Abstract
We report a 70-year-old man with primary (AL) amyloidosis with predominantly vascular deposition of amyloid diagnosed by renal biopsy, who was successfully treated using two chemotherapy regimens. There was rapid elevation of the serum creatinine level without remarkable proteinuria or hematuria. Renal histological examination showed some thickened arterial walls with amyloid fibril accumulation, and only a small amount of amyloid deposition in the glomeruli. Immunohistochemical examination was positive for anti-kappa staining. Serum immunoelectrophoresis and immunofixation testing did not show monoclonal proteins, and urine immunoelectrophoresis did not show Bence-Jones proteins. Serum free light chain (FLC) analysis showed that the serum FLC level and FLC kappa/lambda ratio were abnormally high for his renal function. He received two courses of VAD (vincristine, doxorubicin, and dexamethasone), followed by BD (bortezomib and dexamethasone), resulting in a hematologic partial response. Renal amyloidosis with vascular-limited amyloid deposition has few urinary findings. Early diagnosis of this condition is challenging, because kidney biopsies are not usually performed in patients without significant urinary findings. We suggest several currently available methods of achieving earlier detection of this condition.
Keywords: AL amyloidosis, Vascular deposition, Vascular limited, VAD, Bortezomib
Introduction
Amyloidosis is a generic term used to describe diseases caused by the extracellular deposition of amyloid fibrils. At least 30 protein precursors of amyloid fibrils have now been identified [1]. In primary (AL) amyloidosis, which is the most common type, the fibrils are composed of fragments of monoclonal immunoglobulin light chains. Accumulation of amyloid precursor proteins causes dysfunction of multiple organs, including the heart, liver, gastrointestinal tract, peripheral nerves, skin, and kidneys. Although the precise molecular structure of AL amyloid fibrils has not yet been elucidated, two histopathological patterns of renal amyloid deposition have been reported in AL and AA amyloidosis: diffuse pattern and vascular-limited pattern [2, 3]. The diffuse pattern is characterized by both vascular and glomerular amyloid deposition, with nephrotic range proteinuria in most cases. The vascular-limited pattern is associated with fewer urinary findings, and presents a diagnostic challenge.
Case report
A 70-year-old man was referred to our hospital in October 2010 for investigation of an elevated serum creatinine level (sCr). He had a history of unstable angina, which had been successfully treated by percutaneous coronary intervention several years prior. At the initial visit, his sCr was 1.62 mg/dL, and there was no proteinuria or hematuria. Renal ultrasonography showed slightly reduced thickness of the renal cortices. The initial diagnosis was nephrosclerosis, and he was managed with nutritional guidance and blood pressure control.
Eight months later, his sCr increased to 2.34 mg/dL, and there was still no remarkable proteinuria or hematuria. The loss of renal function seemed to be faster than expected in nephrosclerosis, considering that he had few exacerbating factors. There were no symptoms or physical signs suggesting interstitial nephritis or vascular disease. His medications (aspirin 100 mg/day, pitavastatin 2 mg/day, and silodosin 8 mg/day) had not been changed for at least 4 years.
Further serum and urine analysis and ultrasonography were performed to search for diseases causing renal failure without urinary abnormalities, but the findings were unremarkable. Doppler ultrasonography did not show signs of renal artery stenosis. The patient requested further investigation, and a renal biopsy was performed in September 2011 after temporary cessation of his aspirin.
On admission for the renal biopsy, his blood tests showed: red blood cell count 329 × 106/μL, hemoglobin 9.6 g/dL, hematocrit 29.8 %, leukocyte count 5140/μL, platelet count 29.9 × 104/μL, total protein 6.8 g/dL, albumin 4.4 g/dL, urea nitrogen 28 mg/dL, creatinine 2.45 mg/dL, sodium 141 mEq/L, potassium 4.1 mEq/L, chloride 107 mEq/L, total bilirubin 0.4 mg/dL, aspartate aminotransferase 13 IU/L, alanine aminotransferase 8 IU/L, lactate dehydrogenase 158 IU/L, and total cholesterol 182 mg/dL. Urinalysis was negative for protein and blood, and the 24-h urinary protein excretion was 0.14 g. His C-reactive protein level was 0.1 mg/dL. Coagulation tests were normal. The serum IgG level was 841 mg/dL, IgA level was 64 mg/dL, and IgM level was 43 mg/dL. Serum immunoelectrophoresis did not show monoclonal proteins, and urine immunoelectrophoresis did not show Bence-Jones proteins. The serum levels of angiotensin-converting enzyme, anticardiolipin antibody, and IgG4 were within the normal ranges, and tests for antinuclear antibodies and lupus anticoagulant were negative.
Renal biopsy
Light microscopic examination of the renal biopsy specimen showed global sclerosis of 15 glomeruli out of a total of 26 glomeruli, with 70 % interstitial fibrosis and tubular atrophy. The vascular walls were diffusely thickened and contained weakly periodic acid Schiff-positive material, mainly in the small arteries and arterioles. There was no increase in mesangial cells. Some mesangial areas, as well as the vascular walls, appeared to be slightly expanded with amorphous material that was positive for direct fast scarlet (DFS) staining and showed apple-green birefringence under polarized light after Congo red staining (Fig. 1). Immunofluorescence staining showed predominantly IgM deposits, mainly in the mesangial areas. Immunohistochemical examination showed stronger staining with anti-kappa antibodies than with anti-lambda antibodies. Electron microscopy showed severely thickened vascular walls with amyloid fibril deposition. The randomly arranged non-branching fibrils measured about 15 nm in diameter, indicating amyloid fibrils (Fig. 2). The pathological diagnosis was AL amyloidosis with predominantly vascular amyloid deposition.
Fig. 1.
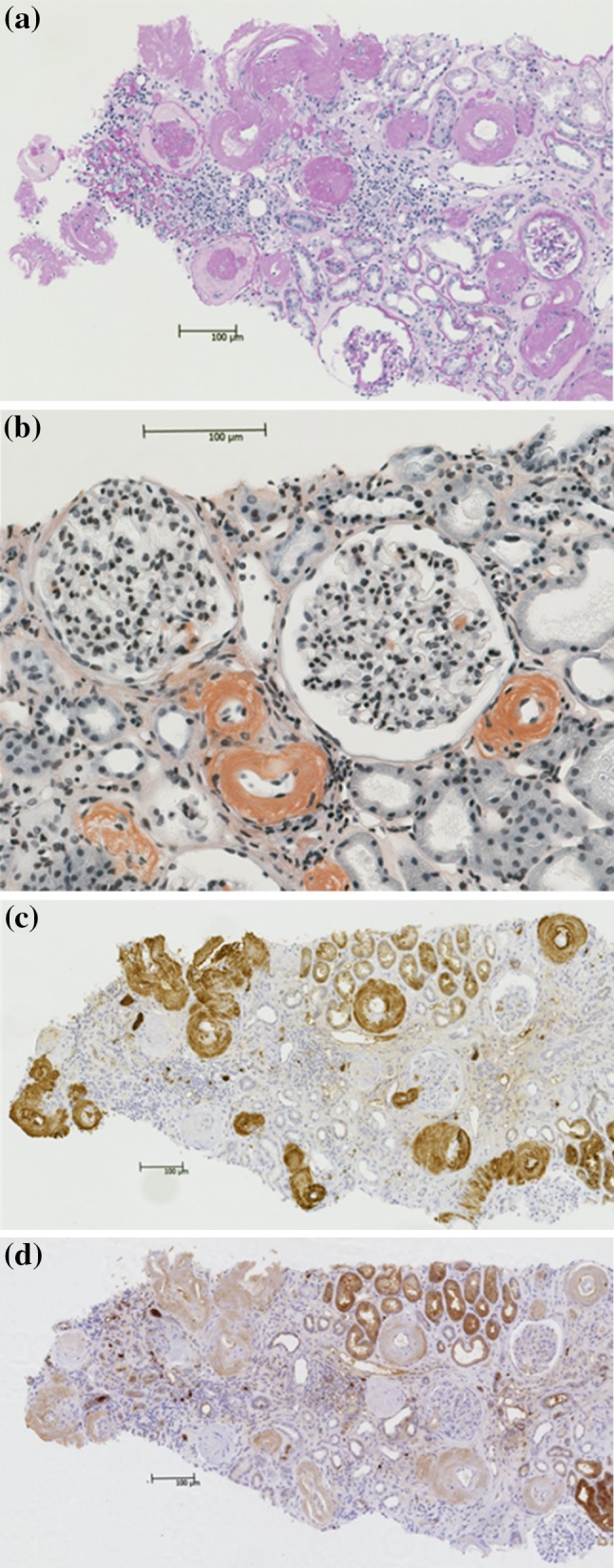
Light microscopy findings. a Periodic acid Schiff (PAS) staining, showing thickening of the walls of the small arteries and arterioles with weakly PAS-positive material, and interstitial invasion by lymphocytes. b Direct fast scarlet staining, showing positive staining of the vascular walls and partial positive staining of the glomeruli including sclerotic glomeruli. c Immunohistochemical staining for kappa chains, showing positive staining of the vascular walls and partial positive staining of the glomeruli including sclerotic glomeruli. d Immunohistochemical staining for lambda chains
Fig. 2.
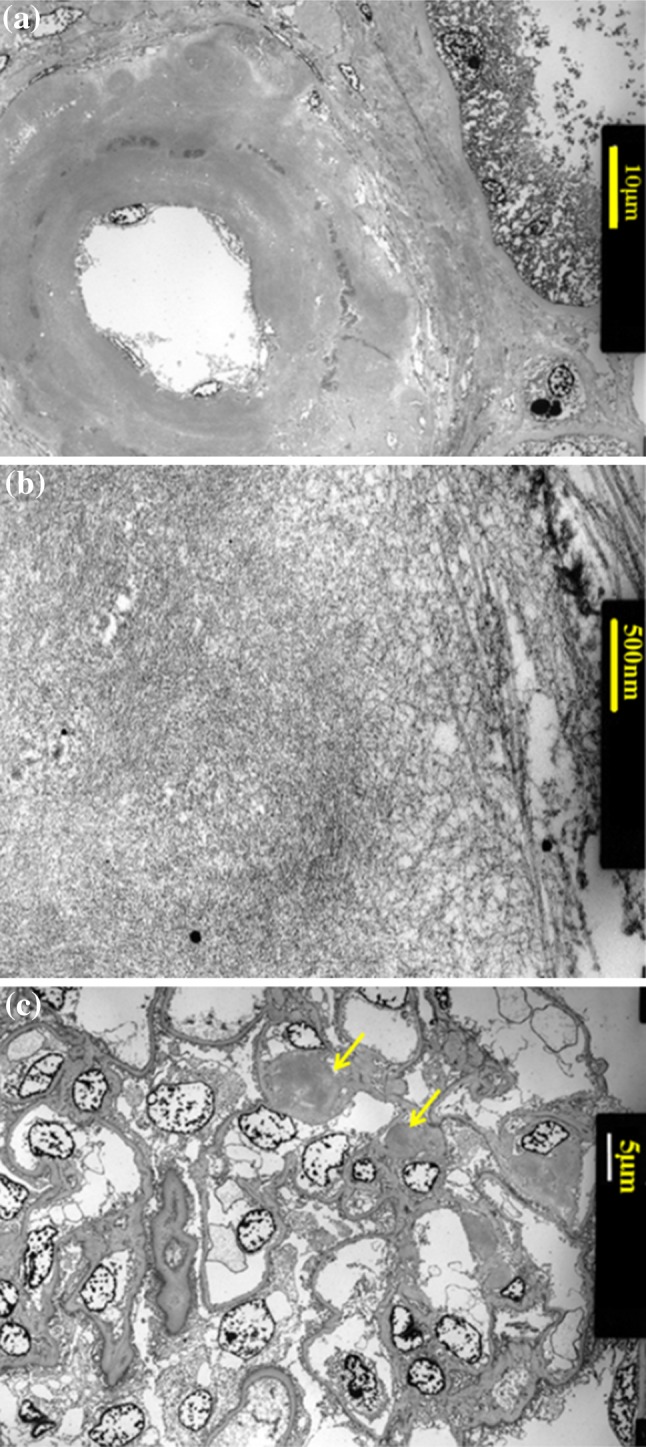
Electron microscopy findings. a Extreme thickening of the vascular walls with amyloid fibrils in the small arteries and arterioles. b Randomly arranged non-branching fibrils measuring about 15 nm in diameter. c A glomerulus with a small amount of amyloid deposition in some mesangial areas
Other investigations
Serum free light chain (FLC) analysis showed a free kappa chain level of 473 mg/L, free lambda chain level of 32.2 mg/L, and FLC kappa/lambda (κ/λ) ratio of 14.7. Bone marrow aspiration showed normal plasma cells (5 %, predominantly kappa positive cells) with no amyloid deposition, including in the vascular tissue. A systemic skeletal survey did not show osteolytic lesions. The patient was diagnosed with AL amyloidosis. Abdominal fat biopsy findings were negative. Cardiac ultrasonography showed no signs of cardiac hypertrophy or fractional shortening. The serum N-terminal B-type natriuretic peptide level was 646 pg/mL (normal range <125 pg/mL), and the cardiac troponin-I level was undetectable.
Clinical course
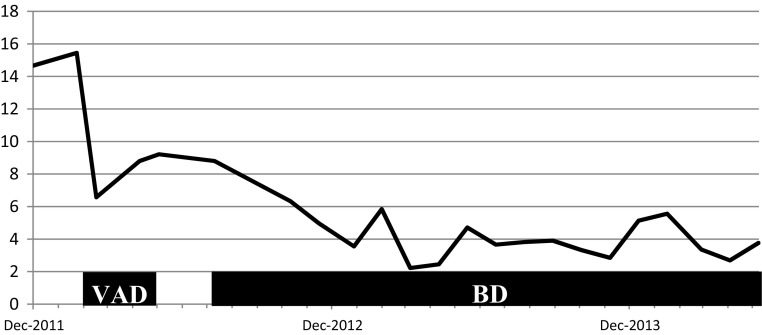
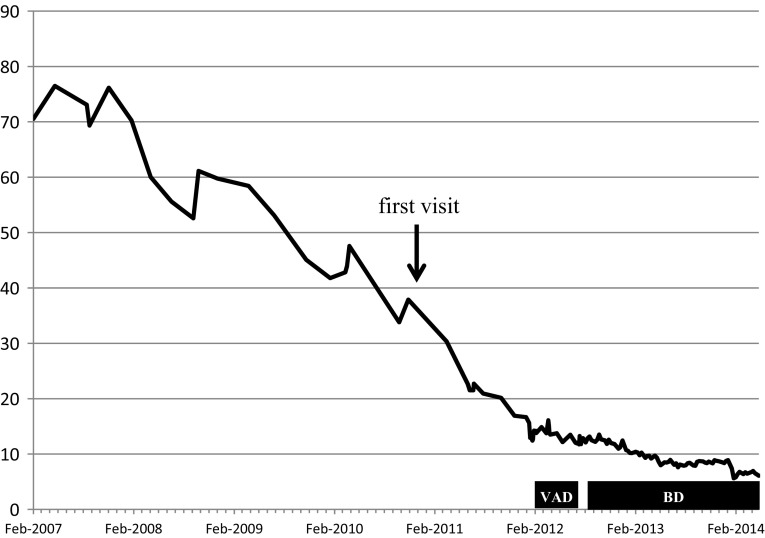
VAD (vincristine 0.28 mg/body and doxorubicin 6.3 mg/m2 by continuous infusion on days 1–4, and dexamethasone 33 mg/body by infusion on days 1–4, 9–12, and 17–20) was started in February 2012. The vincristine and doxorubicin doses were decreased to 70 % of the standard dose, because of the patient’s age and his history of unstable angina. No serious adverse events were observed. This therapy achieved reduction of the serum free kappa chain level from 473 to 124 mg/L, and of the FLC κ/λ ratio from 14.7 to 6.5. After two courses of VAD, the FLC κ/λ ratio began to increase again. As several studies have reported that bortezomib-based regimens are effective for the treatment of newly diagnosed or relapsed AL amyloidosis [4–6], his chemotherapy regimen was changed to BD (bortezomib 1.3 mg/m2 by infusion on days 1, 7, 14, and 21; and dexamethasone 20 mg/day by infusion on days 1–2, 7–8, 14–15, and 21–22). In October 2013, after the 13th course of BD, the sCr was 5.5 mg/dL and the FLC κ/λ ratio was 3.3, indicating a hematologic partial response (Fig. 3). No remarkable adverse effects were observed, except for mild constipation and glucose intolerance. The decline in estimated glomerular filtration rate was about 12 mL/min/1.73 m2 per year before the initiation of treatment, and improved to about 5 mL/min/1.73 m2 per year after the initiation of chemotherapy in February 2012 (Fig. 4). At the time of writing in May 2014, the patient was still receiving chemotherapy on an outpatient basis, with bortezomib administered by subcutaneous infusion and dexamethasone administered orally. His sCr was 7.68 mg/dL, and initiation of hemodialysis was planned. Cardiac ultrasonography still showed no signs of cardiac hypertrophy or fractional shortening.
Fig. 3.
Serum FLC κ/λ ratio
Fig. 4.
Clinical course (estimated glomerular filtration rate, mL/min/1.73 m2)
Discussion
Two histopathological patterns of renal amyloid deposition in AL amyloidosis have previously been reported: diffuse pattern and vascular-limited pattern [2]. According to some definitions, amyloid deposition is observed only in the blood vessels in the vascular-limited pattern, and in both the glomeruli and blood vessels in the diffuse pattern. The pathophysiological mechanisms underlying these different patterns are currently unclear. In the diffuse pattern, renal insufficiency may result from mechanical disruption of the glomeruli and interstitial damage caused by the proteinuria. In the vascular-limited pattern, Falck et al. [3] considered that narrowing and occlusion of the vessels by amyloid deposition in the vessel walls may cause ischemia. In our case, we think that the vascular narrowing is remarkable and the glomerular and interstitial changes may be consistent with ischemic damage. It has been hypothesized that some amyloidogenic precursor proteins have direct cellular toxicity, [7] but such toxicity could not be proven pathologically or clinically in our case. In a study of 234 patients with AL amyloidosis, only 12 (5.1 %) had a vascular-limited pattern [2]. Uda et al. [8] reported 38 patients with rheumatoid arthritis and renal secondary (AA) amyloidosis, of which 11 (28.9 %) had a vascular-limited pattern. Falck et al. [3] used the term ‘predominantly vascular’ deposition, which could also be applied to our case, as there was a small amount of amyloid deposition in the glomeruli.
Patients with a vascular-limited pattern of amyloid deposition at the time of diagnosis have a significantly higher sCr than those with a diffuse pattern of amyloid deposition [2]. This is probably because the diagnosis is delayed in patients with a vascular-limited pattern, who have less proteinuria than patients with a diffuse pattern. Yao et al. [9] studied 186 patients with renal AL amyloidosis, and found that the extent of glomerular amyloid deposition was positively correlated with the degree of proteinuria. A significant difference in survival has also been reported between the two patterns, with a median survival time of 77.2 months in patients with diffuse amyloid deposition and 40.6 months in patients with vascular-limited amyloid deposition [2]. Early diagnosis of patients with vascular-limited amyloid deposition is usually challenging, because kidney biopsies are not usually performed in patients without significant urinary findings. We propose several methods of achieving earlier detection of AL amyloidosis with vascular-pattern amyloid deposition, as follows.
Detection of monoclonal proteins is an important aspect of the diagnosis of AL amyloidosis. Serum FLC analysis can now be performed using a commercially available assay, and has a high sensitivity for the detection of monoclonal proteins [10]. In our case, serum and urine immunoelectrophoresis did not detect monoclonal proteins, and the serum immunofixation test, which is reported to be the most sensitive test, was negative. The combination of these tests has a reported sensitivity of 99 % [10, 11]. Serum FLC analysis is useful in patients with renal insufficiency, even though worsening of renal function is known to be associated with an increase in the uninvolved serum FLC level. Bochtler et al. [12] reported that 21 out of 24 (88 %) patients with a creatinine clearance of <30 mL/min had a positive FLC test. Hutchison et al. [13] studied 688 patients with chronic kidney disease from various causes, and found that the serum FLC level and FLC κ/λ ratio increased progressively with increasing stages of kidney disease. Although the reference ranges for the serum FLC level and FLC κ/λ ratio in patients with chronic kidney disease are not clearly defined, the reference ranges obtained from that study can be helpful in patients with renal failure. FLC can also be used to assess the response to treatment. Our patient achieved a hematologic partial response, with a decrease in the serum free kappa chain level of >50 % (Fig. 3) [13, 14].
Abdominal fat pad biopsy is less invasive and easier to perform than renal or hepatic biopsy. This test is positive in 57–93 % of patients with amyloidosis, but the results can vary depending on the number of samples, observers, and smears [15–18]. In some situations, bone marrow biopsy (positive in 50–55 % of patients with amyloidosis), rectal biopsy (positive in 50–70 % of patients), or skin biopsy (positive in 50 % of patients) are recommended because of their convenience [18–20]. However, we are still hesitant to perform these tests immediately in patients without significant urinary findings, because the benefits of testing are insufficient in most cases. Most elderly patients with deteriorating renal function but no significant urinary findings only have nephrosclerosis. The incidence of AL amyloidosis is approximately 8.9 cases per million person-years [21], and the vascular-limited pattern only accounts for a small proportion of these cases.
The pretest probability of AL amyloidosis may increase when renal symptoms occur in combination with other abnormalities of unknown cause such as chronic heart failure, hepatosplenomegaly, carpal tunnel syndrome, purpura, peripheral neuropathy, and macroglossia. Coexisting symptoms of multiple myeloma can sometimes help the diagnostic process. Ultrasonography in renal amyloidosis typically shows enlarged kidneys with increased cortical thickness but maintenance of a normal shape, and very fine echogenic texture [22]. None of these findings were present in our case, but the rate of decline in renal function suggested that renal biopsy might be indicated. The clinical course of our patient was not typical for nephrosclerosis, which has few risk factors (Fig. 4). A large trial found that the cumulative 7-year incidence rate of a twofold increase in sCr to >2 mg/dL had a range of 0.12–0.17 % [23]. Freedman et al. [24] proposed that many patients diagnosed with hypertensive nephrosclerosis actually have intrinsic renal parenchymal disease, renal artery stenosis, unrecognized episodes of accelerated hypertension, or a primary renal microvascular disease.
Further investigation is needed to develop a reliable protocol for earlier detection of AL amyloidosis with vascular-limited amyloid deposition. Our case highlights the importance of further investigation when there is inconsistency between the expected and actual clinical courses, especially in cases with a tentative initial diagnosis.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 2.Eirin A, Irazabal MV, Gertz MA, et al. Clinical features of patients with immunoglobulin light chain amyloidosis (AL) with vascular-limited deposition in the kidney. Nephrol Dial Transplant. 2012;27:1097–1101. doi: 10.1093/ndt/gfr381. [DOI] [PubMed] [Google Scholar]
- 3.Falck HM, Törnroth T, Wegelius O. Predominantly vascular amyloid deposition in the kidney in patients with minimal or no proteinuria. Clin Nephrol. 1983;19:137–142. [PubMed] [Google Scholar]
- 4.Reece DE, Hegenbart U, Sanchorawala V, et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118:865–873. doi: 10.1182/blood-2011-02-334227. [DOI] [PubMed] [Google Scholar]
- 5.Reece DE, Sanchorawala V, Hegenbart U, VELCADE CAN2007 Study Group et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. 2009;114:1489–1497. doi: 10.1182/blood-2009-02-203398. [DOI] [PubMed] [Google Scholar]
- 6.Kastritis E, Wechalekar AD, Dimopoulos MA, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- 7.Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;12:3458–3471. doi: 10.1681/ASN.2006050460. [DOI] [PubMed] [Google Scholar]
- 8.Uda H, Yokota A, Kobayashi K, et al. Two distinct clinical courses of renal involvement in rheumatoid patients with AA amyloidosis. J Rheumatol. 2006;33:1482–1487. [PubMed] [Google Scholar]
- 9.Yao Y, Wang SX, Zhang YK, et al. A clinicopathological analysis in a large cohort of Chinese patients with renal amyloid light-chain amyloidosis. Nephrol Dial Transplant. 2013;28:689–697. doi: 10.1093/ndt/gfs501. [DOI] [PubMed] [Google Scholar]
- 10.Katzmann JA, Abraham RS, Dispenzieri A, et al. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005;51:878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- 11.Bochtler T, Hegenbart U, Heiss C, et al. Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica. 2008;93:459–462. doi: 10.3324/haematol.11687. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–1690. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 14.Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 15.Duston MA, Skinner M, Meenan RF, et al. Sensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosis. Arthritis Rheum. 1989;32:82–85. doi: 10.1002/anr.1780320114. [DOI] [PubMed] [Google Scholar]
- 16.Westermark P, Davey E, Lindbom K, et al. Subcutaneous fat tissue for diagnosis and studies of systemic amyloidosis. Acta Histochem. 2006;108:209–213. doi: 10.1016/j.acthis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 17.van Gameren II, Hazenberg BP, Bijzet J, et al. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54:2015–2021. doi: 10.1002/art.21902. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra S, Krishnani N, Kumari N, et al. Evaluation of abdominal fat pad aspiration cytology and grading for detection in systemic amyloidosis. Acta Cytol. 2007;51:860–864. doi: 10.1159/000325861. [DOI] [PubMed] [Google Scholar]
- 19.Duston MA, Skinner M, Shirahama T, et al. Diagnosis of amyloidosis by abdominal fat aspiration: analysis of four years’ experience. Am J Med. 1987;82:412–414. doi: 10.1016/0002-9343(87)90439-6. [DOI] [PubMed] [Google Scholar]
- 20.Sungur C, Sungur A, Ruacan S, et al. Diagnostic value of bone marrow biopsy in patients with renal disease secondary to familial Mediterranean fever. Kidney Int. 1993;44:834–836. doi: 10.1038/ki.1993.318. [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–1822. [PubMed] [Google Scholar]
- 22.O’Neill WC. Sonographic evaluation of renal failure. Am J Kidney Dis. 2000;35:1021–1038. doi: 10.1016/S0272-6386(00)70036-9. [DOI] [PubMed] [Google Scholar]
- 23.Neaton JD, Kuller LH, Wentworth D, et al. Total and cardiovascular mortality in relation to cigarette smoking, serum cholesterol concentration, and diastolic blood pressure among black and white males followed up for five years. Am Heart J. 1984;108:759–769. doi: 10.1016/0002-8703(84)90669-0. [DOI] [PubMed] [Google Scholar]
- 24.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207–221. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]