Abstract
Neuronal circadian oscillators in the mammalian and Drosophila brain express a circadian clock comprised of interlocking gene transcription feedback loops. The genetic clock regulates the membrane electrical activity by poorly understood signaling pathways to generate a circadian pattern of action potential firing. During the day, Na+ channels contribute an excitatory drive for the spontaneous activity of circadian clock neurons. Multiple types of K+ channels regulate the action potential firing pattern and the nightly reduction in neuronal activity. The membrane electrical activity possibly signaling by changes in intracellular Ca2+ and cyclic adenosine monophosphate (cAMP) regulates the activity of the gene clock. A decline in the signaling pathways that link the gene clock and neural activity during aging and disease may weaken the circadian output and generate significant impacts on human health.
Neurons in the suprachiasmatic nucleus are electrically silent at night but generate action potentials during the day. The ionic and molecular mechanisms driving these rhythmic firing patterns are beginning to be understood.
Neurons in the suprachiasmatic nucleus (SCN) function as part of a central timing circuit that drives daily changes in our behavior and underlying physiology. A hallmark feature of SCN neurons is that they are electrically silent during the night, start firing action potentials near dawn, and then continue to generate action potentials with a slow and steady pace all day long. These rhythms in electrical activity are critical for the function of the circadian timing system. This article reviews what is known about the ionic and molecular mechanisms driving the rhythmic firing patterns in our body’s clock. We raise the hypothesis that a decline in neural activity in the central clock may be a critical mechanism by which aging and disease may weaken the circadian output and contribute to a set of symptoms that impacts human health.
THE IONIC MECHANISMS UNDERLYING NEURAL ACTIVITY RHYTHMS
SCN neurons generate action potentials in the absence of synaptic drive, and can therefore be considered endogenously active neurons. To maintain spontaneous activity, a set of intrinsic currents must interact to depolarize the cell membrane to threshold, elicit an action potential, and return the membrane to negative potentials from which the next spike can be initiated. This “spontaneous” firing arises from specific combinations of intrinsic membrane currents (Bean 2007). Conceptually, it can be useful to divide the ionic mechanisms into, first, currents that are responsible for providing the excitatory drive required for all spontaneously active neurons; second, currents that translate this excitatory drive into a regular pattern of action potentials; and third, currents that are responsible for the nightly silencing of firing because of the hyperpolarization of the membrane (Fig. 1).
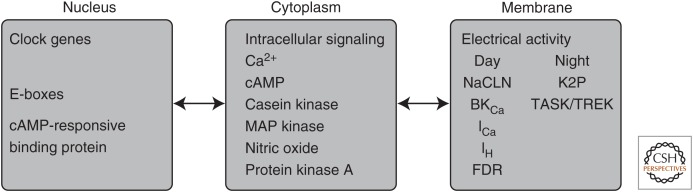
Figure 1.
Cellular signaling domains mediating the generation of circadian rhythms in suprachiasmatic nucleus neurons. Molecular timekeeping signals generated in the nucleus are transmitted via intracellular signaling pathways to alter the activity of ion channels located in the neuronal membrane. Changes in the membrane electrical potential are communicated back to the molecular clock to synchronize and stabilize molecular rhythms. NACLN, Voltage-insensitive nonselective cation channel; cAMP, cyclic adenosine monophosphate; BKCa, large conductance calcium-activated potassium channel; ICa, voltage-gated calcium channels; IH, hyperpolarization-activated channel; K2P, two-pore domain potassium channel; TASK, TWIK (two-pore domain weak inward rectifying potassium channel)-related acid-sensitive K+ channel; TREK, TWIK-related potassium channel; FDR, fast delayed rectifier.
DAILY DEPOLARIZATION
During the day, SCN neurons are more depolarized than neurons that do not show spontaneous activity. They have a resting membrane potential between −50 and −55 mV, which places them close to the threshold for generating an action potential (−45 mV). A subset of SCN neurons may even move to such a depolarized state (−30 mV) during the day that they cannot generate action potentials (Belle et al. 2009). This relatively depolarized resting potential is the result of excitatory drive provided by multiple cation currents (Pennartz et al. 1997; Jackson et al. 2004; Kononenko et al. 2004; Clay 2015). In dissociated SCN neurons, which have been investigated in much detail, the spontaneous interspike depolarization was found to be attributable primarily to sodium (Na+) currents that flow at between −60 and −40 mV (Jackson et al. 2004). Modeling work using data from the action potential clamp also emphasizes the role of the Na+ leak in driving excitability in SCN neurons (Clay 2015). A recent experimental advance has been the description of a voltage-independent Na+ conductance via the NA/NALCN ion channel, which depolarizes both SCN neurons and Drosophila pacemaker neurons (Flourakis et al. 2015). This study represents a significant advance as it provides the first evidence for the circadian regulation of an Na+ current. Also, there are at least three other currents that contribute to the daily depolarization of the SCN neurons including the persistent Na+ current, hyperpolarization-activated (IH) conductance, and voltage-sensitive calcium (Ca2+) currents (see Colwell 2011 for review). In Drosophila, there is also evidence that daily rhythms in inward-rectifier K+ channel expression contributes to daily rhythms in electrical excitability (Ruben et al. 2012).
FREQUENCY MODULATION
In response to the excitatory drive mediated by cation ion channels, SCN neurons show sustained action potential firing for 4–6 h in the subjective day without spike adaptation. The fast delayed rectifier (FDR) K+ current may allow for this type of discharge. Within the SCN, the FDR current is of particular interest because, first, the magnitude of this current shows a circadian rhythm, and, second, sustained pharmacological blockade of the FDR current reduced the amplitude of the daily rhythm in firing in a brain slice preparation (Itri et al. 2005). Two members of this family are expressed in the SCN Kv3.1 (Kcnc1) and Kv3.2 (Kcnc2) and these proteins may be rhythmically regulated. SCN neurons from mice lacking both Kcnc1 and Kcnc2 genes (double knockout, dKO) show reduced spontaneous activity during the day and reduced N-methyl-d-aspartate (NMDA)-evoked excitatory responses during the night (Kudo et al. 2011a). Also, the width of the action potential in SCN neurons is almost doubled in the dKO mice. Thus, the FDR K+ current is necessary for the circadian modulation of electrical activity in SCN neurons and represents an important part of the ionic basis for the generation of rhythmic output. The subthreshold-operating A-type K+ current (IA) is mainly involved in the regulation of neuronal excitability and the timing of action potential firing (Huang 1993; Bouskila and Dudek 1995; Alvado and Allen 2008; Itri et al. 2010). The magnitude of the IA current shows a diurnal rhythm that peaks during the day in the dorsal region of the mouse SCN (Itri et al. 2010). This rhythm is driven by a subset of SCN neurons with a larger peak current and a longer decay constant (Itri et al. 2010). A detailed analysis of SCN neurons in mice deficient in the pore-forming (α) subunits of IA channels Kv1.4 or Kv4.2 α subunits found that these neurons have altered excitability but still show circadian rhythms in repetitive firing (Granados-Fuentes et al. 2012). Finally, Ca2+ activated K+ channels (large conductance potassium channel [BK]) are a major contributor to repolarization of the membrane after an action potential in the SCN. A study in which BK currents were inhibited with iberiotoxin suggests that this current may contribute 40% of the afterhyperpolarization that occurs after an action potential in the SCN (Cloues and Sather 2003). Blocking the BK current (iberiotoxin) can reduce daytime peak firing rate in at least some SCN neurons (Pitts et al. 2006) and genetic manipulation of the BK channel also impacts daytime firing frequency (Montgomery and Meredith 2012; Montgomery et al. 2013). Recent work has found that inactivating BK currents predominate during the day in the SCN. Loss of the β2 subunit eliminates inactivation and decreases daytime firing. Importantly, selective restoration of inactivation via the β2 amino-terminal “ball-and-chain” domain rescues BK current levels and SCN daytime firing rate (Whitt et al. 2016). These studies provide firm evidence that the BK current contributes to the daytime “upstate” in SCN neurons. Therefore, FDR, IA, and BK K+ currents all appear to play a role in the regulation of spontaneous action potential firing in SCN neurons during the day.
NIGHTLY SILENCING
During the night, SCN neurons move into “downstate” in which the membrane potential is hyperpolarized (6–10 mV) and the cells are electrically inactive (De Jeu et al. 1998; Kuhlman and McMahon 2004; Belle et al. 2009). This day–night difference in resting membrane potential is largely mediated by a hyperpolarizing K+-dependent conductance that is active at night at resting membrane potential and inactive during the day (Kuhlman and McMahon 2004, 2006). Resting membrane potentials of neurons are mainly set by a class of two-pore-domain K channels (K2P and TASK/TREK) (Bayliss and Barrett 2008). These K+ channels are active over the whole voltage range (unlike other K+ channels) and are referred to as “leak” or background currents. The K2P channels are coded for by the Kcnk gene family. The transcripts for Kcnk1 and Kcnk2 are expressed in the SCN (Lein et al. 2007), with transcripts for Kcnk1 showing a robust rhythm in the SCN (Panda et al. 2002). Unfortunately, specific pharmacological blockers are not available, but the rhythmic pattern of expression in the SCN makes the K2P channels a promising candidate for driving the nightly hyperpolarization in membrane potential. BK currents are also involved in the nightly silencing of SCN neurons. The expression of Kcnma1, the gene encoding the pore-forming subunit of the BK channel, peaks in the middle of the night (Panda et al. 2002; Meredith et al. 2006; Pitts et al. 2006) and the relative contribution of the BK current to the outward currents is larger in the night than in the day. Also, deletion of Kcnma1 increases nighttime firing in SCN neurons, although it does not completely abolish the day–night difference in firing rate (Meredith et al. 2006; Kent and Meredith 2008). Again, the recent work from Meredith’s group indicates that the kinetic properties of the BK current shifts from day to night (Whitt et al. 2016). During the day, the current is inactivating, whereas during the night this inactivation is lost. The net result is a more sustained BK current during the night, which drives the hyperpolarization.
SCN NEURONAL HETEROGENEITY
SCN neurons are not a homogenous population but show regional variation in the expression of neuropeptides, rhythmic expression of clock genes, responses to light, and neuronal activity. In particular, many SCN neurons do not fire action potential in a circadian manner or have rhythmic expression of clock genes (Albrecht et al. 1997; Shigeyoshi et al. 1997; Yan et al. 1999; Hamada et al. 2001; Yamamoto et al. 2001). In mice, Per1 messenger RNA (mRNA) expression is not uniformly distributed across the SCN but localized in light-sensitive and -insensitive compartments (Albrecht et al. 1997; Shigeyoshi et al. 1997). In the rat SCN, under constant conditions, Per1 and Per2 show strong oscillations in the dorsomedial SCN and weaker oscillations in the ventrolateral SCN (Yan et al. 1999). Similarly in the hamster, Per1 and Per2 mRNA show rhythmic expression primarily in the dorsolateral SCN but not in the compact subnucleus (CBsn) (Hamada et al. 2001).
Rhythmic and nonrhythmic clock components could also underly the functional diversity among individual SCN neurons (Hamada et al. 2001). Using a transgenic mouse in which the expression of green fluorescent protein was driven by the mPer1 promoter, Kuhlman et al. (2000) observed that the molecular clocks of individual neurons are not synchronized. This heterogenous pattern of clock gene expression suggests that SCN circadian output is the result of a complex network of rhythmic and nonrhythmic SCN neurons. To understand the generation of SCN circadian output requires knowledge of the functional properties of these different SCN neuronal populations.
The mean action potential firing frequency of SCN neurons shows a peak at CT6 and nadir near CT18 (Inouye and Kawamura 1979; Green and Gillette 1982). Similarly, Drosophila circadian clock neurons show peak excitability in the morning and trough in the evening (Sheeba et al. 2007; Cao and Nitabach 2008; Cao et al. 2013; Kunst et al. 2014). However, not all SCN neurons fire in a rhythmic pattern and some do not fire action potentials spontaneously (Jobst and Allen 2002). In dispersed cell culture, 25%–50% of rat SCN neurons do not display a circadian rhythm in spontaneous firing rate (SFR) (Welsh et al. 1995; Bina et al. 1998). In organotypic SCN cultures, 87% of dorsal SCN neurons showed a circadian rhythm in firing rate, whereas in the ventral SCN, only 62% of neurons were rhythmic (Nakamura et al. 2001). Also, in organotypic SCN neurons, Herzog et al. (1997) observed a group of neurons that fired in antiphase to neighboring neurons, suggesting a multiplicity of firing phenotypes (Herzog et al. 1997). Interestingly, this out-of-phase pattern is similar to that observed in the per-GFP mouse and in our studies of the intracellular Ca2+ rhythms (Kuhlman et al. 2000; Ikeda et al. 2003). To date, the neurophysiological rhythmicity of only a limited number of phenotypically identified SCN neuronal types has been investigated. In the rat, vasopressin neurons, which are primarily located in the dorsomedial region, show a circadian rhythmicity in SFR (Pennartz et al. 1998; Schaap et al. 1999). In contrast, calbindin-expressing SCN neurons fire action potentials in a rhythmic manner (Jobst and Allen 2002). Similarly, gastrin-releasing peptide (GRP)-expressing neurons do not show a circadian pattern of clock gene expression, suggesting they may not fire action potentials in a circadian pattern (Drouyer et al. 2010). The circadian output is the combination of rhythmically and nonrhythmically firing neurons present in individual compartments of the SCN. It is important, therefore, that the action potential firing patterns of additional groups of phenotypically identified SCN neurons be described.
ACTION POTENTIAL ACTIVATION OF SIGNALING PATHWAYS
Currently, the identity of the signaling pathways mediating the interactions between membrane potential and clock gene expression are largely unknown and their identity remains a key unanswered question in circadian neurobiology. Of particular interest is whether the circadian pattern of action potential firing is an output of the circadian clock or an integral component of the molecular timekeeping system. When an SCN neuron fires an action potential, the resulting sequence of membrane depolarization and hyperpolarization alters the activity of multiple types of voltage-dependent ion channels (Jackson et al. 2004). One of the most important of these is the voltage-gated Ca2+ channel, which, when opened, will allow Ca2+ to enter the neuron and increase the intracellular Ca2+ concentration. Ca2+ is a widely used signaling molecule linking membrane electrical activity to intracellular signaling pathways. The magnitude and duration of the Ca2+ signal is directly related to the action potential firing frequency (Irwin and Allen 2007). Therefore, the tetrodotoxin (TTX)-sensitive intracellular Ca2+ levels are higher during the day than during the night, secondary to the SCN neurons firing action potentials, which are faster during the day than during the night (Colwell 2000; Irwin and Allen 2007). Altering the membrane potential or inhibiting Ca2+ entry into the neuron modifies clock gene activity. An early study found that TTX infusion in the rat SCN eliminated free-running locomotor rhythms, but without altering clock phase on restoration of rhythms following cessation of TTX infusion, suggesting that TTX did not interfere with the timekeeping mechanism itself (Schwartz et al. 1987). However, more recently it was shown that TTX treatment of SCN organotypic slice cultures caused severe damping of circadian gene transcription rhythms and disruption of clock phase (Yamaguchi et al. 2003). In addition, lowering membrane potential, lowering extracellular Ca2+, or inhibiting Ca2+ entry with Ca2+ channel antagonists inhibits the rhythmic expression of the clock genes per1 and per2 in cultured SCN neurons (Lundkvist et al. 2005). These data are consistent with the extracellular Ca2+ that enters the neuron being required for the action of the circadian clock (McMahon and Block 1987; Lundkvist and Block 2005; Lundkvist et al. 2005). In addition, SCN neurons also express a TTX-insensitive Ca2+ rhythm dependent on Ca2+ release from intracellular stores (Fig. 2A) (Ikeda et al. 2003). The relationship between molecular and electrical circadian rhythms was recently examined with optogenetic manipulation of SCN firing rate with bioluminescence imaging (Jones et al. 2015). Manipulating firing rate reset circadian rhythms both ex vivo and in vivo, and this resetting required action potentials. These findings add weight to the suggestion that SCN firing rate is fundamental to circadian pacemaking.
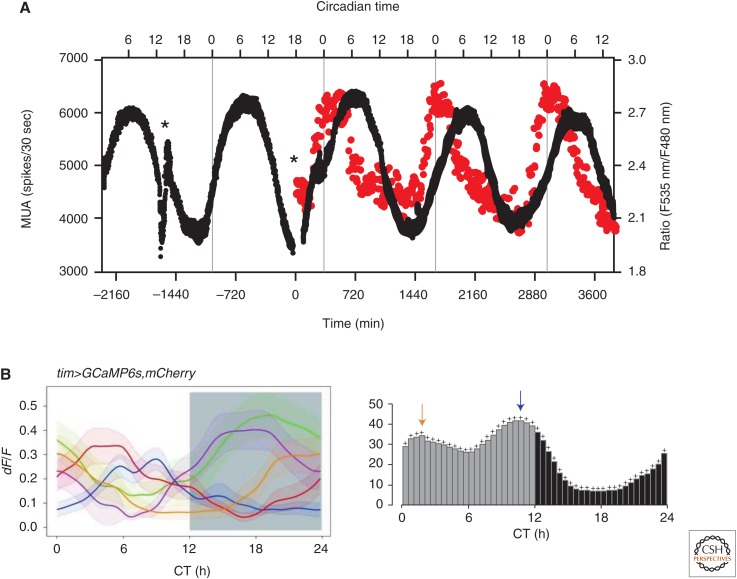
Figure 2.
Intracellular calcium rhythms are present in mouse and Drosophila pacemaker neurons. (A) Long-duration intracellular calcium levels recorded cameleon expressed in a ventral SCN neuron (red dots). Simultaneous recording of multiunit activity was performed using a multielectrode array. The peak of the calcium rhythm of neuron preceded the peak of the multiunit activity by 6 h. Asterisks indicate the timing of the culture medium exchange, during which the firing frequency became temporarily unstable. (Panel A from Ikeda et al. 2003; reprinted, with permission, from Elsevier © 2003.) (B) Average calcium transients in five identified groups of circadian pacemaker neurons in the Drosophila brain. The calcium transients were measured using GCaMP6s. Note that the calcium levels peak at different times of the day in different neuron populations. (Panel B from Liang et al. 2016; reprinted, with permission, from AAAS © 2016.)
Interestingly, similar roles for membrane activity and Ca2+ signals in circadian timekeeping have been established in circadian pacemaker neurons in the Drosophila brain (Fig. 2B). Electrical silencing of fly pacemaker neurons via transgenic expression of the hyperpolarizing potassium channel Kir2.1 stops free-running per and tim rhythms, while leaving diurnal molecular rhythms intact (Nitabach et al. 2002). This genetic manipulation was shown to both eliminate action potentials and hyperpolarize the plasma membrane to ∼−80 mV, near the reversal potential for potassium (Wu et al. 2008). Intracellular Ca2+ signaling mediated through calmodulin and CaMKII plays a key role in setting the pace of the Drosophila circadian timekeeping system (Harrisingh et al. 2007). A more recent study showed that adult-specific expression of Kir2.1, using a temporally controllable transgenic expression system, eliminated free-running rhythmic locomotor rhythms without stopping molecular timekeeping rhythms (Depetris-Chauvin et al. 2011). However, the relevance of this to the earlier results obtained with constitutive Kir2.1 expression during both development and adulthood (Nitabach et al. 2002) is rendered uncertain by the failure to confirm that the adult-specific expression of Kir2.1 was at a level sufficient to not only block action potentials, but also to hyperpolarize the plasma membrane to ∼−80 mV. This distinction is expected to be very important, given the key role for Ca2+ in cellular timekeeping and the likelihood that low-voltage activated Ca2+ channels are activated by synaptic inputs even in the absence of action potentials.
Neuronal gene expression, mediated by Ca2+-dependent signaling pathways, is activated by the membrane electrical activity changes produced by synaptic transmission (Impey et al. 1998, 1999). L-type channels appear to play a critical role in signaling neuronal activity to the nucleus to alter gene expression (Bading et al. 1993; Hardingham et al. 1999; Zhang et al. 2006). For example, in the hippocampus, activation of L-type Ca2+ channels is required for cyclic adenyl monophosphate (cAMP)-response element-binding (CREB)-responsive element (CRE)-mediated gene expression (Bading et al. 1993). CRE activation is thought to be a key step in light-induced changes of circadian clock phase (Ginty et al. 1993). A significant portion of the increased intracellular Ca2+ that follows an action potential in SCN neurons flows through open L-type Ca2+ channels (De Jeu et al. 1998; Jackson et al. 2004; Irwin and Allen 2007). Depolarization of cerebellar granule cells induced Per1 but not Per2 expression, and inhibition of L-type Ca2+ channels reduced the Per1 expression (Akiyama et al. 2001). Nimodipine, an L-type Ca2+ channel antagonist, significantly reduced the amplitude of Per1 rhythms but did not completely block them (Lundkvist et al. 2005). In Drosophila, electrical hyperexcitation or silencing induced by ectopic ion channel expression stabilizes the transcriptional state of pacemaker neurons in the “morning” or “evening” states, respectively (Mizrak et al. 2012).
Ca2+ acts as a second messenger by activating a number of intracellular signaling pathways, including adenyl cyclases and Ca2+-dependent kinases. Ca2+ modulation of adenyl cyclase activity is one possible Ca2+-mediated signaling pathway linking membrane electrical activity to molecular clock gene activity. cAMP is an important component of the circadian clock in addition to being a key regulator of the synchrony of individual SCN neurons and nonphotic entrainment (O’Neill et al. 2008). O’Neill et al. (2008) observed that reducing or increasing cAMP levels reduces rhythmic Per2 expression (O’Neill et al. 2008). cAMP can phase shift the circadian clock, an effect that is mediated by CREB (Kako et al. 1997; Tischkau et al. 2003). Three adenyl cyclase isoforms (AC1, AC3, AC8) are activated by Ca2+ in a calmodulin-dependent manner, whereas two isoforms (AC5, AC6) are inhibited by Ca2+ in a calmodulin-independent manner (Sunahara and Taussig 2002).
In this signaling pathway, Ca2+ binds to and activates calmodulin, which then activates adenyl cyclase increasing the production of cAMP (Fukushima et al. 1997). Whereas adenylyl cyclase II is the predominant cyclase in the SCN adenylyl cyclases I and III have also been found in the SCN Allen Brain Atlas (Fukuhara et al. 2004). All three isoforms are activated by Gsα with ACI and ACIII additionally activated by Ca2+-calmodulin (Sunahara and Taussig 2002). Of particular interest in this type of model is adenylyl cyclase isoform-1. Peptide receptors for vasoactive intestinal peptide (VIP) and GRP, which are believed to be critical for synchronization of individual SCN neurons, activate Gs-type G proteins. Current models suggest that G proteins mediate VIP synchronization of neuronal oscillators. Consistent with this mechanism, the synchronizing effects of VIP are blocked by cholera toxin (Aton et al. 2006). VIP acts with high affinity on VPAC2 receptors, which are coupled to Gs-type G proteins (Laburthe et al. 2002; Dickson et al. 2006). Activation of VPAC2 receptors will increase adenylyl cyclase activity and intracellular cAMP concentrations (Rea 1990; Laburthe et al. 2002). However, AC1 is not activated by Gs subunits alone but by Gs and an increase of intracellular Ca2+. These isoforms of adenyl cyclase are frequently located close to the voltage-gated Ca2+ channels so they can respond rapidly to the Ca2+ entering the cell through the open channel. Thus, the AC1 serves as a coincidence detector coupling action potential firing to activation of Gs-coupled receptors.
Regulators of G protein signaling (RGS) are a family of proteins that increase the rate of guanosine triphosphate (GTP) hydrolysis by GTPases to turn off G protein–mediated signaling pathways. In a well-described case, the levels of RGS16, which inactivates Giα, increase at a circadian time to produce a time-dependent elevation of intracellular cAMP signaling in the SCN. Deletion of the Rgs16 gene eliminates the circadian rhythm of cAMP generation and lengthens circadian period. The loss of the cAMP signal disrupts the phase relationship between neurons in the dorsomedial ventrolateral SCN (Doi et al. 2011). Also, knocking out the RGS16 reduces the food-anticipatory behavior observed when feeding is restricted to the daytime (Hayasaka et al. 2011). RGS4 regulates melatonin activity mediated by type 1 melatonin receptors (MT1) in the pars tuberalis and could mediate melatonin signaling in the SCN (Dupre et al. 2011).
In Drosophila, the neuropeptide pigment dispersing factor (PDF) plays a similar role in coordinating oscillation between circadian clock neurons (Renn et al. 1999; Lin et al. 2004; Stoleru et al. 2004, 2005, 2007; Choi et al. 2009, 2012), and its receptor PDFR also signals through Gsα and ACIII or ACVIII (Hyun et al. 2005; Lear et al. 2005; Mertens et al. 2005; Duvall and Taghert 2012, 2013). Interestingly, PDFR signaling through ACIII is limited to one subset of circadian clock neurons, while signaling through ACVIII in another (Duvall and Taghert 2013). PDFR activation in a target cell induces two parallel signaling pathways downstream of cAMP: protein kinase A (PKA)-dependent regulation of the clock component TIMELESS and PKA-independent regulation of target neurons electrical excitability (Seluzicki et al. 2014). Perhaps related to the independence of these two pathways, it was recently shown that Drosophila circadian clock neurons with the same phase of molecular oscillation can show very different phases of intracellular Ca2+, and their specific phase relationships are determined by PDF/PDFR signaling (Liang et al. 2016).
HOW THE MOLECULAR CLOCKWORK REGULATES NEURAL ACTIVITY?
The clearest links between the molecular clockwork and neural activity comes from several studies that have explored the impact of mutations in the core clockwork on electrical activity rhythms. The Tau (casein kinase 1ɛ) mutation in hamsters shortens the period of wheel-running activity and neural activity rhythms (Liu et al. 1997). Similarly, homozygote Clock mutant mice are behaviorally arrhythmic, whereas heterozygote animals show lengthened behavioral rhythms, findings that are paralleled by physiological recordings from the SCN (Herzog et al. 1998; Nakamura et al. 2002). Furthermore, Cry1/2 double mutants show behavioral arrhythmicity and loss of rhythms in SCN neural activity (Albus et al. 2002). In Drosophila period null-mutant flies, which are behaviorally arrhythmic, the daily rhythm of excitability of pacemaker neurons is also eliminated (Cao and Nitabach 2008). Even outside of central clocks, neurons in the medial habenula express rhythms in electrical activity that could be measured in a brain slice and these rhythms are lost in the Cry1/2 dKO (Sakhi et al. 2014a,b); although, in the hippocampus, the loss of Per2 did not alter excitability of CA1 neurons (Wang et al. 2009). Still, the finding in the habenula raises the possibility that the molecular clock may influence the membrane properties of neurons throughout the nervous system. Together, these studies provide clear evidence that the molecular clockwork can drive neural activity as an output.
Some of the mechanisms by which the molecular clockwork drives rhythms in neural activity are beginning to be identified. The expression of the transcripts for the clock genes Per1 and Per2 peak in the midday (CT 4–6) in the SCN, a little before the peak in neural activity. So, at dawn, when electrical activity is rising, CLOCK/BMAL are starting to drive transcription of Per and Cry genes. A critical issue is whether blocking this increase in Per or Cry transcripts would alter the increase in neural activity. A recent study transiently and selectively decreased levels of PER1 through use of an antisense oligodeoxynucleotide. This treatment effectively reduced SCN neural activity and altered the BK current (Kudo et al. 2015). In addition, work in Drosophila suggests that CRY can alter membrane potential through a redox-based regulation of a K+ channel conductance (Fogle et al. 2011). In the SCN, the redox state also regulates excitability through modulation of multiple potassium currents (Wang et al. 2012). In addition, the molecular clockwork is a potent regulator of transcription and there is evidence of the rhythmic transcription of several ion channels, including L- and T-type Ca2+ channels, BK channels, and K2P K+ channels and the Na+ leak current (Panda et al. 2002; Colwell 2011). The genes encoding these channels could be under regulation from the Clock/BMAL protein complex that acts directly on E-box or other elements present in the regulatory sequences of these genes. To provide one example in mammals, the expression of one of the genes coding for L-type Ca2+ channels (Cav1.2, Cacna1c) is rhythmic (peaking during the late night) and is regulated by the circadian clock component REV-ERBα (Schmutz et al. 2014). In Drosophila, circadian rhythms in mRNA encoding a regulatory protein associated with BK channels have been described (McDonald and Rosbash 2001; Duffield 2003). A recent study from the Allada laboratory (Flourakis et al. 2015) showed that the circadian clock regulates excitability via an Na+ leak conductance (as described above). This sodium leak current depends on the expression of nonspecific cross-reacting antigen (NCA) localization factor 1. This localization factor NFL-1 is under clock control and so this work provides a concrete mechanism by which the molecular clock can regulate membrane excitability.
Besides transcriptional regulation and circadian trafficking of membrane proteins, posttranslational modifications of channels and associated auxiliary proteins are perhaps the most likely explanation for the circadian variation in the electrical activity of SCN neurons. In chick photoreceptors, circadian oscillations in the expression of cone cGMP-gated channels have been well described (Ko et al. 2004). Recent work from Meredith’s group indicates that the kinetic properties of the BK current shifts from day to night (Whitt et al. 2016). During the day the current is inactivating, whereas during the night this inactivation is lost. The net result is a more sustained BK current during the night, which drives the hyperpolarization. This work clearly implicates the regulation of the β2 subunit as a driver of the rhythm in SCN firing but the levels of the β2 subunit appears to be stable through time. So this suggests that the circadian clock regulation of the subunit is through posttranslational regulation. Within the SCN, there is clear evidence for a daily rhythm in the levels of cAMP and Ca2+ (reviewed in Colwell 2011). More recent work used fluorescence/bioluminescence imaging to visualize GCaMP3-reported circadian oscillations of intracellular Ca2+ alongside activation of Ca2+/cAMP-responsive elements (Brancaccio et al. 2013). This work illustrates that key intracellular signaling pathways that are known to alter neural excitability are rhythmically regulated within the SCN. The daily rhythms in these signaling pathways would be expected to drive daily rhythms in the activity of ion channels and membrane currents.
EFFECTS OF AGING AND DISEASE ON NEURAL ACTIVITY
Disruptions in the circadian system are commonly associated with aging (Duffy et al. 2015). Low amplitude, fragmented locomotor activity has been documented in humans (Czeisler et al. 1992; Oosterman et al. 2009), nonhuman primates (Zhdanova et al. 2011), rodents (Farajnia et al. 2012), and insects (Giebultowicz and Long 2015). Although undoubtedly many factors contribute to these changes, a variety of data is emerging that is consistent with the hypothesis that an age-related decline in the neural output of the central circadian clock may be key. Several studies have shown the electrophysiological activity of aged SCN neurons in vitro is altered (Satinoff et al. 1993; Watanabe et al. 1995; Aujard et al. 2001; Nygard et al. 2005; Biello 2009). To provide one example, in vivo multiunit recordings were performed from the SCN and a brain region that receives robust innervation from the SCN (subparaventricular zone, SPZ) in freely moving animals. The amplitude of the day–night difference in neural activity was substantially reduced in both brain regions of middle-aged mice (10–12 mo) (Nakamura et al. 2011). Another striking feature was the increase in variation in the levels of the spontaneous activity. The mechanisms underlying the age-related decline in SCN neural activity are starting to be identified. The fast delayed rectifier K+ current and also the transient A-type K+ current (described above) lost their circadian modulation in aged SCN neurons (>2 years) (Farajnia et al. 2012). In addition, the circadian modulation of BK channel activity was lost because of a reduction in BK currents during the night. This reduced current diminished the after-hyperpolarization, depolarized the resting membrane potential, widened the action potential, and increased calcium in aged SCN neurons (Farajnia et al. 2012).
Evidence for age-related disruption of circadian oscillations of clock genes in the SCN has been equivocal. Some studies found robust rhythms in the expression of Period genes in old SCN neurons (Asai et al. 2001), whereas other studies report age-related disruption in Per2 mRNA (Weinert et al. 2001) as well as Clock and Bmal1 mRNA (Kolker et al. 2003; Wyse and Coogan 2010). A recent study found a reduction of sirtuins (SIRT1) expression together with a reduction in BMAL1 and CLOCK proteins in the SCN of old mice (Chang and Guarente 2013). SIRT1 activates the transcription of Clock and Bmal1 and SIRT1-deficient young mice showed age-typical changes in circadian behavior such as fragmented activity and reduced entrainment capacity. In our own hands, the molecular clockwork in the SCN as measured by PERIOD2 levels was not disrupted in middle-aged mice held in a light–dark (LD) cycle (Nakamura et al. 2011). However, when these mice were placed in constant dark (DD) for at least 2 weeks, differences in the bioluminescence rhythms (PER2::LUC) emerged (Nakamura et al. 2015). In our view, the reduction in the amplitude of the circadian timing signal produced by the central clock will result in a weakening in the control of peripheral oscillators as well as a decrease in amplitude and precision of daily rhythms in physiology and behavior. Support for this model comes from work in Drosophila (Luo et al. 2012; Gill et al. 2015), which also found that the molecular central clock continues to function during aging even as the outputs weaken.
Patients suffering from neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) commonly show sleep disorders. These patients have difficulty sleeping at night and staying awake during the day. These symptoms have a major impact on the quality of life of the patients and on their caregivers. There is some evidence linking these timing problems to the central clock. In humans, prior work by Swaab and colleagues has shown that there is SCN pathology in aging and neurodegenerative diseases (e.g., Swaab et al. 1985; Zhou et al. 1995; van Wamelen et al. 2013). One recent study reported that the circadian rhythm amplitude of motor activity in both AD subjects and age-matched controls is correlated with the number of VIP-expressing SCN neurons (Wang et al. 2015). AD was additionally associated with delayed circadian phase compared to cognitively healthy subjects. Mouse models of neurodegenerative disorders are used to determine whether SCN physiology is impacted by these disease processes (Fig. 3). In both HD and PD models, the SCN neural activity during the daytime peak is reduced (Kudo et al. 2011a,b, 2014). Behaviorally, these models show low-amplitude fragmented rhythms, which resemble those seen in the aging mouse. Together, this data in mouse models of AD, HD, and PD in combination with the clinical symptoms in humans raises the possibility that a weakening of circadian neural output is a core feature of neurodegenerative diseases.
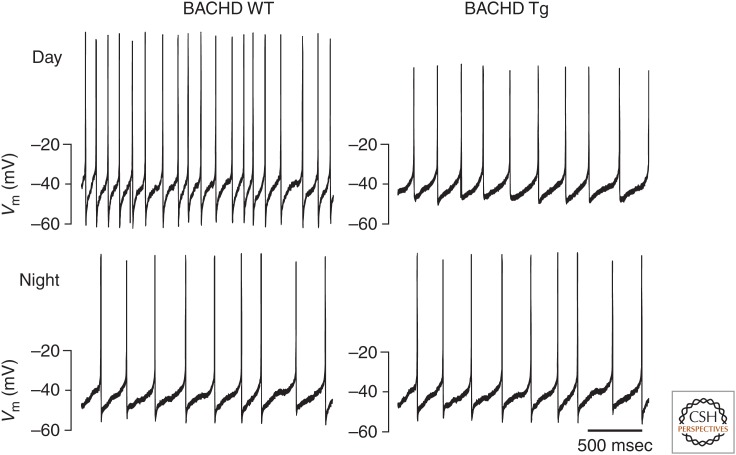
Figure 3.
Suprachiasmatic nucleus (SCN) neuronal activity in a mouse model of Huntington’s disease. The spontaneous action potential firing frequency is reduced in the SCN of mice expressing the entire human huntingtin gene (HTT) with 97 mixed CAA-CAG repeats (BACHD). The current-clamp recording technique was used in the cell-attached configuration to record the spontaneous firing rate (SFR) of dorsal SCN neurons during the day (ZT4-6, top row) and night (ZT16-18, bottom row). The reduced excitability of SCN neurons in the BACHD mice are consistent with the hypothesis that reduced circadian output signals from the SCN contribute to the phenotypes observed in the BACHD mice.
ACKNOWLEDGMENTS
The work is supported by National Institutes of Health (NIH) Grants R01 NS036607 to C.N.A. and National Institute of Neurological Disorders and Stroke (NINDS) R01NS091070 and National Institute of General Medical Sciences (NIGMS) R01GM098931 to M.N.N.
Footnotes
Editors: Paolo Sassone-Corsi, Michael W. Young, and Akhilesh B. Reddy
Additional Perspectives on Circadian Rhythms available at www.cshperspectives.org
REFERENCES
- Akiyama M, Minami Y, Nakajima T, Moriya T, Shibata S. 2001. Calcium and pituitary adenylate cyclase-activating polypeptide induced expression of circadian clock gene mPer1 in the mouse cerebellar granule cell culture. J Neurochem 78: 499–508. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC. 1997. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, Meijer JH. 2002. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol 12: 1130–1133. [DOI] [PubMed] [Google Scholar]
- Alvado L, Allen CN. 2008. Tetraethylammonium (TEA) increases the inactivation time constant of the transient K+ current in suprachiasmatic nucleus neurons. Brain Res 1221: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. 2001. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66: 1133–1139. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. 2006. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci 103: 19188–19193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujard F, Herzog ED, Block GD. 2001. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106: 255–261. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. 1993. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181–186. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Barrett PQ. 2008. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. 2007. The action potential in mammalian central neurons. Nat Rev 8: 451–465. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. 2009. Daily electrical silencing in the mammalian circadian clock. Science 326: 281–284. [DOI] [PubMed] [Google Scholar]
- Biello SM. 2009. Circadian clock resetting in the mouse changes with age. Age (Dordr) 31: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina KG, Rusak B, Wilkinson M. 1998. Daily variation of muscarinic receptors in visual cortex but not suprachiasmatic nucleus of Syrian hamsters. Brain Res 797: 143–153. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. 1995. A rapidly activating type of outward rectifier K+ current and A-current in rat suprachiasmatic nucleus neurones. J Physiol (Lond) 488: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon AS, Hastings MH. 2013. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron 78: 714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. 2008. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci 28: 6493–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Platisa J, Pieribone VA, Raccuglia D, Kunst M, Nitabach MN. 2013. Genetically targeted optical electrophysiology in intact neural circuits. Cell 154: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L. 2013. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153: 1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Fortin JP, McCarthy E, Oksman L, Kopin AS, Nitabach MN. 2009. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol 19: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Cao G, Tanenhaus AK, McCarthy EV, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JC, Nitabach MN. 2012. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep 2: 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay JR. 2015. Novel description of ionic currents recorded with the action potential clamp technique: Application to excitatory currents in suprachiasmatic nucleus neurons. J Neurophysiol 114: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. 2003. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci 23: 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. 2000. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci 12: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. 2011. Linking neural activity and molecular oscillations in the SCN. Nat Rev 12: 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sanchez R, Rios CD, Ronda JM. 1992. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 340: 933–936. [DOI] [PubMed] [Google Scholar]
- De Jeu M, Hermes N, Pennartz C. 1998. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. NeuroReport 9: 3725–3729. [DOI] [PubMed] [Google Scholar]
- Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF. 2011. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol 21: 1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson L, Aramori I, Sharkey J, Finlayson K. 2006. VIP and PACAP receptor pharmacology: A comparison of intracellular signaling pathways. Ann NY Acad Sci 1070: 239–242. [DOI] [PubMed] [Google Scholar]
- Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, Kimura I, Tsuchiya S, Kori H, Seo K, et al. 2011. Circadian regulation of intracellular G protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun 2: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouyer E, LeSauter J, Hernandez AL, Silver R. 2010. Specializations of gastrin-releasing peptide cells of the mouse suprachiasmatic nucleus. J Comp Neurol 518: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield GE. 2003. DNA microarray analyses of circadian timing: The genomic basis of biological time. J Neuroendocrinol 15: 991–1002. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zitting KM, Chinoy ED. 2015. Aging and circadian rhythms. Sleep Med Clin 10: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre SM, Dardente H, Birnie MJ, Loudon AS, Lincoln GA, Hazlerigg DG. 2011. Evidence for RGS4 modulation of melatonin and thyrotrophin signalling pathways in the pars tuberalis. J Neuroendocrinol 23: 725–732. [DOI] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. 2012. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol 10: e1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. 2013. E and M circadian pacemaker neurons use different PDF receptor signalosome components in Drosophila. J Biol Rhythms 28: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, Vanderleest HT, Houben T, Rohling JH, Ramkisoensing A, Yasenkov R, Meijer JH. 2012. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci 32: 5891–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flourakis M, Kula-Eversole E, Hutchison AL, Han TH, Aranda K, Moose DL, White KP, Dinner AR, Lear BC, Ren D, et al. 2015. A conserved bicycle model for circadian clock control of membrane excitability. Cell 162: 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. 2011. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 331: 1409–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC, Storm DR, Iuvone PM, Tosini G. 2004. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci 24: 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Shimazoe T, Shibata S, Watanabe A, Ono M, Hamada T, Watanabe S. 1997. The involvement of calmodulin and Ca2+/calmodulin-dependent protein kinase II in the circadian rhythms controlled by the suprachiasmatic nucleus. Neurosci Lett 227: 45–48. [DOI] [PubMed] [Google Scholar]
- Giebultowicz JM, Long DM. 2015. Ageing and circadian rhythms. Curr Opin Insect Sci 7: 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, Panda S. 2015. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347: 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. 1993. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260: 238–241. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Norris AJ, Carrasquillo Y, Nerbonne JM, Herzog ED. 2012. IA channels encoded by Kv1.4 and Kv4.2 regulate neuronal firing in the suprachiasmatic nucleus and circadian rhythms in locomotor activity. J Neurosci 32: 10045–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Gillette R. 1982. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res 245: 198–200. [DOI] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. 2001. Expression of Period genes: Rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci 21: 7742–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H. 1999. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron 22: 789–798. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. 2007. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci 27: 12489–12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka N, Aoki K, Kinoshita S, Yamaguchi S, Wakefield JK, Tsuji-Kawahara S, Horikawa K, Ikegami H, Wakana S, Murakami T, et al. 2011. Attenuated food anticipatory activity and abnormal circadian locomotor rhythms in Rgs16 knockdown mice. PLoS ONE 6: e17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Geusz ME, Khalsa SBS, Straume M, Block GD. 1997. Circadian rhythms in mouse suprachiasmatic nucleus explants on multimicroelectrode plates. Brain Res 757: 285–290. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. 1998. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci 1: 708–713. [DOI] [PubMed] [Google Scholar]
- Huang RC. 1993. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J Neurophysiol 70: 1692–1703. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. 2005. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 48: 267–278. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS. Yoshioka T, Miyawaki A, Allen CN. 2003. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron 38: 253–263. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. 1998. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21: 869–883. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. 1999. Making new connections: Role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23: 11–14. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. 1979. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci 76: 5962–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. 2007. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci 27: 11748–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. 2005. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci 8: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. 2010. Circadian regulation of A-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol 103: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. 2004. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci 24: 7985–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. 2002. Calbindin neurons in the hamster suprachiasmatic nucleus do not show a circadian variation in spontaneous firing rate. Eur J Neurosci 16: 2469–2474. [DOI] [PubMed] [Google Scholar]
- Jones JR, Tackenberg MC, McMahon DG. 2015. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat Neurosci 18: 373–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kako K, Banasik M, Lee K, Ishida N. 1997. Regulation of cAMP response element binding protein (CREB) binding in the mammalian clock pacemaker by light but not a circadian clock. Mol Brain Res 44: 39–45. [DOI] [PubMed] [Google Scholar]
- Kent J, Meredith AL. 2008. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS ONE 3: e3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. 2004. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: Role of cAMP and Ras. J Neurosci 24: 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. 2003. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms 18: 159–169. [DOI] [PubMed] [Google Scholar]
- Kononenko NI, Medina I, Dudek FE. 2004. Persistent subthreshold voltage-dependent cation single channels in suprachiasmatic nucleus neurons. Neuroscience 129: 85–92. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. 2011a. Fast delayed rectifier potassium current: Critical for input and output of the circadian system. J Neurosci 31: 2746–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS. 2011b. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol 228: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Tahara Y, Truong D, Hernandez-Echeagaray E, Colwell CS. 2014. Circadian dysfunction in response to in vivo treatment with the mitochondrial toxin 3-nitropropionic acid. ASN Neuro 6: e00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Block GD, Colwell CS. 2015. The circadian clock gene Period1 connects the molecular clock to neural activity in the suprachiasmatic nucleus. ASN Neuro 10.1177/1759091415610761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. 2004. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci 20: 1113–1117. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. 2006. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms 21: 470–481. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG. 2000. GFP fluorescence reports Period1 circadian gene regulation in the mammalian biological clock. NeuroReport 11: 1479–1482. [PubMed] [Google Scholar]
- Kunst M, Hughes ME, Raccuglia D, Felix M, Li M, Barnett G, Duah J, Nitabach MN. 2014. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol 24: 2652–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. 2002. VPAC receptors for VIP and PACAP. Receptors Channels 8: 137–153. [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. 2005. A G protein–coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 48: 221–227. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176. [DOI] [PubMed] [Google Scholar]
- Liang X, Holy TE, Taghert PH. 2016. Synchronous Drosophila circadian pacemakers display nonsynchronous Ca2+ rhythms in vivo. Science 351: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. 2004. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24: 7951–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. 1997. Cellular construction of a circadian clock: Period determination in the suprachiasmatic nuclei. Cell 91: 855–860. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Block GD. 2005. Role of neuronal membrane events in circadian rhythm generation. Meth Enzymol 393: 623–642. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. 2005. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci 25: 7682–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Chen WF, Yue Z, Chen D, Sowcik M, Sehgal A, Zheng X. 2012. Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell 11: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MJ, Rosbash M. 2001. Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107: 567–578. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Block GD. 1987. The Bulla ocular circadian pacemaker I. Pacemaker neuron membrane potential controls phase through a calcium-dependent mechanism. J Comp Physiol 161: 335–346. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. 2006. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 9: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. 2005. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48: 213–219. [DOI] [PubMed] [Google Scholar]
- Mizrak D, Ruben M, Myers GN, Rhrissorrakrai K, Gunsalus KC, Blau J. 2012. Electrical activity can impose time of day on the circadian transcriptome of pacemaker neurons. Curr Biol 22: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR, Meredith AL. 2012. Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proc Natl Acad Sci 109: 18997–19002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL. 2013. Mis-expression of the BK K+ channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am J Physiol Cell Physiol 304: C299–C311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. 2001. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci 14: 666–674. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma KI. 2002. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci 5: 399–400. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. 2011. Age-related decline in circadian output. J Neurosci 31: 10201–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD. 2015. Age-related changes in the circadian system unmasked by constant conditions. eNeuro 10.1523/ENEURO.0064-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. 2002. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109: 485–495. [DOI] [PubMed] [Google Scholar]
- Nygard M, Hill RH, Wikstrom MA, Kristensson K. 2005. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res Bull 65: 149–154. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. 2008. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. 2009. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res 18: 129–135. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Bierlaagh MA, Geurtsen AMS. 1997. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: Involvement of a slowly inactivating component of sodium current. J Neurophysiol 78: 1811–1825. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Bos NPA, De Jeu MTG, Geurtsen AMS, Mirmiran M, Sluiter AA, Buijs RM. 1998. Membrane properties and morphology of vasopressin neurons in slices of rat suprachiasmatic nucleus. J Neurophysiol 80: 2710–2717. [DOI] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. 2006. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res 1071: 54–62. [DOI] [PubMed] [Google Scholar]
- Rea MA. 1990. VIP-stimulated cyclic AMP accumulation in the suprachiasmatic hypothalamus. Brain Res Bull 25: 843–847. [DOI] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. 1999. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802. [DOI] [PubMed] [Google Scholar]
- Ruben M, Drapeau MD, Mizrak D, Blau J. 2012. A mechanism for circadian control of pacemaker neuron excitability. J Biol Rhythms 27: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, Belle MD, Gossan N, Delagrange P, Piggins HD. 2014a. Daily variation in the electrophysiological activity of mouse medial habenula neurones. J Physiol 592: 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, Wegner S, Belle MD, Howarth M, Delagrange P, Brown TM, Piggins HD. 2014b. Intrinsic and extrinsic cues regulate the daily profile of mouse lateral habenula neuronal activity. J Physiol 592: 5025–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, Gillette MU. 1993. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones. Am J Physiol 265: R1216–R1222. [DOI] [PubMed] [Google Scholar]
- Schaap J, Bos NPA, De Jeu MTG, Geurtsen AMS, Meijer JH, Pennartz CMA. 1999. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res 815: 154–166. [DOI] [PubMed] [Google Scholar]
- Schmutz I, Chavan R, Ripperger JA, Maywood ES, Langwieser N, Jurik A, Stauffer A, Delorme JE, Moosmang S, Hastings MH, et al. 2014. A specific role for the REV-ERBα-controlled L-type voltage-gated calcium channel CaV1.2 in resetting the circadian clock in the late night. J Biol Rhythms 29: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Gross RA, Morton MT. 1987. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci 84: 1694–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. 2014. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol 12: e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. 2007. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol 99: 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, et al. 1997. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. 2004. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431: 862–868. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. 2005. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438: 238–242. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernandez MP, Menet JS, Ceriani MF, Rosbash M. 2007. The Drosophila circadian network is a seasonal timer. Cell 129: 207–219. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Taussig R. 2002. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol Interv 2: 168–184. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. 1985. The suprachiamatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res 342: 37–44. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. 2003. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem 278: 718–723. [DOI] [PubMed] [Google Scholar]
- van Wamelen DJ, Aziz NA, Anink JJ, van Steenhoven R, Angeloni D, Fraschini F, Jockers R, Roos RA, Swaab DF. 2013. Suprachiasmatic nucleus neuropeptide expression in patients with Huntington’s disease. Sleep 36: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O’Dell TJ, Colwell CS. 2009. Expression of the circadian clock gene Period2 in the hippocampus: Possible implications for synaptic plasticity and learned behaviour. ASN Neuro 1: e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU. 2012. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 337: 839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB. 2015. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol 78: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. 1995. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res 695: 237–239. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. 2001. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int 18: 559–565. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14: 697–706. [DOI] [PubMed] [Google Scholar]
- Whitt JP, Montgomery JR, Meredith AL. 2016. BK channel inactivation gates daytime excitability in the circadian clock. Nat Commun 7: 10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cao G, Nitabach MN. 2008. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms 23: 117–128. [DOI] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. 2010. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res 1337: 21–31. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302: 1408–1412. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. 2001. Expression of the Per1 gene in the hamster: Brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol 430: 518–532. [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. 1999. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: Circadian profile and the compartment-specific response to light. Neuroscience 94: 141–150. [DOI] [PubMed] [Google Scholar]
- Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. 2006. Ca1.2 and CaV1.3 neuronal L-type calcium channels: Differential targeting and signaling to pCREB. Eur J Neurosci 23: 2297–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Masuda K, Quasarano-Kourkoulis C, Rosene DL, Killiany RJ, Wang S. 2011. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms 26: 149–159. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. 1995. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 16: 571–576. [DOI] [PubMed] [Google Scholar]