Abstract
Hypopharyngeal carcinoma is one of the worst prognostic malignancies among head and neck carcinomas. Therefore, a good biomarker should be identified to predict the best therapeutic option before starting the treatment. In cell models, p62/SQSTM1 levels affected the Nrf2-Keap1 pathway, ROS levels, GSH/GSSG ratios and cell growth, especially under irradiation rather than under CDDP exposure, which was toxic despite p62/SQSTM1 status. In a clinical cohort of hypopharyngeal carcinomas, high levels of p62/SQSTM1 significantly predicted poor prognosis (log-rank test, Chi-square value = 6.750, P = 0.0094) and maximum critical risk (Cox proportional hazard ratio = 4.405, P = 0.0086), especially in the radiotherapy group. Therefore, when p62/SQSTM1 is elevated in the biopsy section, hypopharyngeal carcinoma should be treated with surgical and/or chemotherapeutic options.
Keywords: p62/SQSTM1, hypopharyngeal carcinoma, head and neck cancer, radiotherapy resistance
Introduction
Hypopharyngeal carcinoma is the malignancy with the worst prognosis among all head and neck squamous cell carcinomas (HNSCCs) [1-3]. Low-grade small tumors are usually treated with ionizing radiation for larynx preservation, whereas few cases can be treated with partial pharyngectomy; other in situ cases in the mucosal surface are treated with endoscopic submucosal dissection [4]. However, recurrence leading to poor prognosis is often observed after the initial treatment, and surgical salvage after failure of radiation therapy has a lower success rate for hypopharyngeal cancers than for cancers in other sites [1,2]. In addition, the recurrent cancer is usually more malignant than the starting one and is often unresectable [1,3]. So, it is necessary to evaluate the susceptibility to radiotherapy and chemotherapy of each case of hypopharyngeal carcinoma and its prognosis, before starting various treatments.
In oral squamous cell carcinomas, p62/SQSTM1 expression is a potential marker to identify carcinogenesis, therapeutic resistance or poor prognosis, and it is a key player in glutathione (GSH) induction [5]. Defective autophagic lysosomal pathway leads to accumulation of p62/SQSTM1, which promotes oncogenesis by itself and genome instability [6]. Otherwise, p62/SQSTM1 stabilizes Nrf2, thus inducing the associated antioxidant proteins, NAD(P)H dehydrogenase quinone 1 (NQO1) and heme oxygenase-1 (HO-1) and leading to the resistance to antioxidative stress [7]. Cumulative Nrf2 mutations have been frequently observed in head and neck cancers, and alterations in the Nrf2 pathway might play an important role in tissues exposed to abundant reactive oxygen species (ROS), such as oral, nasopharyngeal and tracheal epithelium [8].
In the present study, we investigated whether p62/SQSTM1 could be a potential marker of prognosis or a therapeutic option in a cohort of hypopharyngeal carcinomas. In hypopharyngeal carcinoma cell models, p62/SQSTM1 downregulation was not correlated with chemotherapy resistance, but with radiotherapy resistance. Therefore, we propose p62/SQSTM1 status as a predictive biomarker for radiotherapy resistance in hypopharyngeal carcinomas.
Materials and methods
Tissue samples
Fifty-four primary hypopharyngeal carcinomas were obtained by biopsy at the Kyoto Prefectural University of Medicine from 1997 to 2008. The tumor samples were all squamous cell carcinomas, obtained from 7 females and 47 males with a mean age of 65.1 years (range 45-81 yrs). Four cases were stage I, six cases were stage II, four cases were stage III and forty cases were stage IV.
Twenty-two cases were treated with total pharyngolaryngectomy followed by irradiation (R); 19 cases were stage IV, and 2 cases were stage III. One case, T2N2bM0, was treated with partial pharyngectomy followed by chemoradiotherapy. Twenty cases were treated primarily with R without surgical resection; four cases were stage I, six were stage II, two were stage III, and eight were stage IV. Twelve cases were firstly treated with chemoradiotherapy and followed by neck dissection; all cases were stage IV. Data were collected from clinical and pathological records with the written informed consent from individual patients and after approval by the Ethics Committee of the institute.
Immunohistochemistry
Surgical specimens were transferred to 10% buffered formalin and fixed overnight. The fixed samples were embedded in paraffin, and serially sliced into 5-µm sections. After dewaxing, sections were autoclaved at 120°C for 1 min in 10 mM sodium citrate buffer (pH 6.0) and immersed in 0.3% H2O2. They were then incubated overnight at 4°C with primary antibodies against p62/SQSTM1 (diluted 1:500, 5F2, MBL, Aichi, Japan), Ki67 (diluted 1:50, MM-1, Leica Microsystems, Tokyo, Japan) and Nrf2 (diluted 1:200, ab31163, Abcam, Tokyo, Japan). The sections were rinsed with PBS and incubated with the secondary antibody conjugated with horseradish peroxidase (Simple Stain MAX-PO; Nichirei, Tokyo, Japan) at room temperature for 1 h. The sections were then stained with 3.3’-diaminobenzidine tetrahydrochloride and counter-stained with hematoxylin.
Microscopic evaluation
The stainings were evaluated by two blinded independent observers. A positive expression of p62/SQSTM1 was defined when the cytoplasm and/or nuclei were stained with DAB. Positive staining for Ki67 was defined when more than 40% of tumor cells showed nuclear expression of the antigen. The staining for Nrf2 was classified as positive, according to nuclear staining of cancer cells (Figure 1).
Figure 1.
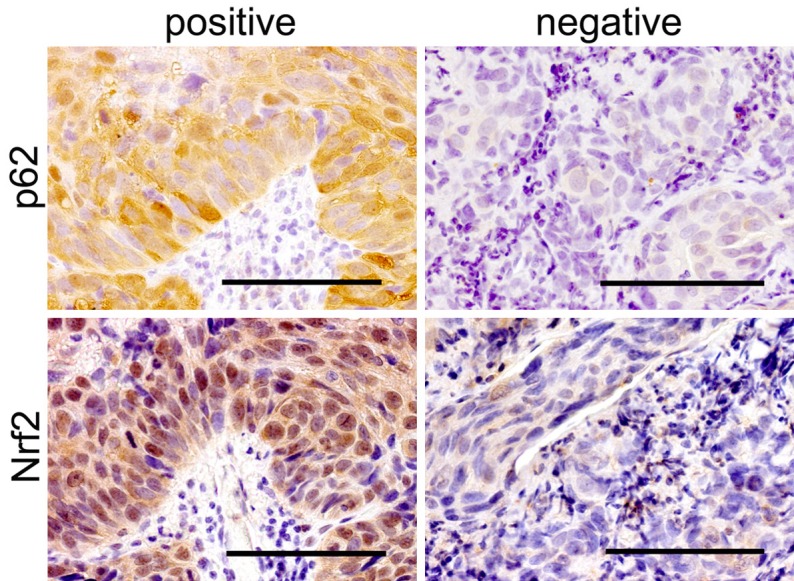
Representative staining of p62/SQSTM1 and Nrf2. Representative pictures showing positive (left) and negative (right) cases of p62/SQSTM1 (upper) and Nrf2 (lower). Scale bar, 100 µm.
Cell cultures
Human hypopharyngeal carcinoma cell lines, FaDu and D-562, were purchased from American Type Culture Collection. These cell lines were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (DMEM/10% FBS). Cell culture media were supplemented with penicillin (50 units/mL) and streptomycin (50 mg/mL), and incubated at 37°C in a humidified chamber supplemented with 5% CO2.
Preparation of recombinant lentiviruses encoding p62/SQSTM1 shRNA
Vectors for p62/SQSTM1 shRNA (sh-p62 (1) and (2)) and for control (sh-control) were purchased from Dharmacon (GE Healthcare UK Ltd, England). Mature antisense sequences for p62/SQSTM1 were ATCAACTTCAATGCCCAGAGG and TTCTCTTTAATGTAGATTCGG (available on the Dharmacon website). Lentivirus for each shRNA was prepared with each packaging mixture, according to the manufacturer’s instruction. FaDu and D562 cells transduced with > 10 MOI of each lentivirus were selected in the presence of 3 μg/mL puromycin, expanded and used in the experiments.
X-ray irradiation
For X-ray irradiation, subconfluent FaDu or D562 cancer cells in 6-well plates or 96-well plates containing DMEM/10% FBS were exposed to X-rays (5-10 Gy, at 2 Gy/min) using a 150-kVp X-ray generator (Model MBR-1520R, Hitachi, Tokyo, Japan) with a total filtration of 0.5 mm aluminum filter, plus a 0.1-mm copper filter, and then incubated at 37°C in a conventional humidified 5% CO2 incubator.
Cis-platinum (II) diammine dichloride (CDDP) exposure
CDDP was provided by NIPPON KAYAKU (Tokyo, Japan), dissolved in N, N-dimethylformamide, and diluted 1:200 with purified water. The final concentration of 1.25-10 nM CDDP was added to FaDu and D-562 cells.
WST-8 assay for cell viability
FaDu and D-562 cells were cultured in 96-well plates at a density of 1×103 per well, and cell viability assays were carried out with a Cell Counting Kit-8 (Dojindo Molecular Technologies Inc, Kumamoto, Japan). WST-8 reagent solution was added to each well, and absorbance at 450 nm (OD450) was then measured by a microplate reader after incubation with the reagent for 2 h at 37°C, in accordance with the manufacturer’s instructions.
Protein extractions and Western blot analysis
The proteins of each cell line were analyzed by immunoblotting. Proteins were extracted in ice-cold lysis buffer (40 mM HEPES [pH 7.5], 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, 0.5 mM orthovanadate, and EDTA-free protease inhibitors; Roche, Switzerland) containing 1% Triton X-100. Cell extracts were kept on ice for 20 min and centrifuged at 12,000 rpm for 10 min (4°C). The supernatants were boiled with SDS sample buffer. Proteins separated by SDS-PAGE were transferred to polyvinylidene difluoride filters. After blocking the filters with TBS-T (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.1% Tween 20) containing 5% bovine serum albumin (BSA), the filters were incubated for 1-2 h with the primary antibodies in TBS-T containing 2% BSA. Primary antibodies for p62/SQSTM1 (5F2, MBL), Keap1 (10503-2-AP, Proteintech, IL, USA), Nrf2 (EP1808Y, Epitomics, CA, USA), NQO1 (ab28947, Abcam, MA, USA), HO-1 (ab13248, Abcam) and α-tubulin (DM 1A, Sigma, MO, USA) were used with 1:250-2000 dilutions. The filters were then washed in TBS-T and incubated for 30 min in horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (GE Healthcare) diluted 1:10,000 in TBS-T containing 2% BSA. After several washes with TBS-T, immunoreactivity was detected using the Luminata Classico Western HRP substrate (Merck Millipore Corporation, Germany) with LAS4000 (Fujifilm, Tokyo, Japan).
ROS assays
ROS levels were detected with CellROX® Green reagent (Life technologies, Thermo, CA, USA) at 485/520 nm. The cells were stained with 5 μM of CellROX® Green Reagent by adding it to the complete medium and incubating the cells at 37°C for 30 min. The cells were then washed with PBS, harvested after trypsinization, immersed in Live cell imaging® reagents (Life technologies), and analyzed on Attune® Acoustic Focusing Cytometer (Life technologies).
GSH/GSSG assay
GSH and GSH-S-S-GSH (GSSG) were determined with a quantification kit (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. The treated cells were lysed by the addition of 80 μL of 10 mM HCl and by freezing and thawing, twice. Salicylsalicylic acid (20 μL, 5%) was added to the homogenate, and the mixture was centrifuged at 8,000×g for 10 min. GSH and GSSG levels in the supernatant were determined by measuring absorbance at 405 nm with a microplate reader, according to the manufacturer’s protocol.
Statistical analysis
Disease-free survival (DFS) interval was defined as the period from the diagnosis to cancer recurrence or metastasis. Bivariate analyses of association between covariables and p62/SQSTM1 status included Chi-square and Fisher’s exact tests. Survival curves were estimated by the Kaplan-Meier method with a log-rank test to assess significance. The univariate Cox proportional hazard regression models were used to evaluate any independent prognostic effect of the variables with a 95% confidence interval. DFS intervals were used as indicators for the relative hazards. To analyze assays for WST-8 and GSH/GSSG among shRNA cells, one-way factorial ANOVA accompanied by Scheffe’s significance test was used. A p-value < 0.05 was considered statistically significant. All the statistical analyses were performed with StatView Version 5.0 for Windows (SAS institute Inc, Cary, NC, USA).
Results
p62/SQSTM1 can be a prognostic factor for hypopharyngeal carcinomas
The relation between p62/SQSTM1 abundance and clinicopathological parameters is shown in Table 1. p62/SQSTM1 status correlates with nuclear Nrf2 positivity, but not significantly. The other parameters, including age, gender, TNM stage, T grade, N grade, therapeutic option, histological differentiation, Ki67 values and Nrf2 status were not significantly related to p62/SQSTM1 levels. In addition, Cox proportional hazard model (Table 2; hazard ratio = 2.762, P = 0.0392) and Kaplan-Meier survival curves (Figure 2; Chi-square value = 4.698, P = 0.0302) indicated that p62/SQSTM1 status was a potential prognostic marker in hypopharyngeal carcinoma.
Table 1.
Relationship between p62 and clinicopathological parameters in 54 hypopharyngeal carcinomas
| p62/SQSTM1 | ||||
|---|---|---|---|---|
|
|
||||
| Parameters | No. of cases | Negative | Positive | p-values |
| Age at diagnosis | ||||
| ≤ 65 years | 27 | 8 | 19 | 0.7613 |
| > 65 years | 27 | 7 | 20 | |
| Gender | ||||
| Male | 47 | 14 | 33 | 0.6586 |
| Female | 7 | 1 | 6 | |
| Stage: TNM class | ||||
| ≤ II | 10 | 4 | 6 | 0.4376 |
| ≥ III | 44 | 11 | 33 | |
| T classification | ||||
| ≤ T2 | 24 | 7 | 17 | 0.8385 |
| ≥ T3 | 30 | 8 | 22 | |
| Nodes status | ||||
| Negative | 18 | 4 | 14 | 0.5192 |
| Positive | 36 | 11 | 25 | |
| Treatment | ||||
| Surgery: S | 22 | 6 | 16 | 0.9452 |
| Radiation: R | 32 | 9 | 23 | |
| Differentiation | ||||
| Well and moderately | 27 | 7 | 20 | 0.7613 |
| Poorly | 27 | 8 | 19 | |
| Ki67 | ||||
| ≤ 40% | 30 | 8 | 22 | 0.8385 |
| > 40% | 24 | 7 | 17 | |
| Nrf2 | ||||
| Negative | 25 | 10 | 15 | 0.0626 |
| Positive | 29 | 5 | 24 | |
Chi-square and Fisher’s exact tests were used to evaluate the relationship between clinicopathological parameters and p62 expression.
Table 2.
Univariate disease-free-survival analysis of clinicopathological data of 54 hypopharyngeal carcinomas
| Variables | Hazard ratio (95% confidence interval) | p-value | |
|---|---|---|---|
| Age at diagnosis (≤ 65 vs > 65) | 0.751 | (0.364-1.548) | 0.4385 |
| Gender (female vs male) | 1.009 | (0.351-2.899) | 0.9870 |
| TNM stage (≤ II vs ≥ III) | 0.983 | (0.375-2.584) | 0.9735 |
| T classification (≤ T2 vs ≥ T3) | 0.921 | (0.449-1.894) | 0.8242 |
| Nodes (negative vs positive) | 1.126 | (0.514-2.463) | 0.7674 |
| Differentiation (well or moderately vs poorly) | 1.241 | (0.602-2.558) | 0.5598 |
| Treatment (R vs S) | 0.543 | (0.248-1.189) | 0.1269 |
| Ki67 (≤ 40% vs > 40%) | 0.572 | (0.267-1.225) | 0.1508 |
| Nrf2 (negative vs positive) | 1.580 | (0.758-3.300) | 0.2222 |
| p62/SQSTM1 (negative vs positive) | 2.762 | (1.052-7.246) | 0.0392* |
The Cox proportional hazards regression model was used to evaluate the effects of clinicopathological parameters on disease-free-survival (DFS) with a 95% confidence interval (95% CI). DFS intervals were used as indicators for the relative hazards.
P < 0.05, statistically significant.
Figure 2.
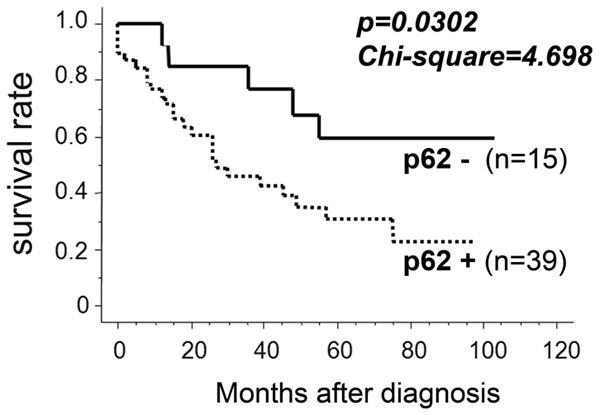
p62/SQSTM1 status can predict prognosis in hypopharyngeal carcinomas. Fifty-four cases of hypopharyngeal carcinomas were immunohistochemically evaluated for p62/SQSTM1 and statistically analyzed. Kaplan-Meier curve with log-rank tests was used to evaluate disease-free survival in relation to p62/SQSTM1 levels (Chi-Square value = 4.698, P = 0.0302).
Cell growth was significantly affected by p62/SQSTM1 expression under irradiation in hypopharyngeal carcinoma cells, but not under CDDP exposure
The growth of hypopharyngeal carcinoma cells treated with shRNA (sh-control, sh-p62 (1) and (2)) was evaluated with/without X-ray irradiation or CDDP exposure. Under X-ray irradiation, the growth of cells treated with p62/SQSTM1 shRNAs was significantly affected (sh-p62 (1) and (2); one-way factorial ANOVA accompanied by Scheffe’s significance test, P < 0.05; Figure 3A). On the other hand, CDDP exposure affected hypopharyngeal carcinoma cells regardless of the status of p62/SQSTM1. These data suggest that p62/SQSTM1 plays a more relevant role in survival after irradiation rather than after chemotherapeutic treatment.
Figure 3.
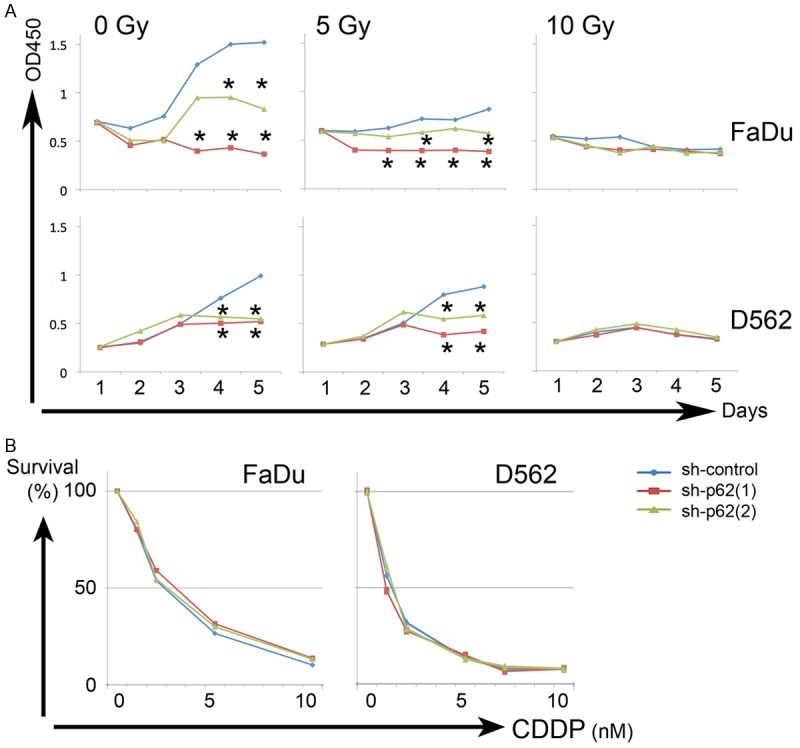
Cell growth was significantly affected by p62/SQSTM1 status under irradiation in hypopharyngeal carcinoma cells, but not under CDDP exposure. The growth of FaDu and D562 carcinoma cells treated with shRNA (sh-control, sh-p62 (1) and (2)) was evaluated with/without X-ray irradiation (A) or CDDP exposure (B). (A) Upper and lower panels indicate the data on FaDu and D562 cells; *, P < 0.05, to sh-control cells, one-way factorial ANOVA accompanied by Scheffe’s significance test. (B) Left and right graphs indicate the data on FaDu and D562 cells. Sh-control (blue), sh-p62 (1) (red) and (2) (green).
p62/SQSTM1 knockdown affected the Nrf2-Keap1 pathway under irradiation
To explore p62/SQSTM1 contribution to the Nrf2-Keap1 pathway, knockdown experiments were performed in FaDu cancer cells. p62/SQSTM1 was significantly repressed by the shRNAs (sh-p62 (1) and (2)). Keap1 was abundantly detected even after p62/SQSTM1 reduction in all the experimental conditions. Under X-ray irradiation, p62/SQSTM1 knockdown affected the pathway, especially Nrf2 and HO-1 expression, or moderately NQO1 (Figure 4; left half). However, CDDP exposure affected the pathway in a dose-dependent manner, regardless of the p62/SQSTM1 status (Figure 4; right half). These data suggest that p62/SQSTM1 could play an important role in the signaling from Nrf2-Keap1 to HO-1 and/or NQO1, under irradiation, but not under CDDP exposures.
Figure 4.
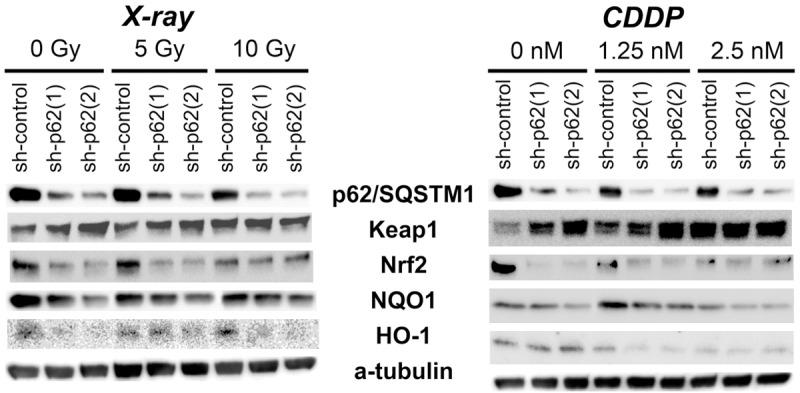
p62/SQSTM1 knockdown affected Nrf2-Keap1 pathway under X-ray irradiation. Under X-ray irradiation (left panels), the contents of Nrf2 and HO-1 were especially decreased in p62/SQSTM1 knockdown (sh-p62 (1) and (2)) cells, and those of NQO1 were moderately decreased. CDDP exposure (right panels) caused dose-dependently reduction of HO-1, Nrf2 and/or NQO1, regardless of the differences of p62/SQSTM1 status. Keap1 was abundantly detected in almost all the experimental conditions.
p62/SQSTM1 knockdown significantly affected ROS levels and GSH/GSSG ratios after irradiation
In order to analyze whether ROS levels were affected by p62/SQSTM1 status, ROS levels were quantified in the knockdown and control FaDu cancer cells. ROS levels were higher in p62/SQSTM1 knockdown cells than in the control ones, both in control and irradiated conditions. CDDP exposure promoted ROS accumulations in all the knockdowns and the controls (Figure 5A).
Figure 5.
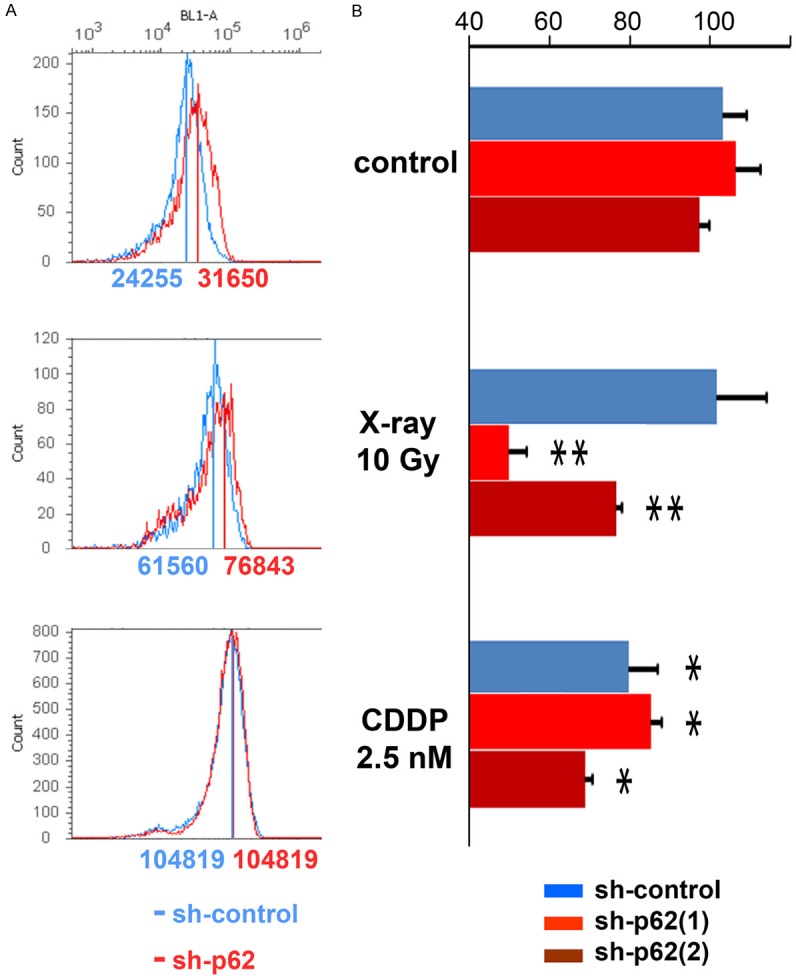
p62/SQSTM1 repression caused ROS accumulation and GSH/GSSG reduction especially under X-ray irradiation. A. ROS levels were higher in p62/SQSTM1 knockdown cells (red lines) than in the control ones (blue lines), under normal cultured conditions (control; upper: sh-p62 vs sh-control = 31650 vs 24255) and irradiation (10 Gy X-ray; middle: sh-p62 vs sh-control = 76843 vs 61560) conditions, whereas CDDP exposure (lower) promoted ROS accumulation in both the knockdown and control cells (sh-p62 vs sh-control = 104819 vs 104819). B. GSH/GSSG ratio wasn’t affected by p62/SQSTM1 knockdowns under control conditions in FaDu cells. The ratio was significantly reduced under X-ray irradiation in p62/SQSTM1 knockdown cells. **, P < 0.05, significant in one-way factorial ANOVA accompanied by Scheffe’s significance test, referring to sh-control cells in control and X-ray-irradiated conditions. CDDP exposure (2.5 nM) affected GSH/GSSG ratio in all the shRNA cells regardless of p62/SQSTM1 status; *, P < 0.05, significant, only referring to sh-control cells in control cultured condition.
To evaluate whether p62/SQSTM1 was essential to induce a GSH response after X-ray irradiation and CDDP exposure, GSH/GSSG ratio was analyzed in p62/SQSTM1 knockdown cells. In control conditions, GSH/GSSG ratio wasn’t affected by p62/SQSTM1 knockdowns in FaDu cancer cells. The ratio was significantly reduced after X-ray irradiation (10 Gy) in knockdown cells, and after CDDP exposure (2.5 nM) in all cells (Figure 5B, P < 0.05). These data indicate that p62/SQSTM1 is significantly involved in GSH/GSSG maintenance under irradiation, but that chemotherapeutic agents like CDDP are toxic for hypopharyngeal carcinoma cells regardless of the p62/SQSTM1 status.
p62/SQSTM1 expression can predict radioresistance in hypopharyngeal carcinoma cases
At this point, we re-evaluated whether p62/SQSTM1 excess was associated with poor prognosis after radiotherapy in our clinical cohort. We divided the 54 hypopharyngeal carcinoma cases into two groups according to the therapeutic options: one treated primarily with radiation (32 cases), and the other treated primarily with surgical resection (22). In the irradiated group, p62+ (with an excess of p62/SQSTM1) cases showed significantly worse prognosis than p62- cases (Figure 6A; Chi-square value = 6.750, P = 0.0094). On the other hand, there was no significant difference for the prognosis between p62+ and -cases in the surgically operated group (Figure 6B; Chi-square value = 0.251, P = 0.6164).
Figure 6.
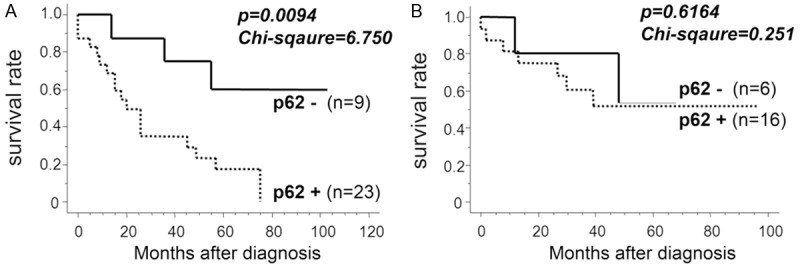
p62/SQSTM1 excess can predict radiotherapy resistance in hypopharyngeal carcinoma cases. A. In 32 cases of hypopharyngeal carcinomas treated primarily with radiation, excessive p62+ cases (high p62/SQSTM1 levels) showed significantly worse prognosis than p62- cases. B. There was no significant difference for the prognosis between p62+ and -cases treated primarily with surgical procedure. The association between p62/SQSTM1 excess and clinical DFS was evaluated statistically using the Kaplan-Meier curve together with log-rank test.
In addition, the relative hazards for the carcinoma-related adverse events were individually evaluated by Cox proportional hazards analysis in the irradiation group. High levels of p62/SQSTM1 showed significantly higher hazard ratio for DFS in the 32 hypopharyngeal carcinoma cases treated with irradiation (Table 3; hazard ratio = 4.405, P = 0.0186), compared with the total carcinoma cases (Table 2; hazard ratio = 2.762, P = 0.0392).
Table 3.
Univariate disease-free-survival analysis of clinicopathological data of 32 hypopharyngeal carcinomas treated with radiation
| Variables | Hazard ratio (95% confidence interval) | p-value | |
|---|---|---|---|
| Age at diagnosis (≤ 65 vs > 65) | 1.171 | (0.484-2.833) | 0.7256 |
| Gender (female vs male) | 1.202 | (0.160-9.009) | 0.8580 |
| TNM stage (≤ II vs ≥ III) | 1.247 | (0.452-3.436) | 0.6695 |
| T classification (≤ T2 vs ≥ T3) | 1.634 | (0.644-4.132) | 0.3013 |
| Nodes (negative vs positive) | 0.958 | (0.389-2.358) | 0.9257 |
| Differentiation (well or moderately vs poorly) | 0.978 | (0.405-2.364) | 0.9622 |
| Ki67 (≤ 40% vs > 40%) | 0.725 | (0.277-1.894) | 0.5117 |
| Nrf2 (negative vs positive) | 2.101 | (0.850-5.181) | 0.1079 |
| p62/SQSTM1 (negative vs positive) | 4.405 | (1.280-15.152) | 0.0186* |
The Cox proportional hazards regression model was used to evaluate the effects of clinicopathological parameters on disease-free-survival (DFS) with a 95% confidence interval (95% CI). DFS intervals were used as indicators for the relative hazards.
P < 0.05, statistically significant.
Discussion
The present study demonstrates that high levels of p62/SQSTM1 are significantly associated with clinical poor prognosis and predict radiotherapy resistance in hypopharyngeal carcinoma. p62/SQSTM1 is a multifunctional protein involved in signal transduction, protein degradation and cell transformation [9]. The tissue expression of p62/SQSTM1 and the appearance of serum autoantibodies against p62/SQSTM1 might be related to metastasis [10]. p62/SQSTM1 increase was reported in malignancies of the digestive tract [10] and prostate [11]; moreover, it contributes to the carcinogenesis of oral squamous cells [5], and potentially predicts poor prognosis of oral [5,12], ovarian [13], endometrial cancers [14] and gliomas [15].
In our study, in hypopharyngeal carcinoma cells, p62/SQSTM1 status affected the Nrf2-Keap1 pathway, ROS levels, GSH/GSSG ratio and cell growth under X-ray irradiation but not under CDDP exposure, which was toxic regardless of p62/SQSTM1 status. It has been reported that p62/SQSTM1 accumulates and activates Nrf2 by competing with Nrf2 for binding to Keap1 [7]. Then, the excess of Nrf2 translocates to the nucleus and upregulates antioxidant response element-dependent cytoprotective genes [7,16,17]. Similarly to these reports, under irradiated conditions, p62/SQSTM1 affected Nrf2 expression and its downstream activities in hypopharyngeal carcinomas, whereas CDDP was toxic regardless of p62/SQSTM1 status. Changes in the GSH/GSSG ratio can broadly affect signaling pathways participating in physiological responses associated with cell proliferation, autophagy and apoptosis [18]. Inclusion formation of p62/SQSTM1 also reflects a defect of autophagic lysosomal pathways [19,20], although ionizing radiation induces excessive autophagy, resulting in cytotoxicity in some types of cancer cells [21]. Together with these previous reports, our experimental data indicate that p62/SQSTM1 abundance influences GSH/GSSG maintenance and ROS reduction under X-ray irradiation, and p62/SQSTM1 excess could cause radiotherapy resistance and worse prognosis in hypopharyngeal carcinomas.
CDDP can cause cytotoxicity through various biological events, by inducing DNA damage and resulting in necrosis and/or apoptosis [22-24]. It was shown that CDDP treatment led to apoptosis in the presence of a functional wild-type p53, but that mitotic catastrophe occurred in the absence of functional p53 [25]. Although further study may be needed, CDDP results in Nrf2-Keap1 signaling inhibition, ROS accumulation, GSH/GSSG decrease and finally cell growth reduction of hypopharyngeal carcinoma cells, regardless of p62/SQSTM1 status.
Hypopharyngeal carcinoma is one of the worst prognostic malignancies, and the salvage rate is less than 50%, even though various therapeutic efforts [2]. Our clinical cohort of hypopharyngeal carcinomas clearly indicated the significant relevance of p62/SQSTM1 status in predicting prognosis after radiotherapy. Therefore, it is beneficial to predict the radio- and/or chemotherapy resistance or disease prognosis of each hypopharyngeal carcinoma case, before starting various treatments.
In conclusion, according to our findings, hypopharyngeal carcinoma should be treated primarily with surgical and/or chemotherapy when p62/SQSTM1 is highly expressed in the tumor; In contrast, radiotherapy should be recommended when p62/SQSTM1 levels are low.
Acknowledgements
The authors are grateful to Hiroko Kita for experimental assistance. This study was partly supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research, n. 25293130 to T.C.).
Disclosure of conflict of interest
None.
Authors’ contribution
T.C. designed the research. A.A. and T.C. wrote the paper. A.A., T.C. and Y.H. did the biological experiments and the data analyses. K.I. and T.S. prepared the clinical cancer samples; and A.A., T.C., Y.O., H.T. performed the analyses of the clinical cohort.
References
- 1.Gourin CG, Johnson JT. A contemporary review of indications for primary surgical care of patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2009;119:2124–2134. doi: 10.1002/lary.20619. [DOI] [PubMed] [Google Scholar]
- 2.Kuo P, Sosa JA, Burtness BA, Husain ZA, Mehra S, Roman SA, Yarbrough WG, Judson BL. Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: Analysis of the national cancer data base. Cancer. 2016;122:1853–1860. doi: 10.1002/cncr.29962. [DOI] [PubMed] [Google Scholar]
- 3.Vandenbrouck C, Eschwege F, De la Rochefordiere A, Sicot H, Mamelle G, Le Ridant AM, Bosq J, Domenge C. Squamous cell carcinoma of the pyriform sinus: retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10:4–13. doi: 10.1002/hed.2890100103. [DOI] [PubMed] [Google Scholar]
- 4.Shiotani A, Tomifuji M, Araki K, Yamashita T. Transoral videolaryngoscopic surgery for en bloc resection of supraglottic and hypopharyngeal cancers. Otolaryngol Head Neck Surg. 2011;144:288–289. doi: 10.1177/0194599810392148. [DOI] [PubMed] [Google Scholar]
- 5.Inui T, Chano T, Takikita-Suzuki M, Nishikawa M, Yamamoto G, Okabe H. Association of p62/SQSTM1 excess and oral carcinogenesis. PLoS One. 2013;8:e74398. doi: 10.1371/journal.pone.0074398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson S, Ryan KM. Autophagy: an adaptable modifier of tumourigenesis. Curr Opin Genet Dev. 2010;20:57–64. doi: 10.1016/j.gde.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 8.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pursiheimo JP, Rantanen K, Heikkinen PT, Johansen T, Jaakkola PM. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28:334–344. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Qian H, Zhang J, Wang S, Shi P, Peng X. The diversity expression of p62 in digestive system cancers. Clin Immunol. 2005;116:118–123. doi: 10.1016/j.clim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura H, Torigoe T, Asanuma H, Hisasue SI, Suzuki K, Tsukamoto T, Satoh M, Sato N. Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Histopathology. 2006;48:157–161. doi: 10.1111/j.1365-2559.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu JL, Chen FF, Lung J, Lo CH, Lee FH, Lu YC, Hung CH. Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br J Cancer. 2014;111:944–954. doi: 10.1038/bjc.2014.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwadate R, Inoue J, Tsuda H, Takano M, Furuya K, Hirasawa A, Aoki D, Inazawa J. High expression of SQSTM1/p62 protein is associated with poor prognosis in epithelial ovarian cancer. Acta Histochem Cytochem. 2014;47:295–301. doi: 10.1267/ahc.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwadate R, Inoue J, Tsuda H, Takano M, Furuya K, Hirasawa A, Aoki D, Inazawa J. High expression of p62 protein is associated with poor prognosis and aggressive phenotypes in endometrial cancer. Am J Pathol. 2015;185:2523–2533. doi: 10.1016/j.ajpath.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Xu H, Zhang B, Hong B, Yan W, Zhang J. Impact of nuclear factor erythroid-derived 2-like 2 and p62/sequestosome expression on prognosis of patients with gliomas. Hum Pathol. 2015;46:843–849. doi: 10.1016/j.humpath.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 16.McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky L, McBride WH. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–6105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 22.Topping RP, Wilkinson JC, Scarpinato KD. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. J Biol Chem. 2009;284:14029–14039. doi: 10.1074/jbc.M809303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu DD, Wang CT, Shi HS, Li ZY, Pan L, Yuan QZ, Leng F, Wen Y, Chen X, Wei YQ. Enhancement of cisplatin sensitivity in Lewis Lung carcinoma by liposome-mediated delivery of a survivin mutant. J Exp Clin Cancer Res. 2010;29:46. doi: 10.1186/1756-9966-29-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo-Avila W, Piulats JM, Garcia Del Muro X, Vidal A, Condom E, Casanovas O, Mora J, Germa JR, Capella G, Villanueva A, Vinals F. Sunitinib inhibits tumor growth and synergizes with cisplatin in orthotopic models of cisplatinsensitive and cisplatin-resistant human testicular germ cell tumors. Clin Cancer Res. 2009;15:3384–3395. doi: 10.1158/1078-0432.CCR-08-2170. [DOI] [PubMed] [Google Scholar]
- 25.Vakifahmetoglu H, Olsson M, Tamm C, Heidari N, Orrenius S, Zhivotovsky B. DNA damage induces two distinct modes of cell death in ovarian carcinomas. Cell Death Differ. 2008;15:555–566. doi: 10.1038/sj.cdd.4402286. [DOI] [PubMed] [Google Scholar]


