Abstract
Airway hyperresponsiveness (AHR) and inflammation are key pathophysiological features of asthma. Enhanced contraction of bronchial smooth muscle (BSM) is one of the causes of the AHR. It is thus important for development of asthma therapy to understand the change in the contractile signaling of airway smooth muscle cells associated with the AHR. In addition to the Ca2+-mediated phosphorylation of myosin light chain (MLC), contractile agonists also enhance MLC phosphorylation level, Ca2+-independently, by inactivating MLC phosphatase (MLCP), called Ca2+ sensitization of contraction, in smooth muscle cells including airways. To date, involvements of RhoA/ROCKs and PKC/Ppp1r14a (also called as CPI-17) pathways in the Ca2+ sensitization have been identified. Our previous studies revealed that the agonist-induced Ca2+ sensitization of contraction is markedly augmented in BSMs of animal models of allergen-induced AHR. In BSMs of these animal models, the expression of RhoA and CPI-17 proteins were significantly increased, indicating that both the Ca2+ sensitizing pathways are augmented. Interestingly, incubation of BSM cells with asthma-associated cytokines, such as interleukin-13 (IL-13), IL-17, and tumor necrosis factor-α (TNF-α), caused up-regulations of RhoA and CPI-17 in BSM cells of naive animals and cultured human BSM cells. In addition to the transcription factors such as STAT6 and NF-κB activated by these inflammatory cytokines, an involvement of down-regulation of miR-133a, a microRNA that negatively regulates RhoA translation, has also been suggested in the IL-13- and IL-17-induced up-regulation of RhoA. Thus, the Ca2+ sensitizing pathways and the cytokine-mediated signaling including microRNAs in BSMs might be potential targets for treatment of allergic asthma, especially the AHR.
Keywords: bronchial asthma, airway hyperresponsiveness, Ca2+ sensitization, RhoA, CPI-17 (Ppp1r14a)
Introduction
Pathophysiology of allergic asthma is characterized by the combination of airway hyperresponsiveness (AHR), inflammation, and remodeling (1-3). The AHR is defined by increased airway narrowing in response to a wide range of stimuli, and is responsible for recurrent episodes of wheezing and breathlessness. Enhanced bronchial smooth muscle (BSM) contraction is one of the causes of AHR. In addition, the AHR correlates with the severity of asthma (4) and with the amount of treatment needed to control symptoms (5). The severity of hyperresponsiveness is associated with severer symptoms and a steeper fall in forced expiratory volume in 1 second (FEV1) (6).
Allergic asthma is a Th2 lymphocyte-mediated inflammatory airway disease. Cytokines derived from Th2 lymphocytes play a key role in the pathophysiology of asthma through the induction of eosinophilic airway inflammation. These lead to variable airway obstruction and AHR to nonspecific stimuli (7). The β adrenergic drugs are the most potent dilators of BSMs currently approved for clinical use against asthma. Among the β adrenergic agonists, the individual agents vary in their rapidity of onset and action duration. Inhaled, short-acting, selective β2 adrenergic agonists (SABAs) are the mainstay of acute asthma therapy, whereas inhaled, long-acting, selective β2 adrenergic agonists (LABAs), in combination with inhaled glucocorticoids, play a role in long-term control of moderate to severe asthma. Rapid relief from airway limitation in asthmatic patients by SABA inhalation suggests the involvement of augmented airway smooth muscle contraction in the airway obstruction. Thus, it is important to understand the changes in the contractile signaling of airway smooth muscle cells associated with AHR for the development of asthma therapy. In this review, we will describe the pathophysiological mechanisms of augmented BSM contraction in AHR.
Involvement of augmented Ca2+ sensitization in BSM hyper-contraction in allergic asthma
To elucidate the pathogenesis of allergic bronchial asthma, various animal models have been used by investigators including us. In an allergic asthma model using rats (8), that were actively sensitized with 2,4-dinitrophenylated Ascaris suum extract antigen and repeatedly challenged with aerosolized antigen, a marked augmentation of airway responsiveness to inhaled acetylcholine (ACh), i.e., the AHR, was observed. In this animal model of asthma, the ACh responsiveness of the isolated BSMs was also enhanced significantly. Similarly, in a mouse model of allergic asthma in which ovalbumin was used as an antigen, both the in vivo AHR and the in vitro BSM hyperresponsiveness have also been shown (9, 10). These observations remind us of an idea that the hyper-contractility of BSM per se is a cause of the AHR. Indeed, the hyperresponsiveness of airway smooth muscles was also suggested in asthmatics (11). At least, the BSM hyperresponsiveness to ACh observed in the antigen-induced AHR animals is not explained simply by changes in its receptor number: no significant difference in the muscarinic receptor density was observed between the AHR and control animals (12). Furthermore, the ACh-mediated increase in cytosolic Ca2+ concentration measured using the Fura-2-loaded BSMs was within normal level whereas the contraction induced by ACh was much enhanced in the AHR animals (13).
We have tried to uncover the mechanism of the BSM hyperresponsiveness in allergic asthma. We shows evidence that the agonist-induced Ca2+ sensitization of contraction is augmented in BSMs of the AHR animals (14). In BSMs that were pre-incubated with ACh (10-3 M) under Ca2+-free condition (in the presence of 10-6 M nicardipine and 0.05 mM EGTA), addition of Ca2+ induced a concentration-dependent contraction. The contractile response to Ca2+ of the ACh-stimulated BSMs isolated from the OA-challenged mice was markedly augmented as compared to that from the sensitized control animals. By contrast, no significant difference in the response to Ca2+ of BSMs depolarized with 60 mM K+ (in the absence of nicardipine and presence of 0.05 mM EGTA and 10-6 M atropine) was observed between the groups. These findings suggest that, although the contraction mediated by Ca2+ itself is not changed, the ACh-mediated contractile signaling independent of cytosolic Ca2+ concentration, i.e., Ca2+ sensitization of contraction, is augmented in BSMs of the AHR animals. To confirm it in more detail, the BSM contractility was also determined by using α-toxin-permeabilized BSM preparations in mice (9). When the Ca2+ concentration was clamped at pCa 6.0, application of ACh (10-5–10-3 M) in the presence of GTP (10-4 M) caused a further contraction, i.e., ACh-induced Ca2+ sensitization, in an ACh concentration-dependent manner. The ACh-induced Ca2+ sensitization was significantly greater in BSMs of the AHR mice as compared to those of control animals. Similar results were also obtained when a rat model of antigen-induced AHR was used (15). It is thus strongly suggested that the Ca2+ sensitization of contraction is augmented in BSMs of the AHR animals.
RhoA/ROCK pathway as a therapeutic target of asthma
Increased bronchial tone plays an important role in the pathophysiology of airway diseases including asthma. Bronchial tone is mainly regulated by the contraction of BSM cells (BSMCs). Smooth muscle contraction is mediated by the phosphorylation of the regulatory myosin light chain (MLC). The MLC phosphorylation level increases when MLC kinase (MLCK) is activated, whereas the level decreases when MLC phosphatase (MLCP) is activated (16). MLCP dephosphorylates MLC, leading to the smooth muscle relaxation (17). The MLCP activity is highly regulated both by contraction and relaxation signaling pathways. A monomeric GTPase RhoA plays a key role in the Ca2+ sensitization of contraction in smooth muscles. Contractile agonists, such as G protein-coupled receptor (GPCR) agonists, have an ability to activate RhoA. The precise nature of the activation of RhoA by GPCR is not yet uncovered but involves guanine nucleotide exchange factors RhoGEFs, such as p115RhoGEF, PDZ-RhoGEF and LARG (18). The RhoGEFs activate RhoA by exchanging GDP- to GTP-bound form of RhoA (19). The activated GTP-bound form of RhoA activates its downstream Rho associated coiled-coil containing protein kinases (ROCKs: also called as Rho-kinases) (20,21,22), which in turn phosphorylates myosin phosphatase targeting protein (MYPT), leading to an inhibition of MLCP activity (23). When the MLC phosphatase is inhibited, the phosphorylated MLC cannot be dephosphorylated, resulting in a promotion of contractile state, that is Ca2+ sensitization of smooth muscle contraction.
As described above, the ACh-induced Ca2+ sensitization was significantly augmented in BSMs of mice with allergic asthma. The augmented Ca2+ sensitization was sensitive to Clostridiumbotulinum C3 exoenzyme, an inhibitor of RhoA, and Y-27632, an inhibitor of ROCK (15, 24), indicating that the RhoA/ROCK pathway is involved in the Ca2+ sensitizing signaling. Interestingly, protein expression of RhoA in the BSMs was markedly increased in rat models of allergic asthma (9, 15). In addition, when BSMs were stimulated with contractile GPCR agonists such as ACh and endothelin-1, a higher expression of active form of RhoA was observed in the AHR animals (25). An augmented RhoA-mediated Ca2+ sensitization in smooth muscle contraction has been reported in experimental animal models of diseases such as hypertension (11, 15, 26), and coronary (9, 27, 28) and cerebral (29,30,31) vasospasms. Thus, the signaling of RhoA and its downstream ROCKs are now considered as a therapeutic target of asthma (28,29,30,31), although the exact mechanism of up-regulation of RhoA is still unclear.
Inflammatory cytokines upregulate RhoA expression in BSMs
Pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) could mediate the inflammatory response in asthma and are linked to the disease severity. IL-1β and TNF-α have been shown to play a prominent role in developing airway responsiveness and airway inflammation in bronchial asthma. Increased amounts of these cytokines have been detected in bronchoalveolar lavage (BAL) fluid (32, 33), and in the culture supernatants of alveolar macrophages from asthmatic patients (34, 35). Furthermore, it has been reported that inhaled TNF-α enhanced airway responsiveness to methacholine in humans (36). Therefore, we examined the effect of TNF-α on the BSM contractility. Treatment of rat BSMs with TNF-α resulted in a significant upward shift of the concentration-response curve to ACh, but not to high K+, compared with control tissues. The TNF-α-induced hyperresponsiveness to ACh was completely blocked by a p42/44 MAPK inhibitor, U0126, and a protein synthesis inhibitor, cycloheximide, but not by a p38 MAPK inhibitor, SB203580. TNF-α also caused a phosphorylation of p42/44 MAPK and an upregulation of RhoA in the BSMs: the TNF-α-induced upregulation of RhoA was abolished by U0126 pretreatment (37).
In addition, cytokines derived from Th2 lymphocytes, including IL-4, IL-5, IL-9, IL-13 and IL-25, play a key role in the pathophysiology of. In the AHR rat model, IL-4, IL-6 and IL-13 in the BAL fluid were markedly and significantly increased compared with the control rats (38). An increased expression of IL-4 has been demonstrated in the BAL fluid after segmental allergen challenge to asthmatic patients (39). IL-4 promotes eosinophilic airway inflammation by increasing eotaxin expression and inhibiting eosinophil apoptosis (40). IL-4 induces mucus hypersecretion (41), which also contributes to the airway obstruction. Interestingly, IL-4 also acts on airway smooth muscles directly, and can cause hyperresponsiveness of airway smooth muscles (42). We thus examined the effect of IL-4 on the expression level of RhoA in cultured human BSMCs (hBSMCs). Incubation of hBSMCs with IL-4 induced a distinct phosphorylation of signal transducer and activator of transcription 6 (STAT6), a major signal transducer activated by IL-4, indicating that IL-4 is capable of activating signal transduction in hBSMCs directly. IL-4 also induced a significant increase in the expression level of RhoA (43).
There is increasing evidence that IL-13 is also a central mediator of AHR induction (44,45,46,47). The human IL-13 gene is located on chromosome 5q in a region that has been linked to asthma (48, 49). An increased expression of IL-13 has been demonstrated in BAL cells obtained from patients with symptomatic asthma (50, 51). In addition, overexpression of IL-13 in mouse airway epithelial cells using the Clara cell 10-kD protein gene promoter induced AHR to aerosolized methacholine (52). Intratracheal instillation of recombinant IL-13 to naive mice also evoked AHR to inhaled methacholine (53) and intravenously administered ACh (44). To elucidate the role of IL-13 in the induction of BSM hyperresponsiveness, the effects of IL-13 on contractility and RhoA expression in BSMs were investigated. In vivo treatment of airways with IL-13 by intranasal instillation induced a BSM hyperresponsiveness with RhoA upregulation in BSMs of naive mice. Moreover, IL-13 induced RhoA upregulation. The IL-13-induced upregulation of RhoA was inhibited by leflunomide, a STAT6 inhibitor, in cultured hBSMCs (54).
Involvement of microRNAs (miRNA) in RhoA expression in smooth muscle cells
MicroRNAs (miRNAs), a class of small non-coding RNA, are associated with a variety of basic biological processes (55,56,57,58). Mature miRNAs regulate the expression of protein-coding genes by targeting their mRNA, leading to translational inhibition or RNA degradation (59). Interestingly, recent studies also revealed a possible involvement of miRNAs in the contraction of vascular smooth muscle cells (60) and myometrial cells (61) by modulating gene expression.
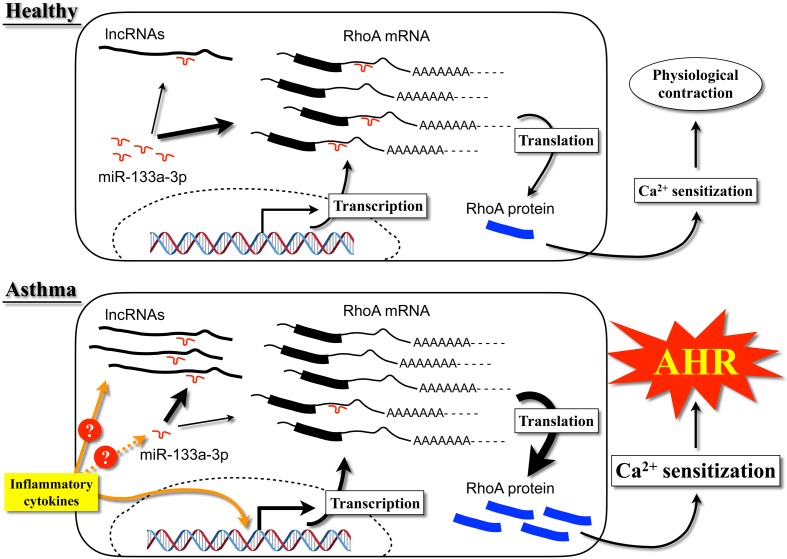
The transcriptional/translational mechanism of RhoA is not well understood. However, it has been suggested that miR-133 negatively regulates RhoA expression in cardiomyocytes (62). RNA-hybrid analysis (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) (63) of human and mouse RhoA mRNA revealed putative binding sites of miR-133a in the 3′-untranslated region (64). Based on this information, we tested the hypothesis that downregulation of miR-133 could induce RhoA upregulation in BSMs. As a result, transfection of BSM cells with a miR-133a antagomir upregulated RhoA protein expression, whereas transfection with pre-miR133a downregulated it (64), suggesting that RhoA protein expression is negatively regulated by miR-133a in BSMs. Interestingly, our data also demonstrated that IL-13 directly acts on BSM cells to cause downregulation of miR-133a and upregulation of RhoA protein (54, 65). It is thus possible that the increased IL-13 level in the airways of asthmatics causes a downregulation of miR-133a, resulting in an upregulation of RhoA protein to induce the augmented BSM contractility, one of the causes of the AHR in allergic bronchial asthma. These findings provide new insight into the role of miR-133a in the BSM contractility and suggest that the miR-133a/RhoA pathway is a putative therapeutic target for asthma (Fig. 1).
Fig. 1.
Schematic diagram of the mechanism of upregulation of RhoA protein in BSM cells of asthmatics.
Involvement of CPI-17 in smooth muscle contraction
PKC-potentiated inhibitory protein for heterotrimeric MLCP of 17 kDa (CPI-17), which is activated by PKC and acts on an MLCP-specific target, was isolated from pig aorta smooth muscle extracts (66). CPI-17 expression is highly limited to smooth muscle tissues (67). When a contractile agonist binds to GPCR, PKC is activated via an increase in diacylglycerol (DG) and in turn phosphorylates CPI-17. Activated CPI-17 induces MLCP inhibition. CPI-17 is therefore important for the PKC-mediated Ca2+ sensitization in rabbit arterial smooth muscles (68, 69), human bladder smooth muscles (70), intestinal smooth muscles of rat (71) and mouse (72), human myometrium (73) and rat BSMs (74,75,76).
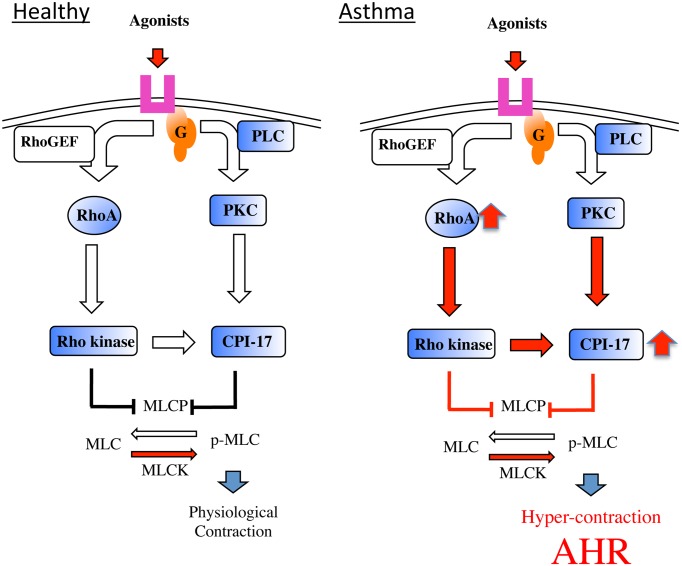
In BSM of AHR animals, the mRNA and protein levels of CPI-17 were significantly increased compared with the controls (77). Upon contractile agonist stimulation of hyperresponsive BSMs PKC/CPI-17 signaling activity increased by CPI-17 upregulation. Indeed, the phorbol 12,13-dibutyrate-mediated contraction was markedly augmented in BSMs of AHR animals (78). ACh-induced phosphorylation of CPI-17 at Thr38 was significantly increased in BSMs of AHR animals. Interestingly, pretreatment of AHR animals with Y-27632 or calphostin C, a PKC inhibitor, inhibited the ACh-induced phosphorylation of CPI-17 in bronchial tissues. Moreover, this pretreatment inhibited the ACh-induced phosphorylation of MLC (74). The inhibitory effects of Y-27632 and calphostin C on agonist-induced phosphorylation of CPI-17 have also been examined in rabbit femoral arterial smooth muscles (79). Treatment with glucocorticoids (prednisolone or beclomethasone) significantly inhibited the AHR, and markedly reduced both the protein and mRNA levels of CPI-17 in BSMs. The ACh-induced activation of CPI-17 was also significantly inhibited by glucocorticoids. Glucocorticoids prevented the augmented ACh-induced MLC phosphorylation observed in rat AHR (80). Therefore, glucocorticoids may inhibit AHR through inhibition of CPI-17 overexpression and activation (80). Although the transcription factors involved in CPI-17 expression in BSMs are unclear, an increase in the PKC/CPI-17-mediated signaling might be involved in the augmented BSM. Thus, in addition to the RhoA-mediated signaling described above, the CPI-mediated signaling is also a putative therapeutic target for the AHR in asthmatics (Fig. 2).
Fig. 2.
Schematic diagram of the mechanism of BSM hyper-contraction, one of the causes of the airway hyperresponsiveness (AHR) in asthmatics.
Conclusions
We propose that both the upregulations of RhoA and CPI-17 are associated with increased BSM contraction in asthma. Therefore, MLCP inhibitory signaling pathways via RhoA/ROCK, PKC/CPI-17 and their combination may be potential targets for new treatment of AHR in asthma.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001; 344(5): 350–62. doi: 10.1056/NEJM200102013440507 [DOI] [PubMed] [Google Scholar]
- 2.Girodet PO, Ozier A, Trian T, Begueret H, Ousova O, Vernejoux JM, Chanez P, Marthan R, Berger P, Tunon de Lara JM. Mast cell adhesion to bronchial smooth muscle in asthma specifically depends on CD51 and CD44 variant 6. Allergy. 2010; 65(8): 1004–12. doi: 10.1111/j.1398-9995.2009.02308.x [DOI] [PubMed] [Google Scholar]
- 3.Denis D, Fayon MJ, Berger P, Molimard M, De Lara MT, Roux E, Marthan R. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr Res. 2001; 50(4): 515–9. doi: 10.1203/00006450-200110000-00015 [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977; 7(3): 235–43. doi: 10.1111/j.1365-2222.1977.tb01448.x [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Frith PA, Hargreave FE. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981; 36(8): 575–9. doi: 10.1136/thx.36.8.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis. 1987; 70(3): 171–9. [PubMed] [Google Scholar]
- 7.James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989; 139(1): 242–6. doi: 10.1164/ajrccm/139.1.242 [DOI] [PubMed] [Google Scholar]
- 8.Misawa M, Chiba Y. Repeated antigenic challenge-induced airway hyperresponsiveness and airway inflammation in actively sensitized rats. Jpn J Pharmacol. 1993; 61(1): 41–50. doi: 10.1254/jjp.61.41 [DOI] [PubMed] [Google Scholar]
- 9.Chiba Y, Ueno A, Shinozaki K, Takeyama H, Nakazawa S, Sakai H, Misawa M. Involvement of RhoA-mediated Ca2+ sensitization in antigen-induced bronchial smooth muscle hyperresponsiveness in mice. Respir Res. 2005; 6: 4. doi: 10.1186/1465-9921-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato Y, Manabe T, Tanaka Y, Mochizuki H. Effect of an orally active Th1/Th2 balance modulator, M50367, on IgE production, eosinophilia, and airway hyperresponsiveness in mice. J Immunol. 1999; 162(12): 7470–9. [PubMed] [Google Scholar]
- 11.Seow CY, Schellenberg RR, Paré PD. Structural and functional changes in the airway smooth muscle of asthmatic subjects. Am J Respir Crit Care Med. 1998; 158(5 Pt 3): S179–86. doi: 10.1164/ajrccm.158.supplement_2.13tac160 [DOI] [PubMed] [Google Scholar]
- 12.Chiba Y, Misawa M. Characteristics of muscarinic cholinoceptors in airways of antigen-induced airway hyperresponsive rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995; 111(3): 351–7. doi: 10.1016/0742-8413(95)00061-5 [DOI] [PubMed] [Google Scholar]
- 13.Chiba Y, Sakai H, Suenaga H, Kamata K, Misawa M. Enhanced Ca2+ sensitization of the bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Res Commun Mol Pathol Pharmacol. 1999; 106(1-2): 77–85. [PubMed] [Google Scholar]
- 14.Chiba Y, Misawa M. Alteration in Ca2+ availability involved in antigen-induced airway hyperresponsiveness in rats. Eur J Pharmacol. 1995; 278(1): 79–82. doi: 10.1016/0014-2999(95)00132-5 [DOI] [PubMed] [Google Scholar]
- 15.Chiba Y, Takada Y, Miyamoto S, MitsuiSaito M, Karaki H, Misawa M. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br J Pharmacol. 1999; 127(3): 597–600. doi: 10.1038/sj.bjp.0702585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372(6503): 231–6. doi: 10.1038/372231a0 [DOI] [PubMed] [Google Scholar]
- 17.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004; 279(36): 37211–4. doi: 10.1074/jbc.R400018200 [DOI] [PubMed] [Google Scholar]
- 18.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 19.Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K, Takuwa Y. Involvement of rho in GTP gamma S-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Lett. 1995; 367(3): 246–50. doi: 10.1016/0014-5793(95)00573-R [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996; 15(9): 2208–16. [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997; 404(2-3): 118–24. doi: 10.1016/S0014-5793(97)00107-5 [DOI] [PubMed] [Google Scholar]
- 22.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995; 270(49): 29051–4. doi: 10.1074/jbc.270.49.29051 [DOI] [PubMed] [Google Scholar]
- 23.Murányi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005; 579(29): 6611–5. doi: 10.1016/j.febslet.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 24.Chiba Y, Takeyama H, Sakai H, Misawa M. Effects of Y-27632 on acetylcholine-induced contraction of intact and permeabilized intrapulmonary bronchial smooth muscles in rats. Eur J Pharmacol. 2001; 427(1): 77–82. doi: 10.1016/S0014-2999(01)01225-0 [DOI] [PubMed] [Google Scholar]
- 25.Chiba Y, Sakai H, Misawa M. Augmented acetylcholine-induced translocation of RhoA in bronchial smooth muscle from antigen-induced airway hyperresponsive rats. Br J Pharmacol. 2001; 133(6): 886–90. doi: 10.1038/sj.bjp.0704137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin JG, Duguet A, Eidelman DH. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J. 2000; 16(2): 349–54. doi: 10.1034/j.1399-3003.2000.16b25.x [DOI] [PubMed] [Google Scholar]
- 27.Schembri F, Sridhar S, Perdomo C, Gustafson AM, Zhang X, Ergun A, Lu J, Liu G, Zhang X, Bowers J, Vaziri C, Ott K, Sensinger K, Collins JJ, Brody JS, Getts R, Lenburg ME, Spira A. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci USA. 2009; 106(7): 2319–24. doi: 10.1073/pnas.0806383106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosens R, Schaafsma D, Nelemans SA, Halayko AJ. Rho-kinase as a drug target for the treatment of airway hyperrespon-siveness in asthma. Mini Rev Med Chem. 2006; 6(3): 339–48. doi: 10.2174/138955706776073402 [DOI] [PubMed] [Google Scholar]
- 29.Schaafsma D, Gosens R, Zaagsma J, Halayko AJ, Meurs H. Rho kinase inhibitors: a novel therapeutical intervention in asthma? Eur J Pharmacol. 2008; 585(2-3): 398–406. doi: 10.1016/j.ejphar.2008.01.056 [DOI] [PubMed] [Google Scholar]
- 30.Schaafsma D, Roscioni SS, Meurs H, Schmidt M. Monomeric G-proteins as signal transducers in airway physiology and pathophysiology. Cell Signal. 2008; 20(10): 1705–14. doi: 10.1016/j.cellsig.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 31.Kume H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem. 2008; 15(27): 2876–85. doi: 10.2174/092986708786242831 [DOI] [PubMed] [Google Scholar]
- 32.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992; 89(5): 958–67. doi: 10.1016/0091-6749(92)90218-Q [DOI] [PubMed] [Google Scholar]
- 33.Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, Durham SR, Kay AB, Hamid Q. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy. 1991; 21(6): 745–50. doi: 10.1111/j.1365-2222.1991.tb03205.x [DOI] [PubMed] [Google Scholar]
- 34.Gosset P, Tsicopoulos A, Wallaert B, Vannimenus C, Joseph M, Tonnel AB, Capron A. Increased secretion of tumor necrosis factor alpha and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol. 1991; 88(4): 561–71. doi: 10.1016/0091-6749(91)90149-I [DOI] [PubMed] [Google Scholar]
- 35.Azevedo I, de Blic J, Dumarey CH, Scheinmann P, Vargaftig BB, Bachelet M. Increased spontaneous release of tumour necrosis factor-alpha by alveolar macrophages from wheezy infants. Eur Respir J. 1997; 10(8): 1767–73. doi: 10.1183/09031936.97.10081767 [DOI] [PubMed] [Google Scholar]
- 36.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995; 152(1): 76–80. doi: 10.1164/ajrccm.152.1.7599866 [DOI] [PubMed] [Google Scholar]
- 37.Sakai H, Otogoto S, Chiba Y, Abe K, Misawa M. Involvement of p42/44 MAPK and RhoA protein in augmentation of ACh-induced bronchial smooth muscle contraction by TNF-alpha in rats. J Appl Physiol 1985. 2004; 97(6): 2154–9. doi: 10.1152/japplphysiol.00752.2003 [DOI] [PubMed] [Google Scholar]
- 38.Chiba Y, Arima J, Sakai H, Misawa M. Lovastatin inhibits bronchial hyperresponsiveness by reducing RhoA signaling in rat allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2008; 294(4): L705–13. doi: 10.1152/ajplung.00531.2007 [DOI] [PubMed] [Google Scholar]
- 39.Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004; 34(3): 437–44. doi: 10.1111/j.1365-2222.2004.01885.x [DOI] [PubMed] [Google Scholar]
- 40.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001; 2(2): 66–70. doi: 10.1186/rr40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999; 162(10): 6233–7. [PubMed] [Google Scholar]
- 42.Bryborn M, Adner M, Cardell LO. Interleukin-4 increases murine airway response to kinins, via up-regulation of bradykinin B1-receptors and altered signalling along mitogen-activated protein kinase pathways. Clin Exp Allergy. 2004; 34(8): 1291–8. doi: 10.1111/j.1365-2222.2004.02031.x [DOI] [PubMed] [Google Scholar]
- 43.Chiba Y, Sakai H, Wachi H, Sugitani H, Seyama Y, Misawa M. Upregulation of rhoA mRNA in bronchial smooth muscle of antigen-induced airway hyperresponsive rats. J Smooth Muscle Res. 2003; 39(6): 221–8. doi: 10.1540/jsmr.39.221 [DOI] [PubMed] [Google Scholar]
- 44.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282(5397): 2258–61. doi: 10.1126/science.282.5397.2258 [DOI] [PubMed] [Google Scholar]
- 45.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282(5397): 2261–3. doi: 10.1126/science.282.5397.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001; 167(8): 4668–75. doi: 10.4049/jimmunol.167.8.4668 [DOI] [PubMed] [Google Scholar]
- 47.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004; 304(5677): 1678–82. doi: 10.1126/science.1095336 [DOI] [PubMed] [Google Scholar]
- 48.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995; 333(14): 894–900. doi: 10.1056/NEJM199510053331402 [DOI] [PubMed] [Google Scholar]
- 49.Palmer LJ, Daniels SE, Rye PJ, Gibson NA, Tay GK, Cookson WO, Goldblatt J, Burton PR, LeSöuef PN. Linkage of chromosome 5q and 11q gene markers to asthma-associated quantitative traits in Australian children. Am J Respir Crit Care Med. 1998; 158(6): 1825–30. doi: 10.1164/ajrccm.158.6.9804037 [DOI] [PubMed] [Google Scholar]
- 50.Bodey KJ, Semper AE, Redington AE, Madden J, Teran LM, Holgate ST, Frew AJ. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy. 1999; 54(10): 1083–93. doi: 10.1034/j.1398-9995.1999.00889.x [DOI] [PubMed] [Google Scholar]
- 51.Prieto J, Lensmar C, Roquet A, van der Ploeg I, Gigliotti D, Eklund A, Grunewald J. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir Med. 2000; 94(8): 806–14. doi: 10.1053/rmed.2000.0826 [DOI] [PubMed] [Google Scholar]
- 52.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999; 103(6): 779–88. doi: 10.1172/JCI5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol. 2001; 25(4): 522–30. doi: 10.1165/ajrcmb.25.4.4620 [DOI] [PubMed] [Google Scholar]
- 54.Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009; 40(2): 159–67. doi: 10.1165/rcmb.2008-0162OC [DOI] [PubMed] [Google Scholar]
- 55.Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trends Genet. 2004; 20(12): 617–24. doi: 10.1016/j.tig.2004.09.010 [DOI] [PubMed] [Google Scholar]
- 56.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005; 33(4): 1290–7. doi: 10.1093/nar/gki200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005; 102(50): 18081–6. doi: 10.1073/pnas.0506216102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA. 2005; 102(6): 1865–70. doi: 10.1073/pnas.0409764102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010; 466(7308): 835–40. doi: 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, Lieberman J, Van Aelst L, Lagna G, Hata A. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012; 287(6): 3976–86. doi: 10.1074/jbc.M111.303156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012; 26(11): 1857–67. doi: 10.1210/me.2012-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007; 13(5): 613–8. doi: 10.1038/nm1582 [DOI] [PubMed] [Google Scholar]
- 63.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004; 10(10): 1507–17. doi: 10.1261/rna.5248604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009; 180(8): 713–9. doi: 10.1164/rccm.200903-0325OC [DOI] [PubMed] [Google Scholar]
- 65.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009; 41(5): 516–24. doi: 10.1165/rcmb.2008-0163OC [DOI] [PubMed] [Google Scholar]
- 66.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995; 118(6): 1104–7. doi: 10.1093/oxfordjournals.jbchem.a124993 [DOI] [PubMed] [Google Scholar]
- 67.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001; 535(Pt 2): 553–64. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003; 546(Pt 3): 879–89. doi: 10.1113/jphysiol.2002.029306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007; 100(1): 121–9. doi: 10.1161/01.RES.0000253902.90489.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol. 2004; 172(2): 748–52. doi: 10.1097/01.ju.0000130419.32165.6b [DOI] [PubMed] [Google Scholar]
- 71.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003; 278(49): 48794–804. doi: 10.1074/jbc.M310166200 [DOI] [PubMed] [Google Scholar]
- 72.Ihara E, Chappellaz M, Turner SR, MacDonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil. 2012; 24(1): e15–26. doi: 10.1111/j.1365-2982.2011.01821.x [DOI] [PubMed] [Google Scholar]
- 73.Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol. 2003; 140(7): 1303–12. doi: 10.1038/sj.bjp.0705552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakai H, Hirano T, Takeyama H, Chiba Y, Misawa M. Acetylcholine-induced phosphorylation of CPI-17 in rat bronchial smooth muscle: the roles of Rho-kinase and protein kinase C. Can J Physiol Pharmacol. 2005; 83(4): 375–81. doi: 10.1139/y05-022 [DOI] [PubMed] [Google Scholar]
- 75.Sakai H, Hirano T, Chiba Y, Misawa M. Acetylcholine-induced phosphorylation and membrane translocation of CPI-17 in bronchial smooth muscle of rats. Am J Physiol Lung Cell Mol Physiol. 2005; 289(6): L925–30. doi: 10.1152/ajplung.00054.2005 [DOI] [PubMed] [Google Scholar]
- 76.Sakai H, Chiba Y, Misawa M. Role of Rho kinase in endothelin-1-induced phosphorylation of CPI-17 in rat bronchial smooth muscle. Pulm Pharmacol Ther. 2007; 20(6): 734–9. doi: 10.1016/j.pupt.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 77.Sakai H, Chiba Y, Hirano T, Misawa M. Possible involvement of CPI-17 in augmented bronchial smooth muscle contraction in antigen-induced airway hyper-responsive rats. Mol Pharmacol. 2005; 68(1): 145–51. [DOI] [PubMed] [Google Scholar]
- 78.Sakai H, Kurihara Y, Hashimoto Y, Chiba Y, Misawa M. Augmented PDBu-mediated contraction of bronchial smooth muscle of mice with antigen-induced airway hyperresponsiveness. J Smooth Muscle Res. 2010; 46(5): 259–66. doi: 10.1540/jsmr.46.259 [DOI] [PubMed] [Google Scholar]
- 79.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000; 275(14): 9897–900. doi: 10.1074/jbc.275.14.9897 [DOI] [PubMed] [Google Scholar]
- 80.Goto K, Chiba Y, Sakai H, Misawa M. Glucocorticoids inhibited airway hyperresponsiveness through downregulation of CPI-17 in bronchial smooth muscle. Eur J Pharmacol. 2008; 591(1-3): 231–6. doi: 10.1016/j.ejphar.2008.06.021 [DOI] [PubMed] [Google Scholar]