Abstract
In bucillamine-treated patients, persistent proteinuria caused by membranous nephropathy (MN) is a major adverse effect affecting the kidneys. We experienced a case of acute interstitial nephritis (AIN) with MN caused by bucillamine. An 81-year-old Japanese woman with a past medical history of rheumatoid arthritis and hypertension presented with a fever, epigastric pain, and nausea of 1 week’s duration. She had commenced bucillamine 4 months earlier. At the time of admission, her baseline creatinine (0.8 mg/dl) had risen to 6.8 mg/dl. A renal biopsy revealed AIN with concomitant MN. Renal function gradually improved after bucillamine administration was stopped. In addition to MN, bucillamine can cause AIN, which requires a renal biopsy for definitive diagnosis. Given the host of pathological findings that tend to develop in patients using bucillamine, patients receiving the drug who present with symptoms of acute kidney injury should undergo a renal biopsy to determine the presence of AIN.
Keywords: Acute interstitial nephritis, Bucillamine, Membranous nephropathy, Acute kidney injury
Introduction
Bucillamine is a disease-modifying antirheumatic drug (DMARD) widely prescribed in Asian countries for use against rheumatoid arthritis (RA). Early administration of DMARD is recommended for all RA patients, and therapeutic strategies for RA in Japan often use bucillamine prior to administering methotrexate [1]. While adverse reactions associated with DMARDs are expected in up to 50 % of cases, a higher rate than seen with many other drugs, the mechanism of the adverse reactions is generally understood [2]. In particular, proteinuria caused by membranous nephropathy (MN) is the major renal side effect associated with use of bucillamine [3]. We report herein a rare instance of bucillamine causing acute interstitial nephritis (AIN) with MN. To the best of our knowledge, this is the first report of bucillamine-induced AIN occurring concomitantly with MN in the English literature.
Case report
An 81-year-old Japanese woman with a past medical history of hypertension and RA presented with intermittent fever, epigastric pain, and nausea of 1 week’s duration. Four months prior to her visit to our clinic, the patient had commenced bucillamine for the treatment of RA. A list of her medications is shown in Table 1. Physical examination revealed a blood pressure of 150/70 mmHg, mild tenderness in the epigastric area, and a rash on the lower extremities. An upper endoscopy disclosed gastritis. Urinary tract infection was ruled out by negative blood cultures while bacteriuria from 1 year ago was still evident (Table 2). Laboratory results showed Cr 6.8 mg/dl, BUN 63 mg/dl, eGFR 5 ml/min/1.73 m2, and 17 mEq/l (Table 3).
Table 1.
Medications
| Medication | Initiation |
|---|---|
| Bucillamine 150 mga | 4 months ago |
| Urapidil 60 mg | 10 months ago |
| Doxazosin mesylate 1 mga | 12 months ago |
| Alfacalcidol 0.5 μg | 1.5 years ago |
| Calcium l-aspartate hydrate 200 mg | 1.5 years ago |
| Prednisolone 5 mg | 1.5 years ago |
| Carvedilol 10 mg | 3 years ago |
| Torasemide 2 mga | 4 years ago |
| Amlodipine besilate 5 mg | >5 years ago |
| Bofutsushosan 5 ga | >5 years ago |
| Imidapril hydrochloride 5 mg | >5 years ago |
| Lansoprazole 30 mg | >5 years ago |
| Magnesium oxide 990 mga | >5 years ago |
| Mosapride citrate hydrate 15 mg | >5 years ago |
| Pravastatin sodium 10 mg | >5 years ago |
aDrugs withdrawn on admission
Table 2.
Urinalysis
| pH | 6.0 | WBC | 30–49/HPF |
| Specific gravity | 1.009 | Bacteria | 3+ |
| Osmolarity (mOsm/kg) | 320 | Hyaline casts | 1–4/HPF |
| Protein | + | Epithelial cell casts | <1/HPF |
| Occult blood | ± | β2-microglobulin (μg/l) | 6800 |
| RBC | 1–4/HPF | NAG (U/l) | 15 |
Table 3.
Serum laboratory results
| WBC (/μl) | 3000 | IgG (mg/dl) | 1300 |
| Neut (%) | 52 | IgA (mg/dl) | 220 |
| Eosino (%) | 8.2 | IgM (mg/dl) | 62 |
| Mono (%) | 10 | C3 (mg/dl) | 120 |
| Lympho (%) | 29 | C4 (mg/dl) | 45 |
| RBC (104/μl) | 350 | C1q (mg/dl) | 8.1 |
| Hb (g/dl) | 10 | CH50 (U/ml) | 44 |
| Plt (104/μl) | 23 | ASK | ×40 |
| Na (mEq/l) | 130 | ASO (IU/ml) | 7 |
| K (mEq/l) | 6.0 | ANA | (−) |
| Cl (mEq/l) | 100 | Anti-DNA | (−) |
| Ca (mEq/l) | 9.4 | RF (IU/ml) | 15 |
| UA (mg/dl) | 7.6 | HBs-Ag | (−) |
| BUN (mg/dl) | 63 | HCV-Ab | (−) |
| Cr (mg/dl) | 6.8 | MPO-ANCA | (−) |
| Total protein (g/dl) | 6.8 | PR3-ANCA | (−) |
| Alb (g/dl) | 3.2 | Anti-GBM | (−) |
| Total cholesterol (mg/dl) | 230 | Cryoglobulin | (−) |
| CRP (mg/dl) | 18 |
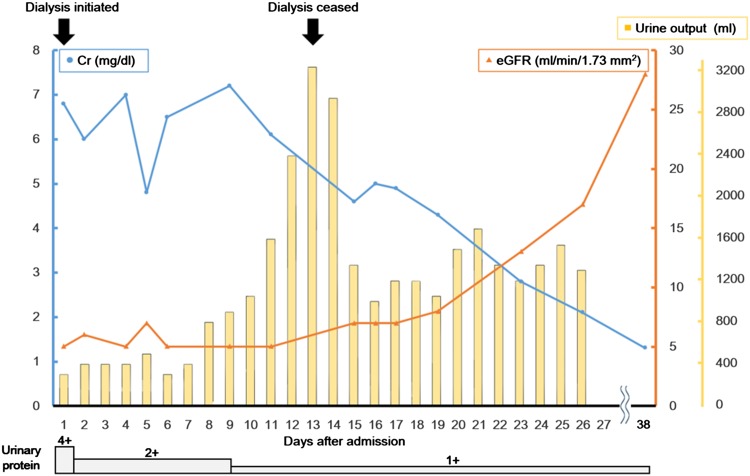
Hemodialysis was initiated on admission, and a renal biopsy was performed on day 4. Histopathological examination of the renal biopsy revealed mixed interstitial inflammatory cell infiltrates consisting of lymphocytes and eosinophils accompanied by tubulitis, suggesting AIN (Fig. 1). Furthermore, mild thickening of the glomerular basement membrane without glomerular hypercellularity was seen. The immunofluorescence staining showing mild granular staining for IgG and C3 along the glomerular capillary walls suggested AIN with concomitant MN (Fig. 2). After the offending drugs were withdrawn, her kidney function gradually improved so that dialysis was discontinued on day 13. The patient was discharged on day 27. At the time of her discharge, her Cr level was 2.1 mg/dl. At outpatient follow-up on day 38, she had a Cr level of 1.3 mg/dl (Fig. 3).
Fig. 1.
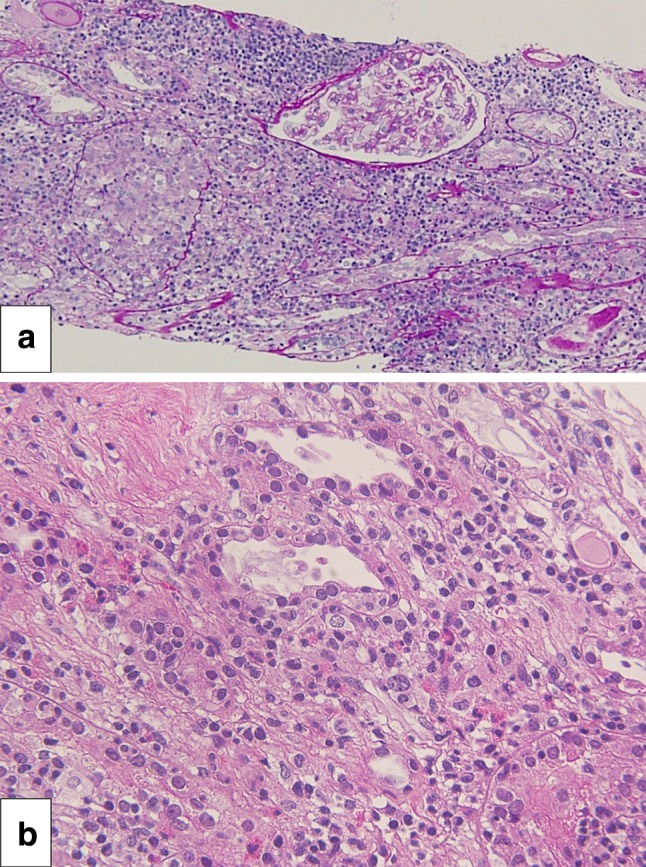
Acute interstitial nephritis. Interstitial inflammatory infiltrates seen in hematoxylin and eosin staining. Original magnification ×100 (a). The infiltrate consists of eosinophils and lymphocytes accompanied by tubulitis (b)
Fig. 2.
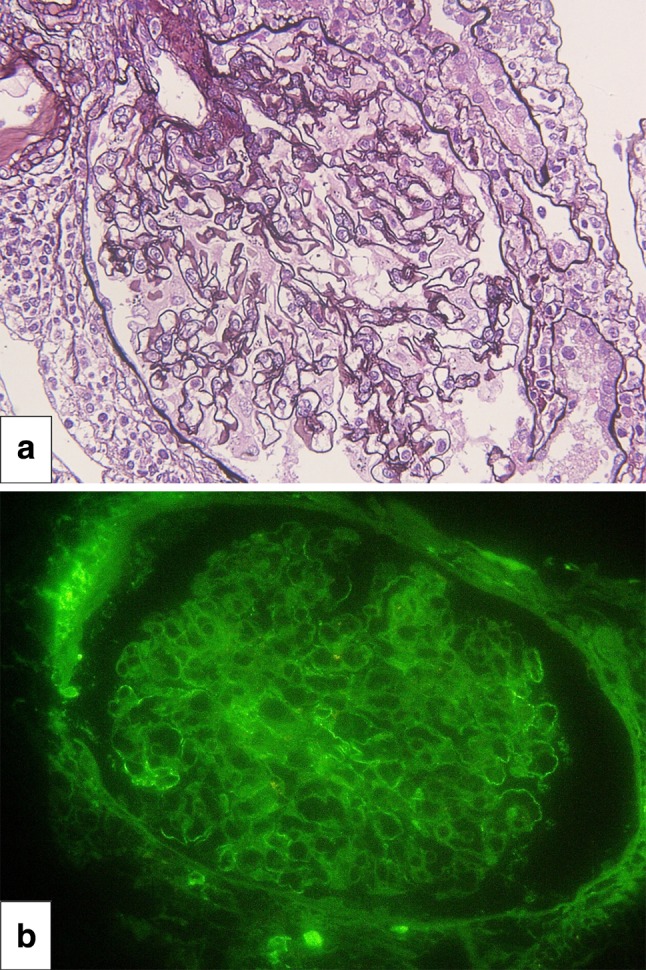
Membranous nephropathy. Periodic acid-methenamine silver staining showing mild thickening of basement membranes suggestive of secondary membranous nephropathy (a). Immunofluorescence staining for IgG shows mild granular immune complex deposition along the basement membranes (b)
Fig. 3.
Clinical course. Figure shows trends in Cr, eGFR, urine output, and urinary protein after admission. Dialysis was initiated on day 1 and discontinued on day 13. Improvement of kidney function was seen as Cr and urinary protein decreased, while urine output and eGFR increased over time. The patient was discharged on day 27, with outpatient follow-up on day 38
Discussion
The clinical course of this patient led to two important conclusions. First, not only MN, which is commonly associated with bucillamine, but also AIN can result from the use of this drug. Second, histological examination of a renal biopsy is instrumental in diagnosing AIN.
Bucillamine is a DMARD frequently prescribed in Japan for moderately active RA. Its efficacy has been demonstrated in a recent review, underscoring the importance of this drug in therapeutic strategies for RA in Japan [1]. However, an analysis of 158 RA patients in Japan found that 49 patients developed MN that was confirmed by biopsy. Of these patients, 40 (82 %) developed symptoms after receiving DMARD therapy, with 27 out of the 40 (68 %) developing symptoms during bucillamine therapy [4]. To date, there have been no reports of bucillamine in association with AIN [5]. The present case is the first report of bucillamine-induced AIN with concomitant MN in the English literature.
Histological examination of a renal biopsy was instrumental in diagnosing bucillamine-induced AIN with MN. The renal histopathology of RA patients varies widely, with MN, mesangial proliferative glomerulonephritis, and amyloidosis occurring frequently among Japanese RA patients [4]. In terms of MN pathology, a number of studies have demonstrated characteristic distribution patterns of glomerular IgG subclass deposits that differentiate idiopathic from secondary MN. In bucillamine-induced MN, glomerular deposition of IgG2 and/or IgG3 components occurs with dense, subepithelial, segmentally distributed deposits visible on electron microscopy. On the other hand, IgG4, the most commonly deposited IgG subclass, is distributed diffusely in idiopathic MN [6]. In the present case, because the quantity of the specimen was insufficient and the fluorescence staining was performed via an indirect assay, the subclasses of IgG could not be determined. The formalin-fixed paraffin-embedded sample was reprocessed and IgG subclass staining by immunohistochemistry method was performed as well, but no visible staining was observed nor were there glomeruli available for electron microscopic observation. A recent report of bucillamine causing rapidly progressive glomerulonephritis [7] suggests that a renal biopsy is crucial in differentiating rapidly progressive glomerulonephritis with AIN in bucillamine users presenting with acute kidney injury (AKI), as these two conditions require different treatments. Recent studies also have report better prognosis with faster time-to-biopsy in patients with AIN [8].
The occurrence of AIN has been reported with many other drugs. Drug-induced AIN is the most common form of AIN reported in up to 75 % of the cases [9]. Antibiotics and nonsteroidal anti-inflammatory drugs are the most frequent causes of drug-induced AIN [10]. As the prevalence of AIN has increased in recent years, and incidence of drug-induced AIN remains high among the elderly, the issue remains a matter of serious concern [11]. In the present case, it is not certain whether the actual cause of membranous nephropathy is bucillamine, as effect of rechallenge was not determined and other in vitro tests such as drug-induced lymphocyte stimulation test were not performed. However, using the causality assessment criteria proposed by the World Health Organization [12] for deciding on the contribution of the drug toward the adverse event, bucillamine would be classified as a likely drug of choice with its clinical event occurring within a reasonable time sequence to its administration. In a reported review of PPI-associated AIN, timing from initiation of drug to presentation with kidney involvement occurred anywhere from 1 week to 9 months, averaging 10 weeks after starting PPI therapy [13]. In this case, the only drug initiated during this time frame is bucillamine, and therefore considered the most likely causative agent. Other possible offending drugs including bofutsushosan (a Chinese herbal mixture), torasemide, imidapril hydrochloride, and magnesium oxide were stopped, although these drugs were unlikely to be causative due to the timing of the symptoms.
Furthermore, as many of the previously reported papers do not include CRP in its review, it is difficult to interpret the high value of CRP upon admission, but is most likely due to AIN per se with possible contribution of a chest contusion from a fall 3 days prior to admission. A review of PPI-induced AIN [14] reports an average of CRP 8 mg/dl, while a case of cimetidine-induced AIN [15] reports a maximum of CRP 34 mg/dl, and a review of tubulointerstitial nephritis and uveitis syndrome [16] reports a maximum CRP of 18 mg/dl. There seems to be no direct significance in test value differences. Following admission, CRP values gradually decreased despite antibiotic treatment with CRP 8 mg/dl on day 5, CRP 3 mg/dl on day 15, and CRP 0.7 mg/dl on day 21. Concerning the natural decrease from a high inflammatory state, an infectious cause seems unlikely, and the patient’s medical history of arthritis seems noncontributory.
In conclusion, bucillamine can cause MN alone or in association with AIN. In the latter case, a renal biopsy is instrumental for reaching the diagnosis. We must be aware of the adverse drug reaction profile of the DMARD family of drugs, MN in particular, and the risk of AIN in bucillamine users with AKI. As milder forms of AKI/AIN are difficult to detect, this condition may be overlooked. As more cases of AIN are likely to occur with an increase in the variety of drugs, further studies are needed to aid the physician in judging which patients require a renal biopsy for evaluation.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Sekiguchi N, Kameda H, Amano K, Takeuchi T. Efficacy and safety of bucillamine, a d-penicillamine analogue, in patients with active rheumatoid arthritis. Mod Rheumatol. 2006;16:85–91. doi: 10.3109/s10165-005-0466-y. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka E, Yamanaka H. DMARDs (disease-modifying antirheumatic drugs) Nippon Rinsho. 2013;71:1199–1206. [PubMed] [Google Scholar]
- 3.Isozaki T, Kimura M, Ikegaya N, et al. Bucillamine (a new therapeutic agent for rheumatoid arthritis) induced nephrotic syndrome: a report of two cases and review of the literature. Clin Investig. 1992;70:1036–1042. doi: 10.1007/BF00180315. [DOI] [PubMed] [Google Scholar]
- 4.Nakano M, Ueno M, Nishi S, et al. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol. 1998;50:154–160. [PubMed] [Google Scholar]
- 5.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60:804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagahama K, Matsushita H, Hara M, Ubara Y, Hara S, Yamada A. Bucillamine induces membranous glomerulonephritis. Am J Kidney Dis. 2002;39:706–712. doi: 10.1053/ajkd.2002.31987. [DOI] [PubMed] [Google Scholar]
- 7.Manabe S, Banno M, Nakano M, et al. Bucillamine-induced membranous nephropathy with crescent formation in a patient with rheumatoid arthritis: case report and literature review. Case Rep Nephrol Dial. 2015;5:30–38. doi: 10.1159/000368826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis. 2014;64:558–566. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Muriithi AK, Leung N, Valeri AM, et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int. 2015;87:458–464. doi: 10.1038/ki.2014.294. [DOI] [PubMed] [Google Scholar]
- 10.Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77:956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 11.Goicoechea M, Rivera F, López-gómez JM. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2013;28:112–115. doi: 10.1093/ndt/gfs143. [DOI] [PubMed] [Google Scholar]
- 12.Edwards R, Aronson J. Adverse drugs reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 13.Brewster UC, Perazella MA. Acute kidney injury following proton pump inhibitor therapy. Kidney Int. 2007;71(6):589–593. doi: 10.1038/sj.ki.5002038. [DOI] [PubMed] [Google Scholar]
- 14.Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006;11(5):381–385. doi: 10.1111/j.1440-1797.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 15.Koarada S, Nagano Y, Sakemi T, Syouno Y, Watanabe T. A case of acute interstitial nephritis and nonoliguria acute renal failure induced by cimetidine. Nihon Jinzo Gakkai Shi. 1992;34(11):1227–1232. [PubMed] [Google Scholar]
- 16.Tan Y, Yu F, Qu Z, et al. Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol. 2011;6(1):93–100. doi: 10.2215/CJN.09051209. [DOI] [PMC free article] [PubMed] [Google Scholar]