Abstract
Prions are self-propagating protein conformations that are traditionally regarded as agents of neurodegenerative disease in animals. However, it has become evident that prion-like aggregation of endogenous proteins can also occur under normal physiological conditions (e.g., during memory storage or activation of the immune response). In this review, we focus on the functional prion-related protein TIA-1, an RNA-binding protein that is involved in multiple aspects of RNA metabolism but is best understood in terms of its role in stress granule assembly during the cellular stress response. We propose that stress granule formation provides a useful conceptual framework with which to address the positive role of TIA-1 prion-like aggregation. Elucidating the function of TIA-1 prion-like aggregation will advance our understanding of how prion-based molecular switches are used in normal physiological settings.
The protein TIA-1 is involved in the assembly of stress granules, which translationally repress nonessential mRNAs when a cell is under stress. During this process, TIA-1 aggregates in a prion-like manner.
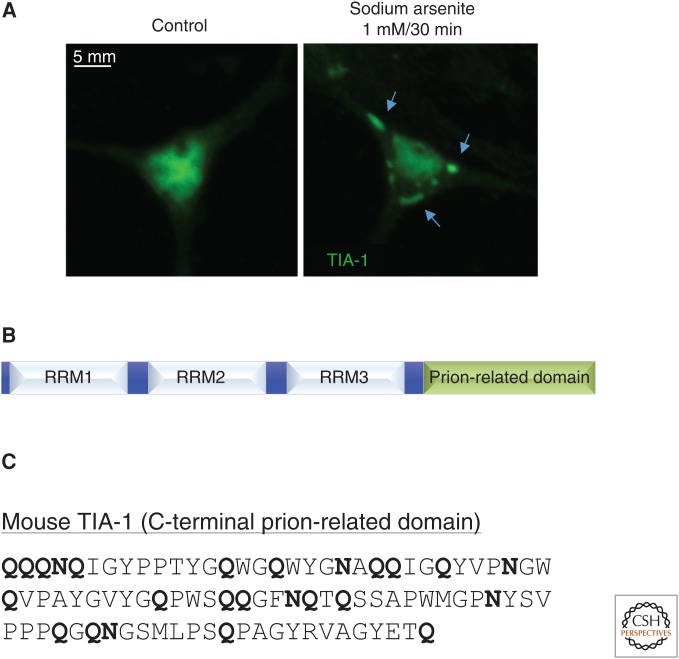
Eukaryotic cells use an array of molecular mechanisms that promote survival and homeostasis in the face of environmental adversity, including the unfolded protein response, the DNA damage response, and the antioxidant defense system. In addition, within minutes of exposure to environmental stressors such as viral infection, oxidative stress, or extremes of temperature, global protein synthesis is curtailed to allow the preferential translation of stress-responsive mRNAs. This redirection of the proteome is accomplished in part by the formation of stress granules (Fig. 1A), nonmembranous structures that are rapidly assembled in the cytoplasm during cellular stress (Kedersha et al. 2013). Stress granules arise from the dynamic accretion of stalled preinitiation complexes that contain translation factors, small ribosomal subunits, and mRNA released from the polysome pool (Anderson and Kedersha 2009; Buchan and Parker 2009). In this manner, stress granules maintain nonessential cellular mRNA in a translationally repressed state during stress, thus facilitating the efficient reallocation of cellular anabolic resources toward restoring homeostasis and minimizing cellular damage. A key component of stress granules is the ubiquitously expressed RNA-binding protein T-cell intracellular antigen-1 (TIA-1), which harbors a C-terminal prion-related domain necessary to drive both protein aggregation and stress granule assembly, while RNA-recognition motifs (RRM1-3) at the N-terminus enable binding of target mRNAs and their recruitment to stress granules (Fig. 1B) (Beck et al. 1996; Gilks et al. 2004). As is common for prions and prion-like proteins, the prion-related domain of TIA-1 is heavily enriched in glutamine and asparagine residues (Fig. 1C). Finally, unlike conventional prions and prion-related proteins, which are generally associated with neurodegenerative disease in animals, TIA-1 aggregation is highly regulated and likely serves a number of important physiological processes in a positive capacity (Fig. 2A,B). Here, we focus primarily on the role of TIA-1 in stress granule assembly, a useful contextual framework to explore prion-like aggregation, which is both amenable to molecular analysis and physiologically relevant. In this review, we also summarize evidence supporting the idea that TIA-1 is a functional prion-like protein and discuss emerging principles that characterize TIA-1 and other members of this new class of protein.
Figure 1.
(A) In cultured mouse hippocampal neurons, endogenous T-cell intracellular antigen-1 (TIA-1) is diffusely present in both the nucleus and cytoplasm, with more prominent staining in the nucleus. Upon brief treatment with sodium arsenite, a potent oxidant, TIA-1 protein dramatically relocalizes into cytoplasmic foci known as stress granules (indicated by arrows). (B) Mammalian TIA-1 contains three RNA-binding domains (RRMs) and a C-terminal prion-related domain. (C) The C-terminal prion-related domain of mouse TIA-1 is highly enriched in the polar amino acids asparagine and glutamine.
Figure 2.
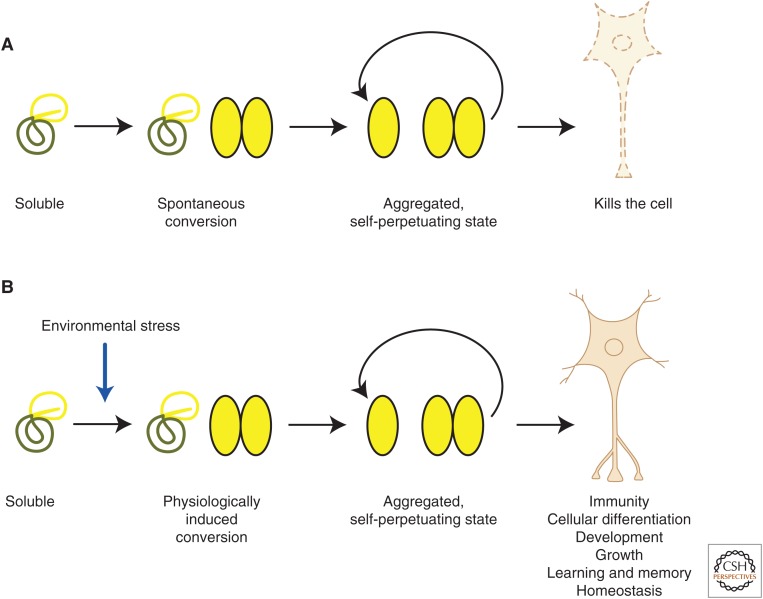
(A) Prions arise from the spontaneous conversion of a soluble protein conformation into an aggregated, self-propagating form that is cytotoxic and causes neurodegenerative disease. (B) In contrast to pathogenic prions, functional prion-like proteins undergo physiologically regulated conversion from soluble to aggregated forms that are likely to contribute in multiple ways to normal biological processes, including viral immunity, development, and cellular homeostasis.
TIA-1 AND STRESS GRANULE FUNCTION
Stress granules were originally viewed as temporary storage depots for RNA transcripts not critically required during the cellular stress response (Kedersha et al. 2013). If a stressor is resolved or eliminated, stress granules are disassembled, and previously bound RNAs are released into the polysome pool, allowing normal protein synthesis to resume. Alternatively, if cellular stress persists beyond a certain threshold, RNAs localized in stress granules may be transferred to processing bodies (P-bodies) and targeted for degradation, which may be accompanied by the initiation of apoptosis (Kedersha and Anderson 2009). However, it is now clear that stress granules are not merely static structures involved in the temporary storage of mRNA but are dynamic and heterogeneous macromolecular assemblies that play an active role in multiple aspects of RNA metabolism during stress (Anderson and Kedersha 2008; Buchan and Parker 2009). First, fluorescence recovery after photobleaching (FRAP) experiments in transfected cells reveal dynamic shuttling of green fluorescent protein-tagged TIA-1 (GFP-TIA-1) into and out of stress granules, with a resident half-life of only 2 sec (Kedersha et al. 2000). Second, in addition to TIA-1, stress granules contain a diversity of proteins involved in various aspects of RNA metabolism, including RNA silencing (e.g., Ago2, Pumilio, and Staufen), message stability (e.g., HuR and PABP), transcription (e.g., SRC3 and Rpb4), alternative splicing (e.g., MLN51), and translational regulation (e.g., Ataxin-2 and FMRP) (for review, see Anderson and Kedersha 2008). Moreover, several of these proteins play important roles in development, cell adhesion, and differentiation in a variety of biological contexts. Stress granules are also heterogeneous with regard to both protein and RNA composition, implying the existence of different granule subclasses that subserve a variety of distinct biological functions (Buchan and Parker 2009). Finally, stress granules interact and dynamically exchange protein and RNA with P-bodies, which are distinguished from stress granules by the presence of decapping enzymes that facilitate RNA degradation (Parker and Sheth 2007; Anderson and Kedersha 2008).
The panoply of RNA and protein found in both stress granules and P-bodies has led to the conclusion that these cytoplasmic structures represent coordinated sites of mRNA triage that allow the proteome to be reprogrammed as a function of the nature and time course of cellular stress (Anderson and Kedersha 2008). In response to a given type of stress, specific mRNAs are shuttled from stress granules into P-bodies for selective degradation or silencing by RNA-binding proteins that escort these mRNAs between the two types of granules in a specific manner (Kawai et al. 2004). The notion that protein synthesis is specifically regulated during stress is supported by the finding that endoplasmic reticulum (ER) stress causes a significant reduction in translation of 25% of mRNAs, whereas another 25% of transcripts, such as those encoding heat shock proteins, is greatly enhanced, and almost all cellular TIA-1 is localized to stress granules (Kawai et al. 2004). It is not yet known, however, if stress granule–dependent reprogramming of the proteome varies according to the type of stress. The precise biological consequences of dynamic routing of protein and RNA components between stress granules and P-bodies are likely to vary according to cellular context.
Furthermore, given the types of proteins found in stress granules, it is highly plausible that stress granule formation alters the stoichiometry of the biochemical processes in which these proteins normally participate. Therefore, stress granule formation could potentially have profound effects on cellular differentiation, development, proliferation, and growth. Taken together, stress granule formation represents a powerful mechanism by which cells may respond to challenging environmental conditions in a rapid, context-appropriate manner that cannot be fulfilled by transcriptional processes, which operate on a much longer time scale.
The biological importance of TIA-1 was shown in a number of studies using TIA-1-deficient cells, whether produced by genetic modification or by siRNA knockdown. For example, mice that lack TIA-1 exhibit partial embryonic lethality, whereas knockout mice that survive into adulthood are susceptible to bacterial endotoxic shock but are otherwise normal (Piecyk et al. 2000). TIA-1 deletion also changes the expression patterns of genes involved in lipid metabolism (Heck et al. 2014) and enhances cell proliferation (Reyes et al. 2009). Finally, TIA-1-depleted cells are more susceptible to viral infection (Albornoz et al. 2014). TIA-1 deficiency is therefore associated with pathophysiological changes at both the cellular and organismal levels under basal conditions and in response to environmental challenge. Presumably, the absence of TIA-1-dependent stress granules explains at least some of these phenotypes. However, it is not clear to what extent these abnormalities are caused by the absence of TIA-1 aggregates per se, which in turn may serve biological functions that may or may not be dependent on stress granule formation. In other words, it is not a simple task to dissociate the contribution of TIA-1 aggregation to stress granule assembly versus its potentially distinct or indirect roles in alternative splicing, translational repression, and message stability, which may all be mechanistically intertwined.
However, a noteworthy physiological context in which TIA-1-dependent stress granule function has been explored in great detail is that of viral pathogenicity. A wealth of data from biochemical and imaging experiments describes an intimate functional connection between stress granules and the viral life cycle. Stress granule formation is important in the cellular defense mechanism against viral infection and is triggered by viral RNA-induced activation of protein kinase R (PKR), a kinase that phosphorylates eIF2α and thus causes depletion of the eIF2-GTP-tRNAiMet ternary complex necessary for the translation of most cellular and viral mRNA (Simpson-Holley et al. 2011). Indeed, a number of viruses inhibit stress granule assembly to circumvent the global shutdown of host translational machinery that could potentially block viral replication (McInerney et al. 2005; Emara and Brinton 2007; Montero et al. 2008). Moreover, viral infectivity of TIA-1-depleted cells is significantly enhanced (Albornoz et al. 2014). Finally, there are examples of viruses that have evolved the ability not only to circumvent but also to take advantage of stress granule assembly to enable replication (Beckham and Parker 2008).
Ultimately, to identify the role of TIA-1 aggregation itself in any biological process, it will be necessary to develop inhibitors (or activators) that specifically disrupt (or enhance) homomeric interaction of the protein, whether in the form of small molecules or oligopeptides. Alternatively, with adequate structural information, it may be possible to create point mutations in TIA-1 that prevent aggregation but do not compromise the three-dimensional structure of the protein in the way that deletion mutants potentially can. Finally, all of these studies are confounded by the possibility of functional compensation by TIA-1-related protein (TIAR) in the absence of TIA-1. Consistent with overlapping functions of TIA-1 and TIAR, mice deficient in both genes are not viable (Piecyk et al. 2000).
REGULATION OF TIA-1 AGGREGATION
Stress granule assembly is a highly regulated and reversible process, in contrast to the aggregation of pathogenic prions and prion-like proteins involved in neurodegeneration. Indeed, stress granules are not visible in the absence of stress. When the cellular stress response is activated, the translation factor eIF2α is phosphorylated by a variety of serine/threonine kinases that respond to different types of stressors. For example, hepatic heme-regulated inhibitor (HRI) phosphorylates eIF2α under conditions of heme depletion (Zhan et al. 2002), while protein kinase RNA-like endoplasmic reticulum kinase (PERK) phosphorylates eIF2α as part of the unfolded protein response during ER stress (Harding et al. 2000). Other eIF2α kinases are sensitive to amino acid deprivation (GCN2) and, as mentioned earlier, viral infection (PKR) (Zhang et al. 2002; Simpson-Holley et al. 2011). In turn, phosphorylation of eIF2α prevents the formation of the initiator ternary complex (eIF2-GTP-tRNAiMet) that is necessary for loading the initiator tRNA onto the 40S ribosomal subunit to generate the 48S preinitiation complex. Thus, reducing formation of the initiator ternary complex during cellular stress leads to the accumulation of stalled 48S preinitiation complexes that can no longer bind to the 60S ribosomal subunit to allow cap-dependent translation to begin (Anderson and Kedersha 2002b). TIA-1 facilitates the localization of these aborted preinitiation complexes into stress granules, maintaining bound RNAs in a functionally transient state as dictated by prevailing environmental conditions.
In addition to eIF2α-dependent regulation of TIA-1 function, the shuttling of TIA-1 from the nucleus into the cytoplasm of stressed cells is regulated (Kedersha et al. 2000; Zhang et al. 2005). Although cytoplasmic translocation of TIA-1 is not strictly required for stress granule formation (Bounedjah et al. 2014), it is conceivable that the normal increase in availability of cytoplasmic TIA-1 during stress has an impact on the kinetics and stoichiometry of stress granule assembly. It is also evident that stress granule formation can occur in an eIF2α phosphorylation-independent manner, for example, when cells are exposed to acute energy starvation (Anderson and Kedersha 2002a). In addition, it will be interesting to explore whether posttranslational modifications such as ubiquitination and sumoylation can modulate TIA-1 aggregation, as has been suggested for the translational regulator CPEB3 (Pavlopoulos et al. 2011; Drisaldi et al. 2015), the first example of a functional prion-like protein in neurons (Si et al. 2003a,b). Although TIA-1 has been shown to undergo posttranslational modifications, including phosphorylation by Fas-activated serine/threonine kinase (FAST) during Fas-mediated apoptosis (Tian et al. 1995), the potential relationship between TIA-1 phosphorylation and stress granule assembly is not known. More recently, TIA-1 is also susceptible to oxidation by reactive oxygen species, leading to the suppression of stress granule formation and neuronal cell death (Arimoto-Matsuzaki et al. 2016). Thus, there are several possible mechanisms by which stress granule formation may be regulated during the cellular stress response.
STRUCTURAL DETERMINANTS OF TIA-1 AGGREGATION
What are the structural features that drive aggregation of TIA-1 during cellular stress? Overexpression of TIA-1, or treating cells with an oxidative stressor such as arsenite, is sufficient to cause the spontaneous formation of stress granules in a variety of cell types. These structures are bona fide stress granules because they contain proteins such as PABP, eIF3, and other stress granule markers, as well as poly(A+) RNA (Gilks et al. 2004). Moreover, inducing oxidative stress can enhance the formation of stress granules in TIA-1-transfected cells even further (Gilks et al. 2004). The ability of TIA-1 to promote stress granule assembly is critically dependent on its C-terminal prion-related domain, which is enriched in glutamine and asparagine. Expansive stretches of these polar residues are known to promote homotypic protein–protein interaction and drive the formation of β-sheet structures that are characteristic of prions and amyloids (Perutz et al. 1994; Michelitsch and Weissman 2000). However, although the prion-related domain itself is capable of aggregation on its own, it only forms microaggregates that are much smaller than stress granules, and these do not contain RNA or other components found in true stress granules (Gilks et al. 2004). Furthermore, although the prion-related domain itself can be recruited to stress granules in low-expressing cells, overexpression produces a dominant-negative effect, such that the stress-induced formation of stress granules is blocked (Gilks et al. 2004). These observations suggest that the prion-related domain alone is capable of interacting with the full-length protein, leading to its sequestration and inability to participate in stress granule assembly. Interestingly, substitution of the TIA-1 prion-related domain with that of the yeast prion Sup35 is sufficient for proper stress granule assembly (Gilks et al. 2004). Together, these results indicate that stress granule formation requires both the prion-related domain as well as RNA-binding activity conferred by one or more of the three RNA-binding domains in the N-terminal region of the protein. The potential role of posttranslational modifications in the induction and maintenance of TIA-1 aggregates has yet to be addressed experimentally, from either the structural or functional perspective.
ADDITIONAL FUNCTIONS OF TIA-1
Like many other RNA-binding proteins, TIA-1 plays multiple roles in RNA metabolism, including alternative splicing, translational repression, and mRNA silencing. Furthermore, these biological functions need not be directly associated with stress granule formation, particularly the regulation of alternative splicing by TIA-1, which is normally found at appreciable levels in the nucleus. TIA-1 recruits the spliceosome complex to splicing regulatory sequences in target RNAs via interaction with U1-C small nuclear ribonucleoprotein (snRNP) (Förch et al. 2002), thus modulating the expression patterns of many genes. However, as indicated earlier, it is conceivable that cytoplasmic localization of TIA-1 during stress could alter the stoichiometry within nuclear splicing complexes that contain TIA-1, with concomitant effects on alternative splicing outcomes for a number of target mRNAs. Indeed, localization of TIA-1 into stress granules would be expected to produce broad effects on other TIA-1-dependent processes that do not involve stress granules per se. Thus, during cellular stress, a number of important biological processes are certainly affected by stress granule formation, both directly and indirectly, which together allow a cell to execute an adaptive physiological response. These possibilities have yet to be examined nor has the question of whether some of these processes require TIA-1 aggregation mediated by the prion-related domain.
EVIDENCE THAT TIA-1 IS A FUNCTIONAL PRION
In animals, pathogenic prions are strictly defined by their ability to be transmitted from one organism to another in an infectious manner. In contrast, yeast prions do not cause infectious disease. Although in both cases the agent of transmission is the prionic conformation of the protein, the cell-to-cell transmission observed in yeast is from mother to daughter cell, and not from one mature cell to another, which is observed in animals. To complicate matters, there is now evidence that a number of prion-like proteins involved in neurodegeneration, including tau and amyloid beta (Aβ), are not only capable of cell-to-cell transmission, but also infectivity (Prusiner 2013). For example, in cell culture, spontaneous uptake of misfolded tau into cells causes the rapid recruitment of endogenous tau into filamentous inclusions (Guo and Lee 2011), whereas tau inclusions can be propagated from one mouse brain to another (Clavaguera et al. 2013). Similar observations have been made for other prion-related models of neurodegeneration (Prusiner 2013). In comparison, functional prions in animals do not appear to be infectious, but exhibit other properties that are characteristic of prions in general, including detergent resistance, β-sheet structure, propensity for aggregation, and cell-to-cell transmissibility (Si et al. 2003b; Gilks et al. 2004; Hou et al. 2011; Li et al. 2014; Fioriti et al. 2015). In addition, these studies provide evidence that the aggregated state is associated with positive function. However, there is no conventional definition of what constitutes a functional prion, and the discussion becomes an exercise in semantics (Prusiner 2013). Here, we define a functional prion as a protein that (1) exists in at least two general conformations, soluble and aggregated; (2) performs a beneficial function in its aggregated conformation; (3) adopts a self-propagating conformation that is transmissible from one cell to another; (4) is dependent on heat shock proteins to transition from one conformation to another; and (5) is not associated with infectious disease in its aggregated state.
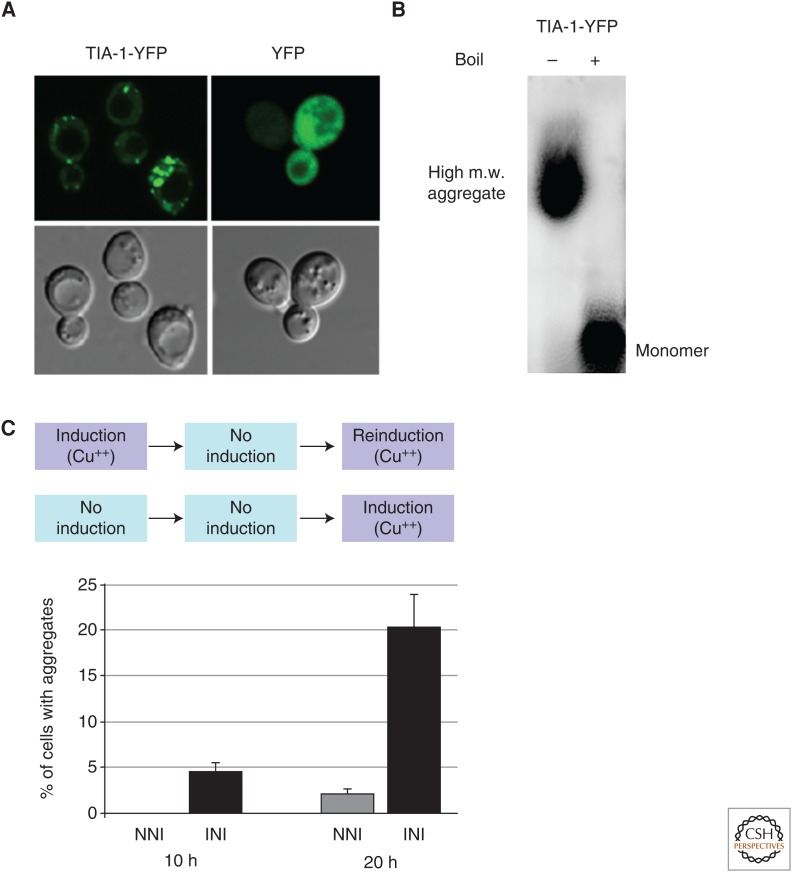
Several lines of evidence indicate that TIA-1 exists in both soluble and insoluble conformations. For example, in transfected cells, TIA-1 becomes increasingly insoluble to detergents as the overexpressed protein accumulates over time (Gilks et al. 2004). In heterologous yeast assays, overexpression of TIA-1 leads to the emergence of stress granule-like structures (Fig. 3A) (Li et al. 2014). In addition, detergent-resistant TIA-1 oligomers are observed by sodium dodecyl sulfate (SDS)-agarose gel analysis (Fig. 3B), a commonly used method to study prion-like aggregation (Li et al. 2014). Also, under specific buffer conditions, recombinant TIA-1 forms amyloid-like fibers that are visible by transmission electron microscopy (Li et al. 2014). Furthermore, preliminary studies in the mouse brain indicate that endogenous TIA-1 transitions from a monomeric state to a higher-order, SDS-resistant structure when animals are exposed to contextual fear conditioning (JB Rayman and ER Kandel, unpubl.). Together, these observations indicate that TIA-1 can adopt at least two different structural conformations that vary in solubility and detergent resistance. However, it is not clear whether the insoluble form of TIA-1 in vivo is composed exclusively of aggregated, homomeric TIA-1, or if associated proteins are necessary to form the observed insoluble complexes. Indeed, Pub1, the yeast homolog of TIA-1, interacts with the classical yeast prion Sup35 to form self-propagating heteroprotein conformations (Li et al. 2014). Also, given that detergent resistance is not an obligatory property of prions and prion-related proteins, and is known to vary among different prion conformations, it will be interesting to determine whether TIA-1 can also adopt a variety of structural conformations with differential solubility and different functional and biophysical properties. Moreover, the relationship between the aggregated conformations observed in the latter experiments and stress granules is unknown. For example, are the TIA-1 oligomers described in the yeast experiments intermediates in the formation of stress granules, or do these entities reflect distinct structures? Notably, experiments performed in yeast show that the formation of oligomeric TIA-1 coincides with the appearance of stress granule–like puncta, arguing that the structures are at least temporally related (Li et al. 2014). In this regard, the development of an in vitro assay for stress granule assembly would be highly informative. It will also be important to obtain experimental evidence that the purified recombinant protein is capable of forming SDS-resistant aggregates that exhibit similar electrophoretic properties as those observed in heterologous yeast experiments.
Figure 3.
(A) Overexpression of yellow fluorescent protein (YFP)-tagged mouse TIA-1 in yeast leads to the formation of stress granule–like structures. In contrast, YFP alone does not produce such structures. (B) TIA-1-YFP forms high-molecular-weight (m.w.) aggregates that are sodium dodecyl sulfate (SDS)-resistant, as observed by SDS-agarose gel electrophoresis. Boiling the extracts causes the collapse of aggregated protein into monomer. (C) Formation of mouse TIA-1 aggregates in a heterologous yeast assay occurs more rapidly in yeast cultures that have been previously induced to express the protein (INI), compared with a culture that is freshly induced in parallel (NNI). No TIA-1 aggregates are observed during the “no induction” phase of INI cultures. The seeding effect is observed at both 10 and 20 h after reinduction (INI) of TIA-1.
Stress granule formation is the most compelling example of a positive physiological function of TIA-1 in its aggregated conformation. That is, the aggregate-prone conformation of TIA-1 is necessary for the localization of stalled preinitiation complexes to stress granules, with concomitant translational repression. In particular, studies investigating the relationship between stress granule formation and the viral life cycle have been especially informative. However, there is now evidence that the formation of self-propagating aggregates of Sup35 and Tia1/Pub1 in yeast is involved in recruiting protein synthesis machinery to the tubulin cytoskeleton, which is a critical aspect of maintaining cytoskeletal integrity (Li et al. 2014). The latter aggregates do not appear as stress granules but rather decorate interpolar microtubules that are formed during mitosis. As mentioned previously, the identification of small molecules that specifically target aggregation in these various cellular contexts will be enormously useful in determining the nature and function of specific conformational states of TIA-1 and whether self-propagation is, in fact, a requisite aspect of stress granule formation.
Heterologous studies in yeast have also provided important new clues as to whether aggregated TIA-1 causes the structural transition of soluble, monomeric TIA-1 into the aggregated form. Specifically, Li and colleagues placed yellow fluorescent protein (YFP)-tagged TIA-1 under the control of a copper-inducible promoter, which supports robust expression of the recombinant protein (Li et al. 2014). Within several hours after induction, the spontaneous formation of stress granule–like structures was evident (Fig. 3A), much like overexpression studies of TIA-1 in mammalian cell lines. If these same yeast cultures are subsequently propagated in media lacking the copper inducer, the stress granules dissolve and are no longer detectable. However, upon reinduction of TIA-1 expression, the emergence of TIA-1-containing foci is dramatically accelerated compared with the expression rate in a freshly induced culture (Fig. 3C). These observations are consistent with a seeding effect of aggregated TIA-1. In other words, the initial induction of TIA-1 expression leads to the formation of TIA-1 aggregates that are not visible by confocal microscopy but remain in a heritable configuration from one generation of cells to the next. A critical research objective in the future is to purify the TIA-1 seed and subject it to various structural analyses to assess its multimerization state and three-dimensional conformation of individual monomers.
As described for other prions and prion-like proteins, TIA-1 aggregation is highly dependent on heat shock proteins. In yeast mutants lacking the heat shock protein Hsp104, TIA-1 does not form visible aggregates (Li et al. 2014). Furthermore, overexpression of Hsp70 in mammalian cells prevents the cytoplasmic aggregation of the prion-related domain of TIA-1 (Gilks et al. 2004). However, more studies in varying contexts are needed to understand the functional interplay between TIA-1 aggregation and heat shock protein activity. For example, the prion-related domain of TIA-1 induces the expression of both Hsp40 and Hsp70 (Gilks et al. 2004), which may represent a feedback mechanism by which the conformational states of aggregation-prone proteins may be dynamically regulated. Ultimately, it will be useful to extend these observations to different types of mammalian cells and then ascertain the contributions of individual heat shock proteins on the formation of TIA-1 aggregates.
Finally, an important feature of a functional prion-like protein is that it does not cause neurodegeneration. At this point, it is not possible to know whether a prionic form of TIA-1 is capable of causing infectious disease. To our knowledge, no one has attempted to inject aggregated TIA-1 protein into live animals to ascertain the effects on neuropathological and psychopathological indices, especially over the long duration that is typically required before signs of prion- and amyloid-related disease appear. Moreover, these experiments are complicated by the fact that the structure of recombinant TIA-1 may not reflect that of the endogenous protein. Indeed, this caveat is a major issue for the prion field in general. It has not been possible to obtain a high-resolution three-dimensional structure of any prion, thus obscuring our ability to elucidate structure–function relationships through the iterative pursuit of in vitro and in vivo experiments. However, new developments in solid-state nuclear magnetic resonance, cryo-electron microscopy, and other structural methodologies should help solve this problem in the near future.
CONCLUSION
In response to cellular stress, global protein synthesis must be rapidly modified to allow the manufacture of specialized pools of proteins that are essential for minimizing cellular damage and promoting homeostasis during the period of stress. The formation of stress granules is an integral part of the cellular stress response, allowing the packaging and translational repression of nonessential mRNAs that were previously associated with polysomes. Without stress granules, mRNAs not only may become more susceptible to degradation, but also may compete with the translation of RNAs that are necessary during the stress response, thus compromising adaptive cellular changes. Here, we propose that prion-like aggregation is a mechanism that allows TIA-1 to respond in a rapid and efficient manner to environmental challenge.
Several lines of evidence are consistent with the idea that TIA-1 is a functional prion-like protein, which constitutes a new and growing class that includes mouse CPEB3 (Stephan et al. 2015), Aplysia CPEB (Si et al. 2003a,b), Drosophila Orb2 (Majumdar et al. 2012), and MAVS (Hou et al. 2011). For example, TIA-1 exists in both soluble and detergent-resistant conformations, and heterologous yeast assays show that only the aggregated form is heritable (Li et al. 2014). Moreover, the aggregated state of TIA-1 is associated with positive functions, including the formation of stress granules and the recruitment of the protein synthesis machinery to the tubulin cytoskeleton (Gilks et al. 2004; Li et al. 2014). Finally, as is true of prions, TIA-1 aggregation is modulated by heat shock proteins, both in yeast and in mammalian cells (Gilks et al. 2004; Li et al. 2014).
An important concept to emerge from studies of functional prion-like proteins is that aggregation is an efficient mechanism by which to achieve rapid cellular response to a physiological input, which is particularly relevant to the stress response (TIA-1), learning and memory storage (CPEB and Orb2), and immune system function (MAVS) (see also Cai et al. 2016; Rayman and Kandel 2016). Aggregation conceivably serves a variety of functions, including the formation of a scaffold that provides novel interaction surfaces with partner binding proteins. TIA-1 aggregation, in particular, is also an efficient means by which to concentrate a diverse population of unrelated mRNAs that are dispensable during stress and is propelled by the aggregation-prone nature of its β-sheet structure. Moreover, the epigenetic transfer of proteomic modulatory information via the transmission of self-propagating TIA-1 conformations may be an adaptive mechanism by which the nature of environmental conditions can be communicated from progenitor to progeny cells, or between neighboring adult cells. For example, it will be interesting to see whether the aggregation state of TIA-1 in neurons can be epigenetically propagated during neurogenesis and neural differentiation in response to stressful environmental stimuli, with adaptive consequences that are evident at the behavioral level. Also, it may be possible to observe exosome-dependent or tunneling nanotube-dependent transfer of aggregated protein between adjacent cells to facilitate population-based responses to certain types of environmental stress (Budnik et al. 2016; Victoria et al. 2016). However, although heritability of TIA-1 aggregates has been observed in yeast (Li et al. 2014), a major question is whether self-propagation is relevant to stress granule formation. For example, it is not clear whether aggregated TIA-1 proteins are capable of directly inducing conformational changes in monomeric protein, leading to dynamic recruitment into stress granules. By extension, it would also be useful to address whether previously stressed cells are capable of reassembling stress granules with enhanced kinetics during a repeated challenge, analogous to the seeding effect described in yeast (Li et al. 2014).
Although a great deal of evidence supports the idea that TIA-1 is a functional prion, there are many other questions that remain unanswered. In their aggregated conformations, functional prion-like proteins facilitate large-scale changes in the proteome and modulate the function of intracellular signaling cascades. Given the profound cellular alterations that these proteins are capable of producing, they must necessarily be regulated by a variety of upstream signaling events and feedback loops. However, although regulation of TIA-1 aggregation is well-characterized, it is not clear how a multimeric, aggregated structure is reversibly disassembled. In other words, is this an active process that requires the participation of chaperone proteins, or is it a kinetic process whose directionality is only dependent on the continuous presence of an inducing signal? Also, when traditional prions undergo conversion from monomer to higher-order conformations, there is generally an increase in β-sheet content. However, for functional prion-like proteins such as MAVS (Hou et al. 2011) and TIA-1 (JB Rayman and ER Kandel, unpubl.), there is no evidence of stimulus-induced enrichment of β-sheet content. It will be interesting to determine whether or not dynamic changes in β-sheet content are a general distinguishing feature between classical prions and functional prion-like proteins, not to mention whether TIA-1 undergoes dynamic structural changes as it enters and exits stress granules over a relatively rapid time scale.
Although stress granules provide a useful conceptual framework for the study of aggregation, TIA-1 can also form high-molecular-weight aggregates that are too small to be resolved by confocal microscopy (JB Rayman and ER Kandel, unpubl.). These subresolution aggregates are likely to be physiologically relevant structures but may or may not be related to stress granules. In the future, it will be necessary to develop more precise approaches to probe the exact role that aggregation plays in TIA-1 function. In concert with new structural methodologies, the development of small molecules that specifically target TIA-1 aggregation will be very helpful, as will the identification of point mutations in the prion-related domain that influence aggregation. These essential tools will enable us to elucidate the function of physiological aggregation per se, which represents a formidable obstacle in the study of amyloids, prions, and prion-like proteins in general. The conceptual advances gained from pursuing these questions are likely to have broad applicability to the growing number of functional prion-like proteins in mammals.
ACKNOWLEDGMENTS
We thank the Howard Hughes Medical Institute (E.R.K.), the Institute for Translational Neuroscience (J.B.R. and E.R.K.), and Cohen Veterans Bioscience (J.B.R. and E.R.K.) for generous support. We thank Paul J. Anderson for critical reading of the manuscript.
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Biology available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Albornoz A, Carletti T, Corazza G, Marcello A. 2014. The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation. J Virol 88: 6611–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. 2002a. Stressful initiations. J Cell Sci 115: 3227–3234. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. 2002b. Visibly stressed: The role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. 2008. Stress granules: The Tao of RNA triage. Trends Biochem Sci 33: 141–150. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. 2009. Stress granules. Curr Biol 19: R397–R398. [DOI] [PubMed] [Google Scholar]
- Arimoto-Matsuzaki K, Saito H, Takekawa M. 2016. TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis. Nat Commun 7: 10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AR, Medley QG, O’Brien S, Anderson P, Streuli M. 1996. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res 24: 3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham CJ, Parker R. 2008. P bodies, stress granules, and viral life cycles. Cell Host Microbe 3: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounedjah O, Desforges B, Wu TD, Pioche-Durieu C, Marco S, Hamon L, Curmi PA, Guerquin-Kern JL, Piétrement O, Pastré D. 2014. Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res 42: 8678–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Parker R. 2009. Eukaryotic stress granules: The ins and outs of translation. Mol Cell 36: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Canada C, Wendler F. 2016. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cai X, Xu H, Chen ZJ. 2016. Prion-like polymerization in immunity and inflammation. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. 2013. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci 110: 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisaldi B, Colnaghi L, Fioriti L, Rao N, Myers C, Snyder AM, Metzger DJ, Tarasoff J, Konstantinov E, Fraser PE, et al. 2015. SUMOylation is an inhibitory constraint that regulates the prion-like aggregation and activity of CPEB3. Cell Rep 11: 1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Brinton MA. 2007. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci 104: 9041–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioriti L, Myers C, Huang YY, Li X, Stephan JS, Trifilieff P, Colnaghi L, Kosmidis S, Drisaldi B, Pavlopoulos E, et al. 2015. The persistence of hippocampal-based memory requires protein synthesis mediated by the prion-like protein CPEB3. Neuron 86: 1433–1448. [DOI] [PubMed] [Google Scholar]
- Förch P, Puig O, Martínez C, Séraphin B, Valcárcel J. 2002. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J 21: 6882–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15: 5383–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM. 2011. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286: 15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904. [DOI] [PubMed] [Google Scholar]
- Heck MV, Azizov M, Stehning T, Walter M, Kedersha N, Auburger G. 2014. Dysregulated expression of lipid storage and membrane dynamics factors in Tia1 knockout mouse nervous tissue. Neurogenetics 15: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. 2011. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146: 448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Fan J, Mazan-Mamczarz K, Gorospe M. 2004. Global mRNA stabilization preferentially linked to translational repression during the endoplasmic reticulum stress response. Mol Cell Biol 24: 6773–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. 2009. Regulation of translation by stress granules and processing bodies. Prog Mol Biol Transl Sci 90: 155–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, Anderson P. 2013. Stress granules and cell signaling: More than just a passing phase? Trends Biochem Sci 38: 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rayman JB, Kandel ER, Derkatch IL. 2014. Functional role of Tia1/Pub1 and Sup35 prion domains: Directing protein synthesis machinery to the tubulin cytoskeleton. Mol Cell 55: 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cesario WC, White-Grindley E, Jiang H, Ren F, Khan MR, Li L, Choi EM, Kannan K, Guo F, et al. 2012. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell 148: 515–529. [DOI] [PubMed] [Google Scholar]
- McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljestrom P. 2005. Importance of eIF2α phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell 16: 3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch MD, Weissman JS. 2000. A census of glutamine/asparagine-rich regions: Implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci 97: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H, Rojas M, Arias CF, López S. 2008. Rotavirus infection induces the phosphorylation of eIF2α but prevents the formation of stress granules. J Virol 82: 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Sheth U. 2007. P bodies and the control of mRNA translation and degradation. Mol Cell 25: 635–646. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Trifilieff P, Chevaleyre V, Fioriti L, Zairis S, Pagano A, Malleret G, Kandel ER. 2011. Neuralized1 activates CPEB3: A function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell 147: 1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz MF, Johnson T, Suzuki M, Finch JT. 1994. Glutamine repeats as polar zippers: Their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci 91: 5355–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, et al. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-α. EMBO J 19: 4154–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 2013. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47: 601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rayman JB, Kandel ER. 2016. Functional prions in the brain. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R, Alcalde J, Izquierdo JM. 2009. Depletion of T-cell intracellular antigen proteins promotes cell proliferation. Genome Biol 10: R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. 2003a. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell 115: 893–904. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. 2003b. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 115: 879–891. [DOI] [PubMed] [Google Scholar]
- Simpson-Holley M, Kedersha N, Dower K, Rubins KH, Anderson P, Hensley LE, Connor JH. 2011. Formation of antiviral cytoplasmic granules during orthopoxvirus infection. J Virol 85: 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan JS, Fioriti L, Lamba N, Colnaghi L, Karl K, Derkatch IL, Kandel ER. 2015. The CPEB3 protein is a functional prion that interacts with the actin cytoskeleton. Cell Rep 11: 1772–1785. [DOI] [PubMed] [Google Scholar]
- Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. 1995. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis. J Exp Med 182: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria GS, Arkhipenko A, Zhu S, Syan S, Zurzolo C. 2016. Astrocyte-to-neuron intercellular prion transfer is mediated by cell-cell contact. Sci Rep 6: 20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan K, Vattem KM, Bauer BN, Dever TE, Chen JJ, Wek RC. 2002. Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol Cell Biol 22: 7134–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, et al. 2002. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Delestienne N, Huez G, Kruys V, Gueydan C. 2005. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci 118: 5453–5463. [DOI] [PubMed] [Google Scholar]