Abstract
A 41-year-old man with a history of multiple sclerosis (MS) developed thrombotic microangiopathy after taking interferon β-1b for 10 years. Although the relapse of his MS was well controlled under normal blood pressure, he had persistent nausea, anorexia, gait disturbance and visual disorder 1 month before admission. He showed lethargy and high blood pressure (180/102 mmHg). Laboratory test results revealed anemia and thrombocytopenia, elevated LDH and renal dysfunction. Urinary dipstick showed a 2+ result for proteinuria and 3+ for hematuria. Schizocyte were present and haptoglobin decreased, and we diagnosed him with possible thrombotic microangiopathy (TMA). Magnetic resonance image indicated posterior reversible encephalopathy syndrome (PRES), which could be accelerated by TMA. After discontinuing interferon β-1b, high dose intravenous methylpredonisolone, anti-hypertension therapy and plasma exchange was started. Because a mild decrease in ADAMTS13 activity and absence of ADAMTS 13 inhibitor could not cause thrombotic thrombocytopenic purpura, plasma exchange was stopped. The patient’s renal function recovered and PRES resolved, and he was discharged with slightly decrease of visual acuity. We suggest that his TMA was likely caused by interferon β-1b, resulting in PRES in a patient with multiple sclerosis. We report this rare case and also review the literature.
Keywords: Thrombotic microangiopathy, Interferon, Multiple sclerosis
Introduction
Thrombotic microangiopathy (TMA) is a rare life-threatening disorder that is characterized by microangiopathic hemolytic anemia, thrombocytopenia and ischemic injury to various organs. TMA is categorized in the following three disorders: (1) thrombotic thrombocytopenic purpura (TTP), caused by congenital or acquired ADAMTA13 deficiency, which functions as a von Willebrand factor (vWF)-cleaving metalloprotease, leading to the platelet aggregation and microvascular thrombus by the large von Willebrand factor multimer; (2) hemolytic uremic syndrome (HUS) caused by Shiga toxin-producing Escherichia coli (STEC-HUS); and (3) atypical hemolytic uremic syndrome (aHUS), which is defined as a syndrome with a TMA that is categorized as neither TTP nor STEC-HUS [1, 2]. aHUS has been further classified as complement-mediated TMA, drug-mediated TMA (immune reaction or toxic dose-related reaction), coagulation-mediated TMA and metabolism-mediated TMA [2]. A diagnostic algorithm for aHUS has been recently proposed [3]. During these steps, a decision is made to immediately initiate plasma exchange (PE) with fresh frozen plasma (FFP) combined with corticosteroid therapy when TTP or TMA that required PE is suspected [2, 4, 5].
Here, we describe the possible case of a successfully treated TMA patient with multiple sclerosis (MS) who had been receiving long-term interferon β-1b. We also performed a literature review.
Case report
A 41-year-old man was referred to the department of Nephrology at the Kyoto University Hospital. He presented with nausea, truncal ataxia and visual disturbance. He was diagnosed with MS in 1993 and has been treated with 9,600,000 IU interferon β-1b (BETAFERON SC inj. 960, Bayer, Osaka, Japan) subcutaneously once daily since 2004 to maintain remission. His blood pressure began to increase, and facial edema and syncopal attack developed 2 months before admission. He did not have diarrhea, fever, purpura or dark urine. His had no family history of disease related to thrombosis. He showed labile consciousness loss, ataxic gait, dysarthria, severe visual loss in his left eye and bilateral directional nystagmus. His blood pressure was 180/102 mmHg. He did not have any abnormal chest or abdominal findings except for a pigmentation and panniculitis on his abdominal wall from the interferon injection. As shown in the Table 1, laboratory test results revealed anemia with schistocytosis, thrombocytopenia, elevated lactic dehydrogenase (LDH) and creatinine (Cr). Those results had been within normal limits 2 months before admission. Urinalysis showed microscopic hematuria and heavy proteinuria, indicating possible involvement of thrombotic microangiopathy. Both plasma renin activity (PRA) and plasma aldosterone concentration (PAC) were significantly elevated, which may have caused his severe hypertension. A broad spectrum of high intensity area was found in bilateral occipital lobe and cerebellum using magnetic resonance imaging (MRI) T2 fluid attenuated inversion recovery (FLAIR) image, which indicated posterior reversible encephalopathy syndrome (PRES; Fig. 1). Since we did not find any typical findings of malignant hypertension on his optic fundi, his visual loss seemed to be due to PRES.
Table 1.
Laboratory data on admission
| CBC | ||
| WBC | 23,600 | /μL |
| Hg | 8.0 | g/dL |
| Plt | 13,300 | /μL |
| Schizocyte | Positive | |
| Blood chemistry | ||
| AST | 51 | IU/L |
| ALT | 34 | IU/L |
| LDH | 970 | IU/L |
| CK | 3,078 | IU/L |
| γ-GTP | 207 | IU/L |
| TP | 5.8 | g/dL |
| Alb | 2.5 | g/dL |
| Cre | 3.8 | mg/dL |
| UA | 16.7 | mg/dL |
| BUN | 122 | mg/dL |
| Na | 132 | mEq/L |
| K | 4.4 | mEq/L |
| Cl | 89 | mEq/L |
| Ca | 8.1 | mg/dL |
| iP | 9.9 | mg/dL |
| CRP | 4.7 | mg/dL |
| Urinalysis | ||
| Protein | (2+) | |
| Sugar | (−) | |
| Occult blood | (3+) | |
| RBC | 5–9 | /HPF |
| Proteinuria | 2.6 | g/day |
| Coagulation test | ||
| PT | 12.6 | s |
| aPTT | 33.9 | s |
| Serological test | ||
| IgG | 718 | mg/dL |
| IgA | 244 | mg/dL |
| IgM | 83 | mg/dL |
| C3 | 105.4 | mg/dL |
| C4 | 30.3 | mg/dL |
| CH50 | >60 | U/mL |
| ANA | ×40 | Speckled |
| Anti-ds-DNA IgG | <10 | IU/mL |
| MPO-ANCA | <10 | EU |
| PR3-ANCA | <10 | EU |
| Anti-Scl-70 antibody | <5 | |
| ADAMTS13 inhibitor | Negative | |
| ADAMTS13 activity | 32.4 | % |
| Lupus anticoagulant | negative | |
| Haptoglobin | <2 | mg/dL |
| Thrombomodulin | 15 (2.1–4.1) | FU/mL |
| Others | ||
| Direct Coombs test | Negative | |
| PRA | 44.6 | ng/mL/hr |
| PAC | 514 | pg/mL |
| BNP | 359.0 | pg/mL |
| Fe | 61 | μg/dL |
| UITC | 135 | μg/dL |
| Ferritin | 1,325.3 | ng/mL |
| Vitamin B12 | 360 | pg/mL |
ANA anti-nuclear antibody, ANCA anti-neutrophil cytoplasmic antibody, MPO myeloperoxidase, PR3 proteinase 3, PRA plasma renin activity, PAC plasma aldosterone concentration, BNP brain natriuretic peptide
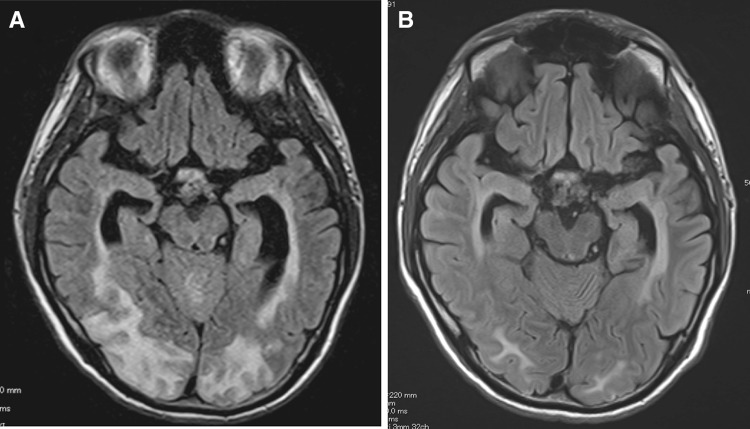
Fig. 1.
Fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging. Bilateral hyperintense subcortical white matter lesions were observed in a predominantly posterior distribution on admission (a). Those lesions were resolved after 12 days (b), suggesting posterior reversible encephalopathy syndrome (PRES)
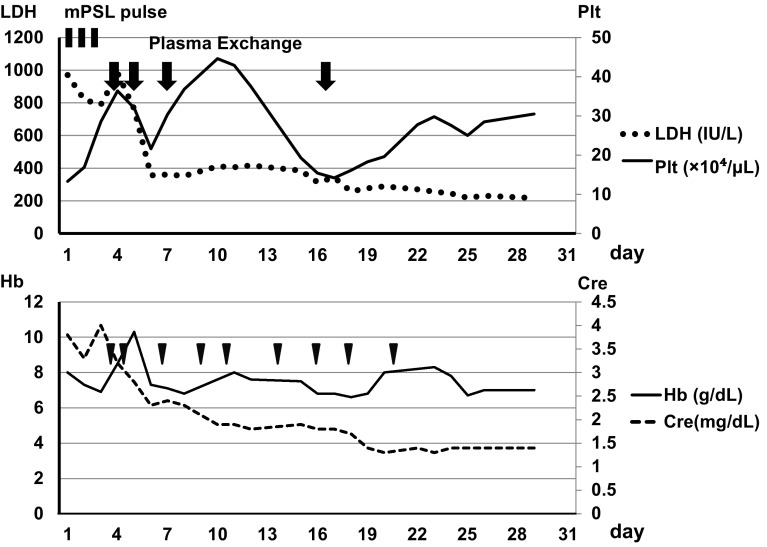
We started anti-hypertension therapy including angiotensin receptor blocker and intravenous calcium channel blocker to control his blood pressure around 130/85, and high-dose intravenous methylpredonisolone therapy for 3 days, as shown in Fig. 2. His visual acuity gradually improved, but he developed oliguria despite medical therapy. We, thus, performed PE using one plasma volume of FFP (2,700 mL each) combined with hemodialysis (HD). After two course of PE, his consciousness became clear although visual acuity did not recover.
Fig. 2.
Clinical course
After three courses of PE, laboratory test results on admission revealed a slight decrease in ADAMTS-13 activity (32.4 %) and the absence of ADAMTS-13 inhibitor, leading to the negative diagnosis of TTP (Table 1). PE then stopped and HD was continued until renal function recovered. We also performed additional PE on day 16 because of thrombocytopenia by unknown cause and the patient’s platelet count gradually returned to normal after the final PE (Fig. 2). His state of consciousness improved and nystagmus disappeared on 6 day of the illness. Laboratory data showed normalized LDH and decreased Cr (1.4 mg/dL) despite persistent schistocytosis one after admission. MRI T2 FLAIR imaging showed regression of the high intensity area (Fig. 1). The patient’s renal function and PRES recovered and he was discharged with a slightly decreased visual acuity. A renal biopsy was not performed. Along with his clinical course, we concluded that this patient had possible TMA that was triggered by interferon β-1b administrated to treat MS, which was complicated by PRES through significant amplification of RAS by TMA in the kidney [6].
Discussion
aHUS was historically determined to distinguish disorders characterized by TMA from TTP and STEC-HUS [2]. After discriminating our case from TTP and STEC-HUS, we had made a differential diagnosis [3]. Abnormal complement activation by gene mutation, which usually presents in the family history or could present recurrent onset of TMA, or autoantibody against factor H is a main cause of aHUS. It is still difficult to examine the abnormality of complement activation using routine laboratory test. Therefore, we could not rule out aHUS caused by abnormal complement activation in our patient. However, we wanted to determine other causes of TMA in this case, as shown in Table 1. Our patient developed TMA with high levels of thrombomodulin. Abnormal cobalamine metabolism, infectious disease, pregnancy-related and organ transplantation-related causes were not probable in this patient. Thus, we suggest that his TMA was induced by interferon β-1b before starting serial therapies, although it is rare. His TMA did not relapse after interferon β-1b cessation. It was still difficult to distinguish his TMA from other condition such as malignant hypertension without renal biopsy. Nevertheless, we concluded that his TMA have been triggered by interferon β-1b that was administered as a treatment for MS. Indeed, interferon β-1a has been shown to cause TMA [6–9]. The difference between interferon β-1a and interferon β-1b is the presence (1a) or absence (1b) of glycosylation. For TMA, the glycosylation is likely not associated with the pathogenicity of interferon β.
It remains unclear why interferon causes TMA [6, 8, 10, 11]. Interferon has been reported to cause endothelial cell damage directly through a decrease in vascular endothelial growth factor production, or to generate ADAMTS-13 inhibitor through T cell activation [12–14]. Although Orvain et al. reported the case of TTP in an MS patient who was treated with interferon β, the pathological mechanism of whether ADAMTS13 inhibitor (autoantibody) is related or not were unclear in many cases [15]. It is unknown why interferon-induced aHUS had develops after long-term use of the drug in patients, including the patient in our case [7, 16]. As a high incidence of TMA in MS patients treated with interferon β from the same manufacturer has been reported, a second factor might be involved in the disease onset [17].
PE with FFP is strongly recommended in the case of TTP [1, 4, 18]. This can supply ADAMTS-13 activity simultaneously removing both large molecules of vWF multimer and ADAMTS-13 inhibitor. Therefore, we started PE with FFP first. When laboratory findings later distinguished his TMA from TTP, we stopped PE and steroid therapy, and continued renal replacement therapy until his renal function recovered. Cessation of interferon might resolve TMA in some patients, and PE has been effective in controlling TMA in other patients. Serious cases might require continuation of dialysis therapy [6–9]. Similar clinical courses have also been reported for tacrolimus in patients with organ transplantation [19, 20]. In our patient, severe hypertension had emerged before admission probably as a result of RAS activation induced by TMA in the kidney. Because severe hypertension can also accelerate endothelial damage, such as malignant hypertension, it is important to treat hypertension by inhibiting RAS system activation [6]. PE might also help to control the TMA activity and organ damage [4].
Recent data on TMA pathogenesis shows a common pathway of complement activation in all TMA patients [21]. In addition, eculizumab, an anti-C5 monoclonal antibody, could be effective in controlling TMA, but it is still unclear whether this monoclonal antibody would also be effective in drug-induced TMA until now [3]. Further information is required to elucidate the mechanism of TMA induced by interferon β-1b.
Conclusion
We report the case of a patient with MS who developed TMA that was possibly caused by interferon β-1b, which resulted in PRES. PE might help to control the disease activity.
Acknowledgments
We thank Dr. Yoshihiro Fujimura and Dr. Masanori Matsumoto (Department of Blood Transfusion Medicine, Nara Medical University) for measuring ADAMTS-13 activity and ADAMTS-13 inhibitor levels.
Abbreviations
- TMA
Thrombotic microangiopathy
- HUS
Hemolytic uremic syndrome
- STEC
Shiga toxin-producing Escherichia coli
- aHUS
Atypical HUS
- vWF
von Willebrand factor
- PE
Plasma exchange
- FFP
Fresh frozen plasma
- HD
Hemodialysis
- PRA
Plasma renin activity
- PAC
Plasma aldosterone concentration
- MRI
Magnetic resonance imaging
- FLAIR
Fluid attenuated inversion recovery
- PRES
Posterior reversible encephalopathy syndrome
- LDH
Lactic dehydrogenase
- Cr
Creatinine
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Rosove MH. Thrombotic microangiopathies. Semin Arthritis Rheum. 2014;43:797–805. doi: 10.1016/j.semarthrit.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 3.Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, Langman CB, Lapeyraque AL, Licht C, Nester C, Pecoraro C, Riedl M, van de Kar NC, Van de Walle J, Vivarelli M, Fremeaux-Bacchi V. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 4.Clark WF. Thrombotic microangiopathy: current knowledge and outcomes with plasma exchange. Semin Dial. 2012;25:214–219. doi: 10.1111/j.1525-139X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, Szczepiorkowski ZM, Williams ME, Wu Y, Shaz BH. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 6.Larochelle C, Grand’maison F, Bernier GP, Latour M, Cailhier JF, Prat A. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome in relapsing-remitting multiple sclerosis patients on high-dose interferon beta. Mult Scler. 2014;20:1783–1787. doi: 10.1177/1352458514523692. [DOI] [PubMed] [Google Scholar]
- 7.Olea T, Diaz-Mancebo R, Picazo ML, Martinez-Ara J, Robles A, Selgas R. Thrombotic microangiopathy associated with use of interferon-beta. Int J Nephrol Renovasc Dis. 2012;5:97–100. doi: 10.2147/IJNRD.S30194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahe J, Meurette A, Moreau A, Vercel C, Jolliet P. Renal thrombotic microangiopathy caused by interferon beta-1a treatment for multiple sclerosis. Drug Des Devel Ther. 2013;7:723–728. doi: 10.2147/DDDT.S42138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vosoughi R, Marriott JJ. Thrombotic microangiopathy in interferon beta treated multiple sclerosis patients: review of literature and report of two new cases. Mult Scler Relat Disord. 2014;3:321–325. doi: 10.1016/j.msard.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Jadoul M, Piessevaux H, Ferrant A, Cosyns JP, de Strihou CVY. Renal thrombotic microangiopathy in patients with chronic myelogenous leukaemia treated with interferon-alpha 2b. Nephrol Dial Transplant. 1995;10:111–113. doi: 10.1093/ndt/10.supp6.111. [DOI] [PubMed] [Google Scholar]
- 11.Galesic K, Bozic B, Racic I, Scukanec-Spoljar M. Thrombotic microangiopathy associated with alpha-interferon therapy for chronic myeloid leukaemia. Nephrology (Carlton) 2006;11:49–52. doi: 10.1111/j.1440-1797.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 12.Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res. 1987;47:5155–5161. [PubMed] [Google Scholar]
- 13.Wu WZ, Sun HC, Shen YF, Chen J, Wang L, Tang ZY, Iliakis G, Liu KD. Interferon alpha 2a down-regulates VEGF expression through PI3 kinase and MAP kinase signaling pathways. J Cancer Res Clin Oncol. 2005;131:169–178. doi: 10.1007/s00432-004-0615-2. [DOI] [PubMed] [Google Scholar]
- 14.Arrambide G. Thrombotic thrombocytopenic purpura-haemolytic uremic syndrome in relapsing-remitting multiple sclerosis patients on high-dose interferon beta. Mult Scler. 2014;20:1788–1789. doi: 10.1177/1352458514529614. [DOI] [PubMed] [Google Scholar]
- 15.Orvain C, Augusto JF, Besson V, Marc G, Coppo P, Subra JF, Sayegh J. Thrombotic microangiopathy due to acquired ADAMTS13 deficiency in a patient receiving interferon-beta treatment for multiple sclerosis. Int Urol Nephrol. 2014;46:239–242. doi: 10.1007/s11255-013-0401-7. [DOI] [PubMed] [Google Scholar]
- 16.Broughton A, Cosyns JP, Jadoul M. Thrombotic microangiopathy induced by long-term interferon-beta therapy for multiple sclerosis: a case report. Clin Nephrol. 2011;76:396–400. doi: 10.5414/CN106523. [DOI] [PubMed] [Google Scholar]
- 17.Hunt D, Kavanagh D, Drummond I, Weller B, Bellamy C, Overell J, Evans S, Jackson A, Chandran S. Thrombotic microangiopathy associated with interferon beta. N Engl J Med. 2014;370:1270–1271. doi: 10.1056/NEJMc1316118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998–2008. Intern Med. 2010;49:7–15. doi: 10.2169/internalmedicine.49.2706. [DOI] [PubMed] [Google Scholar]
- 19.Carson JM, Newman ED, Farber JL, Filippone EJ. Tacrolimus-induced thrombotic microangiopathy: natural history of a severe, acute vasculopathy. Clin Nephrol. 2012;77:79–84. doi: 10.5414/CN107036. [DOI] [PubMed] [Google Scholar]
- 20.Aruch DB, Renteria A. Simultaneous PRES and TMA secondary to tacrolimus after allogeneic bone marrow transplant. Blood. 2015;125:3963. doi: 10.1182/blood-2015-03-634782. [DOI] [PubMed] [Google Scholar]
- 21.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]