, while there were multiple raised petechiae associated with diffuse linear ulcers (arrowheads) with friability in the descending duodenum. Note the presence of scattered residual mucosal tissues (arrows) (c). Total colonoscopy showed scattered ulcerative lesions (arrowheads) associated with sporadic mucosal ecchymosis (arrows) in the terminal ileum (d). Planar images of the anterior abdomen with 99mTc-HSA showed the accumulation of the radiotracer, which indicated presumable jejunum bleeding (arrow), 60 min post-injection (e)
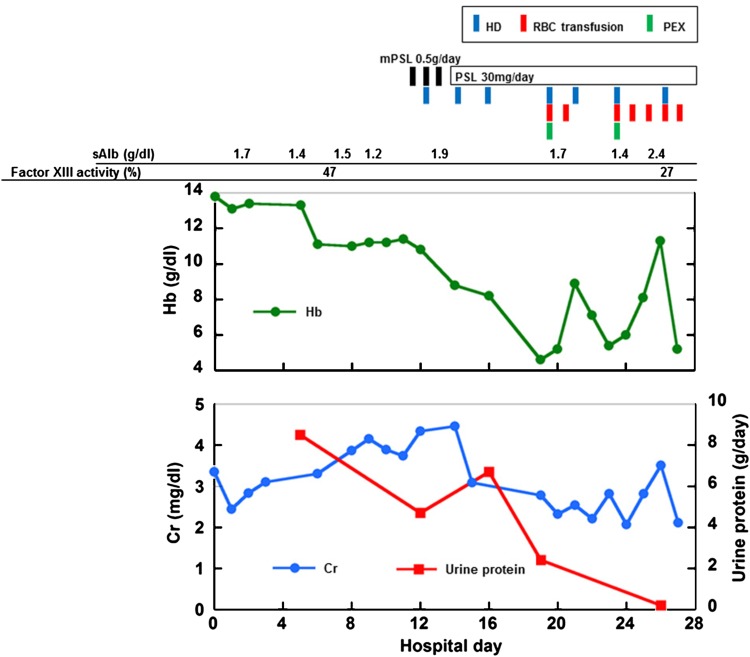
From the eleventh hospital day, the patient was treated with corticosteroids, including intravenous methylprednisolone (mPSL) at 500 mg/day for three consecutive days, followed by oral prednisolone (PSL), combined with entecavir as a prophylactic anti-viral treatment for HB virus reactivation [5–7], while the patient continued to pass tarry stool and required hemodialysis (HD) due to progressive dyspnea as a result of the decrease in his renal function. Despite the improvement of the petechial rash and a gradual decrease in the urinary protein excretion, an abrupt drop in Hb to 4.6 g/dl was confirmed on the nineteenth hospital day when plasma exchange (PEX), which replaced three liters of plasma with the same amount of fresh frozen plasma, was initiated as an adjunct therapy for steroid treatment, and frequent red blood cell (RBC) transfusions had to be carried out for the next 9 days (Fig. 3). Following a period of a few days, the patient showed a transient increase in the Hb level to 11.3 g/dl; however, he bled again, and blood examinations revealed an Hb level of 5.2 g/dl and a decreased activity of coagulation factor XIII (27 %). The patient went into shock and died, despite aggressive RBC transfusion and the administration of coagulation factor XIII. The relatives of the patient declined a postmortem autopsy.
Fig. 3.
The longitudinal changes in the Hb, sCr and the 24-h urinary protein excretion. Note that the patient required frequent RBC transfusions to maintain the level of Hb, despite the aggressive immunomodulatory treatments
Discussion
Vascular injuries, characterized by widespread leukocytoclastic vasculitis of small vessels resulting from vascular entrapment of circulating IgA immune complexes with the activation of complement, have been implicated in the onset of HSP. Therefore, HSP manifests with pleiotropic pathophysiological signs, including skin rashes, arthritis, gastrointestinal disorders and urinary abnormalities [8–10]. Consistently, all such conditions were confirmed in the current patient. It may be argued that another form of glomerulopathy, such as HB virus-associated membranous nephropathy, may also have played a role in the development of the patient’s nephrotic syndrome [5, 6]. Nevertheless, we believe that HSP played a role in the development of the patient’s renal characteristics, since it seems reasonable to consider that the clinical manifestations, including the palpable purpura, acute bowel angina and urinary abnormalities, are more likely to have arisen due to a single pathology than due to different causes.
It has been considered that abdominal involvement, including pain, nausea, vomiting and/or gastrointestinal bleeding, occurs in more than half of patients affected with HSP [3, 9]. The small intestine is the most frequently involved part in the gastrointestinal tract, presumably due to its predilection to ischemic injury [3], while the second portion of the duodenum, as well as the stomach, terminal ileum and colon, are often affected [3, 11–14]. Of note, abdominal symptoms precede purpuric lesions by several days in 12–19 % of patients with HSP [3, 4, 9–11], posing diagnostic challenges to physicians. Endoscopic evaluations have received attention as being one of the most attractive diagnostic modalities, as this technique allows for direct visualization of the gastrointestinal tract [3, 14, 15]. Alternatively, or in addition, the use of abdominal CT can also lead to a prompt diagnosis of the disease, and the detection of segmental mural thickening and luminal narrowing of the gastrointestinal tract during the procedure, as confirmed in the present case, is considered to be a radiographic characteristic of bowel lesions associated with HSP [16]. In this regard, the various gastrointestinal manifestations confirmed in our patient may not be surprising. Indeed, it has been reported that gastrointestinal bleeding may occasionally become severe enough to require transfusion or laparotomy in patients with HSP [3, 11–13]. However, the clinical impact of the current report should be emphasized in that this case illustrates the potential for lethal bowel bleeding, which has been mentioned in only a few previous literature [13, 17–19].
Considering the critical condition of the current patient, one may argue that mucosal injury due to physiological stress, anticoagulation for HD and/or PEX and the use of corticosteroids modulate the hemostatic nature of gastrointestinal manifestations. However, the present patient was administered lansoprazole, a proton pump inhibitor, which may have a benefit in treating ulcerative lesions induced by both stress and corticosteroid therapy [20, 21]. In addition, regional anticoagulation for the extracorporeal circuit was performed using nafamostat mesilate, an optional anticoagulant in patients with a high risk of bleeding [22], during the observation period. Therefore, it may not be possible to precisely evaluate the relative contribution of these factors in causing the disease on a hemorrhagic basis. Otherwise, coagulation factor XIII-dependent mechanisms may instead be implicated. Low plasma activities of coagulation factor XIII have been reported in some subsets of HSP patients, presumably due to degradation by proteolysis, as mediated by the inflammatory cascade, thus resulting in defective local hemostasis [3], and the administration of such agents has received attention anecdotally as an adjunctive treatment for severe gastrointestinal bleeding associated with HSP [23–25]. The delayed administration may have resulted in our failure to confirm the therapeutic benefits of coagulation factor XIII, although a global consensus regarding the appropriate timing and indications for this treatment is lacking.
99mTc-HSA scintigraphy has been focused on as a diagnostic imaging method not only for protein-losing enteropathy, but also for active gastrointestinal bleeding, since the radiotracer is lost into the bowel lumen along with blood [26–28]. These two pathophysiological conditions may occur concurrently in patients with varied underlying diseases [28, 29]. Moreover, HSP may also be complicated with protein-losing enteropathy [30, 31]. Consequently, the findings of the diagnostic 99mTc-HSA scintigraphy in the current patient might require careful evaluation. Nevertheless, we believe that a marked enhancement of the radiotracer at the region of the jejunum did reflect the latent bowel bleeding, which was failed to be confirmed by conventional endoscopic analyses, including esophagogastroduodenoscopy and colonoscopy [32]. Alternatively, or in addition, we speculate that our patient may also have had a concurrent protein-losing condition. Of note, the hypoalbuminuria observed in this case was sustained despite the gradual decrease in the level of urinary protein excretion. The change in the urinary protein level may simply have been due to a presumable concomitant decline in glomerular filtration as a result of instability of the circulatory dynamics requiring recurrent blood transfusions. Otherwise, there may have been a potential discrepancy in the clinical benefit of the therapeutic regimens applied in the current patient with respect to the gastrointestinal and glomerular injuries associated with HSP. Similarly, the deterioration of gastrointestinal symptoms despite improvements in skin, joint and renal manifestations during treatment with prednisolone has been reported [12].
The mainstay of treatment for HSP remains supportive, as the acute symptoms resolve spontaneously in the majority of patients. The potential benefits of immunomodulatory management, including corticosteroids, immunosuppressants and PEX, have demonstrated varied levels of success in numerous clinical experiences among the patients with severe or complicated cases of HSP [8–10, 33], while the abdomen may be tender and distended or similar to an acute abdomen, resulting in unnecessary surgery in some HSP cases [34]. The main indications for surgery in patients with the disease are intussusception, infarction and perforation, while focal arterial bleeding may also be considered as an alternative determinant of the need for surgical management [3, 12, 13]. In the current case, we faced a therapeutic dilemma, as the endoscopic analysis and scintigraphic findings suggested that there were multifocal bleeding sites, including the duodenum, jejunum and terminal ileum, leading us to empirically pursue the use of corticosteroid treatment combined with PEX instead of surgery. The lack of information regarding the demographic characteristics of HSP patients with severe bowel hemorrhage precludes us from understanding the pathogenesis of this condition. In this regard, the recent findings showing that polymorphism of intracellular adhesion molecule-1 is associated with protection against severe gastrointestinal complications in the setting of HSP should be noted [35], implying the presence of a genetic predisposition. Although the functional significance of this polymorphism in subjects with HSP remains to be delineated, it may modulate inflammatory component interactions within specific sites of the vasculature, thereby determining the patient’s susceptibility to the disease [35]. Obviously, the accumulation of more experience with additional cases similar to ours is a matter requiring continuous and careful attention, and we believe that such a strategy would aid the establishment of an optimal management for HSP patients with multifocal gastrointestinal bleeding.
Conflict of interest
The authors declare that they have no conflicts of interest in association with this study.
References
- 1.Cello JP. Gastrointestinal hemorrhage. In: Barret JC, Plum F, editors. Cecil textbook of medicine. 20. Philadelphia: WB Saunders; 1996. pp. 642–645. [Google Scholar]
- 2.Porè G. GI lesions in Henoch–Schönlein purpura. Gastrointest Endosc. 2002;55:283–286. doi: 10.1067/mge.2002.120785. [DOI] [PubMed] [Google Scholar]
- 3.Ebert EC. Gastrointestinal manifestations of Henoch–Schönlein purpura. Dig Dis Sci. 2008;53:2011–2019. doi: 10.1007/s10620-007-0147-0. [DOI] [PubMed] [Google Scholar]
- 4.Raphael CE, Shariff M, Cohen P, Smith G. An unusual case of gastrointestinal bleeding. Postgrad Med J. 2009;85:501–502. doi: 10.1136/pgmj.2008.072959. [DOI] [PubMed] [Google Scholar]
- 5.Akimoto T, Otake T, Tanaka A, Takahashi H, Higashizawa T, Inoue M, Nishino K, Saito O, Isoda N, Muto S, Sugano K, Kusano E. Steroid treatment in patients with membranous nephropathy and hepatitis B virus surface antigenemia: a report of two cases. Clin Exp Nephrol. 2011;15:289–293. doi: 10.1007/s10157-010-0391-z. [DOI] [PubMed] [Google Scholar]
- 6.Numata A, Akimoto T, Toshima M, Iwazu Y, Otani N, Miki T, Sugase T, Saito O, Hamano Y, Takemoto F, Ueda Y, Muto S, Kusano E. Membranous nephropathy in an HIV-positive patient complicated with hepatitis B virus infection. Clin Exp Nephrol. 2011;15:769–773. doi: 10.1007/s10157-011-0477-2. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki T, Akimoto T, Okuda K, Sugase T, Takeshima E, Numata A, Morishita Y, Iwazu Y, Yoshizawa H, Komada T, Iwazu K, Saito O, Takemoto F, Muto S, Kusano E. Purpura with ulcerative skin lesions and mixed cryoglobulinemia in a quiescent hepatitis B virus carrier. Intern Med. 2014;53:115–119. doi: 10.2169/internalmedicine.53.1203. [DOI] [PubMed] [Google Scholar]
- 8.Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch–Schönlein purpura. Arthritis Rheum. 1990;33:1114–1121. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 9.Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637–2644. doi: 10.1681/ASN.V10122637. [DOI] [PubMed] [Google Scholar]
- 10.Saulsbury FT. Henoch–Schönlein purpura. Curr Opin Rheumatol. 2001;13:35–40. doi: 10.1097/00002281-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Wanchu A, Karla N, Singh S, Bambery P. Successful treatment of severe gastrointestinal involvement in adult-onset Henoch–Schönlein purpura. Singapore Med J. 2007;48:1047–1050. [PubMed] [Google Scholar]
- 12.Lippl F, Huber W, Werner M, Nekarda H, Berger H, Weigert N. Life-threatening gastrointestinal bleeding due to a jejunal lesion of Henoch–Schönlein purpura. Endoscopy. 2001;33:811–813. doi: 10.1055/s-2001-16529. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S, Hisamatsu T, Kikuchi J, Adachi M, Yamagishi Y, Imaeda H, Hosoe N, Naganuma M, Ebinuma H, Okamoto S, Kanai T, Ogata H, Hanaoka H, Furuya Y, Kawano Y, Bokuda K, Sasamura H, Uchida H, Endo T, Hashiguchi A, Kameyama K, Mukai M, Hibi T. A case of IgA-related enteropathy complicated with gastrointestinal bleeding and progressive IgA nephropathy: a possible variant Henoch–Schönlein purpura? Intern Med. 2010;49:1755–1761. doi: 10.2169/internalmedicine.49.3678. [DOI] [PubMed] [Google Scholar]
- 14.Esaki M, Matsumoto T, Nakamura S, Kawasaki M, Iwai K, Hirakawa K, Tarumi K, Yao T, Iida M. GI involvement in Henoch–Schönlein purpura. Gastrointest Endosc. 2002;56:920–923. doi: 10.1016/S0016-5107(02)70376-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Huang X. Gastrointestinal involvement in Henoch–Schönlein purpura. Scand J Gastroenterol. 2008;3:1038–1043. doi: 10.1080/00365520802101861. [DOI] [PubMed] [Google Scholar]
- 16.Jeong YK, Ha HK, Yoon CH, Gong G, Kim PN, Lee MG, Min YI, Auh YH. Gastrointestinal involvement in Henoch–Schönlein syndrome. Am J Roenthenol. 1997;168:965–968. doi: 10.2214/ajr.168.4.9124151. [DOI] [PubMed] [Google Scholar]
- 17.Chan JC, Li PK, Lai FM, Lai KN. Fatal adult Henoch–Schönlein purpura due to small intestinal infarction. J Intern Med. 1992;232:181–184. doi: 10.1111/j.1365-2796.1992.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 18.Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch–Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol. 2002;13:1271–1278. doi: 10.1097/01.ASN.0000013883.99976.22. [DOI] [PubMed] [Google Scholar]
- 19.Miniter U, Bae-Harboe YS, Powers JG, Campbell SM, Goldberg LJ. Fatal Henoch–Schönlein purpura in an adult related to bowel perforation: report and review of the literature. Dermatol Online J. 2012;18:9. [PubMed] [Google Scholar]
- 20.Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther. 2004;26:197–213. doi: 10.1016/S0149-2918(04)90019-7. [DOI] [PubMed] [Google Scholar]
- 21.Martínek J, Hlavova K, Zavada F, Seifert B, Rejchrt S, Urban O, Zavoral M. ”A surviving myth” corticosteroids are still considered ulcerogenic by a majority of physicians. Scand J Gastroenterol. 2010;45:1156–1161. doi: 10.3109/00365521.2010.497935. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Muto S, Nakazawa E, Yanagiba S, Masunaga Y, Miyata Y, Tamba K, Kusano E, Matsuo M, Matsuo T, Asano Y. Combined treatment with nafamostat mesilate and aspirin prevents heparin-induced thrombocytopenia in a hemodialysis patient. Clin Nephrol. 2003;59:458–462. doi: 10.5414/CNP59458. [DOI] [PubMed] [Google Scholar]
- 23.Kamitsuji H, Tani K, Yasui M, Taniguchi A, Taira K, Tsukada S, Iida Y, Kanki H, Fukui H. Activity of blood coagulation factor XIII as a prognostic indicator in patients with Henoch–Schönlein purpura. Efficacy of factor XIII substitution. Eur J Pediatr. 1987;146:519–523. doi: 10.1007/BF00441608. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa N, Yamamura F, Akita Y, Sato T, Mitamura K. Gastrointestinal lesions in an adult patient with Henoch–Schönlein purpura. Hepatogastroenterology. 1999;46:2823–2824. [PubMed] [Google Scholar]
- 25.Hosono K, Endo H, Inamori M, Mawatari H, Iida H, Nozaki Y, Yoneda K, Akiyama T, Fujita K, Yoneda M, Takahashi H, Abe Y, Kirikoshi H, Kobayashi N, Kubota K, Saito S, Nakajima A. Severe large-intestine involvement in adult-onset Henoch–Schönlein purpura: successful treatment with factor XIII concentrate. Digestion. 2008;78:9. doi: 10.1159/000151298. [DOI] [PubMed] [Google Scholar]
- 26.Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with 99mTc-labeled human serum albumin scintigraphy. Radiology. 2001;219:86–90. doi: 10.1148/radiology.219.1.r01ap2986. [DOI] [PubMed] [Google Scholar]
- 27.Akimoto T, Saito O, Kusano E, Nagata D. Hypoalbuminemia and technetium-99m-labeled human serum albumin scintigraphy. Intern Med. 2014;53:1723. doi: 10.2169/internalmedicine.53.2538. [DOI] [PubMed] [Google Scholar]
- 28.Herfarth H, Hofstädter F, Feuerbach S, Jürgen Schlitt H, Schölmerich J, Rogler G. A case of recurrent gastrointestinal bleeding and protein-losing gastroenteropathy. Nat Clin Pract Gastroenterol Hepatol. 2007;4:288–293. doi: 10.1038/ncpgasthep0812. [DOI] [PubMed] [Google Scholar]
- 29.Seewoodhary J. Gastrointestinal haemorrhage in protein-losing enteropathy associated with the Fontan circulation. QJ Med. 2010;103:347–349. doi: 10.1093/qjmed/hcp170. [DOI] [PubMed] [Google Scholar]
- 30.Waller DG, Dalziel KL. The site of protein loss in Schönlein–Henoch purpura. Postgrad Med J. 1980;56:361–362. doi: 10.1136/pgmj.56.655.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura A, Fuchigami T, Inamo Y. Protein-losing enteropathy associated with Henoch–Schönlein purpura. Pediatr Rep. 2010;2:e20. doi: 10.4081/pr.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano T, Yamamoto H. Current state of double balloon endoscopy: the latest approach to small intestinal diseases. J Gastroenterol Hepatol. 2009;24:185–192. doi: 10.1111/j.1440-1746.2008.05773.x. [DOI] [PubMed] [Google Scholar]
- 33.Rech J, Fuchs F, Kallert S, Hueber AJ, Requadt C, Manger B, Kalden JR, Amann K, Strauss R, Schulze-Koops H. Plasmapheresis therapy in an elderly patient with rapidly progressive Henoch–Schönlein purpura with disseminated organ involvement. Clin Rheumatol. 2007;26:112–114. doi: 10.1007/s10067-005-0113-1. [DOI] [PubMed] [Google Scholar]
- 34.Agha FP, Nostrant TT, Keren DF. Leucocytoclastic vasculitis (Hypersensitivity angiitis) of the small bowel presenting with severe gastrointestinal hemorrhage. Am J Gastroenterol. 1986;81:195–198. [PubMed] [Google Scholar]
- 35.Amoli MM, Mattey DL, Calvino MC, Garcia-Porrua C, Thomson W, Hajeer AH, Ollier WE, Gonzales-Gay MA. Polymorphism at codon 469 of the intercellular adhesion molecule-1 locus is associated with protection against severe gastrointestinal complications in Henoch–Schönlein purpura. J Rheumatol. 2001;28:1014–1018. [PubMed] [Google Scholar]