Abstract
Myeloma cast nephropathy is a major complication of multiple myeloma. Recent evidence has demonstrated that the earlier induction of bortezomib-based chemotherapy with plasma exchange (PE) provides better results for kidney function and patient survival. Due to its non-selectivity, PE with albumin replacement carries the risk of fibrinogen loss, leading to bleeding. We herein report a case of successful treatment of myeloma cast nephropathy using bortezomib-based chemotherapy and selective PE. A 61-year-old woman who had a 20-year history of type II diabetes mellitus was admitted to our hospital for the evaluation of hypercalcemia, severe kidney dysfunction, and anemia. Subsequent bone marrow evaluation and renal biopsy revealed that she had multiple myeloma (IgG-κ) and myeloma cast nephropathy. Ten days after admission, bortezomib-based chemotherapy with selective PE achieved rapid and thorough free light-chain (FLC) reduction; within a month, her kidney function had been recovered (creatinine level, 1.2 mg/dl). Her serum fibrinogen level was not reduced, and no bleeding complication occurred. Five months later, autologous hematopoietic stem-cell transplantation was performed successfully, and the patient’s kidney function was stable (creatinine level, 1.1 mg/dl) thereafter. This case report demonstrates the importance of early induction therapy with bortezomib-based chemotherapy and PE in a patient with myeloma cast nephropathy, which is especially applicable in patients aged <65 years. In addition, it shows that selective PE is a safe and effective method of FLC removal.
Keywords: Myeloma cast nephropathy, Free light chain, Selective plasma exchange
Introduction
Multiple myeloma is a cancer of plasma cells that produces monoclonal immunoglobulin and invades and destroys adjacent bone tissue [1, 2]. Hypercalcemia, renal failure, anemia, and bone lesions (CRAB) are the four distinctive features of multiple myeloma. Among these factors, renal failure is the most important prognostic factor associated with patient survival [3]. Myeloma cast nephropathy caused by numerous monoclonal free light chains (FLCs) is a major phenotype involving the kidney [4]. A treatment breakthrough occurred with the clinical use of bortezomib as the first proteasome inhibitor. Recent evidence has shown that bortezomib-based chemotherapy with plasma exchange (PE) results in a better outcome, and the authors of these studies emphasized the importance of early induction therapy [5, 6]. However, PE with albumin replacement carried the risk of bleeding after kidney biopsy. In this clinical setting, selective PE using Evacure® (Kawasumi Corp.) [7] may be a useful modality, because it enables the effective maintenance of serum fibrinogen level.
Case report
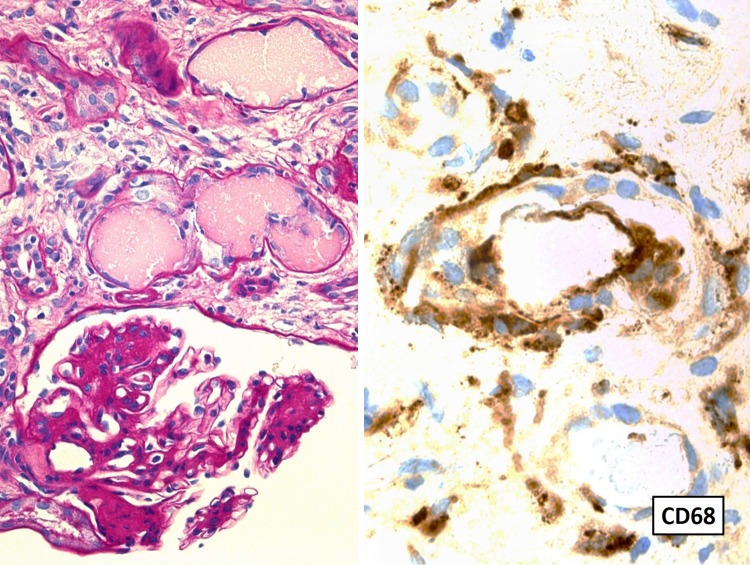
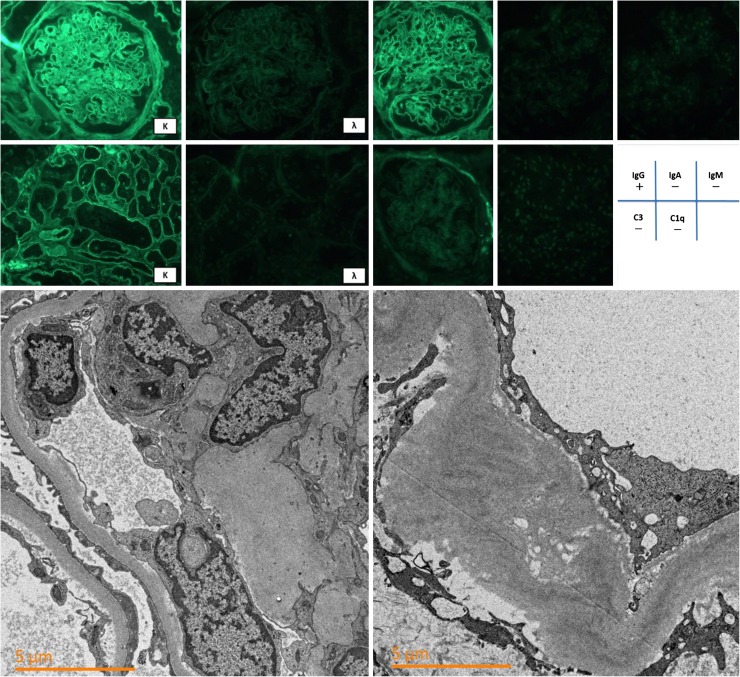
A 61-year-old woman was admitted to our hospital for the evaluation of the chief complaints of general fatigue and appetite loss. She had a 20-year history of type II diabetes mellitus, hypertension, and hyperuricemia. She had started insulin therapy at the age of 50 years, and her diabetic complications included neuropathy and retinopathy, but not nephropathy. She had a serum creatinine level of 0.37 mg/dl and an HbA1c concentration of 7.0 % at the age of 60 years. Five months before admission, her serum calcium and creatinine levels had increased to 12.0 and 0.79 mg/dl, respectively. She had severe anemia, with a hemoglobin level of 7.6 g/dl. Her general fatigue and appetite loss increased gradually, and she was admitted to our hospital. On admission, her height was 155 cm and her body weight had decreased to 47.5 kg (−4.5 kg). Her body temperature was 36.5 °C, and her blood pressure was 130/70 mmHg. Her heart rate was 64/min with regular rhythms. Physical examination showed no remarkable abnormality except for anemic conjunctiva. She had hypercalcemia (s-cCa, 11.8 mg/dl), severe kidney dysfunction (s-creatinine, 2.9 mg/dl), and severe anemia (Hb, 7.0 g/dl). Her serum total protein concentration was 11.4 g/dl, and her albumin level was 3.1 g/dl. In addition, her serum IgG level was 5635 mg/dl, and IgG1 was the predominant subclass (5630 mg/dl; 97.3 %). Serum immunoglobulin electrophoresis showed monoclonality for IgG and κ-FLCs. Her serum κ-FLC level was extremely high (1090 mg/dl). A 24-h urine collection showed dissociation of urine total protein (5.5 g/day) and urine albumin (0.6 g/day). Considering these physical and laboratory examination findings, we strongly suspected multiple myeloma. Subsequent evaluation of the bone marrow showed 53.4 % abnormal plasma cells. To evaluate kidney dysfunction, we performed a kidney biopsy 6 days after admission. Pathological findings showed three distinct features: (1) diffuse interstitial fibrosis and tubular atrophy (85 %), (2) numerous periodic acid-Schiff-negative casts surrounded by macrophages in the distal tubular lumen, and (3) mesangial matrix expansion and mesangial cell proliferation with early nodular lesions (Fig. 1). This case was compatible with myeloma cast nephropathy; however, the glomerular lesions had several differential diagnoses, including amyloid light-chain (AL) amyloidosis, monoclonal immunoglobulin deposition disease (MIDD), proliferative glomerulonephritis with monoclonal immunoglobulin deposition (PGNMID), and diabetic nephropathy. Subsequent immunofluorescence staining showed faint linear and circumferential immunoreactivity for IgG and κ. Congo red staining was negative. Findings for all IgG subclasses (1–4) were negative. Electron microscopy showed no dense deposition in subendothelial spaces, but an average diffuse glomerular capillary basement membrane thickness of 847 nm (Fig. 2). Taken together, these findings led to the diagnosis of the patient with myeloma cast nephropathy and diabetic nephropathy with nodular lesion. The clinical stage of multiple myeloma was IIIB according to the Durie Salmon classification [ 8], and its grade was III according to the international staging system [9].
Fig. 1.
Left panel shows mononuclear inflammatory cell infiltration in the tubulointerstitial area and PAS-negative cast formation in the distal tubular lumen, partly surrounded by mononuclear inflammatory cells. In glomeruli, mesangial cell proliferation and mesangial matrix expansion were evident with focal nodular lesions (magnification, ×200). The right panel shows that the inflammatory cells are predominant for macrophages in the distal tubulus and tubulointerstitial area (CD68-positive cells) (magnification, ×400)
Fig. 2.
Upper panel shows immunoreactivity for κ, λ, IgG, IgA, IgM, C3, and C1q (magnification, ×400). Linear and circumferential faint IgG and κ immunoreactivity were observed along with the glomerular capillaries and tubular basement membranes. The Lower panel shows an electron microscopic image (magnification, ×50,000). No electron-dense deposition is present at the glomerular subendothelial cells. Glomerular basement membrane thickening was predominant and had an average width of 847 nm
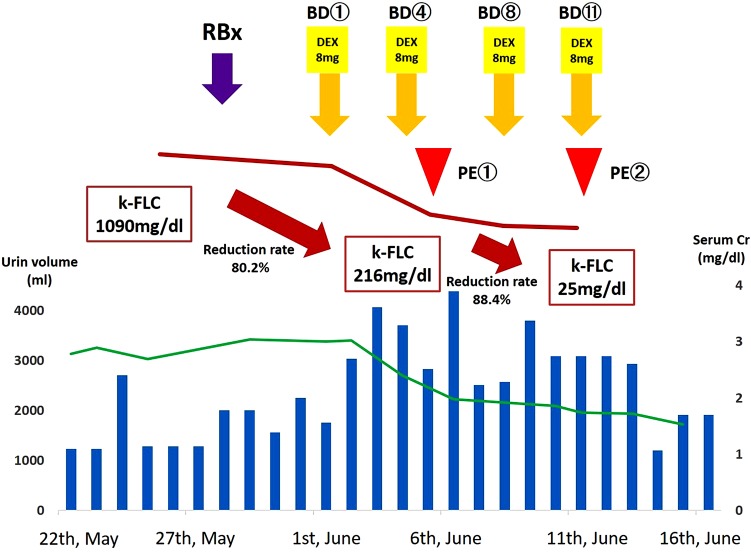
Ten days after admission, we performed combination therapy using bortezomib and dexamethasone at the standard schedule (bortezomib 1.3 mg/m2 on day 1, 4, 8, 11, administered by subcutaneous infusion, and dexamethasone 8 mg/body on day 1, 2, 4, 5, 8, 9, 11, 12 every 21 days). Four days after treatment, the serum κ-FLC level showed a rapid decrease from 1090 to 216 mg/dl. However, as the target FLC level is <50 mg/dl, we additionally performed selective PE using the Evacure® membrane plasma separator (Kawasumi Corp.) and exchange 1.5 plasma volumes using albumin replacement 14 and 21 days, respectively, after admission. We discontinued selective PE only two times because the patient’s serum κ-FLC level was 25 mg/dl and was maintained at <50 mg/dl thereafter. In addition, her serum fibrinogen level was maintained at >200 mg/dl during apheresis. After this therapy, the patient’s urine volume was increased from about 1200 to about 2800 ml/day, and her creatinine level decreased from a maximum of 3.0–1.2 mg/dl in a month (Fig. 3). She was discharged from our hospital and continued bortezomib and dexamethasone in an outpatient setting for a total of four courses; hematopoietic stem cells were then harvested from the patient. Five months after the first admission, high dose of melphalan (70 mg/m2 on day 1, 2) followed by autologous hematopoietic stem-cell transplantation was performed successfully, and her kidney function remained stable (creatinine level, 1.16 mg/dl) thereafter.
Fig. 3.
Clinical course. The horizontal blue bar shows the day-by-day urine volume. The green line graph shows the creatinine level (mg/dl). After the induction of BD and PE, the serum κ-FLC level was decreased significantly. RBx renal biopsy, BD bortezomib and dexamethasone, PE plasma exchange, FLC free light chain
Discussion
Multiple myeloma accounts for 10 % of all hematopoietic tumors. Its estimated incidence is 2–3 patients per 100 thousand people in Japan. Kidney dysfunction is a common complication, occurring in 18–56 % of multiple myeloma cases [10].
Several pathological mechanisms involve the kidney, including those leading to myeloma cast nephropathy, AL amyloidosis, light- and/or heavy-chain deposition disease, and cast-storing histiocytosis. Among these, myeloma cast nephropathy is a major phenotype [1]. A report from the Mayo Clinic concerning 190 cases of multiple myeloma with kidney dysfunction showed that myeloma cast nephropathy comprised the major phenotype (33 %), followed by Randall type MIDD (22 %) and AL amyloidosis (21 %) [4]. In our case, 24-h urine collection showed that the urine total protein (5.5 g/day) to urine albumin (0.6 g/day) ratio was quite low, at 11 % (<25 %), which was strongly suggestive of myeloma cast nephropathy [11]. In addition, her previous serum creatinine was 0.38 mg/dl that is suggestive of hyperfiltration of early diabetic nephropathy. Subsequent kidney biopsy showed the clear presence of myeloma cast nephropathy, but glomerular nodular lesions had several differential diagnoses. Further analysis of Congo red staining ruled out AL amyloidosis. The faint immunoreactivity of IgG and κ-FLCs, along with glomerular capillary findings, suggested that the patient had MIDD or PGNMID. Moreover, detailed analysis by electron microscopy showed no evidence of dense deposition, but very thick glomerular membrane, suggesting diabetic nephropathy. Therefore, we believed that the faint immunoreactivity of IgG and κ-FLCs reflected the insudative change caused by diabetic nephropathy. We confirmed that IgG subclass staining and subdomain (the hinge and CH2 regions of Human IgG1) findings were all negative in this case. Thus, the patient was diagnosed with myeloma cast nephropathy and diabetic nephropathy.
Myeloma cast nephropathy is caused by abundant serum monoclonal FLCs of the κ (25 kDa) or λ (45 kDa) type. Normally, FLCs pass easily through the glomerular capillary and are reabsorbed at the proximal tubulus by the cubilin–megalin complex, located at the proximal apical membrane. When the FLC level exceeds reabsorption ability, excess FLCs reach the distal tubulus. These FLCs bind easily to the Tamm–Horsfall protein at nine amino acids (residues 225–233) in the CDR3 domain [2]. Explanations of the mechanism of kidney dysfunction in myeloma have been based on by two major theories. The first theory involves the endocytosis of FLCs by the cubilin–megalin complex in proximal tubulus-enhanced redox pathways, inducing the NFκB and MAPK pathways. The former is an inflammatory mediator, leading to interleukin (IL)-6, IL-8, and CCL2 activity. The latter is a fibrotic mediator, leading to TGF-β1 activity. The second theory involves the binding of excess FLCs to Tamm–Horsfall protein, which increases intratubular pressure, induces the reduction of the glomerular filtration rate, and enhances the aggregation of macrophages, which promote the inflammatory process [3]. In our case, no electrolytic or glucose imbalance was observed, but the pathological features revealed broad interstitial fibrosis and tubular atrophy, with infiltration of numerous macrophages.
Three different treatment approaches have been considered: (1) elimination or reduction of the FLC burden in patients with myeloma with renal involvement, (2) blockade of the inflammatory pathways that are activated because of FLC toxicity, and (3) blockage of FLC endocytosis. Regarding the third strategy, a recent study conducted by Ying et al. demonstrated that the production of the specific peptide inhibits the binding of FLCs to Tamm–Horsfall protein in a rat cast nephropathy model. This peptide, delivered by intraperitoneal induction, inhibited cast formation in the rat model. However, it is not currently in clinical use. Therefore, the principles of the treatment of myeloma cast nephropathy are the elimination of FLCs and restriction of FLC production using chemotherapy [12]. Patients with multiple myeloma with kidney dysfunction are very likely to develop end-stage renal disease, and the survival rate is quite poor in untreated patients [13]. Recent data showed that survival was better in patients receiving early induction therapy who had recovered kidney function than in those who had not recovered kidney function [14]. A breakthrough occurred with the clinical use of bortezomib as the first proteasome inhibitor. Kidney function recovery was observed in 94 % of 96 cases of previously untreated multiple myeloma within a mean duration of 0.69 months [15]. In addition, Hutchison et al. reported that bortezomib and dexamethasone treatment (<30 and 30–50 ml/min, respectively) recovered kidney function in 113 cases of previously untreated and refractory multiple myeloma. Kidney function recovery rates were 69.5 and 74 %, respectively, in these two groups, and these results were obtained after an average of 2.3 months [5]. Thus, the International Myeloma Working Group recommended bortezomib and dexamethasone administration as first-line therapy for patients with multiple myeloma with kidney dysfunction [16].
Other recent evidence showed that bortezomib-based chemotherapy with PE resulted in a better outcome, and the authors of these studies emphasized the importance of early induction therapy. The guidelines of the American Apheresis Association classify this combined therapy as category II and grade 2B [16]. Randomized controlled trials involving bortezomib and dexamethasone therapy with and without PE are needed. In general, the half-lives of serum FLCs are 3–6 h; however, in patients with prolonged (2–3 days) kidney dysfunction that is considered to be harmful to tubular epithelial cells in the kidney, earlier reduction is needed. More recent studies showed that reduction of serum FLC levels to <80 % in 21 days is correlated with kidney function recovery and patient survival, even in patients with end-stage renal failure [5, 6]. In our case, induction chemotherapy was started 10 days after admission, and the patient showed an early reduction of FLC level from 1090 to 25 mg/dl (97.8 %) and recovery of kidney function from a maximum creatinine level of 3.0–1.6 mg/dl in 21 days.
Considering that PE carries the risk of bleeding due to fibrinogen loss, we performed selective PE using the Evacure® membrane plasma separator (Kawasumi Corp.), which was developed to target IgG. This selective PE enables the removal of small molecules, such as κ-FLCs (25 kDa) and λ-FLCs (45 kDa), with the retention of large molecules, such as fibrinogen (340 kDa). Our case showed rapid FLC reduction without fibrinogen reduction.
In the conclusion, kidney dysfunction in patients with multiple myeloma is an emergent clinical presentation. Early diagnosis and treatment affect kidney function and even patient survival. This case report demonstrates the importance of the early initiation of bortezomib-based chemotherapy with PE in a patient with myeloma cast nephropathy, and this approach enables autologous hematopoietic stem-cell transplantation in patients aged <65 years. Selective PE may be a useful method for FLC reduction without bleeding complication.
Compliance with ethical standards
Conflict of interest
All the authors have declared no competing interest.
Human rights
All procedures performed in studies involving this participant was in accordance with the ethical standards of the institutional and national research committee at which the studies were conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in this study.
References
- 1.Leung N, Rajkumar SV. Renal manifestations of plasma cell disorders. Am J Kidney Dis. 2007;50(1):155–165. doi: 10.1053/j.ajkd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Huang ZQ, Sanders PW. Localization of a single binding site for immunoglobulin light chains on human Tamm–Horsfall glycoprotein. J Clin Invest. 1997;99:732–736. doi: 10.1172/JCI119218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchison CA, Batuman V, Behrens J, International Kidney and Monoclonal Gammopathy Research Group et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8(1):43–51. doi: 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasr SH, Valeri AM, Sethi S, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59(6):786–794. doi: 10.1053/j.ajkd.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison CA, Cockwell P, Stringer S, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22(6):1129–1136. doi: 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364(24):2365–2366. doi: 10.1056/NEJMc1101834. [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo A, Kurashima N, Nakamura A, Miyamoto S, Iimori S, Rai T. Solute removal capacity of high cut-off membrane plasma separators. Ther Apher Dial. 2013;17(5):484–489. doi: 10.1111/1744-9987.12114. [DOI] [PubMed] [Google Scholar]
- 8.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Sohn HJ, Kim S, Kim K, Lee JH, Bang SM, et al. New staging systems can predict prognosis of multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation as first-line therapy. Biol Blood Marrow Transplant. 2006;12:837–844. doi: 10.1016/j.bbmt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, Japan Cancer Surveillance Research Group Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884–91. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 11.Leung N, Gertz M, Kyle RA, et al. Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol. 2012;7:1964–1968. doi: 10.2215/CJN.11161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Investig. 2012;122(5):1777–1785. doi: 10.1172/JCI46490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung N, Gertz MA, Zeldenrust SR, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73(11):1282–1288. doi: 10.1038/ki.2008.108. [DOI] [PubMed] [Google Scholar]
- 14.Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5:e296. doi: 10.1038/bcj.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib–doxorubicin–dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28(30):4635–4641. doi: 10.1200/JCO.2010.28.1238. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28(3):145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]